Treating Intracranial Abscesses in Rats with Stereotactic Injection of Biodegradable Vancomycin-Embedded Microparticles
Abstract
1. Introduction
2. Material and Methods
2.1. Fabrication of VMPs
2.2. Fourier-Transform Infrared Spectroscopy
2.3. Characterization of Electrosprayed Particles
2.4. In Vitro Release
2.5. Surgical Procedure
2.6. In Vivo Antibiotic Pharmacokinetics
2.7. Bacterial Preparation
2.8. Brain Abscess Model Creation and Treatment
2.9. Statistical Analysis
3. Results
3.1. Morphology of the Microparticles
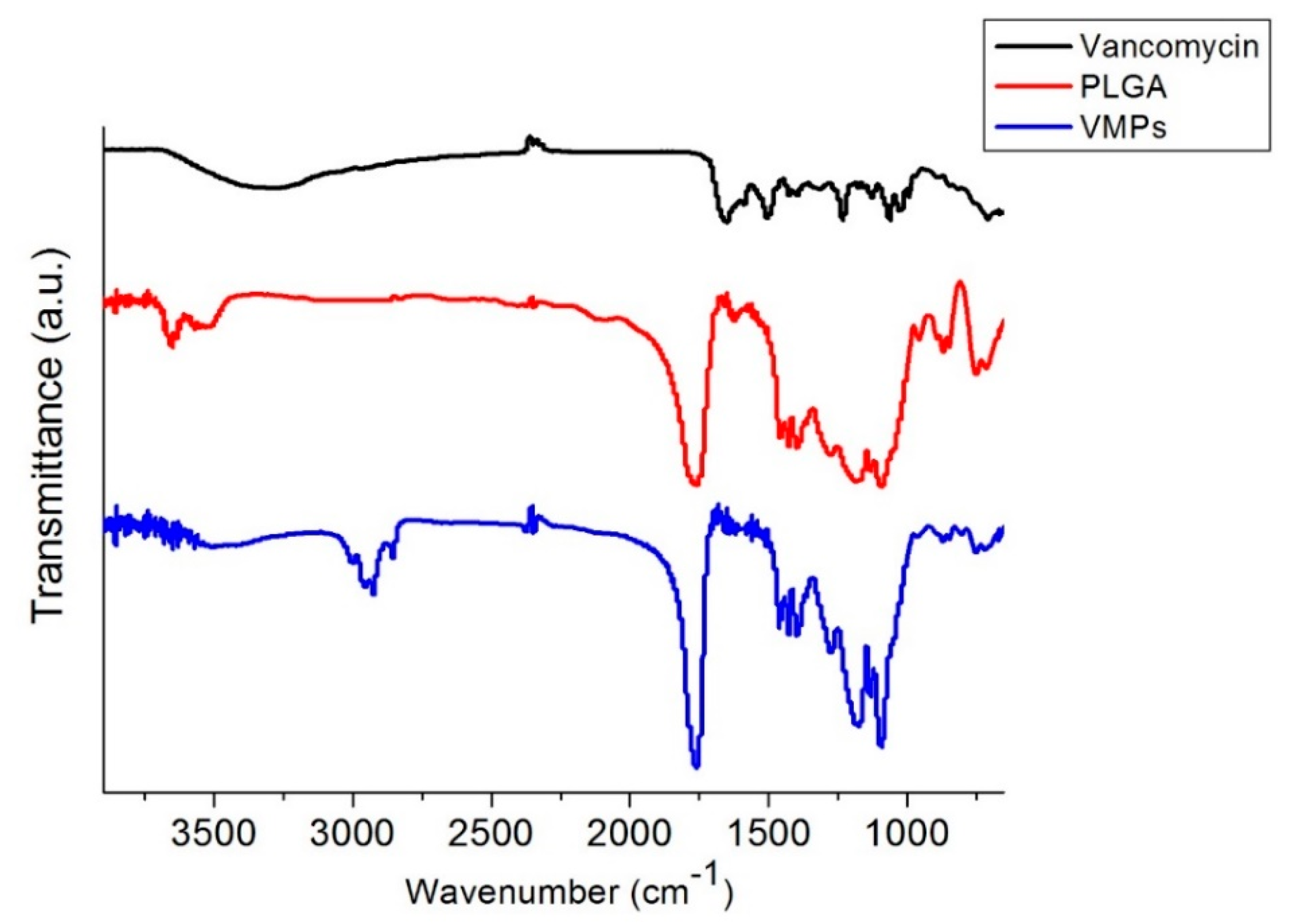
3.2. FTIR Spectroscopy
3.3. In Vivo Elution Behavior of Vancomycin From VMPs
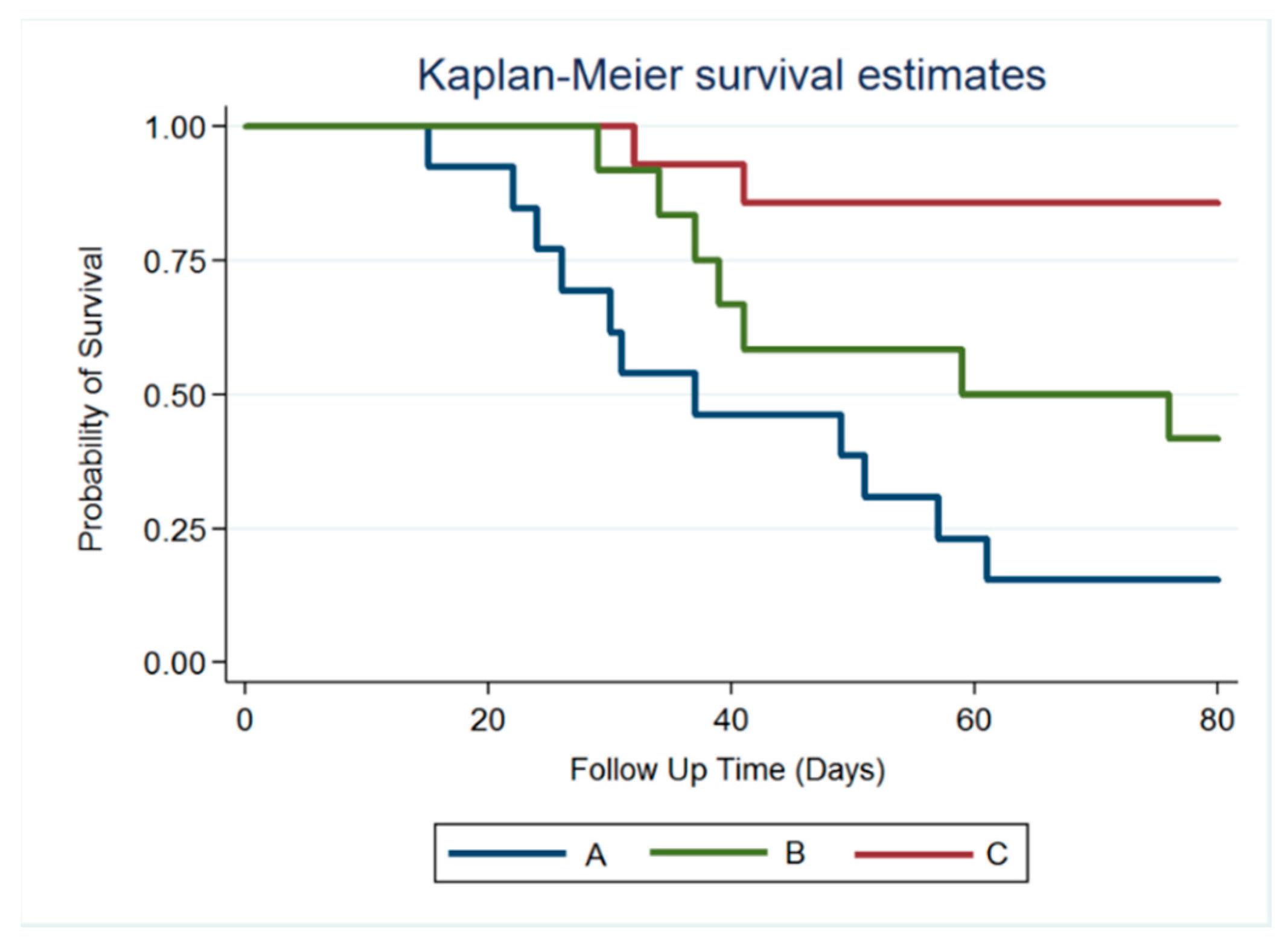
3.4. Survival Rate
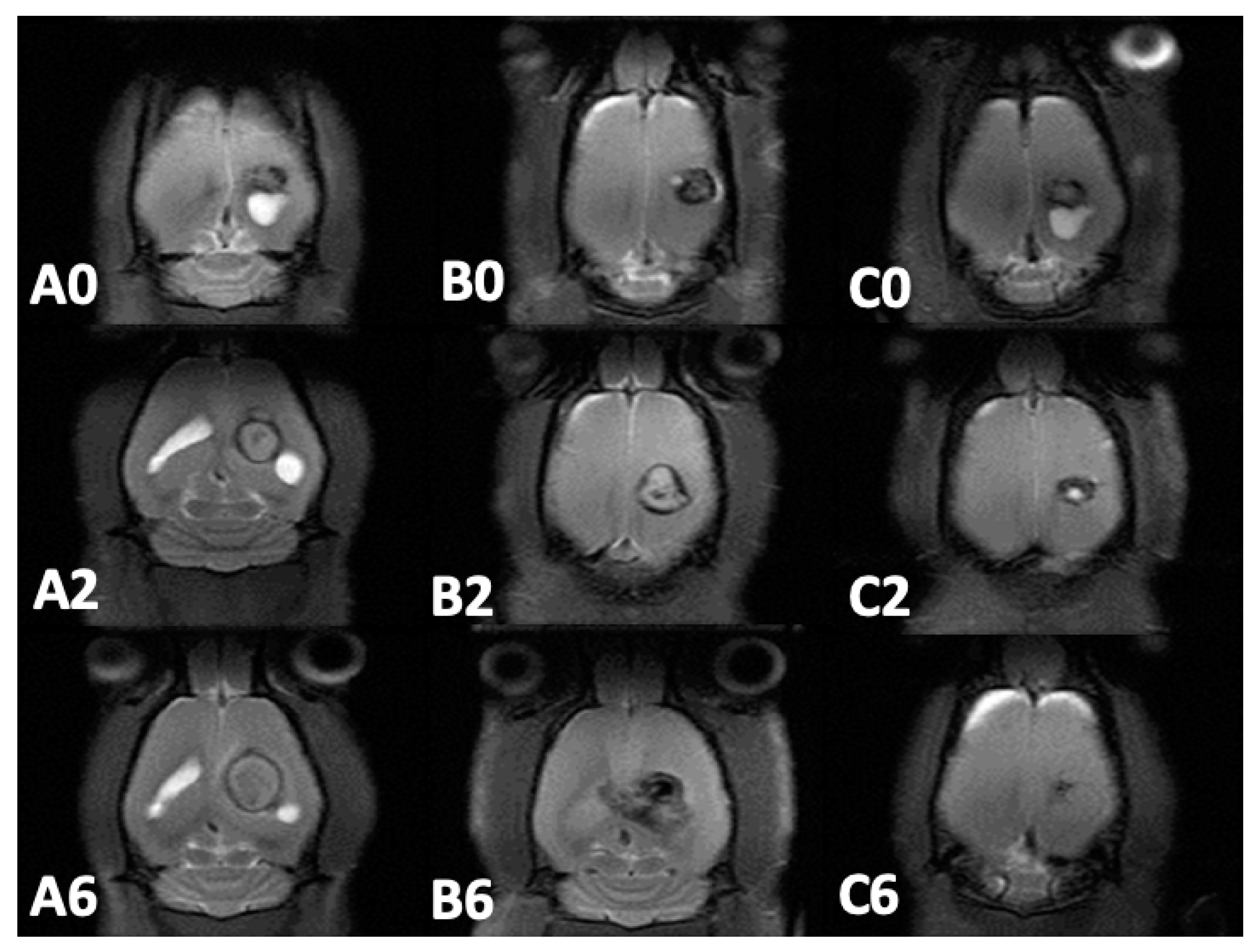
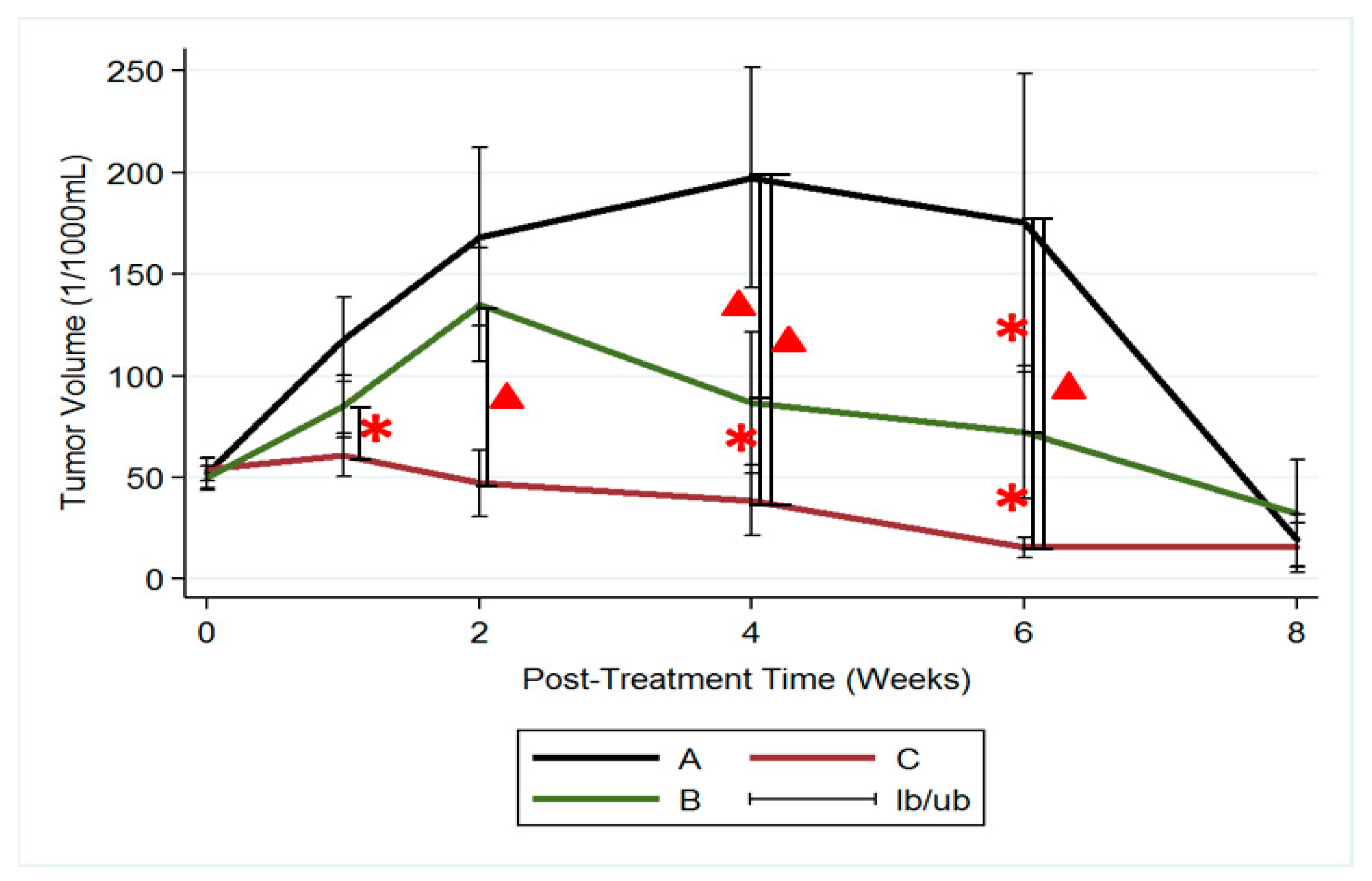
3.5. MRI and Tumor Volume
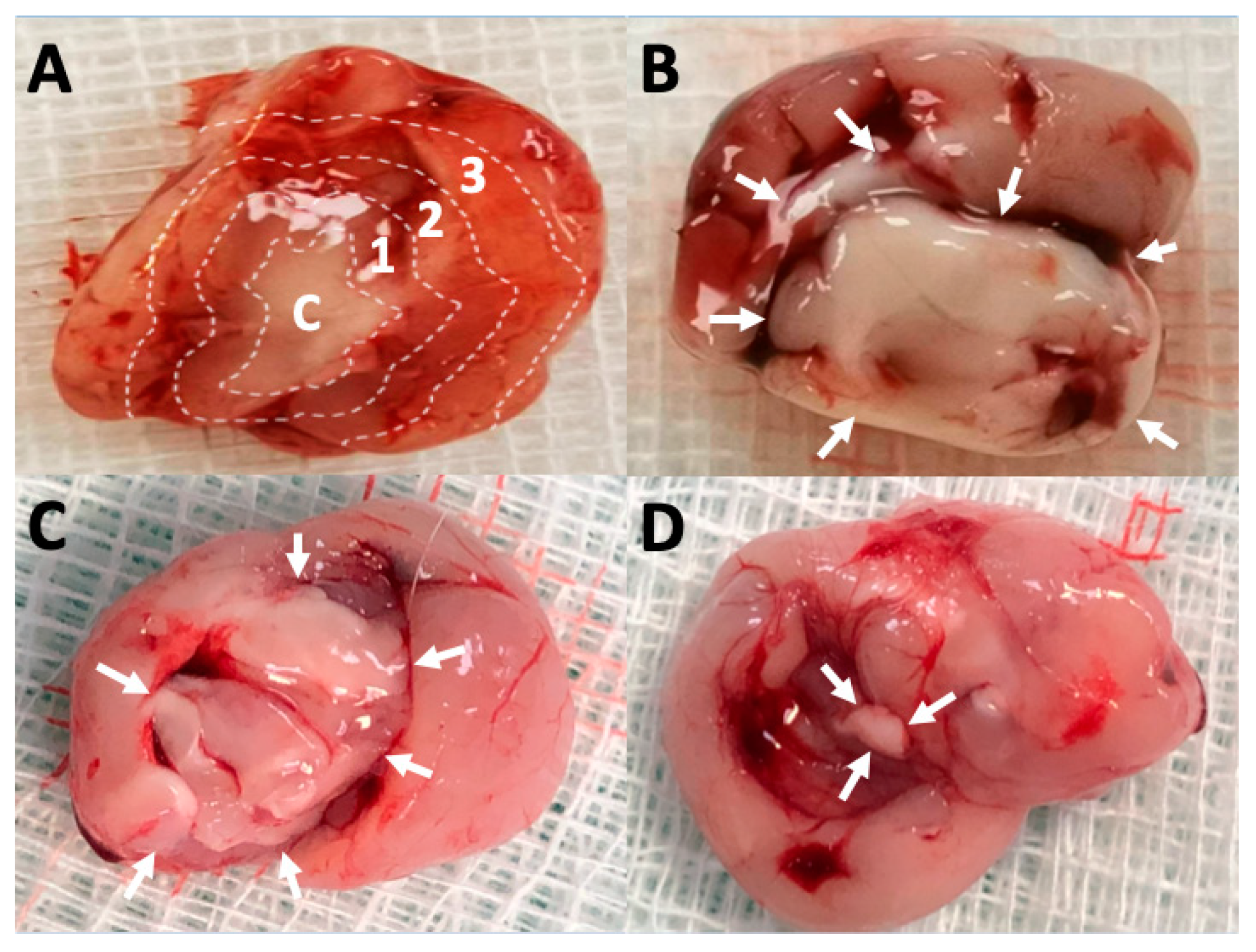
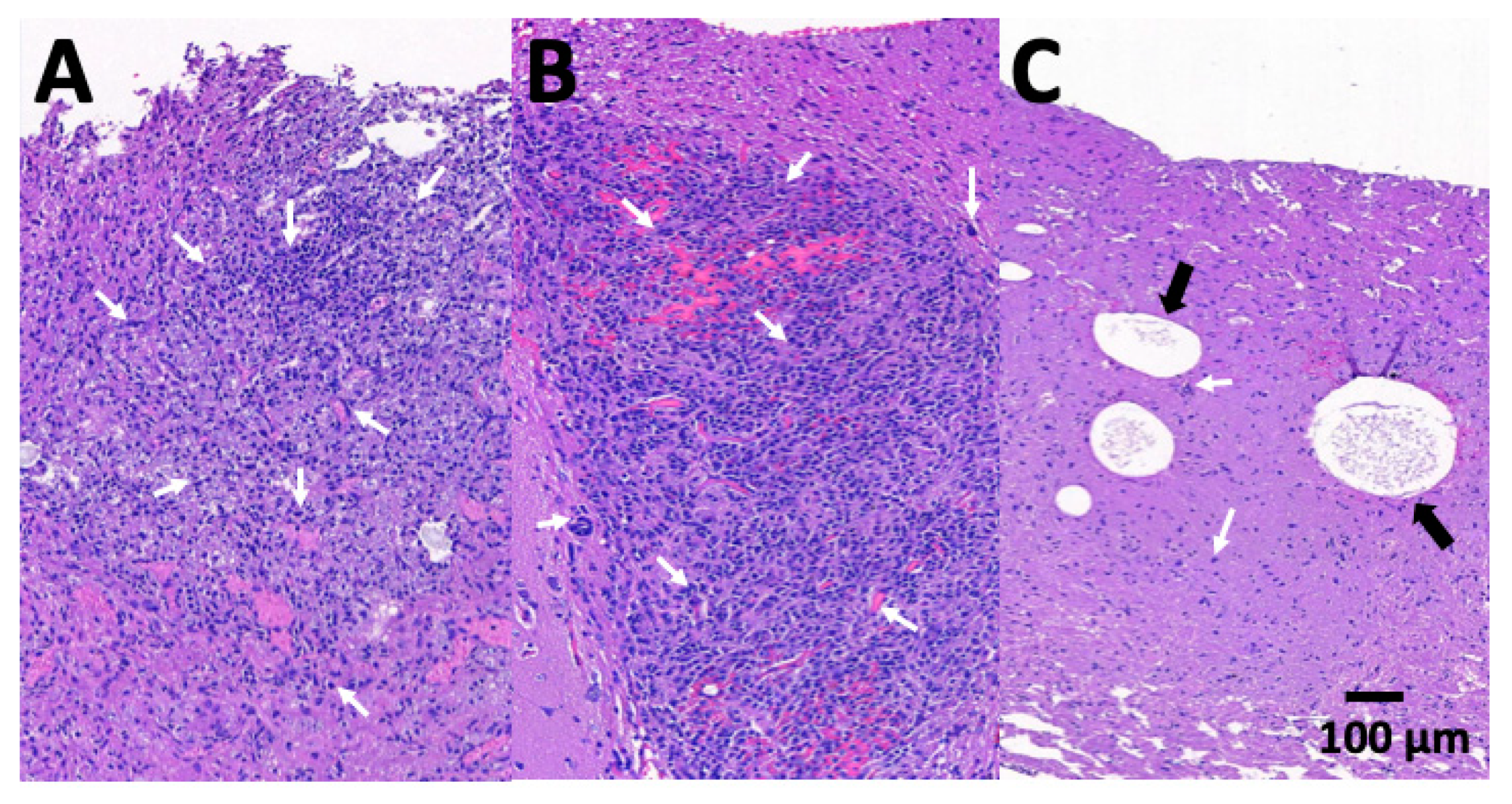
3.6. Pathologic Findings
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mampalam, T.J.; Rosenblum, M.L. Trends in the management of bacterial brain abscesses: A review of 102 cases over 17 years. Neurosurgery 1988, 23, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Brouwer, M.C.; Tunkel, A.R.; McKhann, G.M., 2nd; van de Beek, D. Brain abscess. N. Engl. J. Med. 2014, 371, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Hu, L.; Wu, X.; Hu, G.; Ding, X.; Lu, Y. A retrospective study on the aetiology, management, and outcome of brain abscess in an 11-year, single-centre study from China. BMC Infect. Dis. 2014, 14, 311. [Google Scholar] [CrossRef] [PubMed]
- Moorthy, R.K.; Rajshekhar, V. Management of brain abscess: An overview. Neurosurg. Focus 2008, 24, E3. [Google Scholar] [CrossRef] [PubMed]
- Honda, H.; Warren, D.K. Central nervous system infections: Meningitis and brain abscess. Infect. Dis. Clin. N. Am. 2009, 23, 609–623. [Google Scholar] [CrossRef]
- Brouwer, M.C.; Coutinho, J.M.; van de Beek, D. Clinical characteristics and outcome of brain abscess: Systematic review and meta-analysis. Neurology 2014, 82, 806–813. [Google Scholar] [CrossRef]
- Cavusoglu, H.; Kaya, R.A.; Turkmenoglu, O.N.; Colak, I.; Aydin, Y. Brain abscess: Analysis of results in a series of 51 patients with a combined surgical and medical approach during an 11-year period. Neurosurg. Focus 2008, 24, E9. [Google Scholar] [CrossRef]
- Alderson, D.; Strong, A.J.; Ingham, H.R.; Selkon, J.B. Fifteen-year review of the mortality of brain abscess. Neurosurgery 1981, 8, 1–6. [Google Scholar] [CrossRef]
- Chacko, A.G.; Chandy, M.J. Diagnostic and staged stereotactic aspiration of multiple bihemispheric pyogenic brain abscesses. Surg. Neurol. 1997, 48, 278–282. [Google Scholar] [CrossRef]
- Boviatsis, E.J.; Kouyialis, A.T.; Stranjalis, G.; Korfias, S.; Sakas, D.E. CT-guided stereotactic aspiration of brain abscesses. Neurosurg. Rev. 2003, 26, 206–209. [Google Scholar] [CrossRef]
- Rosenblum, M.L.; Hoff, J.T.; Norman, D.; Edwards, M.S.; Berg, B.O. Nonoperative treatment of brain abscesses in selected high-risk patients. J. Neurosurg. 1980, 52, 217–225. [Google Scholar] [CrossRef] [PubMed]
- De Louvois, J.; Gortavai, P.; Hurley, R. Bacteriology of abscesses of the central nervous system: A multicentre prospective study. Br. Med. J. 1977, 2, 981–984. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.T.; Zhao, Y.Z.; Wong, H.L.; Cai, J.; Peng, L.; Tian, X.Q. Current approaches to enhance CNS delivery of drugs across the brain barriers. Int. J. Nanomed. 2014, 9, 2241–2257. [Google Scholar] [CrossRef] [PubMed]
- Tseng, Y.Y.; Wang, Y.C.; Su, C.H.; Liu, S.J. Biodegradable vancomycin-eluting poly[(d,l)-lactide-co-glycolide] nanofibres for the treatment of postoperative central nervous system infection. Sci. Rep. 2015, 5, 7849. [Google Scholar] [CrossRef] [PubMed]
- Tseng, Y.Y.; Liao, J.Y.; Chen, W.A.; Kao, Y.C.; Liu, S.J. Sustainable release of carmustine from biodegradable poly[(d,l)-lactide-co-glycolide] nanofibrous membranes in the cerebral cavity: In vitro and in vivo studies. Expert Opin. Drug Deliv. 2013, 10, 879–888. [Google Scholar] [CrossRef]
- McClelland, S., 3rd; Hall, W.A. Postoperative central nervous system infection: Incidence and associated factors in 2111 neurosurgical procedures. Clin. Infect. Dis. 2007, 45, 55–59. [Google Scholar] [CrossRef]
- Kaur, A.; Preet, S.; Kumar, V.; Kumar, R.; Kumar, R. Synergetic effect of vancomycin loaded silver nanoparticles for enhanced antibacterial activity. Colloids Surf. B Biointerfaces 2019, 176, 62–69. [Google Scholar] [CrossRef]
- Levy, R.M. Brain abscess and subdural empyema. Curr. Opin. Neurol. 1994, 7, 223–228. [Google Scholar] [CrossRef]
- Lu, C.H.; Chang, W.N.; Lui, C.C. Strategies for the management of bacterial brain abscess. J. Clin. Neurosci. 2006, 13, 979–985. [Google Scholar] [CrossRef]
- Mathisen, G.E.; Johnson, J.P. Brain abscess. Clin. Infect. Dis. 1997, 25, 763–779. [Google Scholar] [CrossRef]
- Yang, S.Y. Brain abscess: A review of 400 cases. J. Neurosurg. 1981, 55, 794–799. [Google Scholar] [CrossRef] [PubMed]
- Skrap, M.; Melatini, A.; Vassallo, A.; Sidoti, C. Stereotactic aspiration and drainage of brain abscesses. Experience with 9 cases. Minim. Invasive Neurosurg. 1996, 39, 108–112. [Google Scholar] [CrossRef] [PubMed]
- Stapleton, S.R.; Bell, B.A.; Uttley, D. Stereotactic aspiration of brain abscesses: Is this the treatment of choice? Acta Neurochir. (Wien) 1993, 121, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, H.; Iwai, Y.; Yamanaka, K.; Kishi, H. Successful treatment of brainstem abscess with stereotactic aspiration. Surg. Neurol. 1999, 52, 445–448. [Google Scholar] [CrossRef]
- Barlas, O.; Sencer, A.; Erkan, K.; Eraksoy, H.; Sencer, S.; Bayindir, C. Stereotactic surgery in the management of brain abscess. Surg. Neurol. 1999, 52, 404–410. [Google Scholar] [CrossRef]
- Kutlay, M.; Colak, A.; Yildiz, S.; Demircan, N.; Akin, O.N. Stereotactic aspiration and antibiotic treatment combined with hyperbaric oxygen therapy in the management of bacterial brain abscesses. Neurosurgery 2005, 57, 1140–1146. [Google Scholar] [CrossRef]
- Ng, P.Y.; Seow, W.T.; Ong, P.L. Brain abscesses: Review of 30 cases treated with surgery. Aust. N. Z. J. Surg. 1995, 65, 664–666. [Google Scholar] [CrossRef]
- Rosenblum, M.L.; Hoff, J.T.; Norman, D.; Weinstein, P.R.; Pitts, L. Decreased mortality from brain abscesses since advent of computerized tomography. J. Neurosurg. 1978, 49, 658–668. [Google Scholar] [CrossRef]
- Rybak, M.J.; Lomaestro, B.M.; Rotschafer, J.C.; Moellering, R.C.; Craig, W.A.; Billeter, M.; Dalovisio, J.R.; Levine, D.P. Vancomycin therapeutic guidelines: A summary of consensus recommendations from the infectious diseases Society of America, the American Society of Health-System Pharmacists, and the Society of Infectious Diseases Pharmacists. Clin. Infect. Dis. 2009, 49, 325–327. [Google Scholar] [CrossRef]
- Rybak, M.J.; Lomaestro, B.M.; Rotschafer, J.C.; Moellering, R.C., Jr.; Craig, W.A.; Billeter, M.; Dalovisio, J.R.; Levine, D.P. Therapeutic monitoring of vancomycin in adults summary of consensus recommendations from the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Pharmacotherapy 2009, 29, 1275–1279. [Google Scholar] [CrossRef]
- Albanese, J.; Leone, M.; Bruguerolle, B.; Ayem, M.L.; Lacarelle, B.; Martin, C. Cerebrospinal fluid penetration and pharmacokinetics of vancomycin administered by continuous infusion to mechanically ventilated patients in an intensive care unit. Antimicrob. Agents Chemother. 2000, 44, 1356–1358. [Google Scholar] [CrossRef] [PubMed]
- Begg, E.J.; Barclay, M.L.; Kirkpatrick, C.J. The therapeutic monitoring of antimicrobial agents. Br. J. Clin. Pharmacol. 1999, 47, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Rybak, M.J. The pharmacokinetic and pharmacodynamic properties of vancomycin. Clin. Infect. Dis. 2006, 42 (Suppl. 1), S35–S39. [Google Scholar] [CrossRef] [PubMed]
- Moise-Broder, P.A.; Forrest, A.; Birmingham, M.C.; Schentag, J.J. Pharmacodynamics of vancomycin and other antimicrobials in patients with Staphylococcus aureus lower respiratory tract infections. Clin. Pharmacokinet. 2004, 43, 925–942. [Google Scholar] [CrossRef] [PubMed]
- Moellering, R.C., Jr. Vancomycin: A 50-year reassessment. Clin. Infect. Dis. 2006, 42 (Suppl. 1), S3–S4. [Google Scholar] [CrossRef]
- Liu, S.J.; Wen-Neng Ueng, S.; Lin, S.S.; Chan, E.C. In vivo release of vancomycin from biodegradable beads. J. Biomed. Mater. Res. 2002, 63, 807–813. [Google Scholar] [CrossRef]
- Le Ray, A.M.; Chiffoleau, S.; Iooss, P.; Grimandi, G.; Gouyette, A.; Daculsi, G.; Merle, C. Vancomycin encapsulation in biodegradable poly(epsilon-caprolactone) microparticles for bone implantation. Influence of the formulation process on size, drug loading, in vitro release and cytocompatibility. Biomaterials 2003, 24, 443–449. [Google Scholar] [CrossRef]
- Pandita, D.; Kumar, S.; Lather, V. Hybrid poly(lactic-co-glycolic acid) nanoparticles: Design and delivery prospectives. Drug Discov. Today 2015, 20, 95–104. [Google Scholar] [CrossRef]
- Tseng, Y.Y.; Huang, Y.C.; Yang, T.C.; Yang, S.T.; Liu, S.C.; Chang, T.M.; Kau, Y.C.; Liu, S.J. Concurrent Chemotherapy of Malignant Glioma in Rats by Using Multidrug-Loaded Biodegradable Nanofibrous Membranes. Sci. Rep. 2016, 6, 30630. [Google Scholar] [CrossRef]
- Tseng, Y.Y.; Kao, Y.C.; Liao, J.Y.; Chen, W.A.; Liu, S.J. Biodegradable drug-eluting poly[lactic-co-glycol acid] nanofibers for the sustainable delivery of vancomycin to brain tissue: In vitro and in vivo studies. ACS Chem. Neurosci. 2013, 4, 1314–1321. [Google Scholar] [CrossRef]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tseng, Y.-Y.; Kao, C.-W.; Liu, K.-S.; Tang, Y.-L.; Liu, Y.-W.; Liu, S.-J. Treating Intracranial Abscesses in Rats with Stereotactic Injection of Biodegradable Vancomycin-Embedded Microparticles. Pharmaceutics 2020, 12, 91. https://doi.org/10.3390/pharmaceutics12020091
Tseng Y-Y, Kao C-W, Liu K-S, Tang Y-L, Liu Y-W, Liu S-J. Treating Intracranial Abscesses in Rats with Stereotactic Injection of Biodegradable Vancomycin-Embedded Microparticles. Pharmaceutics. 2020; 12(2):91. https://doi.org/10.3390/pharmaceutics12020091
Chicago/Turabian StyleTseng, Yuan-Yun, Ching-Wei Kao, Kuo-Sheng Liu, Ya-Ling Tang, Yen-Wei Liu, and Shih-Jung Liu. 2020. "Treating Intracranial Abscesses in Rats with Stereotactic Injection of Biodegradable Vancomycin-Embedded Microparticles" Pharmaceutics 12, no. 2: 91. https://doi.org/10.3390/pharmaceutics12020091
APA StyleTseng, Y.-Y., Kao, C.-W., Liu, K.-S., Tang, Y.-L., Liu, Y.-W., & Liu, S.-J. (2020). Treating Intracranial Abscesses in Rats with Stereotactic Injection of Biodegradable Vancomycin-Embedded Microparticles. Pharmaceutics, 12(2), 91. https://doi.org/10.3390/pharmaceutics12020091






