A Validated IVRT Method to Assess Topical Creams Containing Metronidazole Using a Novel Approach
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Chemicals
2.1.2. Formulations
2.1.3. Equipment
2.2. Methods
2.2.1. UPLC Method Validation
2.2.2. Apparatus Qualification
2.2.3. Performance Verification Test (PVT)
2.2.4. MTZ Solubility and Receptor-Fluid Selection
2.2.5. Membrane Screening
2.2.6. Sampling Duration
2.2.7. IVRT Method
2.2.8. Calculation of Release Rates
- Qn = amount released at time (n) per unit area in µg/cm2;
- Cn = concentration of drug in receptor medium at different sampling times (n) in µg/cm3;
- Vs = volume of the sample in cm3;
- Vc = volume of the cell in cm3;
- Ac = area of the orifice of the cell in cm2.
- Q = the amount absorbed at time t per unit area of exposure µg/cm2;
- A = the concentration of drug expressed in µg/cm3;
- Cs = the solubility of the drug in µg/cm3;
- D = the diffusion constant of the drug molecule.
2.2.9. Validation of the IVRT Method
2.2.10. Comparative IVRT of MTZ Cream Products
3. Results
3.1. Validation of the UPLC Method and Qualification of the IVRT System
3.2. Performance Verification Test
3.3. Receptor-Fluid Selection
3.4. Membrane Screening
3.5. Sampling Duration
3.6. Validation of the IVRT Method
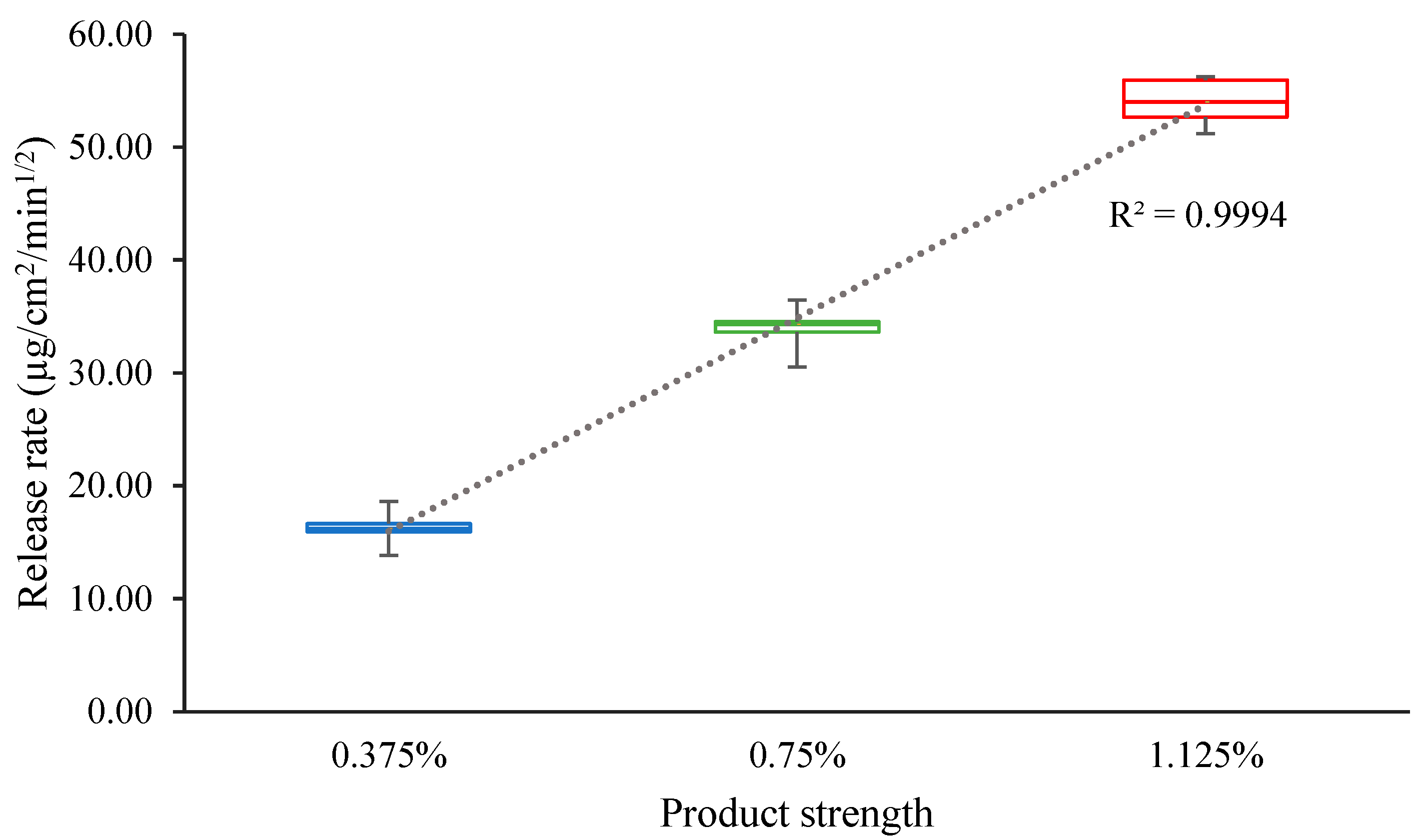
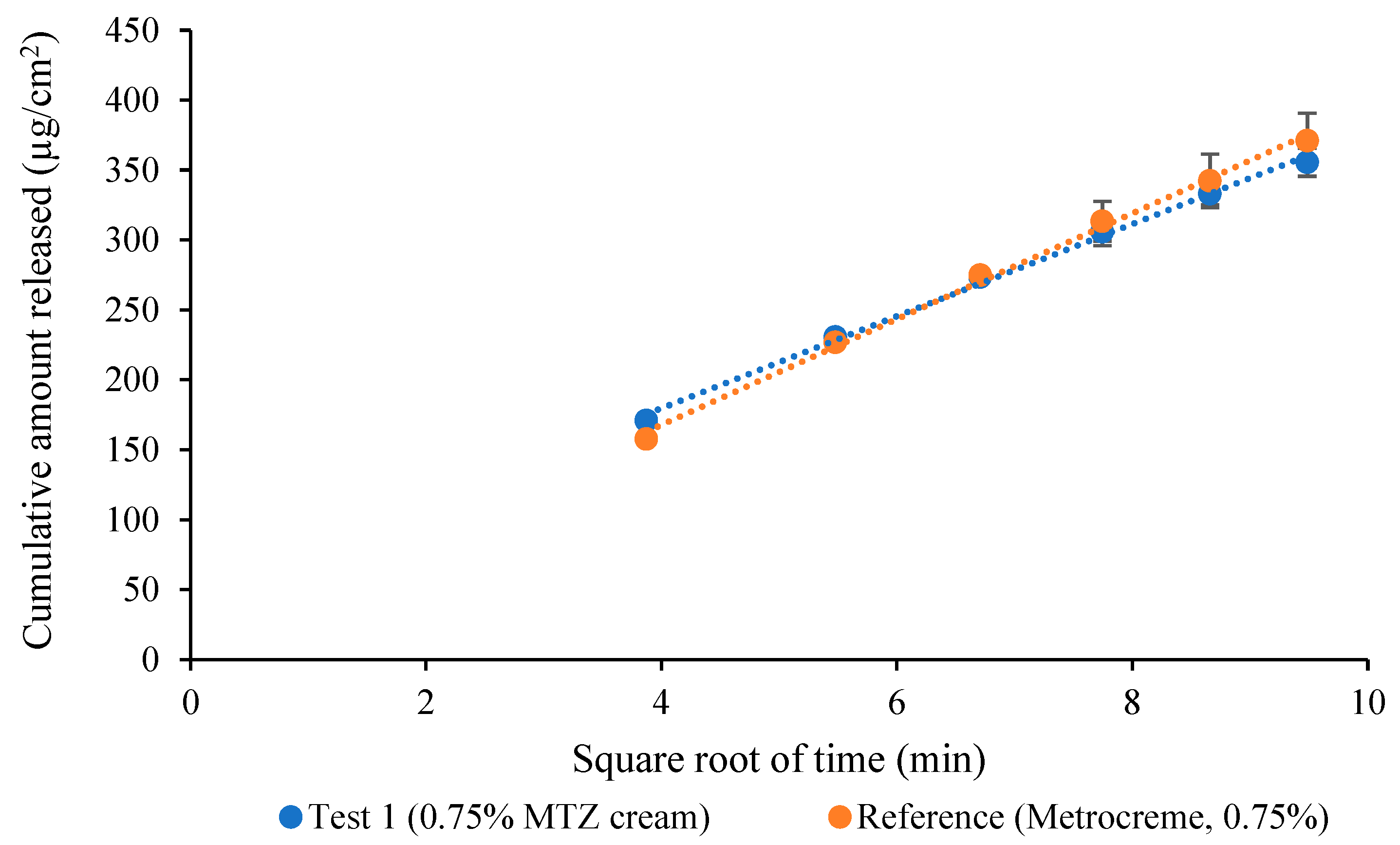
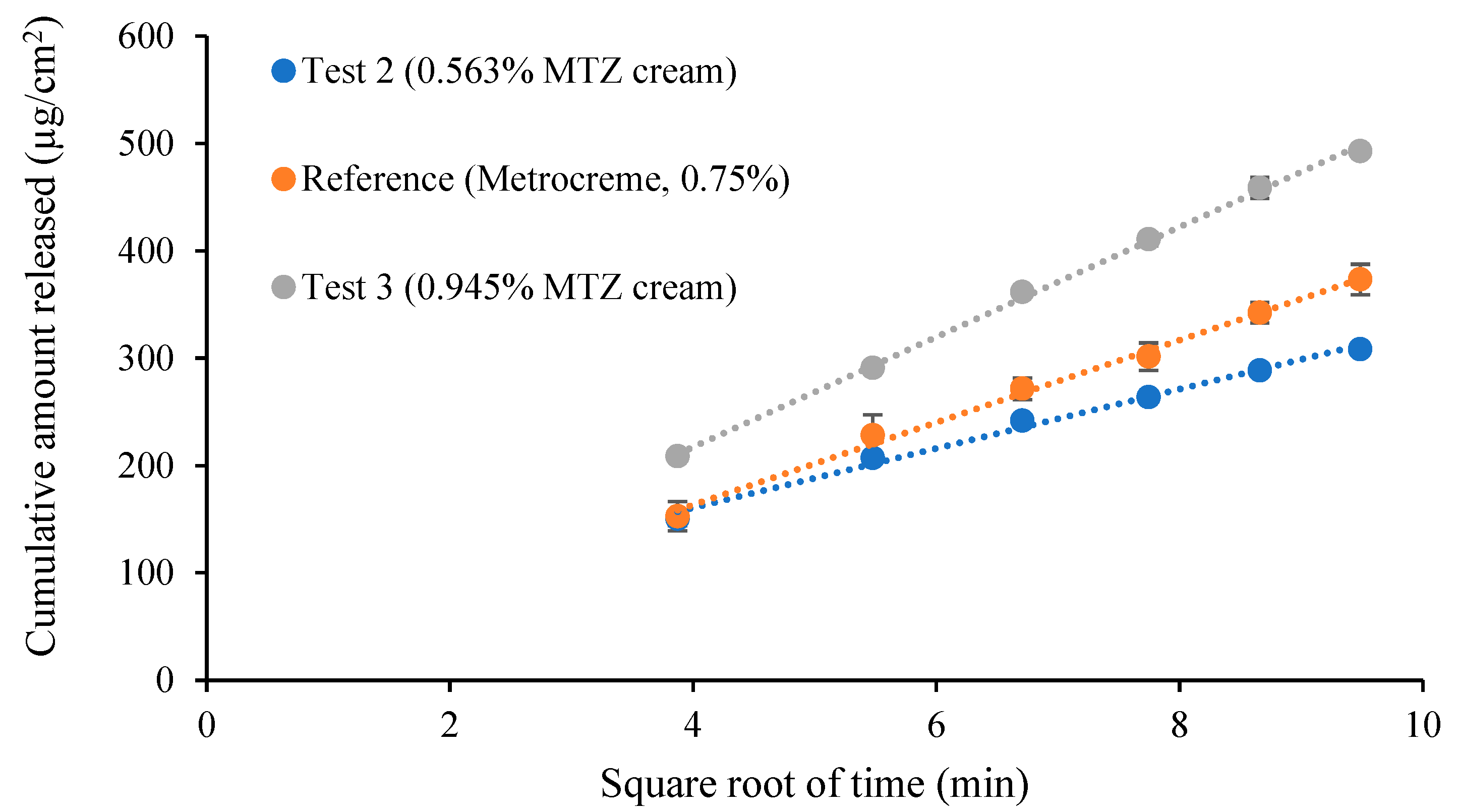
3.7. Comparative IVRT of MTZ Topical Cream Products
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shah, V.P. Progress in Methodologies for Evaluating Bioequivalence of Topical Formulations. Am. J. Clin. Dermatol. 2001, 2, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Olejnik, A.; Goscianska, J.; Nowak, I. Active Compounds Release from Semisolid Dosage Forms. J. Food Drug Anal. 2012, 101, 4032–4045. [Google Scholar] [CrossRef] [PubMed]
- US Food and Drug Administration. Guidance for Industry: Nonsterile Semisolid Dosage Forms, Scale-up and Post Approval Changes, Chemistry, Manufacturing, and Control; In vitro Release Testing and In vivo Bioequivalence Documentation (SUPAC-SS); Center for Drug Evaluation and Research: Rockville, MD, USA, 1997.
- USP General Chapter <1724> Semisolid Drug Products—Performance Tests. In United States Pharmacopeia and National Formulary (USP 41-NF 36); The United States Pharmacopeial Convention: Rockville, MD, USA, 2018; pp. 7944–7956.
- Bashaw, E.D.; Benfeldt, E.; Davit, B.; Ganes, D.; Ghosh, T.; Kanfer, I.; Lionberger, R. Current Challenges in Bioequivalence, Quality, and Novel Assessment Technologies for Topical Products. Pharm. Res. 2014, 31, 837–846. [Google Scholar]
- Ilić, T.; Pantelić, I.; Lunter, D.; Đorđević, S.; Marković, B.; Ranković, D.; Savić, S. Critical quality attributes, in vitro release and correlated in vitro skin permeation—in vivo tape stripping collective data for demonstrating therapeutic (non)equivalence of topical semisolids: A case study of “ready-to-use” vehicles. Int. J. Pharm. 2017, 528, 253–267. [Google Scholar] [CrossRef] [PubMed]
- Aiache, J.; Aoyagi, N.; Bashaw, D.; Brown, C.; Brown, W.; Burgess, D.; Crison, J. FIP/AAPS Guidelines to Dissolution/In Vitro Release Testing of Novel/Special Dosage Forms. AAPS PharmSciTech. 2003, 4, 1–10. [Google Scholar]
- Shah, V.P.; Elkins, J.S.; Williams, R.L. Evaluation of the Test System Used for In Vitro Release of Drugs for Topical Dermatological Drug Products. Pharm. Dev. Technol. 1999, 4, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Klein, R.R.; Heckart, J.L.; Thakker, K.D. In vitro release testing methodology and variability with the vertical diffusion cell (VDC). Dissolution Technol. 2018, 25, 52–61. [Google Scholar] [CrossRef]
- Thakker, K.D.; Chern, W.H. Development and validation of in vitro release tests for semisolid dosage forms—Case study. Dissolution Technol. 2003, 10, 10–15. [Google Scholar] [CrossRef]
- Tiffner, K.I.; Kanfer, I.; Augustin, T.; Raml, R.; Raney, S.G.; Sinner, F. A comprehensive approach to qualify and validate the essential parameters of an in vitro release test (IVRT) method for acyclovir cream, 5%. Int. J. Pharm. 2018, 535, 217–227. [Google Scholar] [CrossRef] [PubMed]
- US Food and Drug Administration. Draft Guidance on Acyclovir. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/psg/Acyclovir_oint_18604_RC03-12.pdf (accessed on 20 October 2019).
- US Food and Drug Administration. Draft Guidance on Acyclovir Cream. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/psg/Acyclovir_topical%20cream_RLD%2021478_RV12-16.pdf (accessed on 20 October 2019).
- US Food and Drug Administration. Draft Guidance on Docosanol Topical Cream. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/psg/Docosanol_topical%20cream_NDA%20020941_RC08-17.pdf (accessed on 20 October 2019).
- US Food and Drug Administration. Draft Guidance on Dapsone Gel. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/psg/Dapsone_Topical%20gel_NDA%20207154_RV%20Nov%202018.pdf (accessed on 20 October 2019).
- European Medicines Agency; Committee for Medicinal Products for Human Use. Draft Guideline on Quality and Equivalence of Topical Products. London, UK, 2018. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/draft-guideline-quality-equivalence-topical-products_en.pdf (accessed on 20 October 2019).
- Ueda, C.T.; Shah, V.P.; Derdzinski, K.; Ewing, G.; Flynn, G.; Maibach, H.; Yacobi, A. Topical and transdermal drug products. Pharmacop. Forum. 2009, 35, 750–764. [Google Scholar] [CrossRef]
- Klein, R.R.; Bechtel, J.L.; Burchett, K.; Thakker, K.D. Hydrocortisone as a Standard for In Vitro Release Testing Hydrocortisone as a Standard for In Vitro Release Testing. Dissolution Technol. 2010, 17, 37–38. [Google Scholar] [CrossRef]
- Higuchi, W.I. Analysis of data on the medicament release from ointments. J. Pharm. Sci. 1962, 51, 802–804. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, T. Rate of Release of Medicaments from Ointment Bases Containing Drugs in Suspension. J. Pharm. Sci. 1961, 50, 874–875. [Google Scholar] [CrossRef] [PubMed]
| Parameter | Acceptance Criteria | Results | Pass |
|---|---|---|---|
| Selectivity and specificity | − < 10% = 0 µg/mL, = 0 µg/mL | 0.063% = 0 µg/mL, = 0 µg/mL | Yes |
| Linearity | 75% of the standards meet the following criteria: For 5–100 μg/mL: [ 15%] For 0.5 μg/mL: [ 20%] R2 0.950 | 30 out of 30 standards (100%) met the acceptance criteria R2 ≥ 0.999 | Yes |
| Accuracy | For and : [ 15%] For : [ 20%] | 0.69% and 1.48% for and 2.08% for | Yes |
| Precision | Intra-day CV < 15% for to Intra-day CV < 20% for | 1.30%, 0.63%, and 0.66% for 1.03%, 0.93%, and 0.11% for 1.07%, 6.29%, and 7.35% for | Yes |
| Inter-day CV < 15% for to Inter-day CV < 20% for | 6.00% for 0.72% for 1.07% for | Yes | |
| Robustness | Inter-run CV < 15% for to Inter-run CV < 20% for | 10.03% for 9.05% for 9.27% for | Yes |
| Stability | For and : [ 15%] For : [ 20%] | Bench-top after 7 days: 0.22% for 0.36% for 5.01% for UPLC machine after 7 days: 0.23% for 0.29% for 0.45% for Refrigerator after 7 days: 0.34% for 0.11% for 0.49% for | Yes |
| LLOQ, LOD | - | LLOQ: 0.5 µg/mL LOD: 0.167 µg/mL |
| Parameter | Acceptance Criteria | Results | Pass | ||
|---|---|---|---|---|---|
| Mean ± Tolerance | Range of Variation | Mean | Range of Variation | ||
| Capacity of the cells | 7.00 ± 0.35 mL | ≤0.21 mL | 7.92 mL | 0.02 mL | No |
| Diameter of the orifice | 15.00 ± 0.75 mm | ≤0.45 mm | 14.98 mm | 0.10 mm | Yes |
| Temperature of the receptor medium | 32 ± 1 °C | - | 32.07 °C | 0.05 °C | Yes |
| Speed of the magnetic stirrer | 600 ± 60 rpm | ≤12 rpm | 601.67 rpm | 2.53 rpm | Yes |
| Dispensed sampling volume | 200 ± 10 µL | - | 203.67 µL | 4.58 µL | Yes |
| Bench top levelness | Not more than 1° | <1° | Yes | ||
| Parameter | Acceptance Criteria | Results |
|---|---|---|
| Intra-run variability | Intra-run CV for the first run (n = 6 VDCs) < 15% | 8.13% |
| Intra-run CV for the second run (n = 6 VDCs) < 15% | 12.85% | |
| Inter-run variability | Inter-run CV for both runs (n = 12 VDCs) < 15% | 10.30% |
| Product “sameness” testing | The 90% CI should fall within the limits of 75–133.33% | Lower limit: 87.82% Upper limit: 116.52% |
| Time (min) | Release Rate (µg/cm2/min1/2) | R2 Value | Recovery (%) |
|---|---|---|---|
| 90 | 39.47 ± 2.30 | 0.998 ± 0.003 | 29.90 ± 1.20 |
| 180 | 34.19 ± 1.97 | 0.988 ± 0.006 | 38.32 ± 1.19 |
| 360 | 27.58 ± 2.09 | 0.994 ± 0.002 | 42.75 ± 3.57 |
| Pairwise Comparison * | Computed 90% CI | |
|---|---|---|
| Lower Limit | Upper Limit | |
| 0.375% MTZ cream vs. 0.75% MTZ cream | 45.10 | 52.47 |
| 1.125% MTZ cream vs. 0.75% MTZ cream | 152.04 | 167.79 |
| Metrocreme®, 0.75% MTZ (run 1) vs. Metrocreme®, 0.75% MTZ (run 2) | 93.61 | 100.22 |
| Metrocreme®, 0.75% MTZ (run 1) vs. Metrocreme®, 0.75% MTZ (run 3) | 87.40 | 97.66 |
| Metrocreme®, 0.75% MTZ (run 2) vs. Metrocreme®, 0.75% MTZ (run 3) | 93.06 | 99.76 |
| Pairwise Comparison * | Computed 90% CI | Decision | |
|---|---|---|---|
| Lower Limit | Upper Limit | ||
| Positive control Metrocreme®, 0.75% MTZ vs. Metrocreme®, 0.75% MTZ | 93.61 | 100.22 | Pass |
| Negative controls | |||
| 0.563% MTZ cream vs. Metrocreme®, 0.75% MTZ | 68.25 | 74.21 | Fail |
| 0.945% MTZ cream vs. Metrocreme®, 0.75% MTZ | 128.36 | 137.11 | Fail |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rath, S.; Kanfer, I. A Validated IVRT Method to Assess Topical Creams Containing Metronidazole Using a Novel Approach. Pharmaceutics 2020, 12, 119. https://doi.org/10.3390/pharmaceutics12020119
Rath S, Kanfer I. A Validated IVRT Method to Assess Topical Creams Containing Metronidazole Using a Novel Approach. Pharmaceutics. 2020; 12(2):119. https://doi.org/10.3390/pharmaceutics12020119
Chicago/Turabian StyleRath, Seeprarani, and Isadore Kanfer. 2020. "A Validated IVRT Method to Assess Topical Creams Containing Metronidazole Using a Novel Approach" Pharmaceutics 12, no. 2: 119. https://doi.org/10.3390/pharmaceutics12020119
APA StyleRath, S., & Kanfer, I. (2020). A Validated IVRT Method to Assess Topical Creams Containing Metronidazole Using a Novel Approach. Pharmaceutics, 12(2), 119. https://doi.org/10.3390/pharmaceutics12020119





