Topical Amphotericin B Semisolid Dosage Form for Cutaneous Leishmaniasis: Physicochemical Characterization, Ex Vivo Skin Permeation and Biological Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Parasite Strains and Cultures
2.3. Gel Preparation
2.4. Physicochemical Characterization of the AmB gel
2.4.1. Morphological Analysis
2.4.2. Swelling and Degradation Tests
2.4.3. Porosity Study
2.5. Stability Studies
2.6. Rheological Studies
2.7. Spreadability Test
2.8. In Vitro Release Studies
2.9. Ex Vivo Permeation Studies
2.10. In Vivo Tolerance Studies
2.11. In Vitro Cytotoxicity Assay
2.12. In Vitro Leishmanicidal Activity against Promastigotes
2.13. In Vitro Leishmanicidal Activity against Amastigotes
2.14. Statistical Analysis
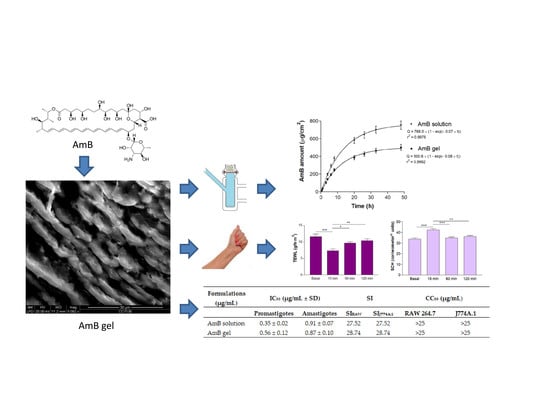
3. Results
3.1. Physicochemical Characterization of the AmB gel
3.2. Stability Studies
3.3. Rheological Studies
3.4. Spreadability Test
3.5. In Vitro Release Studies
3.6. Ex Vivo Permeation Studies
3.7. In Vivo Tolerance Studies
3.8. In Vitro Cytotoxicity Assay
3.9. In Vitro Leishmanicidal Activity against Promastigotes and Amastigotes
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Alvar, J.; Vélez, I.D.; Bern, C.; Herrero, M.; Desjeux, P.; Cano, J.; Janin, J.; den Boer, M. WHO Leishmaniasis Control Team. Leishmaniasis worldwide and global estimates of its incidence. PLoS ONE 2012, 7, e35671. [Google Scholar] [CrossRef]
- Garnier, T.; Croft, S.L. Topical treatment for cutaneous leishmaniasis. Curr. Opin. Investig. Drugs 2002, 3, 538–544. [Google Scholar]
- Reithinger, R.; Dujardin, J.C.; Louzir, H.; Pirmez, C.; Alexander, B.; Brooker, S. Cutaneous leishmaniasis. Lancet Infect. Dis. 2007, 7, 581–596. [Google Scholar] [CrossRef]
- Abu Ammar, A.; Nasereddin, A.; Ereqat, S.; Dan-Goor, M.; Jaffe, C.L.; Zussman, E.; Abdeen, Z. Amphotericin B-loaded nanoparticles for local treatment of cutaneous leihmaniasis. Drug Deliv. Transl. Res. 2019, 9, 76–84. [Google Scholar] [CrossRef]
- Gaspari, V.; Ortalli, M.; Foschini, M.P.; Baldovinci, C.; Lanzoni, A.; Cagarelli, R.; Gaibani, P.; Rossini, G.; Vocale, C.; Tigani, R.; et al. New evidence of cutaneous leishmaniasis in north-eastern Italy. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 1534–1540. [Google Scholar] [CrossRef]
- Alcover, M.M.; Rocamora, V.; Guillén, M.C.; Berenguer, D.; Cuadrado, M.; Riera, C.; Fisa, R. Case Report: Diffuse Cutaneous Leishmaniasis by Leishmania infantum in a Patient Undergoing Immunosuppressive Therapy: Risk Status in an Endemic Mediterranean Area. Am. J. Trop. Med. Hyg. 2018, 98, 1313–1316. [Google Scholar] [CrossRef]
- Aguado, M.; Espinosa, P.; Romero-Maté, A.; Tardío, J.C.; Córdoba, S.; Borbujo, J. Outbreak of cutaneous leishmaniasis in Fuenlabrada, Madrid. Actas Dermosifiliogr. 2013, 104, 334–342. [Google Scholar] [CrossRef]
- Gupta, A.; Sardana, K.; Ahuja, A.; Gautam, R.K. Complete cure of a large complexe cutaneous leihmaniasis with a nonethanolic lipid based-amphotericin B gel. Clin. Exp. Dermatol. 2019, 44, 807–810. [Google Scholar] [CrossRef]
- Santos, C.M.; de Oliveira, R.B.; Arantes, V.T.; Caldeira, L.R.; de Oliveira, M.C.; Egito, E.S.; Ferreira, L.A. Amphotericin B-loaded nanocarriers for topical treatment of cutaneous leishmaniasis: Development, characterization, and in vitro permeation studies. J. Biomed. Nanotechnol. 2012, 8, 322–329. [Google Scholar]
- Jaafari, M.R.; Bavarsad, N.; Fazly-Bazzaz, B.S.; Samiei, A.; Soroush, D.; Ghorbani, S.; Lotfi-Heravi, M.M.; Khamesipour, A. Effect of Topical Liposomes Containing Paromomycin Sulfate in the Course of Leishmania major Infection in Susceptible BALB/c Mice. Antimicrob. Agents Chemother 2009, 53, 2259–2265. [Google Scholar] [CrossRef]
- Cohen, B.E. The role of Signaling via Aqueous Pore Formation in Resistance Responses to Amphotericin B. Antimicrob. Agents Chemother. 2016, 60, 5122–5129. [Google Scholar] [CrossRef]
- Mostafavi, M.; Sharifi, I.; Farajzadeh, S.; Khazaeli, P.; Sharifi, H.; Pourseyedi, E.; Kakooei, S.; Bamorovat, M.; Keyhani, A.; Parizi, M.H.; et al. Niosomal formulation of amphotericin B alone and in combination with glucantime: In vitro and in vivo leishmanicidal effects. Biomed. Pharmacother. 2019, 116, 108942. [Google Scholar] [CrossRef]
- de Carvalho, R.F.; Ribeiro, I.F.; Miranda-Vilela, A.L.; de Souza Filho, J.; Martins, O.P.; Cintra e Silva Dde, O.; Tedesco, A.C.; Lacava, Z.G.; Báo, S.N.; Sampaio, R.N. Leishmanicidal activity of amphotericin B encapsulated in PLGA-DMSA nanoparticles to treat cutaneous leishmaniasis in C57BL/6 mice. Exp. Parasitol. 2013, 135, 217–222. [Google Scholar] [CrossRef]
- Lanza, J.S.; Pomel, S.; Loiseau, P.M.; Frézard, F. Recent advances in amphotericin B delivery strategies for the treatment of leishmaniases. Expert Opin. Drug Deliv. 2019, 16, 1063–1079. [Google Scholar] [CrossRef]
- Singh, P.K.; Jaiswal, A.K.; Pawar, V.K.; Raval, K.; Kumar, A.; Bora, H.K.; Dube, A.; Chourasia, M.K. Fabrication of 3-O-snphosphatidyl-L-serine anchored PLGA nanoparticle bearing amphotericin B for macrophage targeting. Pharm. Res. 2018, 35, 60–71. [Google Scholar] [CrossRef]
- Rochelle Do Vale Morais, A.; Silva, A.L.; Cojean, S.; Balaraman, K.; Bories, C.; Pomel, S.; Barratt, G.; do Egito, E.S.T.; Loiseau, P.M. In-vitro and in-vivo antileishmanial activity of inexpensive Amphotericin B formulations: Heated Amphotericin B and Amphotericin B-loaded microemulsion. Exp. Parasitol. 2018, 192, 85–92. [Google Scholar] [CrossRef]
- Wang, Y.; Ke, X.; Voo, Z.X.; Yap, S.S.L.; Yang, C.; Gao, S.; Liu, S.; Venkataraman, S.; Obuobi, S.A.O.; Khara, J.S.; et al. Biodegradable functional polycarbonate micelles for controlled release of amphotericin B. Acta Biomater. 2016, 46, 211–220. [Google Scholar] [CrossRef]
- Sarwar, H.S.; Sohail, M.F.; Saljoughian, N.; Rehman, A.U.; Akhtar, S.; Nadhman, A.; Yasinzai, M.; Gendelman, H.E.; Satoskar, A.R.; Shahnaz, G. Design of mannosylated oral amphotericin B nanoformulation: Efficacy and safety in visceral leishmaniasis. Artif. Cells Nanomed. Biotechnol. 2018, 46 (Suppl. 1), 521–531. [Google Scholar] [CrossRef]
- Gupta, P.K.; Jaiswal, A.K.; Asthana, S.; Teja, B.V.; Shukla, M.; Sagar, N.; Dube, A.; Rath, S.K.; Mishra, P.R. Synergistic enhancement of parasiticidal activity of amphotericin B using copaiba oil in nanoemulsified carrier for oral delivery: An approach for non-toxic chemotherapy. Br. J. Pharmacol. 2015, 172, 3596–3610. [Google Scholar] [CrossRef]
- Nguyen, A.K.; Yang, K.H.; Bryant, K.; Li, J.; Joice, A.C.; Werbovetz, K.A.; Narayan, R.J. Microneedle-based delivery of Amphotericin B for treatment of cutaneous Leishmaniasis. Biomed. Microdevices 2019, 21, 8. [Google Scholar] [CrossRef]
- Ruiz, H.K.; Serrano, D.R.; Dea-Ayuela, M.A.; Bilbao-Ramos, P.E.; Bolás-Fernández, F.; Torrado, J.J.; Molero, G. New amphotericin B-gamma cyclodextrin formulation for topical use with synergistic activity against diverse fungal species and Leishmania spp. Int. J. Pharm. 2014, 473, 148–157. [Google Scholar] [CrossRef]
- Perez, A.P.; Altube, M.J.; Schilrreff, P.; Apezteguia, G.; Celes, F.S.; Zacchino, S.; de Oliveira, C.I.; Romero, E.L.; Morilla, M.J. Topical amphotericin B in ultradeformable liposomes: Formulation, skin penetration study, antifungal and antileishmanial activity in vitro. Colloids Surf. B Biointerfaces 2016, 139, 190–198. [Google Scholar] [CrossRef]
- Cascone, S.; Lamberti, G. Hydrogel-based commercial products for biomedical applications: A review. Int. J. Pharm. 2019, 118803. [Google Scholar] [CrossRef]
- Sosa, L.; Clares, B.; Alvarado, H.L.; Bozal, N.; Domenech, O.; Calpena, A.C. Amphotericin B releasing topical nanoemulsion for the treatment of candidiasis and aspergillosis. Nanomedicine 2017, 13, 2303–2312. [Google Scholar] [CrossRef]
- Suñer, J.; Calpena, A.C.; Clares, B.; Cañadas, C.; Halbaut, L. Development of Clotrimazole Multiple W/O/W Emulsions as Vehicles for Drug Delivery: Effects of Additives on Emulsion Stability. AAPS PharmSciTech 2017, 18, 539–550. [Google Scholar]
- Council regulation (EC) no 440/2008 Test guideline for skin absorption: In vitro method (B45). J. Eur. Union 2008, 142, 438–443.
- World Medical Association. World medical association declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef]
- Du Plessis, J.; Stefaniak, A.; Eloff, F.; John, S.; Agner, T.; Chou, T.C.; Nixon, R.; Steiner, M.; Franken, A.; Kudla, I.; et al. International guidelines for the in vivo assessment of skin properties in non-clinical settings: Part 2. Transepidermal water loss and skin hydration. Skin. Res. Technol. 2013, 19, 265–278. [Google Scholar] [CrossRef]
- Pujol, A.; Urbán, P.; Riera, C.; Fisa, R.; Molina, I.; Salvador, F.; Estelrich, J.; Fernández-Busquets, X. Application of Quantum Dots to the Study of Liposome Targeting in Leishmaniasis and Malaria. Int. J. Theoret. Appl. Nanotech. 2014, 2, 1–8. [Google Scholar] [CrossRef][Green Version]
- Carrió, J.; de Colmenares, M.; Riera, C.; Gállego, M.; Arboix, M.; Portús, M. Leishmania infantum: Stage-specific activity of pentavalent antimony related with the assay conditions. Exp. Parasitol. 2000, 95, 209–214. [Google Scholar] [CrossRef]
- Carrió, J.; Riera, C.; Gállego, M.; Portús, M. In vitro activity of pentavalent antimony derivates on promastigotes and intracellular amastigotes of Leishmania infantum strains from humans and dogs in Spain. Acta Trop. 2001, 79, 179–183. [Google Scholar] [CrossRef]
- Moon, S.; Jambert, E.; Childs, M.; von Schoen-Angerer, T. A win-win solution? A critical analysis of tiered pricing to improve access to medicines in developing countries. Glob. Helath 2011, 7, 39–49. [Google Scholar] [CrossRef]
- Formariz, T.P.; Sarmento, V.H.; Silva-Junior, A.A.; Scarpa, M.V.; Santilli, C.V.; Oliveira, A.G. Doxorubicin biocompatible O/W microemulsion stabilized by mixed surfactant containing soya phosphatidylcholine. Colloids Surf. B Biointerfaces 2006, 51, 54–61. [Google Scholar] [CrossRef]
- Rolim, A.; Oishi, T.; Maciel, C.P.; Zague, V.; Pinto, C.A.; Kaneko, T.M.; Consiglieri, V.O.; Velasco, M.V. Total flavonoids quantification from O/W emulsion with extract of Brazilian plants. Int. J. Pharm. 2006, 308, 107–114. [Google Scholar] [CrossRef]
- Berenguer, D.; Sosa, L.; Alcover, M.; Sessa, M.; Halbaut, L.; Guillén, C.; Fisa, R.; Calpena-Campmany, A.C.; Riera, C. Development and Characterization of a Semi-Solid Dosage Form of Meglumine Antimoniate for Topical Treatment of Cutaneous Leishmaniasis. Pharmaceutics 2019, 11, 613. [Google Scholar] [CrossRef]
- Sierra, A.F.; Ramirez, M.L.; Campmany, A.C.; Martinez, A.R.; Naveros, B.C. In vivo and in vitro evaluation of the use of a newly developed melatonin loaded emulsion combined with UV filters as a protective agent against skin irradiation. J. Dermatol. Sci. 2013, 69, 202–214. [Google Scholar] [CrossRef]
- Alvarado, H.L.; Abrego, G.; Souto, E.B.; Garduño-Ramírez, M.L.; Clares, B.; García, M.L.; Calpena, A.C. Nanoemulsions for dermal controlled release of oleanolic and ursolic acids: In vitro, ex vivo and in vivocharacterization. Colloids Surf. B Biointerfaces 2015, 130, 40–47. [Google Scholar] [CrossRef]
- Sosa, L.; Calpena, A.C.; Silva-Abreu, M.; Espinoza, L.C.; Rincón, M.; Bozal, N.; Domenech, O.; Rodríguez-Lagunas, M.J.; Clares, B. Thermoreversible Gel-Loaded Amphotericin B for the treatment of Dermal and Vaginal Candidiasis. Pharmaceutics 2019, 11, 312. [Google Scholar] [CrossRef]
- Rizzo, J.A.; Martini, A.K.; Pruskowski, K.A.; Rowan, M.P.; Niece, K.L.; Akers, K.S. Thermal stability of mafenide and amphotericin B topical solution. Burns 2018, 44, 475–480. [Google Scholar] [CrossRef]
- Hussain, A.; Samad, A.; Singh, S.K.; Ahsan, M.N.; Faruk, A.; Ahmed, F.J. Enhanced stability and permeation potential of nanoemulsion containing sefsol-218 oil for topical delivery of amphotericin B. Drug Dev. Ind. Pharm. 2015, 41, 780–790. [Google Scholar] [CrossRef]
- Suñer-Carbó, J.; Calpena-Campmany, A.; Halbaut-Bellowa, L.; Clares-Naveros, B.; Rodriguez-Lagunas, M.J.; Barbolini, E.; Zamarbide-Losada, J.; Boix-Montañés, A. Biopharmaceutical Development of a Bifonazole Multiple Emulsion for Enhanced Epidermal Delivery. Pharmaceutics 2019, 11, 66. [Google Scholar] [CrossRef]
- Martín-Villena, M.J.; Fernández-Campos, F.; Calpena-Campmany, A.C.; Bozal-de Febrer, N.; Ruiz-Martínez, M.A.; Clares-Naveros, B. Novel microparticulate systems for the vaginal delivery of nystatin: Development and characterization. Carbohydr. Polym. 2013, 94, 1–11. [Google Scholar] [CrossRef]
- Potúckova, M.; Vitková, Z.; Tichý, E.; Simunková, V. Indometacin releae in relation to the concentration of pharmaceutical excipients. Pharmazie 2008, 63, 485–486. [Google Scholar]
- Dar, M.J.; Khalid, S.; McElroy, C.A.; Satoskar, A.R.; Khan, G.M. Topical treatment of cutaneous leishmaniasis with novel amphotericin B-miltefosine co-incorporated second generation ultra-deformable liposomes. Int. J. Pharm. 2019, 118900. [Google Scholar] [CrossRef]
- Maes, L.; Beyers, J.; Mondelaers, A.; Van der Kerkhof, M.; Eberhardt, E.; Caljon, G.; Hendrickx, S. In vitro ‘time-to-kill’ assay to assess the cidal activity dynamics of current reference drugs against Leishmania donovani and Leishmania infantum. J. Antimicrob. Chemother. 2017, 72, 428–430. [Google Scholar] [CrossRef]
- Handler, M.Z.; Patel, P.A.; Kapila, R.; Al-Qubati, Y.; Schwarts, R.A. Cutaneous and mucocutaneous leishmaniasis: Differential diagnosis, diagnosis, histopathology, and management. J. Am. Acad. Dermatol. 2015, 73, 911–926. [Google Scholar] [CrossRef]
- Jaafari, M.R.; Hatamipour, M.; Alavizadeh, S.H.; Abbasi, A.; Saberi, Z.; Rafati, S.; Taslimi, Y.; Mohammadi, A.M.; Khamesipour, A. Development of a topical liposomal formulation of Amphotericin B for the treatment of cutaneous leishmaniasis. Drugs Drug Resist. 2019, 11, 156–165. [Google Scholar] [CrossRef]
- Hussain, A.; Singh, V.K.; Singh, O.P.; Shafaat, K.; Kumar, S.; Ahmad, F.J. Formulation and optimization of nanoemulsion using antifungal lipid and surfactant for accentuated accentuated topical delivery of Amphotericin B. Drug Deliv. 2016, 23, 3101–3110. [Google Scholar] [CrossRef]
- Hussain, A.; Singh, S.; Webster, T.J.; Ahmad, F.J. New perspectives in the topical delivery of optimized amphotericin B loaded nanoemulsions using excipients with innate anti-fungal activities: A mechanistic and histopathological investigation. Nanomedicine 2017, 13, 1117–1126. [Google Scholar] [CrossRef]
- Marzulli, F.N.; Callahan, J.F.; Brown, D.W. Chemical structure and skin penetrating capacity of a short series of organic phosphates and phosphoric acid. J. Investig. Dermatol. 1965, 44, 339–344. [Google Scholar] [CrossRef]
- Mehanna, M.M.; Motawaa, A.M.; Samaha, M.W. Nanovesicular carrier-mediated transdermal delivery of tadalafil: I-formulation and physicsochemical characterization. Drug Dev. Ind. Pharm. 2015, 41, 714–721. [Google Scholar] [CrossRef] [PubMed]
- Van Bocxlaer, K.; Yardley, V.; Murdan, S.; Croft, S.L. Drug permeation and barrier damage in Leishmania-infected mouse skin. J. Antimicrob. Chemother. 2016, 71, 1578–1585. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Van Ravenzwaay, B.; Leibold, E. A comparison between in vitro rat and human and in vivo rat skin absorption studies. Hum. Exp. Toxicol. 2004, 23, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Jaulent, C.; Dereure, O.; Raison-Peyron, N. Contact dermatitis caused by polyacrylamide/C13-4 isoparaffin/laureth-7 mix in an emollient cream for atopic skin. Contact Dermat. 2019, 81, 70–71. [Google Scholar] [CrossRef] [PubMed]
- Eskandari, S.E.; Firooz, A.; Nassiri-Kashani, M.; Jaafari, M.R.; Javadi, A.; Miramin Mohammadi, A.; Khamesipour, A. Safety Evaluation of Topical Application of Nano-Liposomal Form of Amphotericin B (SinaAmpholeish) on Healthy Volunteers: Phase I Clinical Trial. Iran J. Parasitol. 2019, 14, 197–203. [Google Scholar] [CrossRef]
- López, L.; Vélez, I.; Asela, C.; Cruz, C.; Alves, F.; Robledo, S.; Arana, B. A phase II study to evaluate the safety and efficacy of topical 3% amphotericin B cream (Anfoleish) for the treatment of uncomplicated cutaneous leishmaniasis in Colombia. PLoS Negl. Trop. Dis. 2018, 12, e0006653. [Google Scholar] [CrossRef]









| AmB (µg/mL ± SD) | ||||
|---|---|---|---|---|
| t0 | t60 RT | t60 37 °C | t60 4 °C | |
| AmB gel | 959.90 ± 84.27 | 199.65 ± 27.99 | 70.95 ± 13.59 | 916.70 ± 99.78 |
| Assay | AmB gel | |
|---|---|---|
| Non-Damaged Skin | Damaged Skin | |
| Permeation (µg/cm2) ± SD | Not detected | Not detected |
| Retention in skin (µg/g/cm2) ± SD | 2484.57 ± 439.12 | 1230.10 ± 331.52 |
| Formulations (µg/mL) | IC50 (µg/mL ± SD) | SI | CC50 (µg/mL) | |||
|---|---|---|---|---|---|---|
| Promastigotes | Amastigotes | SIRAW | SIJ774A.1 | RAW 264.7 | J774A.1 | |
| AmB solution | 0.35 ± 0.02 | 0.91 ± 0.07 | 27.52 | 27.52 | >25 | >25 |
| AmB gel | 0.56 ± 0.12 | 0.87 ± 0.10 | 28.74 | 28.74 | >25 | >25 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berenguer, D.; Alcover, M.M.; Sessa, M.; Halbaut, L.; Guillén, C.; Boix-Montañés, A.; Fisa, R.; Calpena-Campmany, A.C.; Riera, C.; Sosa, L. Topical Amphotericin B Semisolid Dosage Form for Cutaneous Leishmaniasis: Physicochemical Characterization, Ex Vivo Skin Permeation and Biological Activity. Pharmaceutics 2020, 12, 149. https://doi.org/10.3390/pharmaceutics12020149
Berenguer D, Alcover MM, Sessa M, Halbaut L, Guillén C, Boix-Montañés A, Fisa R, Calpena-Campmany AC, Riera C, Sosa L. Topical Amphotericin B Semisolid Dosage Form for Cutaneous Leishmaniasis: Physicochemical Characterization, Ex Vivo Skin Permeation and Biological Activity. Pharmaceutics. 2020; 12(2):149. https://doi.org/10.3390/pharmaceutics12020149
Chicago/Turabian StyleBerenguer, Diana, Maria Magdalena Alcover, Marcella Sessa, Lyda Halbaut, Carme Guillén, Antoni Boix-Montañés, Roser Fisa, Ana Cristina Calpena-Campmany, Cristina Riera, and Lilian Sosa. 2020. "Topical Amphotericin B Semisolid Dosage Form for Cutaneous Leishmaniasis: Physicochemical Characterization, Ex Vivo Skin Permeation and Biological Activity" Pharmaceutics 12, no. 2: 149. https://doi.org/10.3390/pharmaceutics12020149
APA StyleBerenguer, D., Alcover, M. M., Sessa, M., Halbaut, L., Guillén, C., Boix-Montañés, A., Fisa, R., Calpena-Campmany, A. C., Riera, C., & Sosa, L. (2020). Topical Amphotericin B Semisolid Dosage Form for Cutaneous Leishmaniasis: Physicochemical Characterization, Ex Vivo Skin Permeation and Biological Activity. Pharmaceutics, 12(2), 149. https://doi.org/10.3390/pharmaceutics12020149