Oral Treatment of Spontaneously Hypertensive Rats with Captopril-Surface Functionalized Furosemide-Loaded Multi-Wall Lipid-Core Nanocapsules
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Captopril-Functionalized Furosemide-Loaded Nanocapsules Dispersed in Water
2.3. Quantification of Captopril and Furosemide by High Performance Liquid Chromatography (HPLC)
2.3.1. Furosemide and Captopril Analytical Methods
2.3.2. Drug Contents
2.3.3. Furosemide and Captopril Encapsulation Efficiencies
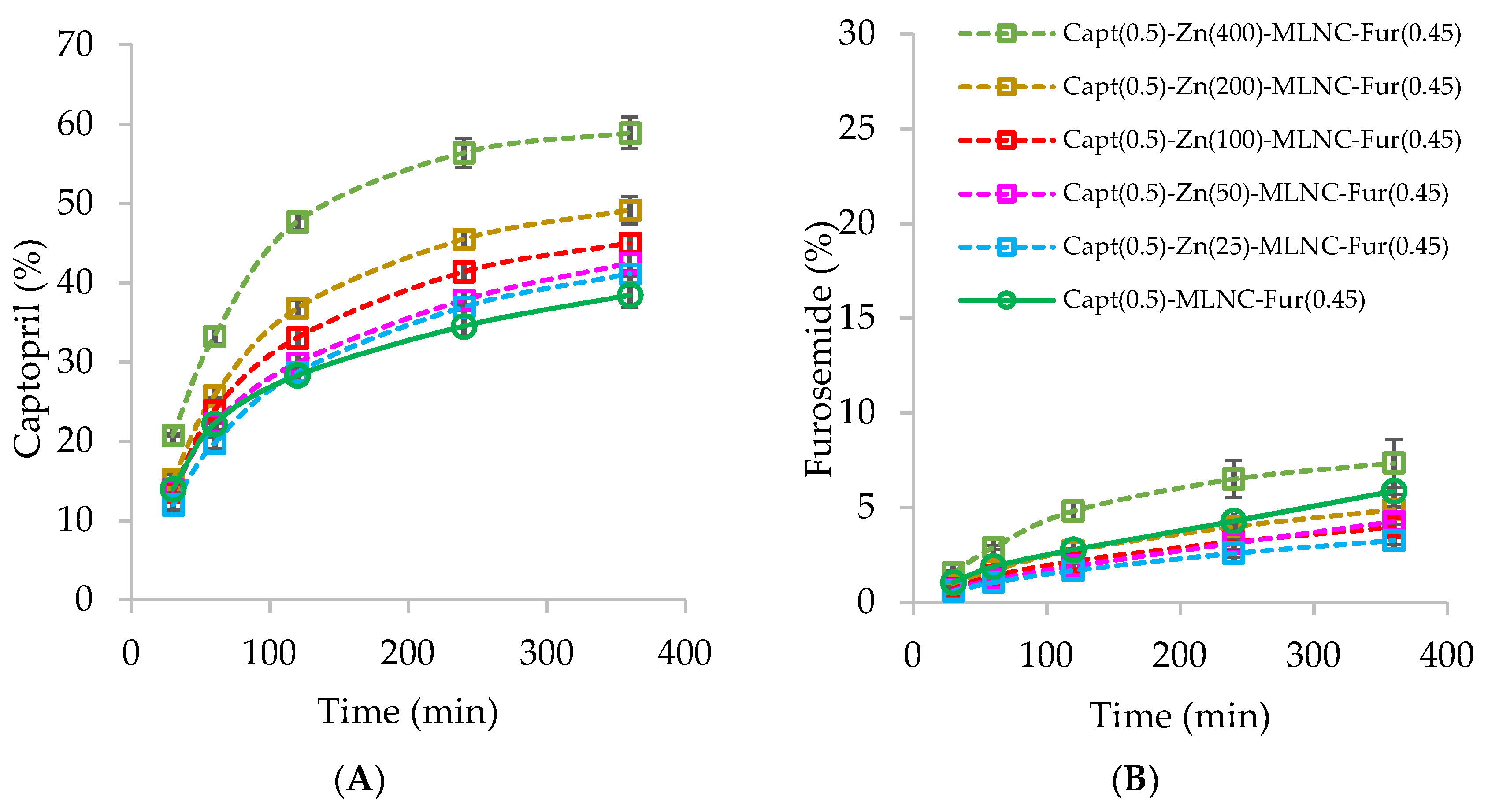
2.4. Captopril and Furosemide Dialysis to Determine the Optimal Concentrations of Zn2+ and Captopril in the Capt(0.5)-Zn-MLNC-Fur(0.45) Formulation
2.5. Particle Sizing
2.5.1. Laser Diffraction
2.5.2. Dynamic Light Scattering
2.5.3. Nanoparticle Tracking Analysis
2.6. Zeta Potential
2.7. Potentiometry
2.8. Particle Size Stability in In Vitro Gastrointestinal Simulated Fluids
2.9. In Vivo Studies
2.9.1. Animals
2.9.2. Treatment
2.9.3. Blood Pressure Measurements
2.9.4. Toxicological Evaluation
Biochemical Analyses
Early Kidney Damage Markers
Oxidative Status Evaluation
2.10. Morphological Analysis and Kinetic Stability of the Selected Formulation
2.10.1. Transmission Electron Microscopy (TEM)
2.10.2. Atomic Force Microscopy (AFM)
2.10.3. Multiple Light Scattering Analysis
2.11. Statistical Analysis
3. Results and Discussion
3.1. Development of Formulations
3.2. Physico-Chemical Characteristics of the Selected Formulations
3.3. Particle Size Stability in Gastrointestinal Simulated Fluids
3.4. In Vivo studies
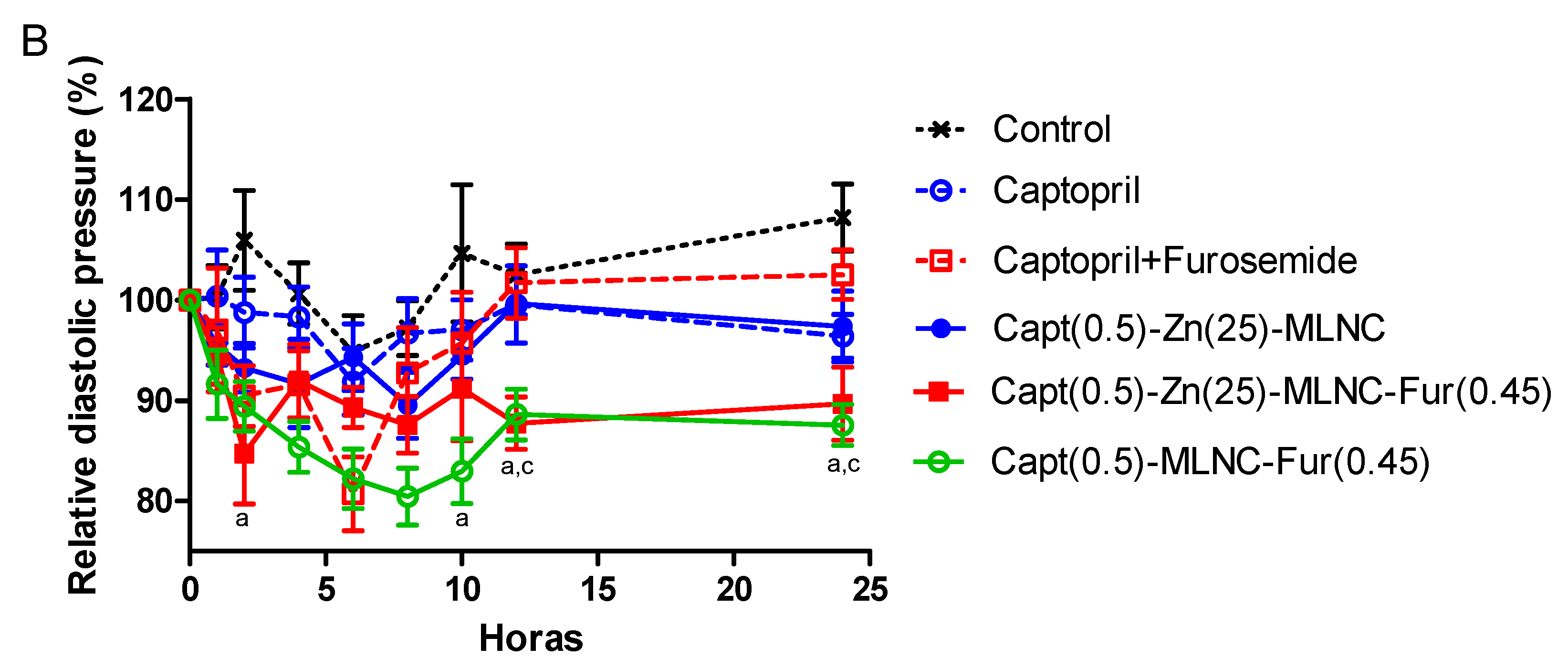
3.4.1. Blood Pressure Measurements
3.4.2. Toxicological Evaluation
Metabolic Cages
Biochemical Analyses
Early Kidney Damage Markers
Macroscopical Organs Analyses
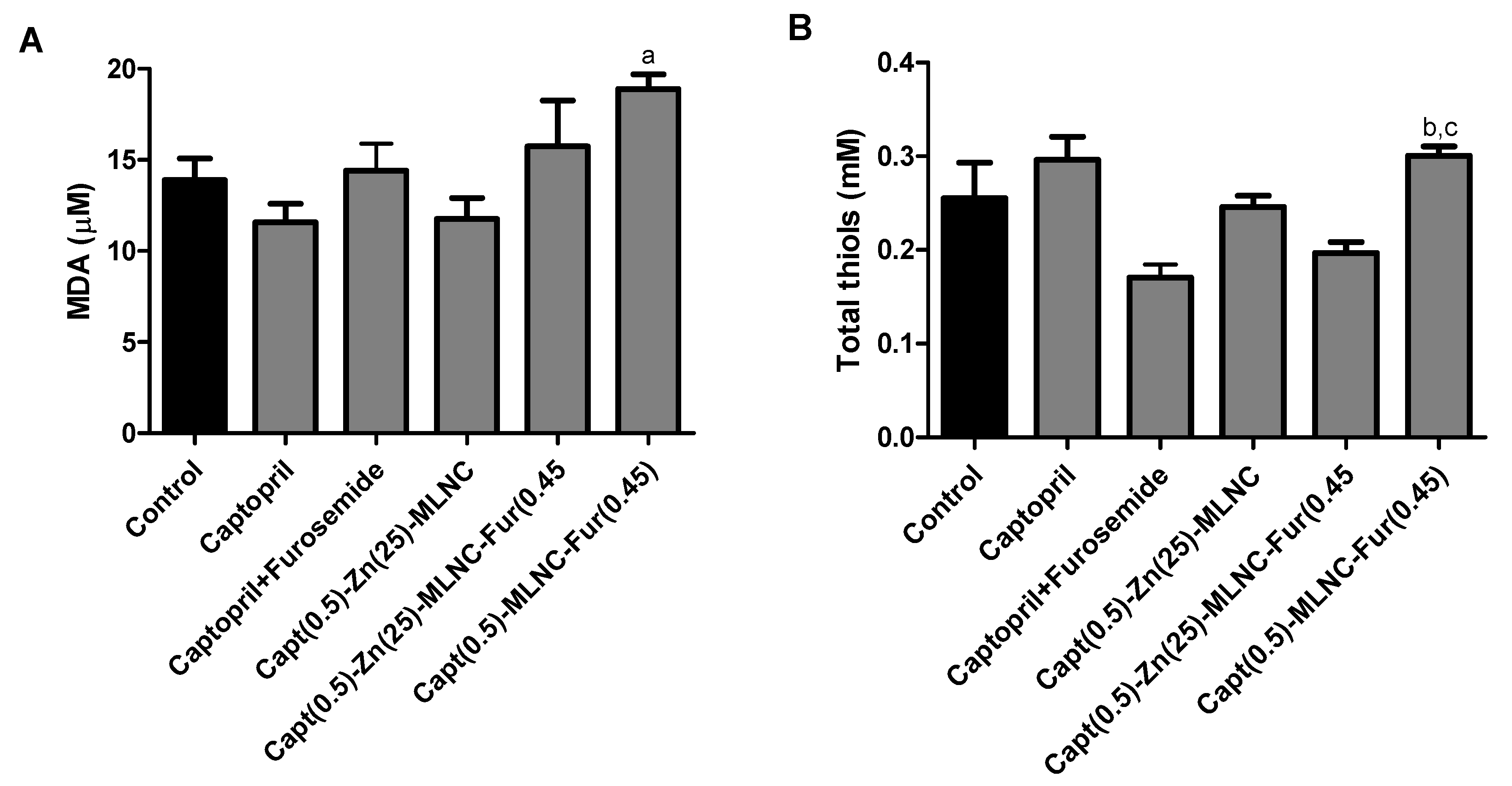
Oxidative Status Evaluation
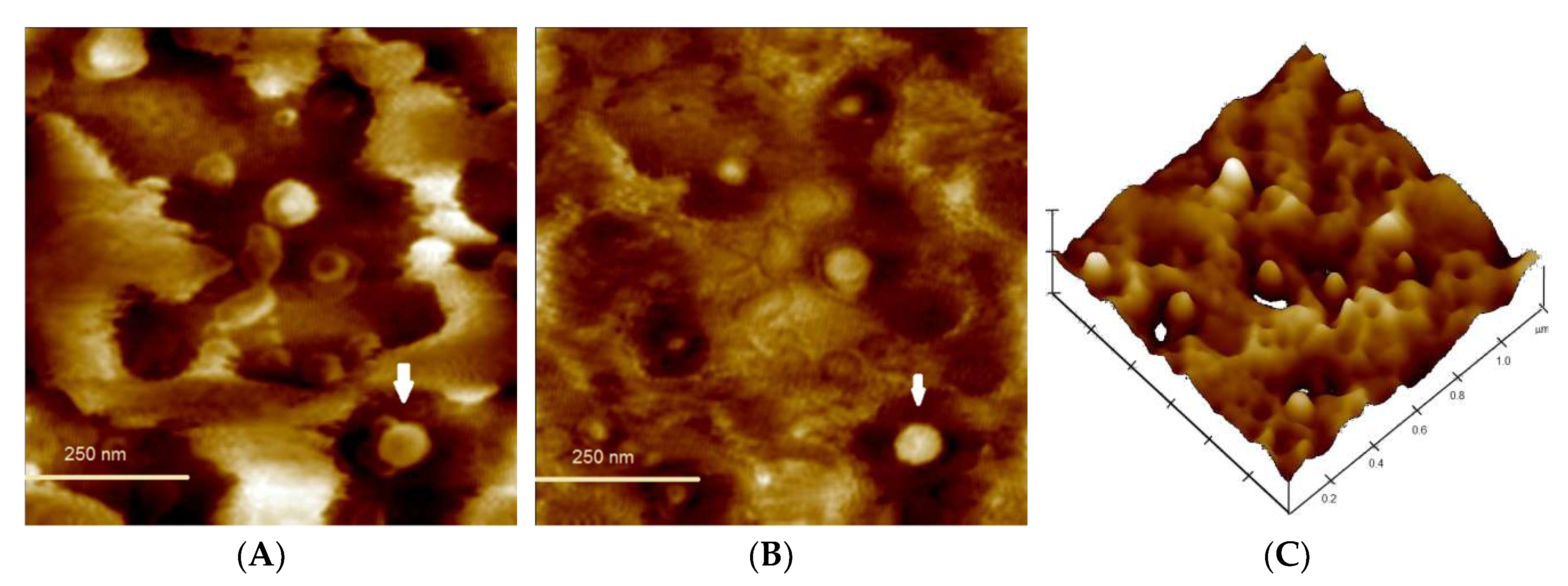
3.5. Morphological Analysis and Kinetic Stability of Capt(0.5)-Zn(25)-MLNC-Fur(0.45)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jouyban, A. Handbook of Solubility Data for Pharmaceuticals; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2010; pp. 1–2. [Google Scholar]
- Nkansah, P.; Antipas, A.; Lu, Y.; Varma, M.; Rotter, C.; Rago, B.; El-Kattan, A.; Taylor, G.; Rubio, M.; Litchfiel, J. Development and evaluation of novel solid nanodispersion system for oral delivery of poorly water-soluble drugs. J. Control. Release 2013, 169, 150–161. [Google Scholar] [CrossRef]
- Radwan, S.E.; Sokar, M.S.; Abdelmonsif, D.A.; El-Kamel, A.S. Mucopenetrating nanoparticles for enhancement of oral bioavailability of furosemide: In vitro and in vivo evaluation/sub-acute toxicity study. Int. J. Pharm. 2017, 526, 366–379. [Google Scholar] [CrossRef]
- Gulsun, T.; Borna, S.E.; Vural, I.; Sahin, S. Preparation and characterization of furosemide nanosuspensions. J. Drug Deliv. Sci. Technol. 2018, 45, 93–100. [Google Scholar] [CrossRef]
- Pohlmann, A.R.; Fonseca, F.N.; Paese, K.; Detoni, C.B.; Coradini, K.; Beck, R.C.; Guterres, S.S. Poly(ϵ-caprolactone) microcapsules and nanocapsules in drug delivery. Expert Opin. Drug Deliv. 2013, 10, 623–638. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, M.B.M.; Tasic, L.; Fattori, J.; Rodrigues, F.H.S.; Cantos, F.C.; Ribeiro, L.P.; Paula, V.; Ianzer, D.; Santos, R.A.S. New formulation of an old drug in hypertension treatment: The sustained release of captopril from cyclodextrin nanoparticles. Int. J. Nanomed. 2011, 6, 1005–1016. [Google Scholar]
- Biollaz, J.; Waeber, B.; Brunner, H.R. Hypertensive Crisis Treated with Orally Administered Captopril. Eur. J. Clin. 1983, 25, 145–149. [Google Scholar] [CrossRef]
- Wilcox, C.S.; Guzman, N.J.; Mitch, W.E.; Kelly, R.A.; Maroni, B.J.; Souney, P.F.; Rayment, C.M.; Braun, L.; Colucci, R.; Loon, N.R. Na+, K+ and BP homeostasis in man during furosemide: Effects of prazosin and captopril. Kidney Int. 1987, 31, 135–141. [Google Scholar] [CrossRef]
- Motwani, J.G.; Fenwick, M.K.; Morton, J.J.; Struthers, A.D. Furosemide-induced natriuresis is augmented by ultra-low-dose captopril but not by standard doses of captopril in chronic heart failure. Circulation 1992, 86, 439–445. [Google Scholar] [CrossRef]
- Sahoo, S.K.; Labhasetwar, V. Enhanced Antiproliferative Activity of Transferrin-Conjugated Paclitaxel-Loaded Nanoparticles Is Mediated via Sustained Intracellular Drug Retention. Mol. Pharm. 2005, 2, 373–383. [Google Scholar] [CrossRef]
- Zhang, Z.; Leeb, S.H.; Feng, S.-S. Folate-decorated poly(lactide-co-glycolide)-vitamin E TPGS nanoparticles for targeted drug delivery. Biomaterials 2007, 28, 1889–1899. [Google Scholar] [CrossRef]
- Rao, K.S.; Keddy, M.K.; Horning, J.L.; Labhasetwar, V. TAT-conjugated nanoparticles for the CNS delivery of anti-HIV drugs. Biomaterials 2008, 29, 4429–4438. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.I.; Lee, J.H.; Kim, J.-Y.; Heo, S.U.; Jeong, Y.Y.; Kim, Y.H.; Tae, G. Targeted antitumor efficacy and imaging via multifunctional nano-carrier conjugated with anti-HER2 trastuzumab. Nanomedicine 2015, 11, 359–368. [Google Scholar] [CrossRef]
- Yang, N.; Ding, Y.; Zhang, Y.; Wang, B.; Zhao, X.; Cheng, K.; Huang, Y.; Taleb, M.; Zhao, J.; Dong, W.F.; et al. Surface Functionalization of Polymeric Nanoparticles with Umbilical Cord-Derived Mesenchymal Stem Cell Membrane for Tumor-Targeted Therapy. ACS Appl. Mater. Interfaces 2018, 11, 22963–22973. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Qiu, L.; Chen, Q.; Hao, T.; Qiao, M.; Zhao, H.; Zhang, J.; Hu, H.; Zhao, X.; Chen, D.; et al. pH-sensitive nanoparticles of poly(L-histidine)–poly(lactide-coglycolide)–tocopheryl polyethylene glycol succinate for anti-tumor drug delivery. Acta Biomater. 2015, 11, 137–150. [Google Scholar] [CrossRef] [PubMed]
- Joseph, E.; Saha, R.N. Investigations on pharmacokinetics and biodistribution of polymeric and solid lipid nanoparticulate systems of atypical antipsychotic drug: Effect of material used and surface modification. Drug Dev. Ind. Pharm. 2017, 22, 678–686. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Teply, B.A.; Sherifi, I.; Sung, J.; Luther, G.; Gu, F.X.; Levy-Nissenbaum, E.; Radovic-Moreno, A.F.; Langer, R.; Farokhzad, O.C. Formulation of functionalized PLGA–PEG nanoparticles for in vivo targeted drug delivery. Biomaterials 2007, 28, 869–876. [Google Scholar] [CrossRef]
- Liu, L.; Guo, K.; Lu, J.; Venkatraman, S.S.; Luo, D.; Ng, K.C.; Ling, E.A.; Moochhala, S.; Yang, Y.Y. Biologically active core/shell nanoparticles self-assembled from cholesterol-terminated PEG-TAT for drug delivery across the blood-brain barrier. Biomaterials 2008, 29, 1509–1517. [Google Scholar] [CrossRef]
- Hwang, D.W.; Son, S.; Jang, J.; Youn, H.; Lee, S.; Lee, D.; Lee, Y.S.; Jeong, J.M.; Kim, W.J.; Lee, D.S. A brain-targeted rabies virus glycoprotein-disulfide linked PEI nanocarrier for delivery of neurogenic microRNA. Biomaterials 2011, 32, 4968–4975. [Google Scholar] [CrossRef]
- Zhang, G.; Gao, J.; Qian, J.; Zhang, L.; Zheng, K.; Zhong, K.; Cai, D.; Zhang, X.; Wu, Z. Hydroxylated Mesoporous Nanosilica Coated by Polyethylenimine Coupled with Gadolinium and Folic Acid: A Tumor-Targeted T1 Magnetic Resonance Contrast Agent and Drug Delivery System. ACS Appl. Mater. Interfaces 2015, 7, 14192–14200. [Google Scholar] [CrossRef]
- Mo, Y.; Lim, L.-Y. Paclitaxel-loaded PLGA nanoparticles: Potentiation of anticancer activity by surface conjugation with wheat germ agglutinin. J. Control. Release 2005, 108, 244–262. [Google Scholar] [CrossRef]
- Narayanan, S.; Binulal, N.S.; Mony, U.; Manzoor, K.; Nair, S.; Menon, D. Folate targeted polymeric ‘green’ nanotherapy for cancer. Nanotechnology 2010, 21, 285107–285120. [Google Scholar] [CrossRef] [PubMed]
- Farokhzad, O.C.; Cheng, J.; Teply, B.A.; Sherifi, I.; Jon, S.; Kantoff, P.W.; Richie, J.P.; Langer, R. Targeted nanoparticle-aptamer bioconjugates for cancer chemotherapy in vivo. PNAS 2006, 103, 6315–6320. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Wang, Y.; Wang, Y.; Zhang, M.; Luo, Y.; Tang, J.; Wang, Z.; Wang, D.; Hao, L.; Wang, Z. Paclitaxel-loaded and A10-3.2 aptamer-targeted poly(lactide-co-glycolic acid) nanobubbles for ultrasound imaging and therapy of prostate cancer. Int. J. Nanomed. 2017, 12, 5313–5330. [Google Scholar] [CrossRef] [PubMed]
- Hrkach, J.; Von Hoff, D.; Mukkaram, A.M.; Andrianova, E.; Auer, J.; Campbell, T.; De Witt, D.; Figa, M.; Figueiredo, M.; Horhota, A.; et al. Preclinical Development and Clinical Translation of a PSMA-Targeted Docetaxel Nanoparticle with a Differentiated Pharmacological Profile. Nanomedicine 2012, 4, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Abstiens, K.; Gregoritza, M.; Goepferich, A.M. Ligand Density and Linker Length are Critical Factors for Multivalent Nanoparticle-Receptor Interactions. ACS Appl. Mater. Interfaces 2019, 11, 1311–1320. [Google Scholar] [CrossRef]
- Bender, E.A.; Cavalcante, M.F.; Adorne, M.D.; Colomé, L.M.; Guterres, S.S.; Abdalla, D.S.P.; Pohlmann, A.R. New strategy to surface functionalization of polymeric nanoparticles: One-pot synthesis of scFv anti-LDL(−)-functionalized nanocapsules. Pharm. Res. 2014, 31, 2975–2987. [Google Scholar] [CrossRef]
- Bender, E.A.; Adorne, M.D.; Colomé, L.M.; Abdalla, D.S.P.; Guterres, S.S.; Pohlmann, A.R. Hemocompatibility of poly(ε-caprolactone) lipid-core nanocapsules stabilized with polysorbate 80-lecithin and uncoated or coated with chitosan. Int. J. Pharm. 2012, 426, 271–279. [Google Scholar] [CrossRef]
- Mayer, F.Q.; Adorne, M.D.; Bender, E.A.; de Carvalho, T.G.; Dilda, A.C.; Beck, R.C.; Guterres, S.S.; Giugliani, R.; Matte, U.; Pohlmann, A.R. Laronidase-Functionalized Multiple-Wall Lipid-Core Nanocapsules: Promising Formulation for a More Effective Treatment of Mucopolysaccharidosis Type I. Pharm. Res. 2015, 32, 941–954. [Google Scholar] [CrossRef]
- Cavalcanti, M.F.; Kazuma, S.M.; Bender, E.A.; Adorne, M.D.; Ullian, M.; Veras, M.M.; Saldiva, P.H.; Maranhão, A.Q.; Guterres, S.S.; Pohlmann, A.R.; et al. A nanoformulation containing a scFv reactive to electronegative LDL inhibits atherosclerosis in LDL receptor knockout mice. Eur. J. Pharm. Biopharm. 2016, 107, 120–129. [Google Scholar] [CrossRef]
- Oliveira, C.P.; Prado, W.A.; Lavayen, V.; Büttenbender, S.L.; Beckenkamp, A.; Martins, B.S.; Lüdtke, D.S.; Campo, L.F.; Rodembusch, F.S.; Buffon, A.; et al. Bromelain-Functionalized Multiple-Wall Lipid-Core Nanocapsules: Formulation, Chemical Structure and Antiproliferative Effect Against Human Breast Cancer Cells (MCF-7)]. Pharm. Res. 2017, 34, 438–452. [Google Scholar] [CrossRef]
- Oliveira, C.P.; Büttenbender, S.L.; Prado, W.A.; Beckenkamp, A.; Asbahr, A.C.; Buffon, A.; Guterres, S.S.; Pohlmann, A.R. Enhanced and Selective Antiproliferative Activity of Methotrexate-Functionalized-Nanocapsules to Human Breast Cancer Cells (MCF-7). Nanomaterials 2018, 8, 24. [Google Scholar] [CrossRef]
- Antonow, M.B.; Franco, C.; Prado, W.; Beckenkamp, A.; Silveira, G.P.; Buffon, A.; Guterres, S.S.; Pohlmann, A.R. Arginylglycylaspartic Acid-Surface-Functionalized Doxorubicin-Loaded Lipid-Core Nanocapsules as a Strategy to Target Alpha(V) Beta(3) Integrin Expressed on Tumor Cells. Nanomaterials 2018, 8, 2. [Google Scholar] [CrossRef] [PubMed]
- Jornada, D.S.; Fiel, L.A.; Bueno, K.; Gerent, J.F.; Petzhold, C.L.; Beck, R.C.R.; Guterres, S.S.; Pohlmann, A.R. Lipid-core nanocapsules: Mechanism of self-assembly, control of size and loading capacity. Soft Matter 2012, 8, 6646–6655. [Google Scholar] [CrossRef]
- Azevedo, R.C.P.; Ribeiro, G.P.; de Araújo, M.B. Desenvolvimento e validação do ensaio de dissolução para captopril em cápsulas magistrais por CLAE. Braz. J. Pharm. 2008, 44, 44261–44269. [Google Scholar] [CrossRef]
- US Pharmacopeia XXIV; USP: Rockville, MD, USA, 2006; Volume 29.
- Roger, E.; Lagarce, F.; Benoit, J.-P. Development and characterization of a novel lipid nanocapsule formulation of Sn38 for oral administration. Eur. J. Pharm. Biopharm. 2011, 79, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Horak, E.; Hopfer, S.M.; Sunderman, F.W., Jr. Spectrophotometric assay for urinary N-acetyl-beta-D-glucosaminidase activity. Clin. Chem. 1981, 27, 1180–1185. [Google Scholar] [PubMed]
- Grotto, D.; Santa Maria, L.D.; Boeira, S.; Valentini, J.; Charão, M.F.; Moro, A.M.; Nascimento, P.C.; Pomblum, V.J.; Garcia, S.C. Rapid quantification of malondialdehyde in plasma by high performance liquid chromatography–visible detection. J. Pharm. Biomed. 2007, 43, 619–624. [Google Scholar] [CrossRef] [PubMed]
- Ellmann, G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef]
- Oliveira, C.P.; Venturini, C.G.; Donida, B.; Poletto, F.S.; Guterres, S.S.; Pohlmann, A.R. An algorithm to determine the mechanism of drug distribution in lipid-core nanocapsule formulations. Soft Matter 2013, 9, 1141–1150. [Google Scholar] [CrossRef]
- Youm, I.; Murowchick, J.B.; Youan, B.C. Entrapment and release kinetics of furosemide from pegylated nanocarriers. Colloids Surf. B 2012, 94, 133–142. [Google Scholar] [CrossRef]
- Keck, C.M.; Muller, R.H. Size analysis of submicron particles by laser diffractometry-90% of the published measurements are false. Int. J. Pharm. 2008, 355, 150–163. [Google Scholar] [CrossRef] [PubMed]
- Bianchin, M.D.; Külkamp-Guerreiro, I.C.; Oliveira, C.P.; Contri, R.V.; Guterres, S.S.; Pohlmann, A.R. Radar charts based on particle sizing as an approach to establish the fingerprints of polymeric nanoparticles in aqueous formulations. J. Drug Deliv. Sci. Technol. 2015, 30, 180–189. [Google Scholar] [CrossRef]
- Saveyn, H.; De Baets, B.; Thas, O.; Hole, P.; Smith, J.; Van der Meeren, P. Accurate particle size distribution determination by nanoparticle tracking analysis based on 2-D Brownian dynamics simulation. J. Colloid Interface Sci. 2010, 352, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Diopa, M.; Aubervalb, N.; Viciglioa, A.; Langloisa, A.; Bietigera, W. Design, characterisation, and bioefficiency of insulin–chitosan nanoparticles after stabilisation by freeze-drying or cross-linking. Int. J. Pharm. 2015, 491, 402–4089. [Google Scholar] [CrossRef]
- Roger, E.; Lagarce, F.; Benoit, J.-P. The gastrointestinal stability of lipid nanocapsules. Pharm. Nanotechnol. 2009, 379, 260–265. [Google Scholar] [CrossRef]
- Garcıa-Fuentes, M.; Torres, D.; Alonso, M.J. Design of lipid nanoparticles for the oral delivery of hydrophilic macromolecules. Colloids Surf. B 2002, 27, 159–168. [Google Scholar] [CrossRef]
- Zimmerman, E.; Müller, R.H. Electrolyte- and pH- stabilities of aqueous solid lipid nanoparticles (SLN) dispersions in artificial gastrointestinal media. Eur. J. Pharm. Biopharm. 2001, 52, 203–210. [Google Scholar] [CrossRef]
- Antal, I.; Kubovcikov, M.; Zavisova, V.; Koneracka, M.; Pechanova, O.; Barta, A.; Cebova, M.; Antala, V.; Diko, P.; Zduriencikova, M.; et al. Magnetic poly(D,L-lactide) nanoparticles loaded with aliskiren: A promising tool for hypertension treatment. J. Magn. Magn. Mater. 2015, 380, 280–284. [Google Scholar] [CrossRef]
- Kim, Y.I.; Fluckiger, L.; Hoffman, M.; Lartaud-Idjouadiene, I.; Atkinson, J.; Maincent, P. The antihypertensive effect of orally administered nifedipine-loaded nanoparticles in spontaneously hypertensive rats. Br. J. Pharmacol. 1997, 120, 399–404. [Google Scholar] [CrossRef]
- Heckmann, U.; Zidek, W.; Schurek, H.J. Sodium reabsorption in the isolated perfused kidney of normotensive and spontaneously hypertensive rats. J. Hypertens. Suppl. 1989, 7, S172–S173. [Google Scholar] [CrossRef]
- Ljutić, D.; Kes, P. The role of arterial hypertension in the progression of non-diabetic glomerular diseases. Nephrol. Dial. Transplant. 2003, 18, v28–v30. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tajima, A.; Hans, F.J.; Livingstone, D.; Wei, L.; Finnegan, W.; DeMaro, J.; Fenstermacher, J. Smaller Local Brain Volumes and Cerebral Atrophy in Spontaneously Hypertensive Rats. Hypertension 1993, 21, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Sumbalová, Z.; Kucharská, J.; Kristek, F. Losartan improved respiratory function and coenzyme Q content in brain mitochondria of young spontaneously hypertensive rats. Cell. Mol. Neurobiol. 2010, 30, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, M.C.; Caldiz, C.; Fantinelli, J.C.; Garciarena, C.D.; Console, G.M.; de Cingolani, G.E.C.; Mosca, S.M. Is cardiac hypertrophy in spontaneously hypertensive rats the cause or the consequence of oxidative stress? Hypertens. Res. 2008, 31, 1465–1476. [Google Scholar] [CrossRef] [PubMed]
- Celia, C.; Trapasso, H.; Cosco, D.; Paolino, D.; Fresta, M. Turbiscan Lab® Expert analysis of the stability of ethosomes® and ultradeformable liposomes containing a bilayer fluidizing agent. Colloids Surf. B 2009, 72, 155–160. [Google Scholar] [CrossRef]





| Group | Water Consumption (%) | Urine Volume (%) | Water/Urine Relation |
|---|---|---|---|
| Control | 9.1 ± 0.5 | 1.8 ± 0.2 | 19.6 ± 2.1 |
| Captopril | 11.3 ± 0.9 | 1.9 ± 0.1 | 17.6 ± 1.5 |
| Captopril + Furosemide | 9.3 ± 0.7 | 2.2 ± 0.2 | 24.3 ± 1.8 |
| Capt(0.5)-Zn(25)-MLNC | 12.0 ± 0.7 | 3.3 ± 0.4 a,b | 27.7 ± 2.9 |
| Capt(0.5)-Zn(25)-MLNC-Fur(0.45) | 8.7 ± 0.5 c | 2.4 ± 0.2 | 28.3 ± 2.5 |
| Capt(0.5)-MLNC-Fur(0.45) | 10.5 ± 0.5 | 2.0 ± 0.2 | 18.9 ± 2.3 |
| Group | ALT (U L−1) | AST (U L−1) | Urea (mg dL−1) | Creatinine (mg dL−1) | Uric Acid (mg dL−1) | Na+ (mmol L−1) | K+ (mmol L−1) | Cl− (mmol L−1) |
|---|---|---|---|---|---|---|---|---|
| Control | 62.2 ± 7.0 | 141.3 ± 5.1 | 98.2 ± 3.2 | 4.4 ± 0.2 | 110.8 ± 15.5 | 0.25 ± 0.01 | 58.2 ± 0.8 | 0.8 ± 0.2 |
| Captopril | 60.7 ± 5.1 | 145.2 ± 2.8 | 102.7 ± 2.0 | 5.1 ± 0.3 | 111.8 ± 10.2 | 0.27 ± 0.02 | 60.7 ± 3.2 | 1.4 ± 0.1 |
| Captopril + Furosemide | 65.5 ± 4.8 | 137.4 ± 7.6 | 85.0 ± 9.6 | 4.5 ± 0.2 | 124.3 ± 20.6 | 0.22 ± 0.02 | 51.5 ± 2.6 | 1.2 ± 0.4 |
| Capt(0.5)-Zn(25)-MLNC | 55.8 ± 3.3 | 143.7 ± 1.9 | 101.8 ± 1.4 | 4.7 ± 0.2 | 105.5 ± 7.5 | 0.27 ± 0.02 | 53.5 ± 2.4 | 1.3 ± 0.2 |
| Capt(0.5)-Zn(25)-MLNC-Fur(0.45) | 76.8 ± 11.9 | 142.0 ± 1.1 | 99.2 ± 0.8 | 4.4 ± 0.1 | 125.0 ± 23.4 | 0.26 ± 0.01 | 60.2 ± 1.2 | 0.8 ± 0.1 |
| Capt(0.5)-MLNC-Fur(0.45) | 58.0 ± 3.9 | 143.4 ± 0.5 | 102.1 ± 9.3 | 4.1 ± 0.1 | 107.4 ± 8.1 | 0.25 ± 0.02 | 58.1 ± 3.9 | 1.1 ± 0.1 |
| Group | Liver (%) | Spleen (%) | Right Kidney (%) | Left Kidney (%) | Lungs (%) | Heart (%) | Brain (%) |
|---|---|---|---|---|---|---|---|
| Control | 4.4 ± 0.14 | 0.17 ± 0.003 | 0.40 ± 0.004 | 0.41 ± 0.012 | 0.51 ± 0.02 | 0.41 ± 0.009 | 0.78 ± 0.017 |
| Captopril | 4.8 ± 0.09 | 0.18 ± 0.002 | 0.39 ± 0.017 | 0.39 ± 0.015 | 0.63 ± 0.07 | 0.41 ± 0.007 | 0.81 ± 0.013 |
| Captopril + Furosemide | 4.3 ± 0.14 | 0.18 ± 0.004 | 0.41 ± 0.006 | 0.40 ± 0.014 | 0.53 ± 0.03 | 0.44 ± 0.004 | 0.81 ± 0.021 |
| Capt(0.5)-Zn(25)-MLNC | 4.6 ± 0.14 | 0.18 ± 0.003 | 0.38 ± 0.004 | 0.39 ± 0.007 | 0.52 ± 0.02 | 0.42 ± 0.008 | 0.86 ± 0.024 |
| Capt(0.5)-Zn(25)-MLNC-Fur(0.45) | 4.3 ± 0.12 | 0.17 ± 0.003 | 0.39 ± 0.006 | 0.39 ± 0.006 | 0.49 ± 0.02 | 0.43 ± 0.012 | 0.76 ± 0.023 |
| Capt(0.5)-MLNC-Fur(0.45) | 4.3 ± 0.05 | 0.18 ± 0.005 | 0.39 ± 0.006 | 0.39 ± 0.006 | 0.57 ± 0.03 | 0.41 ± 0.007 | 0.87 ± 0.009 a,b |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Michalowski, C.B.; Arbo, M.D.; Altknecht, L.; Anciuti, A.N.; Abreu, A.S.G.; Alencar, L.M.R.; Pohlmann, A.R.; Garcia, S.C.; Guterres, S.S. Oral Treatment of Spontaneously Hypertensive Rats with Captopril-Surface Functionalized Furosemide-Loaded Multi-Wall Lipid-Core Nanocapsules. Pharmaceutics 2020, 12, 80. https://doi.org/10.3390/pharmaceutics12010080
Michalowski CB, Arbo MD, Altknecht L, Anciuti AN, Abreu ASG, Alencar LMR, Pohlmann AR, Garcia SC, Guterres SS. Oral Treatment of Spontaneously Hypertensive Rats with Captopril-Surface Functionalized Furosemide-Loaded Multi-Wall Lipid-Core Nanocapsules. Pharmaceutics. 2020; 12(1):80. https://doi.org/10.3390/pharmaceutics12010080
Chicago/Turabian StyleMichalowski, Cecilia B., Marcelo D. Arbo, Louise Altknecht, Andréia N. Anciuti, Angélica S. G. Abreu, Luciana M. R. Alencar, Adriana R. Pohlmann, Solange C. Garcia, and Sílvia S. Guterres. 2020. "Oral Treatment of Spontaneously Hypertensive Rats with Captopril-Surface Functionalized Furosemide-Loaded Multi-Wall Lipid-Core Nanocapsules" Pharmaceutics 12, no. 1: 80. https://doi.org/10.3390/pharmaceutics12010080
APA StyleMichalowski, C. B., Arbo, M. D., Altknecht, L., Anciuti, A. N., Abreu, A. S. G., Alencar, L. M. R., Pohlmann, A. R., Garcia, S. C., & Guterres, S. S. (2020). Oral Treatment of Spontaneously Hypertensive Rats with Captopril-Surface Functionalized Furosemide-Loaded Multi-Wall Lipid-Core Nanocapsules. Pharmaceutics, 12(1), 80. https://doi.org/10.3390/pharmaceutics12010080