Stability and Compatibility Studies of Levothyroxine Sodium in Solid Binary Systems—Instrumental Screening
Abstract
1. Introduction
2. Materials and Methods
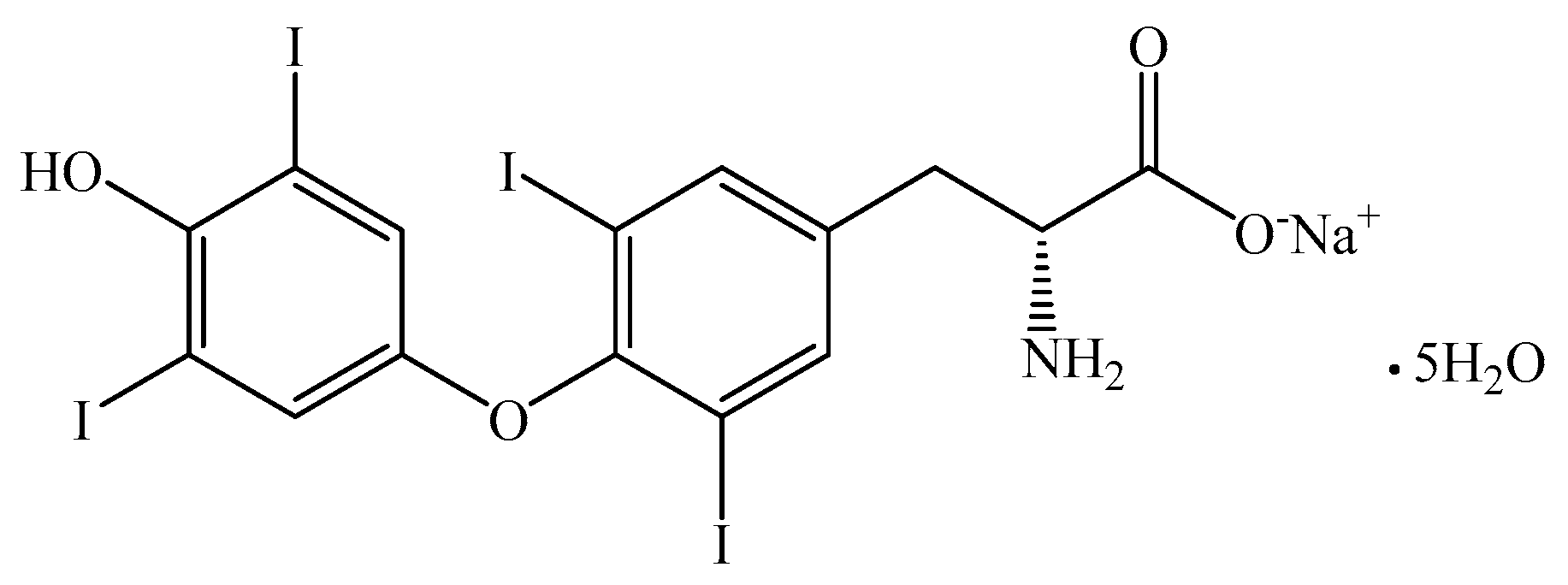
2.1. Reagents
2.2. Preparation of Samples
2.3. Spectroscopic Investigations
2.4. Thermal Stability Investigations
3. Results and Discussion
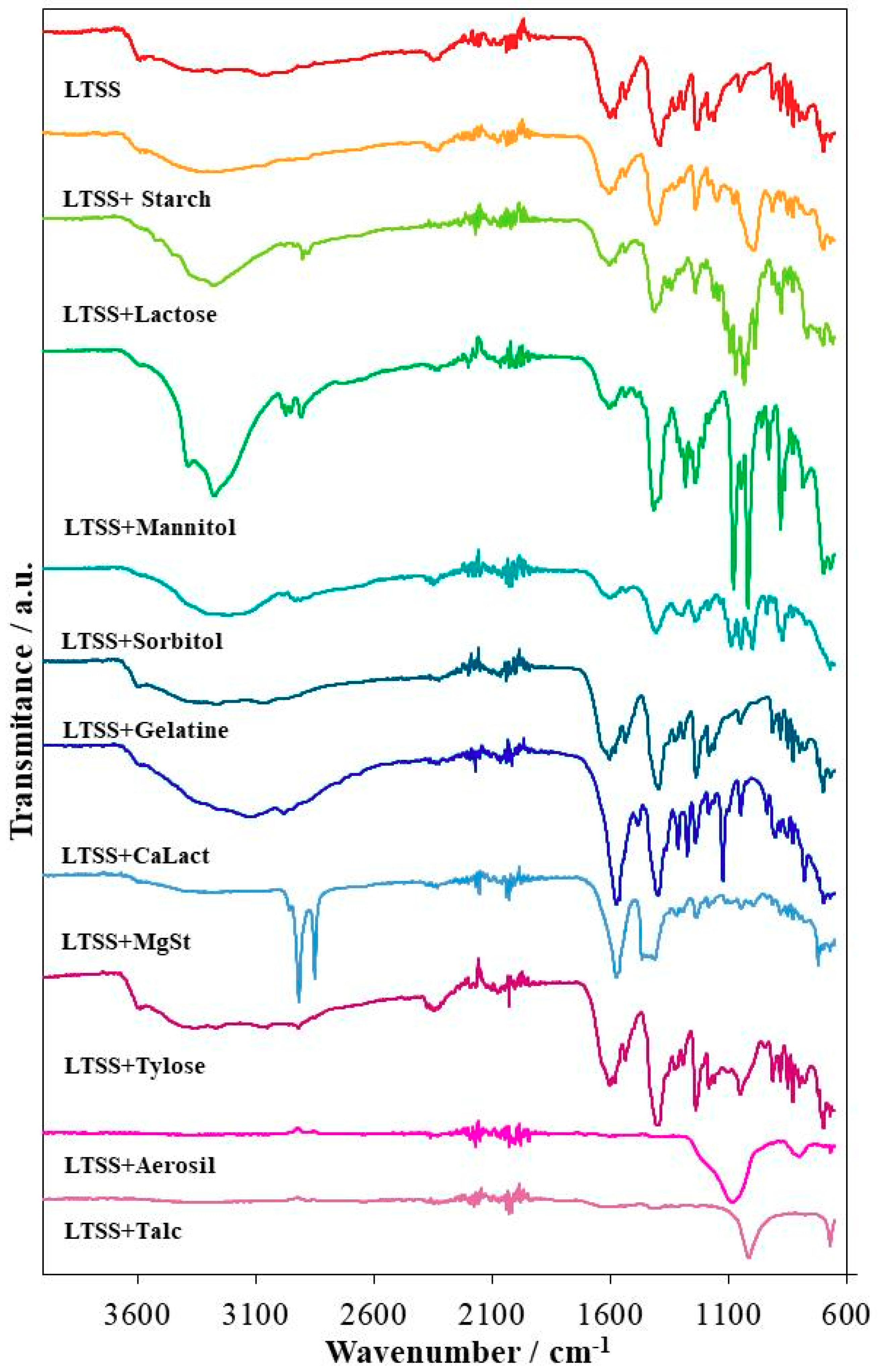
3.1. UATR-FTIR Analysis
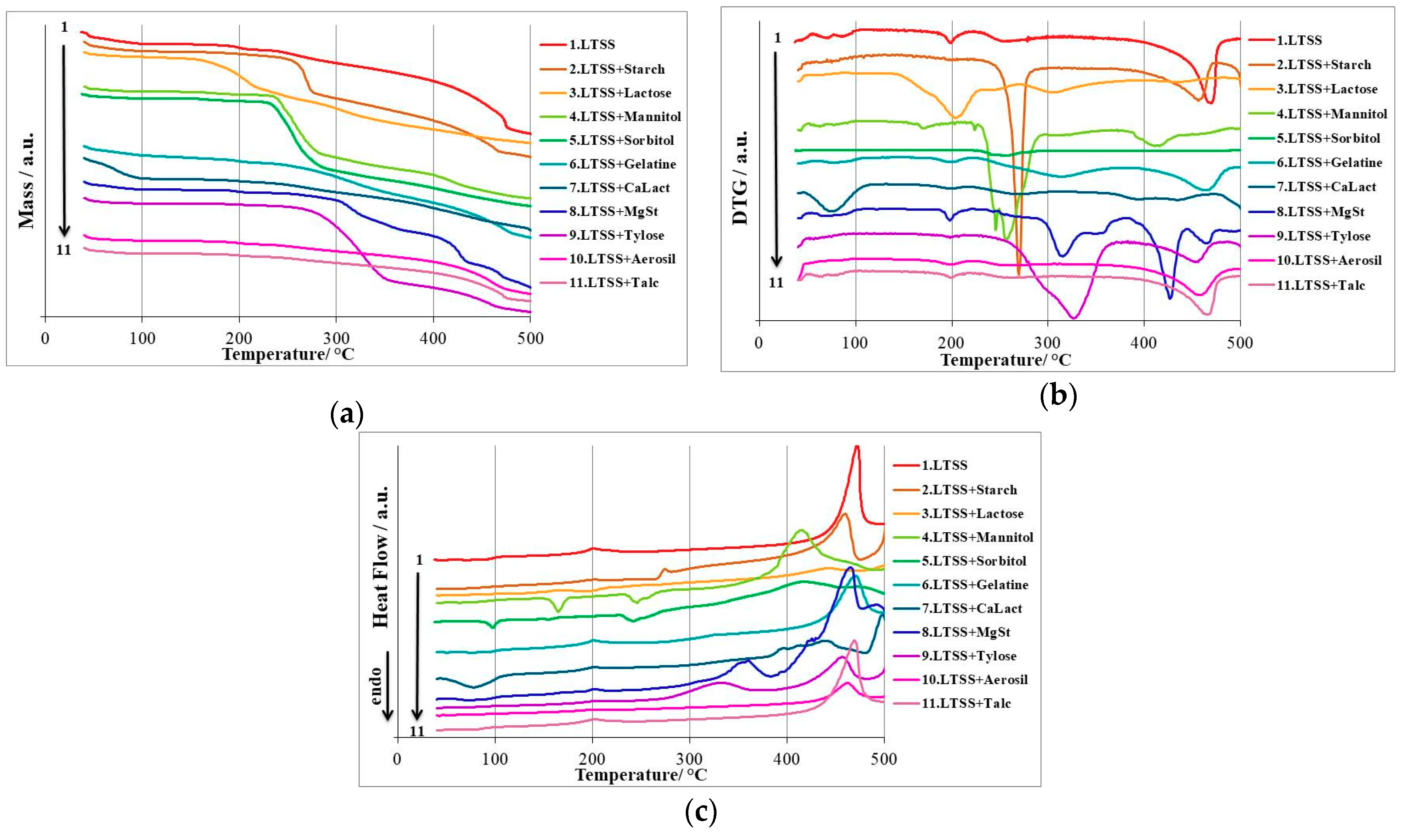
3.2. Thermal Stability Investigations
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Collier, J.W.; Shah, R.B.; Bryant, A.R.; Habib, M.J.; Khan, M.A.; Faustino, P.J. Development and application of a validated HPLC method for the analysis of dissolution samples of levothyroxine sodium drug products. J. Pharm. Biomed. Anal. 2011, 54, 433–438. [Google Scholar] [CrossRef]
- Collier, J.W.; Shah, R.B.; Gupta, A.; Sayeed, V.; Habib, M.J.; Khan, M.A. Influence of formulation and processing factors on stability of levothyroxine sodium pentahydrate. AAPS PharmSciTech 2010, 11, 818–825. [Google Scholar] [CrossRef]
- Hennessey, J.V. The emergence of levothyroxine as a treatment for hypothyroidism. Endocrine 2017, 55, 6–18. [Google Scholar] [CrossRef] [PubMed]
- Colucci, P.; Yue, C.S.; Ducharme, M.; Benvenga, S. A Review of the Pharmacokinetics of Levothyroxine for the Treatment of Hypothyroidism. Eur. Endocrinol. 2013, 9, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Levothyroxine on Drugbank. Available online: https://www.drugbank.ca/drugs/DB00451 (accessed on 15 June 2019).
- Lipp, H.-P.; Hostalek, U. A new formulation of levothyroxine engineered to meet new specification standards. Curr. Med. Res. Opin. 2019, 35, 147–150. [Google Scholar] [CrossRef] [PubMed]
- Ward, L.S. Levothyroxine and the problem of interchangeability of drugs with narrow therapeutic index. Arq. Bras. Endocrinol. Metabol. 2011, 55, 429–434. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Concordet, D.; Gandia, P.; Montastruc, J.-L.; Bousquet-Melou, A.; Lees, P.; Ferran, A.; Toutain, P.-L. Levothyrox((R)) New and Old Formulations: Are they Switchable for Millions of Patients? Clin. Pharmacokinet. 2019, 58, 827–833. [Google Scholar] [CrossRef] [PubMed]
- Rostami, E.; Kashanian, S.; Azandaryani, A.H. Preparation of solid lipid nanoparticles as drug carriers for levothyroxine sodium with in vitro drug delivery kinetic characterization. Mol. Biol. Rep. 2014, 41, 3521–3527. [Google Scholar] [CrossRef]
- Kashanian, S.; Rostami, E. PEG-stearate coated solid lipid nanoparticles as levothyroxine carriers for oral administration. J. Nanopart. Res. 2014, 16. [Google Scholar] [CrossRef]
- Cappelli, C.; Pirola, I.; Gandossi, E.; Formenti, A.; Castellano, M. Oral liquid levothyroxine treatment at breakfast: A mistake? Eur. J. Endocrinol. 2014, 170, 95–99. [Google Scholar] [CrossRef]
- Shah, H.S.; Chaturvedi, K.; Hamad, M.; Bates, S.; Hussain, A.; Morris, K. New Insights on Solid-State Changes in the Levothyroxine Sodium Pentahydrate during Dehydration and its Relationship to Chemical Instability. AAPS PharmSciTech 2019, 20. [Google Scholar] [CrossRef] [PubMed]
- Vakili, H.; Wickstrom, H.; Desai, D.; Preis, M.; Sandler, N. Application of a handheld NIR spectrometer in prediction of drug content in inkjet printed orodispersible formulations containing prednisolone and levothyroxine. Int. J. Pharm. 2017, 524, 414–423. [Google Scholar] [CrossRef] [PubMed]
- Singare, P.U.; Belamkar, N. Applying DMAIC Principles For Improving Method Performance of Quantitative Determination Of Levothyroxine Sodium In Tablet Dosage Form Using High Performance Liquid Chromatography Technique. Res. J. Pharm. Biol. Chem. Sci. 2016, 7, 2041–2052. [Google Scholar]
- Ledeţi, I.; Ledeţi, A.; Vlase, G.; Vlase, T.; Matusz, P.; Bercean, V.; Şuta, L.M.; Piciu, D. Thermal stability of synthetic thyroid hormone l-thyroxine and l-thyroxine sodium salt hydrate both pure and in pharmaceutical formulations. J. Pharm. Biomed. Anal. 2016, 125, 33–40. [Google Scholar] [CrossRef]
- Abdallah, S.; Mohamed, I. Factor Affecting Photo and Thermal Stability of Levothyroxine Sodium. Br. J. Pharm. Res. 2016, 10. [Google Scholar] [CrossRef]
- Hamad, M.L.; Engen, W.; Morris, K.R. Impact of hydration state and molecular oxygen on the chemical stability of levothyroxine sodium. Pharm. Dev. Technol. 2015, 20, 314–319. [Google Scholar] [CrossRef]
- Hamada, Y.; Masuda, K.; Okubo, M.; Nakasa, H.; Sekine, Y.; Ishii, I. Pharmaceutical Studies of Levothyroxine Sodium Hydrate Suppository Provided as a Hospital Preparation. Biol. Pharm. Bull. 2015, 38, 625–628. [Google Scholar] [CrossRef][Green Version]
- Negro, R.; Valcavi, R.; Agrimi, D.; Toulis, K.A. Levothyroxine Liquid Solution Versus Tablet for Replacement Treatment in Hypothyroid Patients. Endocr. Pract. 2014, 20, 901–906. [Google Scholar] [CrossRef]
- Gupta, D.; Odom, C.; Bethea, C.; Plattenbyrg, J. Effect of excipients on the stability of levothyroxine sodium tablets. J. Clin. Pharm. Ther. 1990, 15, 331–336. [Google Scholar] [CrossRef]
- Patel, H.; Stalcup, A.; Dansereau, R.; Sakr, A. The effect of excipients on the stability of levothyroxine sodium pentahydrate tablets. Int. J. Pharm. 2003, 264, 35–43. [Google Scholar] [CrossRef]
- Shah, R.B.; Collier, J.S.; Sayeed, V.A.; Bryant, A.; Habib, M.J.; Khan, M.A. Tablet Splitting of a Narrow Therapeutic Index Drug: A Case with Levothyroxine Sodium. AAPS PharmSciTech 2010, 11, 1359–1367. [Google Scholar] [CrossRef] [PubMed]
- Ledeţi, I.; Vlase, G.; Vlase, T.; Şuta, L.M.; Todea, A.; Fuliaş, A. Selection of solid-state excipients for simvastatin dosage forms through thermal and nonthermal techniques. J. Therm. Anal. Calorim. 2015, 121, 1093–1102. [Google Scholar] [CrossRef]
- Bandas (Ratiu), C.; Orha, C.; Misca, C.; Lazau, C.; Sfirloaga, P.; Olariu, S. Photocatalytical Inactivation of Enterococcus faecalis from Water Using Functional Materials Based on Natural Zeolite and Titanium Dioxide. Chin. J. Chem. Eng. 2014, 22, 38–43. [Google Scholar] [CrossRef]
- Ledeti, I.; Bolintineanu, S.; Vlase, G.; Circioban, D.; Dehelean, C.; Suta, L.M.; Caunii, A.; Ledeti, A.; Vlase, T.; Murariu, M. Evaluation of solid-state thermal stability of donepezil in binary mixtures with excipients using instrumental techniques. J. Therm. Anal. Calorim. 2017, 130, 425–431. [Google Scholar] [CrossRef]
- Ledeţi, I.; Budiul, M.; Matusz, P.; Vlase, G.; Circioban, D.; Dehelean, C.; Şuta, L.M.; Caunii, A.; Ledeţi, A.; Vlase, T.; et al. Preformulation studies for nortriptyline: Solid-state compatibility with pharmaceutical excipients. J. Therm. Anal. Calorim. 2018, 131, 191–199. [Google Scholar] [CrossRef]
- Ledeti, I.; Pusztai, A.M.; Muresan, C.M.; Circioban, D.; Vlase, G.; Murariu, M.; Suta, L.-M.; Vlase, T.; Ledeti, A.; Suciu, O.; et al. Study of solid-state degradation of prochlorperazine and promethazine. J. Therm. Anal. Calorim. 2018, 134, 731–740. [Google Scholar] [CrossRef]
- Trandafirescu, C.; Soica, C.; Ledeti, A.; Borcan, F.; Suta, L.-M.; Murariu, M.; Dehelean, C.; Ionescu, D.; Ledeti, I. Preformulation Studies for Albendazole: A DSC and FTIR analysis of binary mixtures with excipients. Rev. Chim. 2016, 67, 463–467. [Google Scholar]
- Ledeti, I.; Bercean, V.; Alexa, A.; Soica, C.; Suta, L.-M.; Dehelean, C.; Trandafirescu, C.; Muntean, D.; Licker, M.; Fulias, A. Preparation and Antibacterial Properties of Substituted 1,2,4-Triazoles. J. Chem. 2015, 2015. [Google Scholar] [CrossRef]
- Trandafirescu, C.; Gyeresi, A.; Szabadai, Z.; Kata, M.; Aigner, Z. Solid-state characterization of bifonazole-beta-cyclodextrin binary systems. Note I. Farmacia 2014, 62, 513–523. [Google Scholar]
- Ledeţi, A.; Vlase, G.; Vlase, T.; Bercean, V.; Murariu, M.S.; Ledeţi, I.; Şuta, L.M. Solid-state preformulation studies of amiodarone hydrochloride. J. Therm. Anal. Calorim. 2016, 126, 181–187. [Google Scholar] [CrossRef]
- Euthyrox Dosage. Available online: https://www.drugs.com/dosage/euthyrox.html (accessed on 11 May 2019).
- Meira, R.Z.C.; Biscaia, I.F.B.; Nogueira, C.; Murakami, F.S.; Bernardi, L.S.; Oliveira, P.R. Solid-state characterization and compatibility studies of penciclovir, lysine hydrochloride, and pharmaceutical excipients. Materials 2019, 12, 3154. [Google Scholar] [CrossRef] [PubMed]
- Lopes, M.S.; Catelani, T.A.; Nascimento, A.L.C.S.; Garcia, J.S.; Trevisan, M.G. Ketoconazole: Compatibility with pharmaceutical excipients using DSC and TG techniques. J. Therm. Anal. Calorim. 2019. [Google Scholar] [CrossRef]
- Cristea, M.; Baul, B.; Ledeţi, I.; Ledeţi, A.; Vlase, G.; Vlase, T.; Karolewicz, B.; Ştefănescu, O.; Dragomirescu, A.O.; Mureşan, C.; et al. Preformulation studies for atorvastatin calcium: An instrumental approach. J. Therm. Anal. Calorim. 2019, 138, 2799–2806. [Google Scholar] [CrossRef]
- Ding, T.; Chen, L.; Zhai, L.H.; Fu, Y.; Sun, B.W. Compatibility study of rivaroxaban and its pharmaceutical excipients. J. Therm. Anal. Calorim. 2017, 130, 1569–1573. [Google Scholar] [CrossRef]
- Ali, F.; Kumar, R.; Sahu, P.L.; Singh, G.N. Physicochemical characterization and compatibility study of roflumilast with various pharmaceutical excipients. J. Therm. Anal. Calorim. 2017, 130, 1627–1641. [Google Scholar] [CrossRef]
- Matos, A.P.S.; Costa, J.S.; Boniatti, J.; Seiceira, R.C.; Pitaluga, A.; Oliveira, D.L.; Viçosa, A.L.; Holandino, C. Compatibility study between diazepam and tablet excipients. J. Therm. Anal. Calorim. 2017, 127, 1675–1682. [Google Scholar] [CrossRef]
- Ledeti, I.; Bolintineanu, S.; Vlase, G.; Circioban, D.; Ledeti, A.; Vlase, T.; Suta, L.M.; Caunii, A.; Murariu, M. Compatibility study between antiparkinsonian drug Levodopa and excipients by FTIR spectroscopy, X-ray diffraction and thermal analysis. J. Therm. Anal. Calorim. 2017, 130, 433–441. [Google Scholar] [CrossRef]

| Sample | Characteristic UATR-FTIR Bands/cm−1 |
|---|---|
| LTSS | 3650–2750 (wide band); 3589; 3568; 3526; 3448; 3266; 3052; 2939; 2868; 1630; 1603; 1578; 1536; 1500; 1455; 1390; 1327; 1291; 1235; 1183; 1162; 1053; 913; 881; 849; 827; 799; 774; 712; 702; 698; 669 |
| LTSS+Starch | 3650–2750 (wide band); 3589; 3568; 3525; 3447; 3051; 2940; 2879; 1631; 1603; 1581; 1535; 1451; 1406; 1329; 1293; 1239; 1183; 1147; 993; 914; 881; 849; 829; 800; 767; 715; 708; 699; 669 |
| LTSS+Lactose | 3589; 3550–3000 (wide band); 3522; 3448; 3278; 3052; 2977; 2932; 2901; 2876; 1637; 1604; 1579; 1536; 1476; 1449; 1412; 1359; 1341; 1308; 1293; 1241; 1202; 1161; 1142; 1116; 1094; 1070; 1033; 1019; 988; 949; 915; 892; 877; 849; 828; 768; 721; 700; 668 |
| LTSS+Mannitol | 3588; 3500–3000 (wide band); 3385; 3275; 2986; 2972; 2851; 2906; 1603; 1580; 1536; 1488; 1417; 1356; 1314; 1301; 1282; 1240; 1209; 1184; 1078; 1046; 1019; 960; 930; 881; 864; 828; 784; 698; 668 |
| LTSS+Sorbitol | 3588; 3500–3000 (wide band); 2985; 2948; 2931; 2909; 2876; 1602; 1578; 1534; 1458; 1408; 1315; 1295; 1238; 1184; 1133; 1089; 1046; 1015; 999; 938; 914; 883; 872; 849; 828; 769; 669 |
| LTSS+Gelatin | 3589; 3550–3850 (wide band); 3271; 3050; 2917; 2848; 1603; 1579; 1535; 1395; 1328; 1291; 1238; 1183; 1163; 1053; 914; 881; 849; 828; 812; 800; 776; 722; 709; 699; 669 |
| LTSS+CaLact | 3589; 3550–2700 (wide band); 3130; 2979; 2748; 2657; 1571; 1483; 1400; 1313; 1274; 1240; 1184; 1124; 1048; 937; 903; 882; 850; 828; 779; 721; 703; 698; 669 |
| LTSS+MgSt | 3577; 3500–3050 (wide band); 2955; 2917; 2850; 1571; 1466; 1449; 1433; 1411; 1318; 1291; 1237; 1184; 1114; 1044; 994; 914; 881; 849; 828; 799; 721; 698; 669 |
| LTSS+Tylose | 3589; 3530–2800 (wide band); 3269; 3048; 2919; 2850; 1603; 1579; 1536; 1396; 1328; 1292; 1239; 1183; 1162; 1052; 914; 881; 849; 828; 800; 776; 721; 709; 699; 669 |
| LTSS+Aerosil | 1250–990 (broad band—peak:1081); 799; 668 |
| LTSS+Talc | 3580–3180 (wide band); 1630; 1412; 1237; 1080–915 (broad band—peak:1013); 670 |
| Sample | Step | TG | Δm/% | DTG | HF | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Tonset/°C (m% at Tonset) | Toffset/°C (m% at Toffset) | Tonset/°C | Toffset/°C | Tpeak/°C | Tonset/°C | Toffset/°C | Tpeak/°C | |||
| LTSS | I+II | 35 (100%) | 102 (90.52%) | 9.48 | 35 77 | 77 103 | 69 87 | 35 54 | 54 103 | 47 72;92 |
| III | 160 (90.52%) | 223 (86.30%) | 4.22 | 156 | 220 | 198 | 182 | 240 | 201 | |
| IV+V | 223 (86.30%) | 500 (23.05%) | 63.25 | 220 284 | 284 500 | 254 469 | 405 | 500 | 472 | |
| LTSS+Starch | I | 35 (100%) | 227 (88.73%) | 11.27 | - | - | - | 186 258 389 475 | 218 280 475 508 | 201 274 459 504 |
| II-IV | 227 (88.73%) | 508 (10.21%) | 78.52 | 240 390 485 | 285 471 508 | 270 456 503 | ||||
| LTSS+Lactose | I | 40 (99.69%) | 105 (95.97%) | 4.03 | 49 | 83 | 61 | 40 152 413 | 49 191 470 | 43 166 444 |
| II-IV | 125 (95.45%) | 505 (30.45%) | 65 | 143 270 403 | 230 389 470 | 203 305 435 | ||||
| LTSS+Mannitol | I | 40 (99.67%) | 89 (96.04%) | 3.96 | 40 | 93 | 43;62 | 40 151 234 385 | 93 178 270 486 | 43; 62 165 246 414 |
| II | 136 (95.78%) | 200 (93.69%) | 2.09 | 157 | 186 | 170 | ||||
| III-V | 224 (93.14%) | 504 (14.75%) | 78.39 | 227 248 378 | 248 293 439 | 245 256 412 | ||||
| LTSS+Sorbitol | I | 37 (100%) | 83 (97.02%) | 2.98 | 37 | 47 | 41 | 37 83 227 322 454 | 48 106 278 454 505 | 43 97 241 417 471 |
| II | 151 (96.64%) | 187 (95.08%) | 1.56 | 144 | 177 | 156 | ||||
| III | 217 (94.40%) | 505 (15.07%) | 79.33 | 227 | 298 | 255 | ||||
| LTSS+Gelatin | I-IV | 40 (100%) | 505 (29.85%) | 70.15 | 59 167 221 382 | 110 221 382 505 | 77 200 315 463 | 40 186 412 | 108 230 500 | 48; 75; 91 201 470 |
| LTSS+CaLact | I | 40 (99.61%) | 115 (83.93%) | 16.07 | 45 | 117 | 75 | 47 192 383 403 412 480 | 110 214 403 421 480 503 | 78 202 395 414 440 489 |
| II-V | 165 (83.42%) | 508 (42.89%) | 40.53 | 160 216 364 412 | 216 313 412 470 | 199 268 394 435 | ||||
| LTSS+MgSt | I | 40 (99.68%) | 100 (93.94%) | 6.06 | 53 | 99 | 72 | 192 325 398 427 476 | 215 383 427 476 509 | 201 360 426 465 492 |
| II | 177 (93.30%) | 210 (91.65%) | 1.65 | 187 | 220 | 198 | ||||
| III-VI | 237 (91.27%) | 509 (17.59%) | 73.68 | 294 339 398 444 | 340 358 445 476 | 315 350 427 465 | ||||
| LTSS+Tylose | I | 40 (99.57%) | 82 (95.62%) | 4.38 | - | - | - | 192 281 375 | 214 375 480 | 202 334 457 |
| II-III | 187 (94.91%) | 508 (12.09%) | 82.82 | 227 385 | 370 477 | 326 452 | ||||
| LTSS+Aerosil | I | 40 (99.55%) | 86 (95.53%) | 4.47 | - | - | - | 40 192 418 | 46 212 462 | 43 201 491 |
| II-IV | 152 (95.00%) | 504 (54.46%) | 40.54 | 171 222 384 | 222 295 495 | 200 257 458 | ||||
| LTSS+Talc | I | 40 (99.72%) | 90 (95.70%) | 4.30 | 51 | 72 | 65 | 40 186 406 | 90 231 504 | 44; 64; 79 201 469 |
| II-III | 172 (95.15%) | 504 (59.38%) | 35.77 | 176 369 | 216 488 | 199 466 | ||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ledeți, I.; Romanescu, M.; Cîrcioban, D.; Ledeți, A.; Vlase, G.; Vlase, T.; Suciu, O.; Murariu, M.; Olariu, S.; Matusz, P.; et al. Stability and Compatibility Studies of Levothyroxine Sodium in Solid Binary Systems—Instrumental Screening. Pharmaceutics 2020, 12, 58. https://doi.org/10.3390/pharmaceutics12010058
Ledeți I, Romanescu M, Cîrcioban D, Ledeți A, Vlase G, Vlase T, Suciu O, Murariu M, Olariu S, Matusz P, et al. Stability and Compatibility Studies of Levothyroxine Sodium in Solid Binary Systems—Instrumental Screening. Pharmaceutics. 2020; 12(1):58. https://doi.org/10.3390/pharmaceutics12010058
Chicago/Turabian StyleLedeți, Ionuț, Mirabela Romanescu, Denisa Cîrcioban, Adriana Ledeți, Gabriela Vlase, Titus Vlase, Oana Suciu, Marius Murariu, Sorin Olariu, Petru Matusz, and et al. 2020. "Stability and Compatibility Studies of Levothyroxine Sodium in Solid Binary Systems—Instrumental Screening" Pharmaceutics 12, no. 1: 58. https://doi.org/10.3390/pharmaceutics12010058
APA StyleLedeți, I., Romanescu, M., Cîrcioban, D., Ledeți, A., Vlase, G., Vlase, T., Suciu, O., Murariu, M., Olariu, S., Matusz, P., Buda, V., & Piciu, D. (2020). Stability and Compatibility Studies of Levothyroxine Sodium in Solid Binary Systems—Instrumental Screening. Pharmaceutics, 12(1), 58. https://doi.org/10.3390/pharmaceutics12010058










