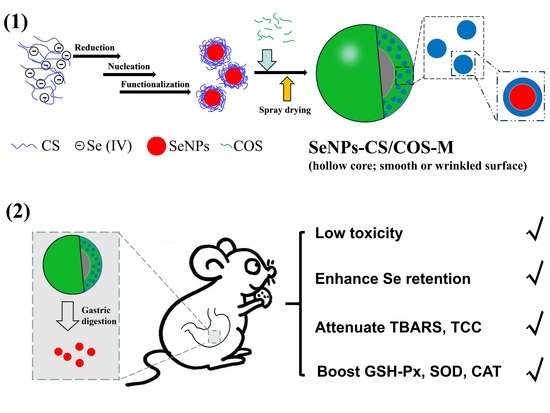
Selenium-Nanoparticles-Loaded Chitosan/Chitooligosaccharide Microparticles and Their Antioxidant Potential: A Chemical and In Vivo Investigation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Animals
2.1.1. Materials
2.1.2. Animals
2.2. Chemicals and Characterization
2.2.1. Synthesis and Characterization of SeNPs
2.2.2. Preparation and Characterization of SeNPs-CS/COS-Ms
2.3. Release Experiment
2.4. Free Radical Scavenging Tests
2.4.1. DPPH Radical (•DPPH) Scavenging Assay
2.4.2. Superoxide Anion Radical (•O2−) Scavenging Assay
2.5. Animal Experiment
2.5.1. Acute Lethal Test In Vivo
2.5.2. Ethanol Challenge Test
2.6. Statistical Analysis
3. Results and Discussion
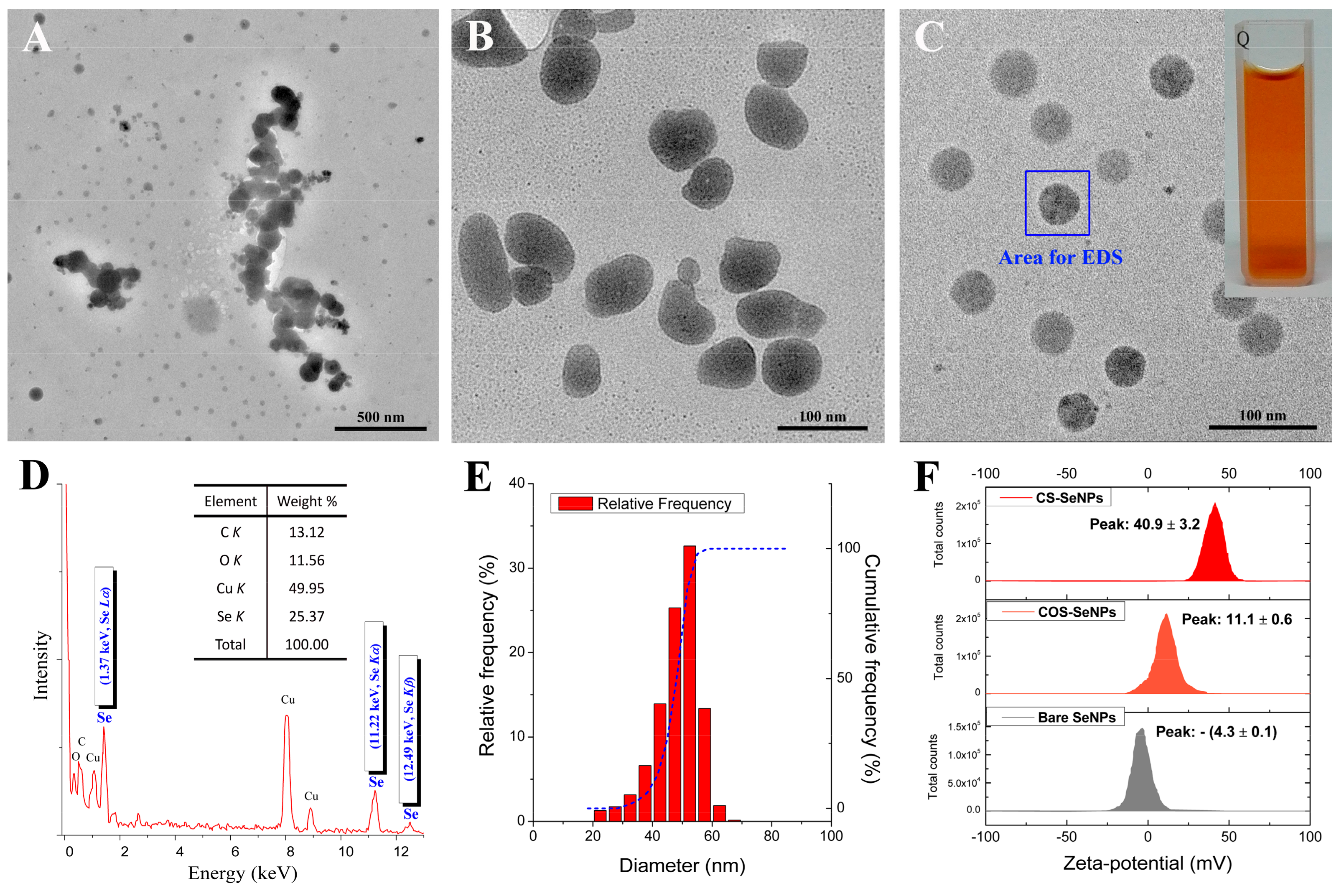
3.1. Characterization of SeNPs
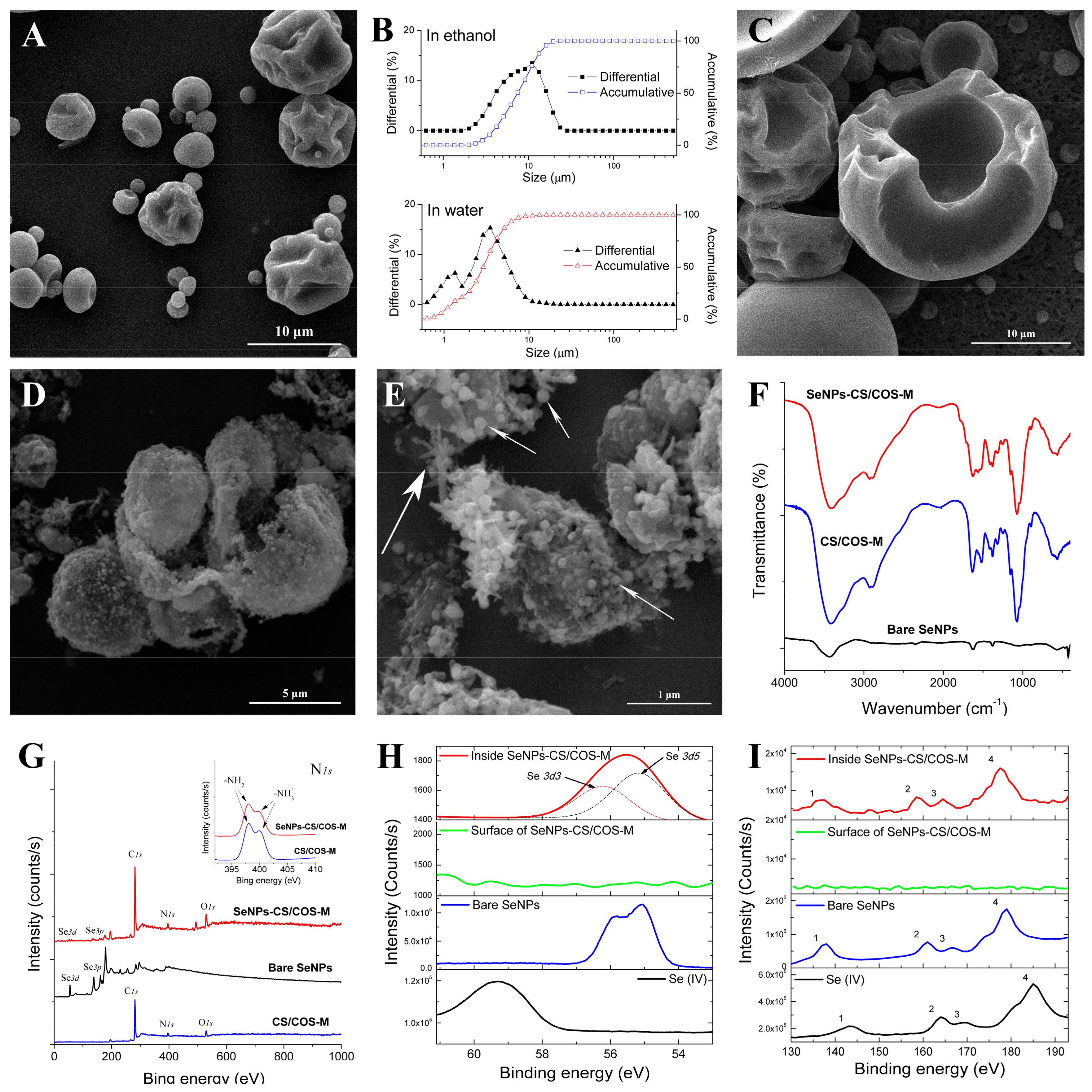
3.2. Characterization of SeNPs-CS/COS-Ms
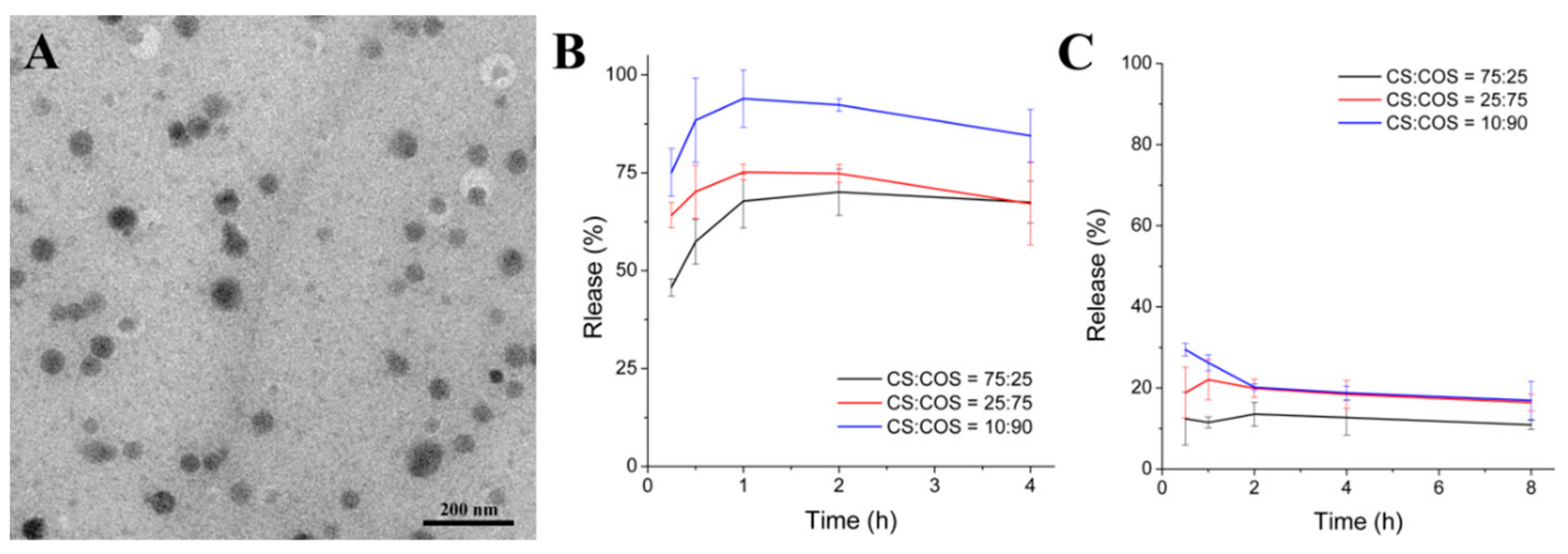
3.3. Release of SeNPs from SeNPs-CS/COS-Ms
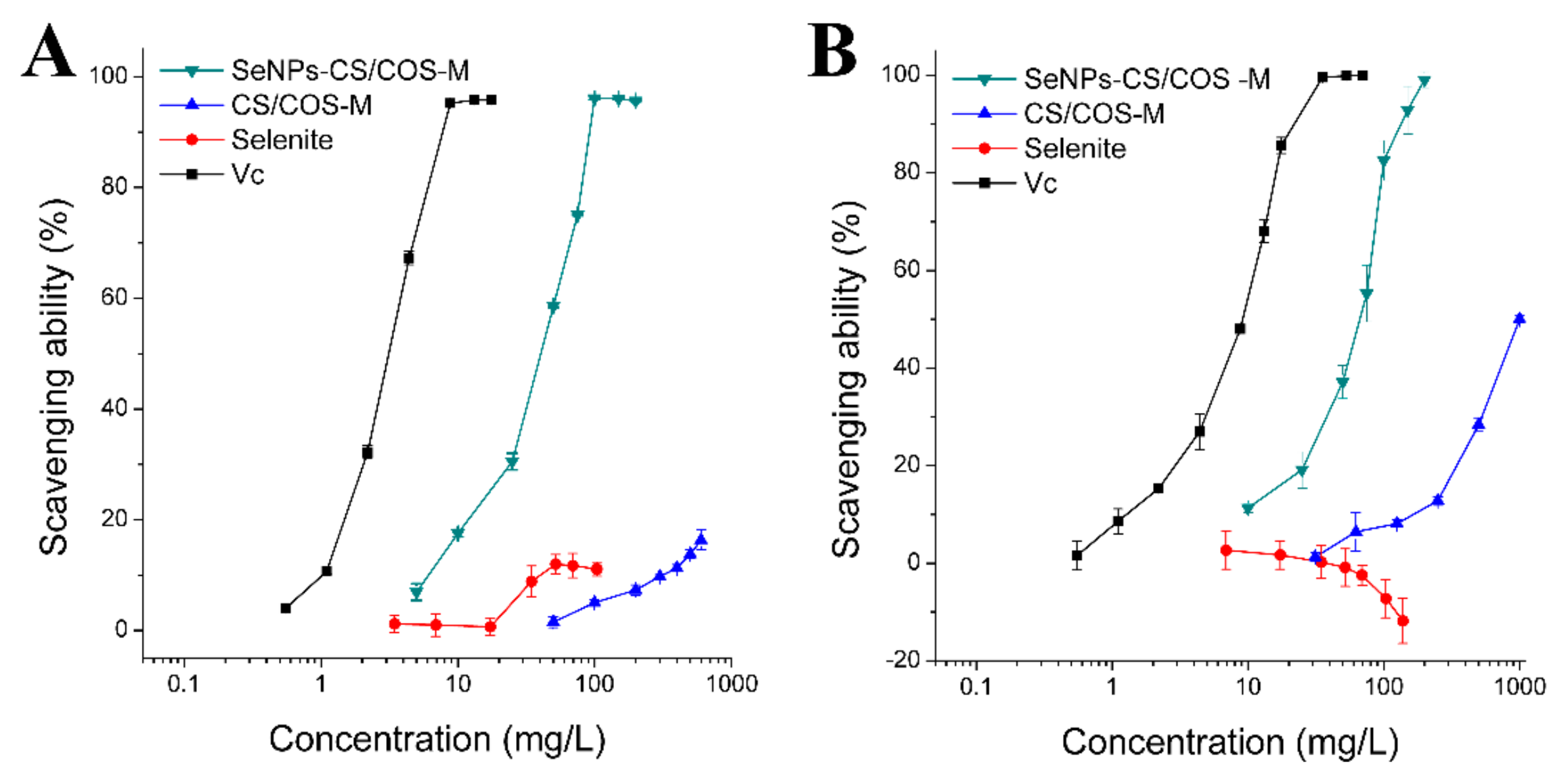
3.4. Free Radical Scavenging Ability of SeNPs-CS/COS-Ms
3.5. Acute Toxicity of SeNPs-CS/COS-Ms
3.6. Protection of SeNPs-CS/COS-Ms against Oxidative Stress Induced by Alcohol
3.6.1. Influence of SeNPs-CS/COS-Ms on Growth
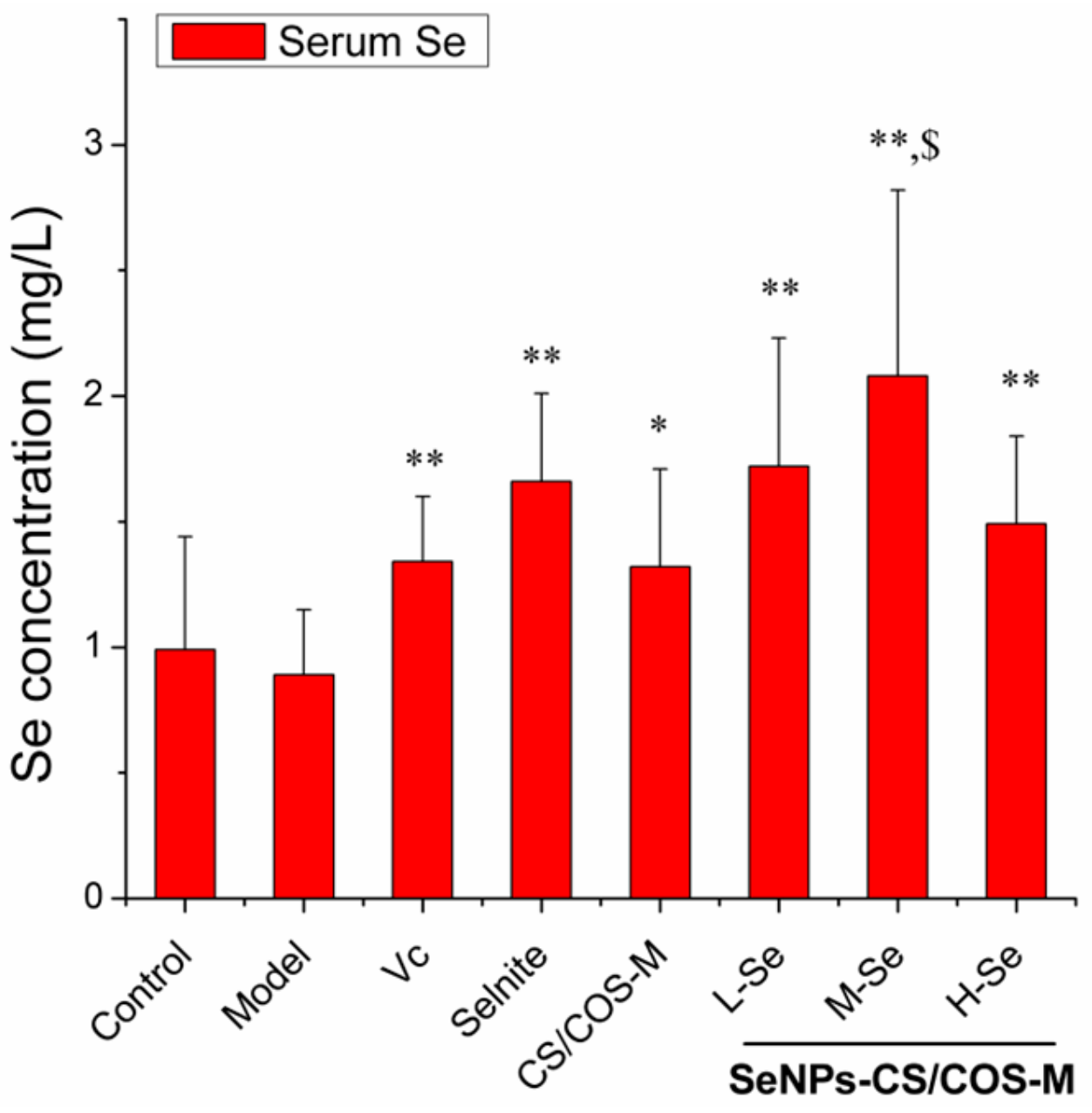
3.6.2. Se Retention by SeNPs-CS/COS-Ms
3.6.3. Biomarkers
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| BSA | bovine serum albumin |
| bw | body weight |
| CAT | catalase |
| CS | chitosan |
| CS/COS-Ms | chitosan/chitooligosaccharide microparticles |
| COS | chitooligosaccharide |
| CS-SeNPs | chitosan-decorated selenium nanopartilcels |
| DLS | dynamic light scattering |
| DPPH | 1,1-diphenyl-2-picrylhydrazyl |
| EC50 | concentration for 50% of maximal effect |
| EDS | energy-dispersive X-ray spectroscopy |
| GSH | glutathione |
| GSH-Px | glutathione peroxidase |
| ICP-MS | inductively coupled plasma mass spectrometry |
| ICR | Institute of Cancer Research |
| KM | Kunming |
| KMnO4 | potassium permanganate |
| LD50 | median lethal dose |
| MW | molecular weight |
| MWCO | molecular weight cut-off |
| PDI | polydispersibility index |
| RH | relative humidity |
| ROS | radical oxygen species |
| Se | selenium |
| SEM | scanning electron microscopy |
| SeNPs | selenium nanoparticles |
| SeNPs-CS/COS-Ms | selenium-nanoparticles-loaded chitosan/chitooligosaccharide microparticles |
| SeNPs-CS-Ms | selenium nanoparticles-embedded chitosan microspheres |
| SGF | simulated gastric fluid |
| SIF | simulated intestinal fluid |
| SOD | superoxide dismutase |
| SPF | specific-pathogen-free |
| TBARS | thiobarbituric acid-reactive substances |
| TCC | total carbonyl compounds |
| TEM | transmission electron microscopy |
| UF | ultrafiltration |
| Vc | ascorbic acid |
References
- Navarro-Alarcon, M.; Cabrera-Vique, C. Selenium in food and the human body: A review. Sci. Total Environ. 2008, 400, 115–141. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Spallholz, J.E. Toxicity of selenium compounds and nano-selenium particles. In General, Applied and Systems Toxicology, 2nd ed.; Casciano, D.A., Sahu, S.C., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2011; pp. 787–802. [Google Scholar] [CrossRef]
- Schrauzer, G.N.; Surai, P.F. Selenium in human and animal nutrition: Resolved and unresolved issues. A partly historical treatise in commemoration of the fiftieth anniversary of the discovery of the biological essentiality of selenium, dedicated to the memory of Klaus Schwarz (1914–1978) on the occasion of the thirtieth anniversary of his death. Crit. Rev. Biotechnol. 2009, 29, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Hosnedlova, B.; Kepinska, M.; Skalickova, S.; Fernandez, C.; Ruttkay-Nedecky, B.; Peng, Q.; Baron, M.; Melcova, M.; Opatrilova, R.; Zidkova, J.; et al. Nano-selenium and its nanomedicine applications: A critical review. Int. J. Nanomed. 2018, 13, 2107–2128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, B.; Zhang, J.; Hou, J.; Chen, C. Free radical scavenging effciency of Nano-Se in vitro. Free Radic Biol. Med. 2003, 35, 805–813. [Google Scholar] [CrossRef]
- Tran, P.A.; Webster, T.J. Selenium nanoparticles inhibit Staphylococcus aureus growth. Int. J. Nanomed. 2011, 6, 1553–1558. [Google Scholar] [CrossRef] [Green Version]
- Chen, T.; Wong, Y.S.; Zheng, W.; Bai, Y.; Huang, L. Selenium nanoparticles fabricated in Undaria pinnatifda polysaccharide solutions induce mitochondria-mediated apoptosis in A375 human melanoma cells. Colloids Surf. B 2008, 67, 26–31. [Google Scholar] [CrossRef]
- Shi, L.; Xun, W.; Yue, W.; Zhang, C.; Ren, Y.; Shi, L.; Wang, Q.; Yang, R.; Lei, F. Effect of sodium selenite, Se-yeast and nano-elemental selenium on growth performance, Se concentration and antioxidant status in growing male goats. Small Rumin. Res. 2011, 96, 49–52. [Google Scholar] [CrossRef]
- Hu, C.H.; Li, Y.L.; Xiong, L.; Zhang, H.M.; Song, J.; Xia, M.S. Comparative effects of nano elemental selenium and sodium selenite on selenium retention in broiler chickens. Anim. Feed Sci. Technol. 2012, 177, 204–210. [Google Scholar] [CrossRef]
- Bai, K.; Hong, B.; He, J.; Hong, Z.; Tan, R. Preparation and antioxidant properties of selenium nanoparticles-loaded chitosan microspheres. Int. J. Nanomed. 2017, 12, 4527–4539. [Google Scholar] [CrossRef] [Green Version]
- Bai, K.; Hong, B.; Hong, Z.; Sun, J.; Wang, C. Selenium nanoparticles-loaded chitosan/citrate complex and its protection against oxidative stress in D-galactose-induced aging mice. J. Nanobiotechnol. 2017, 15, 92–105. [Google Scholar] [CrossRef]
- Zhang, J.S.; Gao, X.Y.; Zhang, L.D.; Bao, Y.P. Biological effects of a nano red elemental selenium. Biofactors 2001, 15, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Li, N.; Chen, J. A subchronic toxicity study of elemental Nano-Se in Sprague-Dawley rats. Life Sci. 2005, 76, 1989–2003. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Yao, H.; Gao, X.; Zhang, Z.; Wang, J.F.; Xu, S.W. The Role of Nitric Oxide and Oxidative Stress in Intestinal Damage Induced by Selenium Deficiency in Chickens. Biol. Trace Elem. Res. 2015, 163, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.P.; Fu, J.; Xu, F.P.; Wang, X.S.; Li, S. The role of heat shock proteins in oxidative stress damage induced by Se deficiency in chicken livers. Biometals 2015, 28, 163–173. [Google Scholar] [CrossRef]
- Klaunig, J.E.; Wang, Z.; Pu, X.; Zhou, S. Oxidative stress and oxidative damage in chemical carcinogenesis. Toxicol. Appl. Pharm. 2011, 254, 86–99. [Google Scholar] [CrossRef]
- Kim, J.H.; Park, S.H.; Nam, S.W.; Choi, Y.H. Gastroprotective Effect of Selenium on Ethanol-Induced Gastric Damage in Rats. Int. J. Mol. Sci. 2012, 13, 5740–5750. [Google Scholar] [CrossRef] [Green Version]
- Salim, S. Oxidative Stress and Psychological Disorders. Curr. Neuropharmacol. 2014, 12, 140–147. [Google Scholar] [CrossRef] [Green Version]
- Sadau, Y.; Adelaiye, A.B.; Magaji, R.A.; Ayo, J.O.; Mabrouk, M.A.; Isa, A.I. Role of selenium and vitamin E on gastric mucosal damage induced by water-immersion restraint stress in wistar rats. IOSR J. Pharm. Biol. Sci. 2015, 10, 34–39. [Google Scholar] [CrossRef]
- Molina, P.E.; Hoek, J.B.; Nelson, S.; Guidot, D.M.; Lang, C.H.; Wands, J.R.; Crawford, J.M. Mechanisms of Alcohol-Induced Tissue Injury. Alcohol. Clin. Exp. Res. 2003, 27, 563–575. [Google Scholar] [CrossRef]
- Wu, C.; Xu, Z.; Huan, K. Effects of Dietary Selenium on Inflammation and Hydrogen Sulfide in the Gastrointestinal Tract in Chickens. Biol. Trace Elem. Res. 2016, 174, 428–435. [Google Scholar] [CrossRef]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Dufailly, V.; Noël, L.; Guérin, T. Determination of chromium, iron and selenium in foodstuffs of animal origin by collision cell technology, inductively coupled plasma mass spectrometry (ICP-MS), after closed vessel microwave digestion. Anal. Chim. Acta 2006, 565, 214–221. [Google Scholar] [CrossRef]
- Van Lierde, V.; Chéry, C.C.; Moens, L.; Vanhaecke, F. Capillary electrophoresis hyphenated to inductively coupled plasma-sector field-mass spectrometry for the detection of chromium species after incubation of chromium in simulated sweat. Electrophoresis 2005, 26, 1703–1711. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Jiang, Y.F.; Du, B.J.; Chai, Z.; Jiao, T.; Zhang, C.Y.; Ren, F.Z.; Leng, X.J. Design and characterization of controlled-release edible packaging films prepared with synergistic whey-protein polysaccharide complexes. J. Agric. Food Chem. 2013, 61, 5824–5833. [Google Scholar] [CrossRef]
- Zhai, X.; Zhang, C.; Zhao, G.; Stoll, S.; Ren, F.; Leng, X. Antioxidant capacities of the selenium nanoparticles stabilized by chitosan. J. Nanobiotechnol. 2017, 15, 4–15. [Google Scholar] [CrossRef] [Green Version]
- Xu, B.J.; Chang, S.K.C. A comparative study on phenolic profles and antioxidant activities of legumes as affected by extraction solvents. J. Food Sci. 2007, 72, S159–S166. [Google Scholar] [CrossRef]
- Liang, X.L.; Wang, X.L.; Li, Z.; Hao, Q.H.; Wang, S.Y. Improved in Vitro Assays of Superoxide Anion and 1,1-Diphenyl-2-picrylhydrazyl (DPPH) Radical-Scavenging Activity of Isoflavones and Isoflavone Metabolites. J. Agric. Food Chem. 2010, 58, 11548–11552. [Google Scholar] [CrossRef]
- Bliss, C.I. The calculation of the dosage-mortality curve. Ann. Appl. Biol. 1935, 22, 134–167. [Google Scholar] [CrossRef]
- Han, Y.; Xu, Q.; Hu, J.; Han, X.; Li, W.; Zhao, L. Maltol, a Food Flavoring Agent, Attenuates Acute Alcohol-Induced Oxidative Damage in Mice. Nutrients 2015, 7, 682–696. [Google Scholar] [CrossRef]
- Zeng, T.; Zhang, C.L.; Zhu, Z.P.; Yu, L.H.; Zhao, X.L.; Xie, K.Q. Diallyl trisulfide (DATS) effectively attenuated oxidative stress-mediated liver injury and hepatic mitochondrial dysfunction in acute ethanol-exposed mice. Toxicology 2008, 252, 86–91. [Google Scholar] [CrossRef]
- Zhang, C.; Zhai, X.; Zhao, G.; Ren, F.; Leng, X. Synthesis, characterization, and controlled release of selenium nanoparticles stabilized by chitosan of different molecular weights. Carbohydr. Polym. 2015, 134, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Ramamurthy, C.; Sampath, K.S.; Arunkumar, P.; Kumar, M.S.; Sujatha, V.; Premkumar, K.; Thirunavukkarasu, C. Green synthesis and characterization of selenium nanoparticles and its augmented cytotoxicity with doxorubicin on cancer cells. Bioprocess. Biosyst. Eng. 2013, 36, 1131–1139. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.S.; Zheng, S.Y.; Zhang, Y.B.; Yu, B.; Zheng, W.; Yang, F.; Chen, T. Sialic acid surface decoration enhances cellular uptake and apoptosis-inducing activity of selenium nanoparticles. Colloid Surf. B 2011, 83, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Kong, H.; Yang, J.; Zhang, Y.; Fang, Y.; Nishinari, K.; Phillips, G.O. Synthesis and antioxidant properties of gum arabic-stabilized selenium nanoparticles. Int. J. Biol. Macromol. 2014, 65, 155–162. [Google Scholar] [CrossRef]
- Yu, B.; Zhang, Y.; Zheng, W.; Fan, C.; Chen, T. Positive surface charge enhances selective cellular uptake and anticancer efficacy of selenium nanoparticles. Inorg. Chem. 2012, 51, 8956–8963. [Google Scholar] [CrossRef]
- Hamed, I.; Özogul, F.; Regenstein, J.M. Industrial applications of crustacean by-products (chitin, chitosan, and chitooligosaccharides): A review. Trends Food Sci. Tech. 2016, 48, 40–50. [Google Scholar] [CrossRef]
- Xia, W.; Liu, P.; Zhang, J.; Chen, J. Biological activities of chitosan and chitooligosaccharides. Food Hydrocolloid 2011, 25, 170–179. [Google Scholar] [CrossRef]
- Liu, W.; Chen, X.D.; Cheng, Z.; Selomulya, C. On enhancing the solubility of curcumin by microencapsulation in whey protein isolate via spray drying. J. Food Eng. 2016, 169, 189–195. [Google Scholar] [CrossRef]
- Hsu, S.; Whu, S.W.; Tsai, C.; Wu, Y.; Chen, H.; Hsieh, K. Chitosan as scaffold materials: Effects of molecular weight and degree of deacetylation. J. Polym. Res. 2004, 11, 141–147. [Google Scholar] [CrossRef]
- Feng, Y.; Su, J.; Zhao, Z.; Zheng, W.; Wu, H.; Zhang, Y.; Chen, T. Differential effects of amino acid surface decoration on the anticancer efficacy of selenium nanoparticles. Dalton Trans. 2014, 43, 1854–1861. [Google Scholar] [CrossRef]
- Peng, D.; Zhang, J.; Liu, Q.; Taylor, E.W. Size effect of elemental selenium nanoparticles (Nano-Se) at supranutritional levels on selenium accumulation and glutathione S-transferase activity. J. Inorg. BioChem. 2007, 101, 1457–1463. [Google Scholar] [CrossRef] [PubMed]
- Clegg, M.; Shafat, A. Energy and macronutrient composition of breakfast affect gastric emptying of lunch and subsequent food intake, satiety and satiation. Appetite 2010, 54, 517–523. [Google Scholar] [CrossRef] [PubMed]
- Willis, H.J.; Thomas, W.; Willis, D.J.; Slavin, J.L. Feasibility of measuring gastric emptying time, with a wireless motility device, after subjects consume fiber-matched liquid and solid breakfasts. Appetite 2011, 57, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, J.; Yu, H. Elemental selenium at nano size possesses lower toxicity without compromising the fundamental effect on selenoenzymes: Comparison with selenomethionine in mice. Free Radic. Biol. Med. 2007, 42, 1524–1533. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Gil, P.; Jimenez de Aberasturi, D.; Wulf, V.; Pelaz, B.; Del Pino, P.; Zhao, Y.; De La Fuente, J.M.; Ruiz De Larramendi, I.; Rojo, T.; Liang, X.J.; et al. The challenge to relate the physicochemical properties of colloidal nanoparticles to their cytotoxicity. Acc. Chem. Res. 2013, 46, 743–749. [Google Scholar] [CrossRef]
- Gil, P.R.; Oberdörster, G.; Elder, A.; Puntes, V.; Parak, W.J. Correlating physicochemical with toxicological properties of nanoparticles: The present and the future. ACS Nano 2010, 4, 5527–5531. [Google Scholar] [CrossRef]
- Barnes, K.M.; Evenson, J.K.; Raines, A.M.; Sunde, R.A. Transcript Analysis of the Selenoproteome Indicates That Dietary Selenium Requirements of Rats Based on Selenium-Regulated Selenoprotein mRNA Levels Are Uniformly Less Than Those Based on Glutathione Peroxidase Activity. J. Nutr. 2009, 139, 199–206. [Google Scholar] [CrossRef] [Green Version]
- Raines, A.M.; Sunde, R.A. Selenium toxicity but not deficient or supernutritional selenium status vastly alters the transcriptome in rodents. BMC Genom. 2011, 12, 26–40. [Google Scholar] [CrossRef] [Green Version]
- Dai, H.C.; Long, L.Q.; Zhang, X.W.; Zhang, W.M.; Wu, X.X. Cloning and expression of the duck leptin gene and the efect of leptin on food intake and fatty deposition in mice. J. Anim. Sci. 2007, 20, 850–855. [Google Scholar] [CrossRef]
- Baltacıoğlu, E.; Yuva, P.; Aydın, G.; Alver, A.; Kahraman, C.; Karabulut, E.; Akalın, F.A. Lipid Peroxidation Levels and Total Oxidant/Antioxidant Status in Serum and Saliva from Patients With Chronic and Aggressive Periodontitis. Oxidative Stress Index: A New Biomarker for Periodontal Disease? J. Periodontol. 2014, 85, 1432–1441. [Google Scholar] [CrossRef]
- Dalle-Donne, I.; Giustarini, D.; Colombo, R.; Rossi, R.; Milzani, A. Protein carbonylation in human diseases. Trends Mol. Med. 2003, 9, 169–176. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, H.; Yan, X.; Zhang, L. Comparison of short-term toxicity between Nano-Se and selenite in mice. Life Sci. 2005, 76, 1099–1109. [Google Scholar] [CrossRef] [PubMed]
- Behne, D.; Alber, D.; Kyriakopoulos, A. Long-term selenium supplementation of humans: Selenium status and relationships between selenium concentrations in skeletal muscle and indicator materials. J. Trace Elem. Med. Biol. 2010, 24, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Burk, R.F.; Hill, K.E.; Motley, A.K. Plasma selenium in specific and non-specific forms. Biofactors 2001, 14, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Burk, R.F.; Hill, K.E.; Motley, A.D.; Winfrey, V.P.; Kurokawa, S.; Mitchell, S.L.; Zhang, W. Selenoprotein P and apolipoprotein E receptor-2 interact at the blood-brain barrier and also within the brain to maintain an essential selenium pool that protects against neurodegeneration. FASEB J. 2014, 28, 3579–3588. [Google Scholar] [CrossRef] [Green Version]
- Burk, R.F.; Hill, K.E. Regulation of Selenium Metabolism and Transport. Annu. Rev. Nutr. 2015, 35, 109–134. [Google Scholar] [CrossRef]
| Group | Pre-Treatment (Once Daily, Day 1–35) | Treatment (Once, Day 36) |
|---|---|---|
| Control | Normal saline | Normal saline |
| Model | Normal saline | 50% Ethanol (12 mL/kg bw) |
| Vc | Vc (80 mg/kg bw) | 50% Ethanol (12 mL/kg bw) |
| Sodium selenite | Sodium selenite (2.65 mg/kg bw) | 50% Ethanol (12 mL/kg bw) |
| CS/COS-M | CS/COS-M (80 mg/kg bw) | 50% Ethanol (12 mL/kg bw) |
| L-Se | SeNPs-CS/COS-M (40 mg/kg bw) | 50% Ethanol (12 mL/kg bw) |
| M-Se | SeNPs-CS/COS-M (80 mg/kg bw) | 50% Ethanol (12 mL/kg bw) |
| H-Se | SeNPs-CS/COS-M (120 mg/kg bw) | 50% Ethanol (12 mL/kg bw) |
| Sample | Z-Average Diameter (nm) | PDI |
|---|---|---|
| Bare SeNPs (fresh) | 102.9 ± 6.4 | 0.355 ± 0.06 |
| COS-SeNPs (fresh) | 97.5 ± 3.6 | 0.305 ± 0.044 |
| CS-SeNPs (fresh) | 103.3 ± 2.3 | 0.262 ± 0.035 |
| Bare SeNPs (after 6 h of storage at 25 °C) | 265.2 ± 15.6 | 0.351 ± 0.040 |
| COS-SeNPs (after 14 days of storage at 25 °C) | 335.7 ± 11.7 | 0.472 ± 0.028 |
| CS-SeNPs (after 14 days of storage at 25 °C) | 80.5 ± 0.6 | 0.116 ± 0.018 |
| Free Radical | Vc | Sodium Selenite | CS/COS-M 1 | SeNPs-CS/COS-M 2 |
|---|---|---|---|---|
| DPPH | 2.90 ± 0.04 | >>103.8 | >>600 | 29.54 ± 1.24 |
| O2− | 5.80 ± 1.00 | >>138.4 | 1082 ± 104 | 34.45 ± 1.57 |
| Sample | Se Content (mg/g) | LD50 # (mg/kg bw) | LD50 (Se) * (mg Se /kg bw) |
|---|---|---|---|
| Sodium selenite 1 | 456.7 | 19.2 (16.2–22.7) $ | 8.8 (7.4–10.4) ^ |
| CS/COS-M | -- | >20 × 103 | -- |
| SeNPs-CS/COS-M | 15.1 | 11.06 × 103 (6.28 × 103 –19.51 × 103) $ | 167 (94.9–294.5) ^ |
| Group | Body Weight (g) | Increase (%) | |
|---|---|---|---|
| Day 1 | Day 35 | ||
| Control | 22.93 ± 1.45 | 43.56 ± 1.20 | 89.97 |
| Model | 23.08 ± 0.79 | 44.84 ± 3.63 | 94.28 |
| Vc | 23.38 ± 0.85 | 43.33 ± 3.98 | 85.33 |
| Sodium selenite | 22.85 ± 1.23 | 41.36 ± 4.18 | 81.01 |
| CS/COS-M | 22.55 ± 1.12 | 42.73 ± 3.94 | 89.49 |
| L-Se | 22.92 ± 0.66 | 44.10 ± 2.41 | 92.41 |
| M-Se | 23.12 ± 0.47 | 43.21 ± 3.85 | 86.89 |
| H-Se | 21.93 ± 1.05 | 41.21 ± 2.22 | 87.92 |
| Group | TBARS (nmol/mL) | SOD (U/mL) | GSH-PX (U/mL) | CAT (U/mL) |
|---|---|---|---|---|
| Control | 10.7 ± 4.0 | 95.9 ± 25.5 | 576 ± 160 | 89.7 ± 8.2 |
| Model | 13.9 ± 6.7 | 82.9 ± 26.2 | 187 ± 186 ## | 84.5 ± 8.0 |
| Vc | 13.1 ± 2.4 | 84.6 ± 22.1 | 431 ± 253 * | 87.2 ± 8.3 |
| Sodium selenite | 12.8 ± 4.6 | 101 ± 14 | 343 ± 189 | 79.7 ± 16.7 |
| CS/COS-M | 11.9 ± 4.7 | 83.7 ± 25.2 | 375 ± 285 | 92.2 ± 10.0 |
| L-Se | 9.2 ± 3.2 * | 108 ± 10 * | 256 ± 171 | 84.9 ± 9.7 |
| M-Se | 9.2 ± 1.8 * | 105 ± 12 * | 451 ± 112 ** | 88.6 ± 9.3 |
| H-Se | 11.8 ± 2.5 | 57.3 ± 15.5 * | 437 ± 196 ** | 72.9 ± 21.2 # |
| Group | TBARS (nmol/mg Prot) | GSH (mg/g Prot) | TCC (nmol/mg Prot) | SOD (U/mg Prot) | CAT (U/mg Prot) |
|---|---|---|---|---|---|
| Control | 3.64 ± 2.03 | 2.91 ± 1.20 | 12.6 ± 5.8 | 90.8 ± 16.5 | 105 ± 36 |
| Model | 3.93 ± 2.55 | 2.01 ± 0.48 # | 20.1 ± 10.1 # | 85.0 ± 16.3 | 69.6 ± 47.5 |
| Vc | 2.71 ± 1.16 | 3.60 ± 1.12 ** | 14.5 ± 4.1 | 109 ± 16 ** | 123 ± 57 * |
| Sodium selenite | 3.16 ± 1.29 | 3.77 ± 1.36 ** | 19.0 ± 6.6 | 107 ± 16 ** | 147 ± 43 ** |
| CS/COS-M | 3.56 ± 0.91 | 2.10 ± 0.34 | 22.7 ± 11.3 | 96.7 ± 17.0 | 90.9 ± 36.3 |
| L-Se | 3.04 ± 0.77 | 2.59 ± 0.33 ** | 13.4 ± 7.8 | 110 ± 18 ** | 128 ± 39 ** |
| M-Se | 2.74 ± 0.96 | 2.88 ± 0.90 * | 7.3 ± 3.3 ** | 92.1 ± 8.0 | 100 ± 50 |
| H-Se | 1.70 ± 0.33 * | 2.53 ± 0.47 * | 8.9 ± 3.5 ** | 98.2 ± 11.1 * | 100 ± 17 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, K.; Hong, B.; Huang, W.; He, J. Selenium-Nanoparticles-Loaded Chitosan/Chitooligosaccharide Microparticles and Their Antioxidant Potential: A Chemical and In Vivo Investigation. Pharmaceutics 2020, 12, 43. https://doi.org/10.3390/pharmaceutics12010043
Bai K, Hong B, Huang W, He J. Selenium-Nanoparticles-Loaded Chitosan/Chitooligosaccharide Microparticles and Their Antioxidant Potential: A Chemical and In Vivo Investigation. Pharmaceutics. 2020; 12(1):43. https://doi.org/10.3390/pharmaceutics12010043
Chicago/Turabian StyleBai, Kaikai, Bihong Hong, Wenwen Huang, and Jianlin He. 2020. "Selenium-Nanoparticles-Loaded Chitosan/Chitooligosaccharide Microparticles and Their Antioxidant Potential: A Chemical and In Vivo Investigation" Pharmaceutics 12, no. 1: 43. https://doi.org/10.3390/pharmaceutics12010043
APA StyleBai, K., Hong, B., Huang, W., & He, J. (2020). Selenium-Nanoparticles-Loaded Chitosan/Chitooligosaccharide Microparticles and Their Antioxidant Potential: A Chemical and In Vivo Investigation. Pharmaceutics, 12(1), 43. https://doi.org/10.3390/pharmaceutics12010043