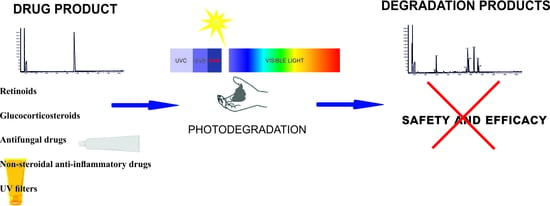
Photostability of Topical Agents Applied to the Skin: A Review
Abstract
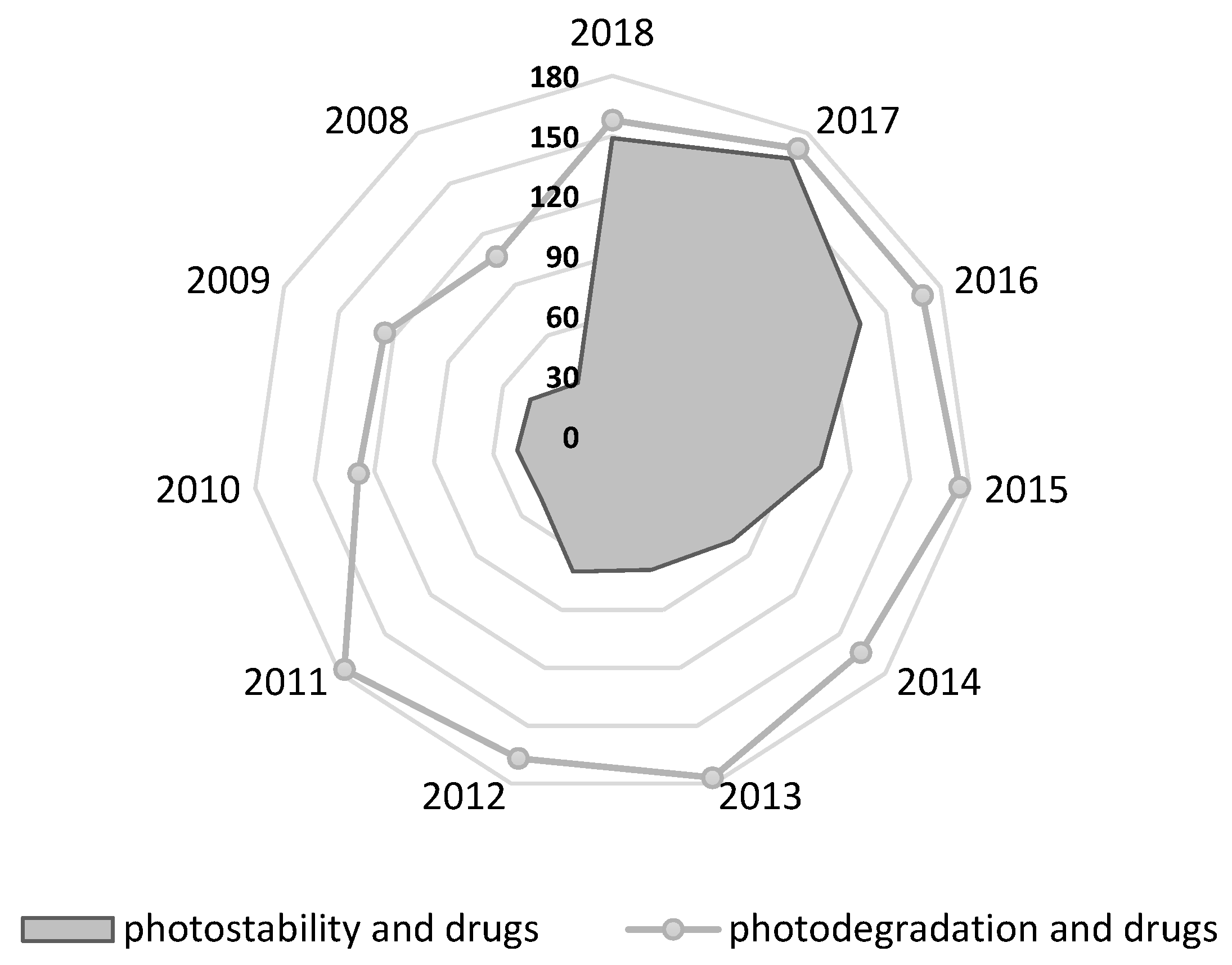
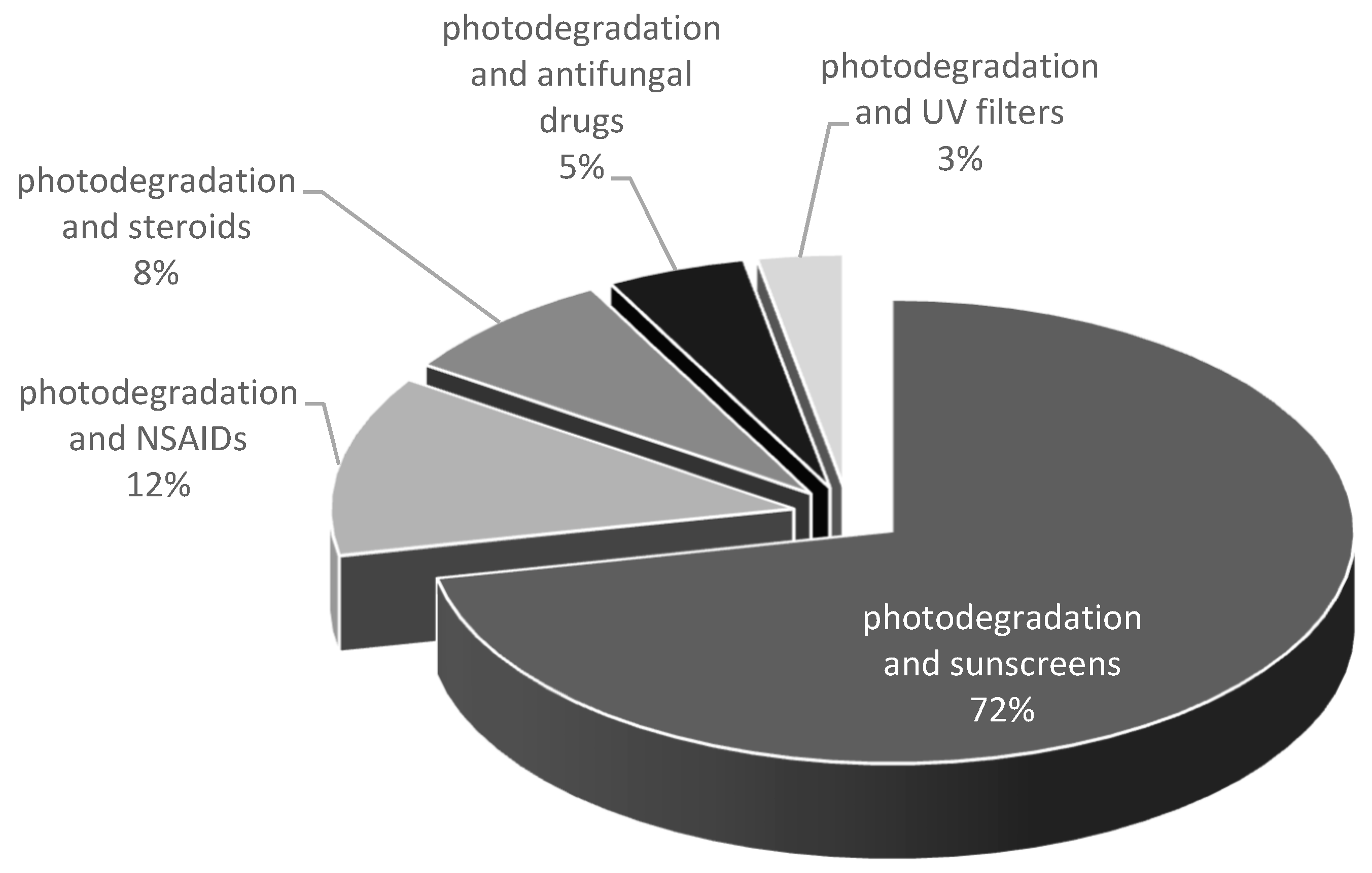
1. Introduction
2. Classes of Drugs
2.1. Topical Glucocorticosteroids
2.2. Retinoids
2.3. Antifungal Drugs
2.4. Non-Steroidal Anti-Inflammatory Drugs (NSAIDs)
2.5. UV Filters
3. Photostabilization Strategies of Selected Dermatological Drugs
4. Drug-Induced Photosensitivity
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chen, Y.C.; Liu, D.Z.; Liu, J.J.; Chang, T.W.; Ho, H.O.; Sheu, M.T. Development of terbinafine solid lipid nanoparticles as a topical delivery system. Int. J. Nanomed. 2012, 7, 4409–4418. [Google Scholar]
- Alkilani, A.Z.; McCrudden, M.T.; Donnelly, R.F. Transdermal Drug Delivery: Innovative Pharmaceutical Developments Based on Disruption of the Barrier Properties of the stratum corneum. Pharmaceutics 2015, 7, 438–470. [Google Scholar] [CrossRef] [PubMed]
- Hagen, M.; Baker, M. Skin penetration and tissue permeation after topical administration of diclofenac. Curr. Med. Res. Opin. 2017, 33, 1623–1634. [Google Scholar] [CrossRef] [PubMed]
- EMA. Draft Guideline on Quality and Equivalence of Topical Products; EMA: London, UK, 2018; Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/draft-guideline-quality-equivalence-topical-products_en.pdf (accessed on 24 September 2019).
- Ghosh, A.; Coondoo, A. A Treatise on Topical Corticosteroids in Dermatology; Lahiri, K., Ed.; Springer: Singapore, 2018; Chapter 25; pp. 241–249. [Google Scholar]
- Alsante, K.M.; Huynh-Ba, K.C.; Baertschi, S.W.; Reed, R.A.; Landis, M.S.; Furness, S.; Olsen, B.; Mowery, M.; Russo, K.; Iser, R.; et al. Recent trends in product development and regulatory issues on impurities in active pharmaceutical ingredient (API) and drug products. Part 2: Safety considerations of impurities in pharmaceutical products and surveying the impurity landscape. AAPS PharmSciTech 2014, 15, 237–251. [Google Scholar] [CrossRef] [PubMed]
- Jamrógiewicz, M. Consequences of New Approach to Chemical Stability Tests to Active Pharmaceutical Ingredients. Front. Pharmacol. 2016, 7, 17. [Google Scholar] [CrossRef][Green Version]
- EMA. ICH Q1A (R2) Stability Testing of New Drug Substances and Drug Products; EMA: London, UK, 2003; Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/ich-q-1-r2-stability-testing-new-drug-substances-products-step-5_en.pdf (accessed on 24 September 2019).
- EMA. ICH Q1B Photostability Testing of New Active Substances and Medicinal Products; EMA: London, UK, 1998; Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/ich-q-1-b-photostability-testing-new-active-substances-medicinal-products-step-5_en.pdf (accessed on 24 September 2019).
- Baertschi, S.W.; Clapham, D.; Foti, C.; Kleinman, M.H.; Kristensen, S.; Reed, R.A.; Templeton, A.C.; Tønnesen, H.H. Implications of In-Use Photostability: Proposed Guidance for Photostability Testing and Labeling to Support the Administration of Photosensitive Pharmaceutical Products, Part 2: Topical Drug Product. J. Pharm. Sci. 2015, 104, 2688–2701. [Google Scholar] [CrossRef]
- Agar, N.S.; Halliday, G.M.; Barnetson, R.S.; Ananthaswamy, H.N.; Wheeler, M.; Jones, A.M. The basal layer in human squamous tumors harbors more UVA than UVB fingerprint mutations: A role for UVA in human skin carci-nogenesis. Proc. Natl. Acad. Sci. USA 2004, 101, 4954–4959. [Google Scholar] [CrossRef]
- Brenner, M.; Hearing, V.J. The protective role of melanin against UV damage in human skin. Photochem. Photobiol. 2008, 84, 539–549. [Google Scholar] [CrossRef]
- Kappes, U.P.; Luo, D.; Potter, M.; Schulmeister, K.; Rünger, T.M. Short- and long-wave UV light (UVB and UVA) induce similar mutations in human skin cells. J. Invest. Dermatol. 2006, 126, 667–675. [Google Scholar] [CrossRef]
- Huang, X.X.; Bernerd, F.; Halliday, G.M. Ultraviolet A within sunlight induces mutations in the epidermal basal layer of engineered human skin. Am. J. Pathol. 2009, 174, 1534–1543. [Google Scholar] [CrossRef]
- Brash, D.E.; Rudolph, J.A.; Simon, J.A.; Lin, A.; McKenna, G.J.; Baden, H.P.; Halperin, A.J.; Pontén, J. A role for sunlight in skin cancer: UV-induced p53 mutations in squamous cell carcinoma. Proc. Natl. Acad. Sci. USA 1991, 88, 10124–10128. [Google Scholar] [CrossRef] [PubMed]
- Allain, L.R.; Pierce, B.C.; Wuelfing, W.P.; Templeton, A.C.; Helmy, R. In-Use Photostability Practice and Regulatory Evaluation for Pharmaceutical Products in an Age of Light-Emitting Diode Light Sources. J. Pharm. Sci. 2019, 108, 1172–1176. [Google Scholar] [CrossRef] [PubMed]
- Baertschi, S.W.; Alsante, K.M.; Tønnesen, H.H. A critical assessment of the ICH guideline on photostability testing of new drug substances and products (Q1B): Recommendation for revision. J. Pharm. Sci. 2010, 99, 2934–2940. [Google Scholar] [CrossRef]
- Baertschi, S.W.; Clapham, D.; Foti, C.; Jansen, P.J.; Kristensen, S.; Reed, R.A.; Templeton, A.C.; Tønnesen, H.H. Implications of in-use photostability: Proposed guidance for photostability testing and labeling to support the administration of photosensitive pharmaceutical products, part 1: Drug products administered by injection. J. Pharm. Sci. 2013, 102, 3888–3899. [Google Scholar] [CrossRef] [PubMed]
- Allain, L.; Baertschi, S.W.; Clapham, D.; Foti, C.; Lantaff, W.M.; Reed, R.A.; Templeton, A.C.; Tønnesen, H.H. Implications of In-Use Photostability: Proposed Guidance for Photostability Testing and Labeling to Support the Administration of Photosensitive Pharmaceutical Products, Part 3. Oral Drug Products. J. Pharm. Sci. 2016, 105, 1586–1594. [Google Scholar] [CrossRef] [PubMed]
- Janga, K.Y.; King, T.; Ji, N.; Sarabu, S.; Shadambikar, G.; Sawant, S.; Xu, P.; Repka, M.A.; Murthy, S.N. Photostability Issues in Pharmaceutical Dosage Forms and Photostabilization. AAPS PharmSciTech. 2018, 19, 48–59. [Google Scholar] [CrossRef] [PubMed]
- CoelhoI, L.; Almeida, I.F.; Sousa Lobo, J.M.; Sousa e Silva, J.P. Photostabilization strategies of photosensitive drugs. Int. J. Pharm. 2018, 541, 19–25. [Google Scholar] [CrossRef]
- European Commission, Annex VI. Available online: https://ec.europa.eu/growth/tools-databases/cosing/pdf/COSING_Annex%20VI_v2.pdf (accessed on 24 September 2019).
- FDA, Sunscreen Drug Products for Over-the-Counter Human Use. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=352&showFR=1%20(dost%C4%99p%2029.04.2019) (accessed on 24 September 2019).
- Ricci, A.; Fasani, E.; Mella, M.; Albini, A. General patterns in the photochemistry of pregna-1,4-dien-3,20-diones. J. Org. Chem. 2004, 68, 4361–4366. [Google Scholar] [CrossRef]
- Miolo, G.; Gallocchio, F.; Levorato, L.; Dalzoppo, D.; Beyersbergen van Henegouwen, G.M.; Caffieri, S. UVB photolysis of betamethasone and its esters: Characterization of photoproducts in solution, in pig skin and in drug formulations. J. Photochem. Photobiol. B 2009, 9, 75–81. [Google Scholar] [CrossRef]
- Saif, K.; Dilnawaz, S.; Ahmad, I.; Usmanghani, K.; Qazi, M.S. In vitro evaluation of betamethasone esters for phototoxic potential. Drug Chem. Toxicol. 2012, 35, 43–47. [Google Scholar]
- Khattak, S.U.; Shaikh, D.; Ahmad, I.; Usmanghani, K.; Sheraz, M.A.; Ahmed, S. Photodegradation and Stabilization of Betamethasone-17 Valerate in Aqueous/Organic Solvents and Topical Formulations. AAPS PharmSciTech. 2013, 14, 177–182. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Teng, X.W.; Cutler, D.C.; Davies, N.M. Degradation kinetics of mometasone furoate in aqueous systems. Int. J. Pharm. 2003, 259, 129–141. [Google Scholar] [CrossRef]
- Caffieri, S.; Dall’Acqua, S.; Castagliuolo, I.; Brun, P.; Miolo, G. UVB photolysis of hydrocortisone 21-acetate. J. Pharm. Biomed. Anal. 2008, 47, 771–777. [Google Scholar] [CrossRef] [PubMed]
- Cacciari, R.D.; Reynoso, E.; Montejano, H.A.; Biasutti, M.A. Photodegradation of prednisolone under UVB solar irradiation. Role of photogenerated ROS in the degradation mechanism. Photochem. Photobiol. Sci. 2017, 16, 1717–1726. [Google Scholar] [CrossRef]
- Miolo, G.; Caffieri, S.; Dalzoppo, D.; Ricci, A.; Fasani, E.; Albini, A. Photochemistry and phototoxicity of fluocinolone 16,17-acetonide. Photochem. Photobiol. 2005, 81, 291–298. [Google Scholar] [CrossRef]
- Miolo, G.; Caffieri, S.; Dalzoppo, D.; Gallocchio, F.; Fasani, E.; Beyersbergen van Henegouwen, G.M. Photoactivation of corticosteroids in UVB-exposed skin. J. Photochem. Photobiol. B 2011, 103, 35–41. [Google Scholar] [CrossRef]
- Dalla Santa, F.; Sperotto, L.E.; Braga, M.P.; Dalcin, T.C.S.; Codevilla, C.S.; Meneghini, L.Z.; Donato, E.M.; Bueno Rolim, C.M.; Bergold, A.M.; Horn Adams, A.I. Development and validation of a simple stability-indicating LC-method and UVA phostability study of desonide hair lotion. Curr. Anal. Chem. 2013, 9, 659–667. [Google Scholar] [CrossRef]
- Rosa, P.; Snovarski Salla, A.P.; de Bona da Silva, C.; Bueno Rolim, C.M.; Horn Adams, A.I. Investigation of the Stabilizing Effects of Antioxidants and Benzophenone-3 on Desonide Photostability. AAPS PharmSciTech. 2014, 15, 11. [Google Scholar] [CrossRef][Green Version]
- Gaspar, L.R.; Campos, P.M. Photostability and efficacy studies of topical formulations containing UV-filters combination and vitamins A, C and E. Int. J. Pharm. 2007, 343, 181–189. [Google Scholar] [CrossRef]
- Tashtoush, B.M.; Jacobson, E.L.; Jacobson, M.K. UVA is the major contributor to the photodegradation of tretinoin and isotretinoin: Implications for development of improved pharmaceutical formulations. Int. J. Pharm. 2008, 352, 123–128. [Google Scholar] [CrossRef]
- Ioele, G.; Cione, E.; Risoli, A.; Genchi, G.; Ragno, G. Accelerated photostability study of tretinoin and isotretinoin in liposome formulations. Int. J. Pharm. 2005, 293, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Ioele, G.; De Luca, M.; Garofalo, A.; Ragno, G. Photosensitive drugs: A review on their photoprotection by liposomes and cyclodextrins. Drug Deliv. 2017, 24, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Brisaert, M.G.; Everaerts, I.; Plaizier-Vercammen, J.A. Chemical stability of tretinoin in dermatological preparations. Pharm. Acta Helv. 1995, 70, 161–166. [Google Scholar] [CrossRef]
- Brisaert, M.G.; Plaizier-Vercammen, J.A. Investigation on the photostability of a tretinoin lotion and stabilization with additives. Int. J. Pharm. 2000, 199, 49–57. [Google Scholar] [CrossRef]
- Schmidt, N.; Gans, E.H. Tretinoin: A Review of Its Anti-inflammatory Properties in the Treatment of Acne. J. Clin. Aesthet. Dermatol. 2011, 4, 22–29. [Google Scholar]
- Martin, B.; Meunier, C.; Montels, D.; Watts, O. Chemical stability of adapalene and tretinoin when combined with benzoyl peroxide in presence and in absence of visible light and ultraviolet radiation. Br. J. Dermatol. 1998, 52, 8–11. [Google Scholar] [CrossRef]
- Del Rosso, J.Q.; Harper, J.; Radhakrishnan, P.; Moore, R. Tretinoin photostability comparison of micronized tretinoin gel 0.05% and tretinoin gel 0.025% following exposure to fluorescent and Solar Light. J. Clin. Aesthet. Dermatol. 2013, 6, 25–28. [Google Scholar]
- Lai, F.; Pireddu, R.; Corrias, F.; Fadda, A.M.; Valenti, D.; Pini, E.; Sinico, C. Nanosuspension improves tretinoin photostability and delivery to the skin. Int. J. Pharm. 2013, 458, 104–109. [Google Scholar] [CrossRef]
- Patel, M.R.; Patel, R.B.; Parikh, J.R.; Patel, B.G. Improving the Isotretinoin Photostability by Incorporating in Microemulsion Matrix. ISRN Pharm. 2011, 2011, 838016. [Google Scholar] [CrossRef]
- Tolba, M.M.; El-Gamal, R.M. Determination of adapalene in gel formulation by conventional and derivative synchronous fluorimetric approaches. Application to stability studies and in vitro diffusion test. Chem. Cent. J. 2016, 10, 33. [Google Scholar] [CrossRef]
- Roy, C.; Panigrahi, L.; Chakrabarty, J. Validated Stability-Indicating RP-HPLC Method for the Estimation of Degradation Behaviour of Organic Peroxide and Third-Generation Synthetic Retinoids in Topical Pharmaceutical Dosage Formulation. Sci. Pharm. 2015, 83, 321–338. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hecker, D.; Worsley, J.; Yueh, G.; Kuroda, K.; Lebwohl, M. Interactions between tazarotene and ultraviolet light. J. Am. Acad. Dermatol. 1999, 41, 927–930. [Google Scholar] [CrossRef]
- OSPAR Commission. OSPAR Background Document on Clotrimazole. 2005. Available online: http://www.ospar.org/documents?v=7317Google Scholar (accessed on 24 September 2019).
- Kryczyk, A.; Żmudzki, P.; Koczurkiewicz, P.; Piotrowska, J.; Pękala, E.; Hubicka, U. The impact of ZnO and TiO2 on the stability of clotrimazole under UVA irradiation: Identification of photocatalytic degradation products and in vitro cytotoxicity assessment. J. Pharm. Biomed. Anal. 2017, 145, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Kryczyk, A.; Żmudzki, P.; Hubicka, U. Determination of bifonazole and identification of its photocatalytic degradation products using UPLC-MS/MS. Biomed Chromatogr. 2017, 31, e3955. [Google Scholar] [CrossRef]
- Nardi, G.; Marin, M.L.; de Souza, P.A.; Lhiaubet-Vallet, V.; Miranda, M.A. Generation of reactive aryl radical intermediates in the reductive photodehalogenation of itraconazole. RSC Adv. 2014, 4, 2687–2693. [Google Scholar] [CrossRef]
- Kryczyk, A.; Żmudzki, P.; Hubicka, U. Determination of itraconazole and its photodegradation products with kinetic evaluation by ultra-performance liquid chromatography/tandem mass spectrometry. Biomed. Chromatogr. 2016, 30, 1733–1743. [Google Scholar] [CrossRef]
- Kryczyk-Poprawa, A.; Żmudzki, P.; Koczurkiewicz, P.; Pękala, E.; Hubicka, U. Photostability of Terbinafine Under UVA Irradiation: The Effect of UV Absorbers. Photochem. Photobiol. 2019, 95, 911–923. [Google Scholar] [CrossRef]
- Bartsch, H.; Eiper Kopelent-Frank, H. Stability indicating assays for the determination of piroxicam comparison methods. J. Pharm. Biomed. Anal. 1999, 20, 531–541. [Google Scholar] [CrossRef]
- Nikolic, V.; Ilic-Stojanovic, S.; Nikolic, L.; Cakic, M.; Zdravkovic, A.; Kapor, A.; Popsavin, M. Photostability of piroxicam in the inclusion complex with 2-hydroxypropyl-β-cyclodextrin. Hem. Ind. 2014, 68, 107–116. [Google Scholar] [CrossRef]
- Glass, B.D.; Brown, M.E.; Daya, S.; Worthington, M.S.; Drummond, P.; Antunes, E.; Lebete, M.; Anoopkumar-Dukie, S.; Maharaj, D. Influence of cyclodextrins on the photostability of selected drug molecules in solution and the solid-state. Int. J. Photoenergy 2011, 3, 205–211. [Google Scholar] [CrossRef]
- Sammartino, M.P.; Castrucci, M.; Ruiu, D.; Visco, G.; Campanella, L. Photostability and toxicity of finasteride, diclofenac and naproxen under simulating sunlight exposure: Evaluation of the toxicity trend and of the packaging photoprotection. Chem. Cent. J. 2013, 7, 181. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.H.; Liou, Y.B.; Lee, J.A.; Chen, C.Y.; Wu, A.B. Assay of naproxen by high-performance liquid chromatography and identification of its photoproducts by LC-ESI MS. Biomed. Chromatogr. 2006, 20, 787–793. [Google Scholar] [CrossRef] [PubMed]
- Arany, E.; Szabó, R.K.; Apáti, L.; Alapi, T.; Ilisz, I.; Mazellier, P.; Dombi, A.; Gajda-Schrantz, K. Degradation of naproxen by UV, VUV photolysis and their combination. J. Hazard Mater. 2013, 262, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Isidori, M.; Lavorgna, M.; Nardelli, A.; Parrella, A.; Previtera, L.; Rubino, M. Ecotoxicity of naproxen and its phototransformation products. Sci. Total Environ. 2005, 348, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Musa, K.A.K.; Eriksson, L.A. Photodegradation mechanism of the common non-steroidanti-inflammatory drug diclofenac and its carbazole photoproduct. Phys. Chem. Chem. Phys. 2009, 11, 4601–4610. [Google Scholar] [CrossRef]
- Szabó, R.K.; Megyeri, C.; Illés, E.; Gajda-Schrantz, K.; Mazellier, P.; Dombi, A. Phototransformation of ibuprofen and ketoprofen in aqueous solutions. Chemosphere 2011, 84, 1658–1663. [Google Scholar] [CrossRef]
- Matamoros, V.; Duhec, A.; Albaiges, J.; Bayona, J.M. Photodegradation ofcarbamazepine, ibuprofen, ketoprofen and 17a-ethinylestradiol in fresh andseawater. Water Air Soil Pollut. 2009, 196, 161–168. [Google Scholar] [CrossRef]
- Bosca, F.; Carganico, G.; Castell, J.V.; Gómez-Lechón, M.J.; Hernandez, D.; Mauleón, D.; Martínez, L.A.; Miranda, M.A. Evaluation of ketoprofen(R, S and R/S) phototoxicity by a battery of in vitro assays. J. Photochem. Photobiol. B Biol. 1995, 31, 133–138. [Google Scholar] [CrossRef]
- Loh, T.Y.; Cohen, P.R. Ketoprofen-induced photoallergic dermatitis. Indian J. Med. Res. 2016, 144, 803–806. [Google Scholar]
- Yan, S.; Song, W. Photo-transformation of pharmaceutically active compounds in the aqueous environment: A review. Environ. Sci. Process. Impacts 2014, 16, 697–720. [Google Scholar] [CrossRef]
- Li, F.H.; Yao, K.; Lv, W.Y.; Liu, G.G.; Chen, P.; Huang, H.P.; Kang, Y.P. Photodegradation of ibuprofen under UV-Vis irradiation: Mechanism and toxicity of photolysis products. Bull. Environ. Contam. Toxicol. 2015, 94, 479–483. [Google Scholar] [CrossRef] [PubMed]
- Ruggeri, G.; Ghigo, G.; Maurino, V.; Minero, C.; Vione, D. Photochemical transformation of ibuprofen into harmful 4-isobutylacetophenone: Pathways, kinetics, and significance for surface waters. Water Res. 2013, 47, 6109–6121. [Google Scholar] [CrossRef] [PubMed]
- Vione, D.; Maddigapu, P.R.; De Laurentiis, E.; Minella, M.; Pazzi, M.; Maurino, V.; Minero, C.; Kouras, S.; Richard, C. Modelling the photochemical fate of ibuprofen in surface waters. Water Res. 2011, 45, 6725–6736. [Google Scholar] [CrossRef] [PubMed]
- Gou, N.; Yuan, S.; Lan, J.; Gao, C.; Alshawabkeh, A.N.; Gu, A.Z. A quantitative toxicogenomics assay reveals the evolution and nature of toxicity during the transformation of environmental pollutants. Environ. Sci. Technol. 2014, 48, 8855–8863. [Google Scholar] [CrossRef] [PubMed]
- Beck, I.; Deflandre, A.; Lang, G.; Arnaud, R.; Lemair, J. Study of the photochemical behaviour of sunscreens-benzylidene camphor and derivatives. Int. J. Cosmet. Sci. 1981, 3, 139–152. [Google Scholar] [CrossRef] [PubMed]
- Hanson, K.M.; Narayanan, S.; Nichols, V.M.; Bardeen, C.J. Photochemical degradation of the UV filter octyl methoxycinnamate in solution and in aggregates. Photochem. Photobiol. Sci. 2015, 14, 1607–1616. [Google Scholar] [CrossRef] [PubMed]
- Broadbent, J.K.; Martincigh, B.S.; Raynor, M.W.; Salter, L.F.; Moulder, R.; Sjoberg, P.; Markides, K.E. Capillary supercritical fluid chromatography combined with atmospheric pressure chemical ionisation mass spectrometry for the investigation of photoproduct formation in the sunscreen absorber 2-ethylhexyl-p-methoxycinnamate. J. Chromatogr. A 1996, 732, 101–110. [Google Scholar] [CrossRef]
- Pattanaargson, S.; Limphong, P. Stability of octyl methoxycinnamate and identification of its photo-degradation product. Int. J. Cosmet. Sci. 2001, 23, 153–160. [Google Scholar] [CrossRef]
- Chatelain, E.; Gabard, B. Photostabilization of Butyl methoxydibenzoylmethane (Avobenzone) and Ethylhexyl methoxycinnamate by Bis-ethylhexyloxyphenol methoxyphenyl triazine (Tinosorb S), a New UV Broadband Filter. Photochem. Photobiol. 2001, 74, 401–406. [Google Scholar] [CrossRef]
- Wang, S.Q.; Balagula, Y.; Osterwalder, U. Photoprotection: A review of the current and future technologies. Dermatol. Ther. 2010, 23, 31–47. [Google Scholar] [CrossRef]
- Mturi, G.J.; Martincigh, B.S. Photostability of the sunscreening agent 4-tert-butyl-4′-methoxydibenzoylmethane (avobenzone) in solvents of different polarity and proticity. J. Photochem. Photobiol. A 2008, 200, 410–420. [Google Scholar] [CrossRef]
- Huong, S.P.; Rocher, E.; Fourneron, J.D.; Charles, L.; Monnier, V.; Bun, H.; Andrieu, V. Photoreactivity of the sunscreen butylmethoxydibenzoylmethane (DBM) under various experimental conditions. J. Photochem. Photobiol. A Chem. 2001, 196, 106–112. [Google Scholar] [CrossRef]
- Almawi, W.Y.; Melemedjian, O.K. Molecular mechanisms of glucocorticoid antiproliferative effects: Antagonism of transcription factor activity by glucocorticoid receptor. J. Leukoc. Biol. 2002, 71, 9–15. [Google Scholar] [PubMed]
- De Bosscher, K.; Haegeman, G. Minireview: Latest perspectives on antiinflammatory actions of glucocorticoids. Mol. Endocrinol. 2009, 23, 281–291. [Google Scholar] [CrossRef]
- Gabros, S.; Zito, P.M. Topical Corticosteroids. Available online: https://www.ncbi.nlm.nih.gov/books/NBK532940/ (accessed on 24 September 2019).
- Albini, A.; Fasani, E. Photostability of Drugs and Drug Formulations; Tonnesen, H.H., Ed.; CRC Press: Boca Raton, FL, USA, 2004; Chapter 4; pp. 68–101. [Google Scholar]
- Thoma, K. Photostabilzation of solid and semisolid dosage forms. In Pharmaceutical Photostability and Stabilization Technology; Piechocki, J.T., Thoma, K., Eds.; Informa Healthcare: New York, NY, USA, 2007; pp. 336–341. [Google Scholar]
- Hongying, Y.; Sukang, Z.; Ning, P. Studying the mechanisms of titanium dioxide as ultraviolet-blocking additive for films and fabrics by an improved scheme. J. Appl. Polym. Sci. 2004, 92, 3201–3210. [Google Scholar]
- Ricci, A.; Fasani, E.; Mella, M.; Albini, A. Noncommunicating photoreaction paths in some pregna-1,4-diene-3,20-diones. J. Org. Chem. 2001, 66, 8086–8093. [Google Scholar] [CrossRef]
- Miolo, G.; Ricci, A.; Caffieri, S.; Levorato, L.; Fasani, E.; Albini, A. In vitro phototoxic properties of triamcinolone 16,17-acetonide and its main photoproducts. Photochem. Photobiol. 2003, 78, 425–430. [Google Scholar] [CrossRef]
- Fox, L.; Csongradi, C.; Aucamp, M.; du Plessis, J.; Gerber, M. Treatment Modalities for Acne. Molecules 2016, 21, 1063. [Google Scholar] [CrossRef]
- Irby, C.E.; Yentzer, B.A.; Feldman, S.R. A review of adapalene in the treatment of acne vulgaris. J. Adolesc. Health 2008, 43, 421–424. [Google Scholar] [CrossRef]
- Beckenbach, L.; Baron, J.M.; Merk, H.F.; Löffler, H.; Amann, P.M. Retinoid treatment of skin diseases. Eur. J. Dermatol. 2015, 25, 384–391. [Google Scholar] [CrossRef]
- Khalil, S.; Bardawil, T.; Stephan, C.; Darwiche, N.; Abbas, O.; Kibbi, A.G.; Nemer, G.; Kurban, M. Retinoids: A Journey from the Molecular Structures and Mechanisms of Action to Clinical Uses in Dermatology and Adverse Effects. J. Dermatolog. Treat. 2017, 28, 684–696. [Google Scholar] [CrossRef] [PubMed]
- Curley, R.W.; Fowble, J.W. Photoisomerization of retinoic acid and its photoprotection in physiologic-like solutions. Photochem. Photobiol. 1988, 47, 831–835. [Google Scholar] [CrossRef] [PubMed]
- Tolleson, W.H.; Cherng, S.H.; Xia, Q.; Boudreau, M.; Yin, J.J.; Wamer, W.G.; Howard, P.C.; Yu, H.; Fu, P.P. Photodecomposition and Phototoxicity of Natural Retinoids. Int. J. Environ. Res. Public Health 2005, 2, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Czernielewski, J.; Michel, S.; Bouclier, M.; Baker, M.; Hensby, J.C. Adapalene biochemistry and the evolution of a new topical retinoid for treatment of acne. J. Eur. Acad. Dermatol. Venereol. 2001, 15, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Jain, S. Topical tretinoin or adapalene in acne vulgaris: An overview. J. Dermatolog. Treat. 2004, 15, 200–207. [Google Scholar] [CrossRef] [PubMed]
- EUROPEAN PHARMACOPOEIA 7.0. Available online: http://www.fptl.ru/biblioteka/farmacop/EP-7.0-2.pdf (accessed on 24 September 2019).
- Koo, J.Y.; Lowe, N.J.; Lew-Kaya, D.A.; Vasilopoulos, A.I.; Lue, J.C.; Sefton, J.; Gibson, J.R. Tazarotene plus UVB phototherapy in the treatment of psoriasis. J. Am. Acad. Dermatol. 2000, 43, 821–828. [Google Scholar] [CrossRef]
- Dayal, S.; Kaura, R.; Sahu, P.; Jain, V.K. Tazarotene gel with narrow-band UVB phototherapy: A synergistic combination in psoriasis. An. Bras. Dermatol. 2018, 93, 385–390. [Google Scholar] [CrossRef]
- Kim, H.; Jung, S.; Yeo, S.; Kim, D.; Na, Y.C.; Yun, G.; Lee, J. Characteristics of Skin Deposition of Itraconazole Solubilized in Cream Formulation. Pharmaceutics 2019, 11, e195. [Google Scholar] [CrossRef]
- Kumar, N.; Shishu. D-optimal experimental approach for designing topical microemulsion of itraconazole: Characterization and evaluation of antifungal efficacy against a standardized Tinea pedis infection model in Wistar rats. Eur. J. Pharm. Sci. 2015, 67, 97–112. [Google Scholar] [CrossRef]
- Hosseini-Yeganeh, M.; McLachlan, A.J. Physiologically based pharmacokinetic model for terbinafine in rats and humans. Antimicrob. Agents Chemother. 2002, 46, 2219–2228. [Google Scholar] [CrossRef]
- Schäfer-Korting, M.; Schoellmann, C.; Korting, H.C. Fungicidal activity plus reservoir effect allow short treatment courses with terbinafine in tinea pedis. Skin Pharmacol. Physiol. 2008, 21, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Faergemann, J.; Zehender, H.; Millerioux, L. Levels of terbinafine in plasma, stratum corneum, dermis-epidermis (without stratum corneum), sebum, hair and nails during and after 250 mg terbinafine orally once daily for 7 and 14 days. Clin. Exp. Dermatol. 1994, 19, 121–126. [Google Scholar] [CrossRef] [PubMed]
- López, L.; Vélez, I.; Asela, C.; Cruz, C.; Alves, F.; Robledo, S.; Arana, B. A phase II study to evaluate the safety and efficacy of topical 3% amphotericin B cream (Anfoleish) for the treatment of uncomplicated cutaneous leishmaniasis in Colombia. PLoS Negl. Trop. Dis. 2018, 12, e0006653. [Google Scholar] [CrossRef] [PubMed]
- Thoma, K.; Kübler, N. Photostability of antifungal agents. 2. Photostability of polyene antibiotics. Pharmazie 1997, 52, 294–302. [Google Scholar]
- Chow, W.S.; Chen, S.C.; Timmins, P. United States Patent. Available online: https://patentimages.storage.googleapis.com/ac/d9/68/c6d22d190158b3/US4883785.pdf (accessed on 24 September 2019).
- Van Haeringen, N.J.; van Sorge, A.A.; Carballosa Coré-Bodelier, V.M. Constitutive cyclooxygenase-1 and induced cyclooxygenase-2 in isolated human iris inhibited by S(+) flurbiprofen. J. Ocul. Pharmacol. 2000, 16, 353–361. [Google Scholar] [CrossRef]
- Bagheri, H.; Lhiaubet, V.; Montastruc, J.L.; Chouini-Lalanne, N. Photosensitivity to ketoprofen: Mechanisms and pharmacoepidemiological data. Drug Saf. 2000, 22, 339–349. [Google Scholar] [CrossRef]
- Aminuddin, M.; Nazim, U.; Ahmad, I. Photo- and Thermal Degradation of Piroxicam in Aqueous Solution. Indian J. Pharm. Sci. 2011, 73, 387–391. [Google Scholar]
- Salgado, R.; Pereira, V.J.; Carvalho, G.; Soeiro, R.; Gaffney, V.; Almeida, C.; Vale Cardoso, V.; Ferreira, E.; Benoliel, M.J.; Ternes, T.A.; et al. Photodegradation kinetics and transformation products of ketoprofen, diclofenac and atenolol in pure water and treated wastewater. J. Hazard. Mater. 2013, 244–245, 516–527. [Google Scholar] [CrossRef]
- Atarashi, K.; Takano, M.; Kato, S.; Kuma, H.; Nakanishi, M.; Tokura, Y. Addition of UVA-absorber butyl methoxy dibenzoylmethane to topical ketoprofen formulation reduces ketoprofen-photoallergic reaction. J. Photochem. Photobiol. B 2012, 113, 56–62. [Google Scholar] [CrossRef]
- Allen, J.M.; Gosset, C.J.; Allen, S.F. Photochemical formation of singlet molecular oxygen in illuminated aqueous solutions of several commercially available sunscreen active ingredients. Chem. Res. Toxicol. 1996, 9, 605–609. [Google Scholar] [CrossRef]
- Nash, J.F.; Tanner, R.P. Relevance of UV filter/sunscreen product photostability to human safety. Photodermatol Photoimmunol Photomed. 2014, 30, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, L.R.; Tharmann, J.; Maia Campos, P.M.; Liebsch, M. Skin phototoxicity of cosmetic formulations containing photounstable and photostable UV-filters and vitamin A palmitate. Toxicol. In Vitro 2013, 27, 418–425. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, L.R.; Maia Campos, P.M. Evaluation of the photostability of different UV filter combinations in a sunscreen. Int. J. Pharm. 2006, 307, 123–128. [Google Scholar]
- Ioele, G.; De Luca, M.; Tavano, L.; Ragno, G. The difficulties for a photolabile drug in topical formulations: The case of diclofenac. Int. J. Pharm. 2014, 465, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Rasul, M.; Martens, W.N.; Brown, R.; Hashib, M. Heterogeneous photocatalytic degradation of phenols in wastewater: A review on current status and developments. Desalination 2010, 261, 3–18. [Google Scholar] [CrossRef]
- Picatonotto, T.; Vione, D.; Carlotti, M.E.; Gallarate, M. Photocatalytic Activity of Inorganic Sunscreens. J. Dispers. Sci. Technol. 2001, 22, 381–386. [Google Scholar] [CrossRef]
- Yi, D.K.; Papaefthymiou, G.C. Nanobiomaterials: Development and Applications; CRC Press, Taylaor& Francis group: Boca Raton, FL, USA, 2013. [Google Scholar]
- Li, M.; Yin, J.J.; Wamer, W.G.; Lo, Y.M. Mechanistic characterization of titanium dioxide nanoparticle-induced toxicity using electron spin resonance. J. Food Drug Anal. 2014, 22, 76–85. [Google Scholar] [CrossRef]
- Manconi, M.; Valenti, D.; Sinico, C.; Lai, F.; Loy, G.; Fadda, A.M. Niosomes as carriers for tretinoin. II. Influence of vesicular incorporation on tretinoin photostability. Int. J. Pharm. 2003, 260, 261–272. [Google Scholar] [CrossRef]
- Drucker, A.M.; Rosen, C.F. Drug-induced photosensitivity: Culprit drugs, management and prevention. Drug Saf. 2011, 34, 821–837. [Google Scholar] [CrossRef]
- Blakely, K.M.; Drucker, A.M.; Rosen, C.F. Drug-Induced Photosensitivity-An Update: Culprit Drugs, Prevention and Management. Drug Saf. 2019, 42, 827–847. [Google Scholar] [CrossRef]
- Kim, K.; Park, H.; Lim, K.M. Phototoxicity: Its Mechanism and Animal Alternative Test Methods. Toxicol. Res. 2015, 31, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Gould, J.W.; Mercurio, M.G.; Elmets, C.A. Cutaneous photosensitivity diseases induced by exogenous agents. J. Am. Acad. Dermatol. 1995, 33, 551–573. [Google Scholar] [CrossRef]
- OECD Guidelines for the Testing of Chemicals, Test No. 432: In Vitro 3T3 NRU Phototoxicity Test. 2019. Available online: https://ntp.niehs.nih.gov/iccvam/suppdocs/feddocs/oecd/oecdtg432-508.pdf (accessed on 24 September 2019).
- Moore, R.A.; Tramer, R.M.; Caroll, D.; Wiffen, P.J.; McQuay, H.J. Quantitative systematic review of topically applied non-steroidal anti-inflammatory drugs. BMJ 1998, 316, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Swedish Adverse Drug Reactions Advisory Committee (SADRAC). Ketoprofen gel contact dermatitis and photosensitivity. Bull. Swed. Advers. Drug React. Advis. Comm. 1998, 67, 4. [Google Scholar]
- Gutierrez-Gonzalez, E.; Rodriguez-Pazos, L.; Rodriguez-Granados, M.T.; Toribio, J. Photosensitivity induced by naproxen. Photodermatol. Photoimmunol. Photomed. 2011, 27, 338–340. [Google Scholar] [CrossRef] [PubMed]
- Diffey, B.L.; Daymond, T.J.; Fairgreaves, H. Phototoxic reactions to piroxicam, naproxen and tiaprofenic acid. Br. J. Rheumatol. 1983, 22, 239–242. [Google Scholar] [CrossRef] [PubMed]
- Fjellner, B. Photosensitivity induced by piroxicam. Acta Derm. Venereol. 1983, 63, 557–558. [Google Scholar] [PubMed]
- Bergner, T.; Przybilla, B. Photosensitization caused by ibuprofen. J. Am. Acad. Dermatol. 1992, 26, 114–116. [Google Scholar] [CrossRef]
- Alvarez-Fernández, J.G.; Castaño-Suárez, E.; Cornejo-Navarro, P.; de la Fuente, E.G.; Ortiz de Frutos, F.J.; Iglesias-Diez, L. Photosensitivity induced by oral itraconazole. J. Eur. Acad. Dermatol. Venereol. 2000, 14, 501–503. [Google Scholar]
- Malani, A.N.; Aronoff, D.M. Voriconazole-induced photosensitivity. Clin. Med. Res. 2008, 6, 83–85. [Google Scholar] [CrossRef]
- Goyal, R.K. Voriconazole-associated phototoxic dermatoses and skin cancer. Expert Rev Anti Infect Ther. 2015, 13, 1537–1546. [Google Scholar] [CrossRef] [PubMed]
- Haylett, A.K.; Felton, S.; Denning, D.W.; Rhodes, L.E. Voriconazole-induced photosensitivity: Photobiological assessment of a case series of 12 patients. Br. J. Dermatol. 2013, 168, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, K.N. Severe photodermatitis during ketoconazole therapy. Clin. Exp. Dermatol. 1988, 13, 54. [Google Scholar] [CrossRef] [PubMed]
- Kawabe, Y.; Mizuno, N.; Miwa, N.; Sakakibara, S. Photosensitivity induced by griseofulvin. Photodermatology 1988, 5, 272–274. [Google Scholar]
- Seishima, M.; Shibuya, Y.; Kato, G.; Watanabe, K. Photoleukomelanoderma possibly caused by etretinate in a patient with psoriasis. Acta Derm. Venereol. 2010, 90, 85–86. [Google Scholar] [CrossRef]
- Ferguson, J.; Johnson, B.E. Photosensitivity due to retinoids: Clinical and laboratory studies. Br. J. Dermatol. 1986, 115, 275–283. [Google Scholar] [CrossRef]
- Wong, R.C.; Gilbert, M.; Woo, T.Y.; Kang, S.; Petersen, C.; Ellis, C.N. Photosensitivity and isotretinoin therapy. J. Am. Acad. Dermatol. 1986, 14, 1095–1096. [Google Scholar] [CrossRef]
- Kullavanijaya, P.; Lim, H.W. Photoprotection. J. Am. Acad. Dermatol. 2005, 52, 937–958. [Google Scholar] [CrossRef]
- Schauder, S.; Ippen, H. Contact and photocontact sensitivity to sunscreens. Review of a 15-year experience and of the literature. Contact Dermat. 1997, 37, 221–232. [Google Scholar] [CrossRef]
| Drug Class | Active Pharmaceutical Ingredient | Ref. |
|---|---|---|
| Glucocorticosteroids | pregna-1,4-dien-3,20-diones | [24] |
| betamethasone and its esters | [25,26] | |
| betamethasone-17 valerate | [27] | |
| mometasone furoate | [28] | |
| hydrocortisone 21-acetate | [29] | |
| prednisolone | [30] | |
| fluocinolone 16,17-acetonide | [31,32] | |
| desonide | [33,34] | |
| Retinoids | vitamin A | [35] |
| tretinoin | [36,37,38,39,40,41,42,43,44] | |
| isotretinoin | [36,37,45] | |
| adapalene | [46,47] | |
| tazarotene | [48] | |
| Antifungal drugs | clotrimazole | [49,50] |
| bifonazole | [51] | |
| itraconazole | [52,53] | |
| terbinafine | [54] | |
| Non-steroidal anti-inflammatory drugs | piroxicam | [55,56,57] |
| naproxen | [58,59,60,61] | |
| diclofenac | [57,58,62] | |
| ketoprofen | [63,64,65,66] | |
| ibuprofen | [67,68,69,70,71] | |
| UV filters | 4-methylbenzylidene camphor | [72] |
| octyl methoxycinnamate | [73,74,75] | |
| avobenzone | [76,77,78,79] |
| Retinoid | Presentation of Samples | Light Source | Irradiation Time/Dose | Ref. |
|---|---|---|---|---|
| Adapalene |
| CAMAG UV-lamp, S/N 29000, dual wavelength 254/366 nm (Switzerland) | day light, UV-light 254 nm UV-light 366 nm irradiation time—12 h distance—15 cm | [46] |
| Adapalene with benzoyl peroxide |
| monochromatic sodium lamp type NA 55 W (Osram), and fluorescent lighting tubes for normal room lighting | inactinic light actinic light 24 h | [42] |
| Adapalene |
| photostability chamber (SUN TEST XLS+, Atlas, USA). | visible light for 240 h (1.2 million lux h), UV light for 250 h (200 W h/m2) temp. 25 °C | [47] |
| Tazarotene |
| UVB—Light Sources FS72 T12-UVB-HO bulbs UVA—FS72 T12-BL HO/50R bulbs covered with filters blocking UVB and lower wavelengths. | phototherapy UVB 100 to 150 mJ/cm2 UVA 15 to 22 J/cm2 | [48] |
| Tretinoin with benzoyl peroxide |
| monochromatic sodium lamp type NA 55 W (Osram), and fluorescent lighting tubes for normal room lighting | inactinic light actinic light 24 h | [42] |
| Tretinoin |
| XBO 450 W high pressure xenon lamp | distance of 28 cm temperature in the cuvette never exceeded 36 °C | [40] |
| Tretinoin |
| light testing cabinet Suntest CPS+ (Heraeus, Milan, Italy), equipped with a Xenon lamp | light dose of 21 kJ min−1 m−2, temperature of 25 °C. 0.5–240 min | [37] |
| Tretinoin |
| solar simulator, model 91293, (Oriel Corporation, Stratford, CT, USA) equipped with 1000 W Xenon lamp Luzchem expo Panels composed of 5 Sylvania 8 W cool white light tubes | distance 20 cm at 365 nm from the source, the SSL dose was 7.63 mJ/cm2/sec UVA and 0.40 mJ/cm2/sec UVB radiation, UVB/UVC blocking filter the dose at 365 nm from the source was 5.39 mJ/cm2/sec UVA radiation with residual UVB dose of 3.16 µJ/cm2/sec. | [36] |
| Isotretinoin |
| light testing cabinet Suntest CPS+ (Heraeus), equipped with a Xenon lamp Luzchem expo Panels composed of 5 Sylvania 8-W cool white light tubes | light dose of 21 kJ min−1 m−2, temperature of 25 °C. 0.5–240 min | [37] |
| Isotretinoin |
| solar simulator, model 91293, (Oriel Corporation) equipped with 1000 W Xenon lamp | distance 20 cm at 365 nm from the source, the SSL dose was 7.63 mJ/cm2/sec UVA and 0.40 mJ/cm2/sec UVB radiation; distance 20 cm at 365 nm from the source, the dose was 5.39 mJ/cm2/sec UVA radiation with residual UVB dose of 3.16 µJ/cm2/sec (UVB and UVC blocking filter) | [36] |
| Vitamin A |
| 96000 Oriel 150 W Xenon arc solar simulator (Oriel Corporation) | UVA/UVB irradiation (280–400 nm) UVB dose of approximately 334.8 mJ/cm2 30 min | [35] |
| Active Pharmaceutical Ingredients | Photostabilizers/Excipients | Form | Percent Loss | Irradiation Dose/Time/Type/Source | Ref |
|---|---|---|---|---|---|
| Betamethasone valerate | control | cream | 49.2 ± 0.92 | UV lamp (300 W, Ultra-Vitalux Osram) 300–400 nm, the intensity of light-16 000 lx, up to 2 h of irradiation | [27] |
| titanium dioxide (light scattering) | 17.78 ± 1.24 | ||||
| vanillin (radical scavenger) | 27.6 ± 1.36 | ||||
| butyl hydroxytoluene (radical scavenger) | 31.0 ± 1.22 | ||||
| Betamethasone valerate | control | gel | 42.5 ± 1.64 | UV lamp (300–400 nm) the intensity of light-16 000 lx | [27] |
| titanium dioxide (light scattering) | 7.2 ± 0.98 | ||||
| vanillin (radical scavenger) | 13.8 ± 1.44 | ||||
| butyl hydroxytoluene (radical scavenger) | 21.9 ± 1.60 | ||||
| Betamethasone valerate cream | control (without the preservative) | topical ointment 0.1% | about 30% | UVB (5 J/cm2)– Philips PL-S 9W/12 lamp mainly emitting at 312 nm | [25] |
| chlorocresol (excipient-preservative) | less than 10% | ||||
| Hydrocortisone 21-acetate | control (without the preservatives) | commercial formulation (cream) | 40% | UVB (15 J/cm2)– Philips PL-S 9W/12 lamp mainly emitting at 312 nm | [29] |
| parabens: methyl- and propyl p-hydroxybenzoates (excipients—preservatives) | 20% | ||||
| Triamcinolone acetonide | control (without the preservatives) | basis cream DAC | 38% | 3 h of irradiation, Suntest CPS+, 415 W/m2 | [84] |
| pigmented creams (ZnO, TiO2) | 95% | ||||
| Desonide | control | hair solution (0.1%) | 61% | UVA irradiation (1350 W h/m2) 15 h of irradiation | [34] |
| benzophenone-3 (UV-filter, 0.3%) | 1.49% | ||||
| Vitamin A | control: 0.6% (w/w) vitamin A palmitate (1,700,000 UI/g) | topical formulation | n.d. | 30 min UVA/UVB irradiation (280–400 nm) 96000 Oriel 150 W xenon arc solar simulator (Oriel Corporation), 0.186 mW/cm2, UVB dose 334.8 mJ/cm2 | [35] |
| octyl methoxycinnamate, avobenzone, 4-methylbenzilidene camphor | enhanced vitamin A stability | ||||
| octyl methoxycinnamate, benzophenone-3, octocrylene | enhanced vitamin A stability | ||||
| Tretinoin | solution | ethanolic solution | 92% | Sunset CPS+ (Heraeus)-xenon lamp (300–800 nm) 250 W/m2 for 240 min | [37] |
| liposomes | liposomes | 40% | |||
| Tretinoin | micronized tretinoin (0.05%) | gel | 9% | UVA light (315–400 nm) 22 W/m2 | [43] |
| tretinoin (0.025%) | gel | 72% | |||
| Tretinoin | control: | Methanolic solution | 63% | 1 h of irradiation, 30 W lamp-366 nm (Min UVIS, Desaga, GmbH, Germany) | [44] |
| Nanosuspension tretinoin (0.035%) | nanosuspension | 17% | |||
| Nanoemulsion tretinoin (0.035%) | nanoemulsion | 48% | |||
| Tretionoin | control: | methanolic solution | incorporation in vesicles always improved the photostability of tretinoin | UV lamp set at 366 nm (Min UVIS, Desaga, GmbH) fluorescent light | [121] |
| niosomes tretinoin | vesicular suspensions | ||||
| Isotretinoin | control | methanol solution | 84% | natural sunlight (>20,000 Lux) | [45] |
| micro-emulsion tretinoin (0.035%) | micro-emulsion formulation | 25% | |||
| Diclofenac | control | solution | the drug appears to be more stable than the complex for T < 30 min and thereafter degrades rapidly (the complex is more stable) | 400 W mercury lamp | [57] |
| 2-hydroxypropyl-β-cyclodextrin | cyclodextrin | ||||
| Piroxicam | control | piroxicam | not affected the rate of photodegradation | n.d. | [57] |
| 2-hydroxypropyl-β-cyclodextrin | piroxicam-β-cyclodextrin | ||||
| Piroxicam | control | piroxicam | complex improved photostability | daylight up to 30 days | [56] |
| 2-hydroxypropyl-β-cyclodextrin | piroxicam:2-hydroxypropyl-β-cyclodextrin complex | ||||
| Avobenzone | control | prepared formulation | 56%–70% (different concentration of avobenzone) | optically filtered xenon arc source (Multiport Solar UV simulator, Solar light, Philadelphia, PA, USA) UV irradiance adjusted at 1 mean effective dose [MED]/min | [76] |
| tinosorb S | formulation with tinosorb S | 5%–15% |
| Class of Drug | Active Pharmaceutical Ingredient | Photosensitivity | Action Spectra | Ref. |
|---|---|---|---|---|
| NSAID | Ketoprofen | Phototoxic reaction Photoallergic reaction | UVA | [108,127,128] |
| Naproxen | Phototoxic reaction Photoallergic reaction | UVA | [129] | |
| Piroxicam | Photoallergic reaction | UVA | [130,131] | |
| Ibuprofen | Phototoxic reaction | UVA | [132] | |
| Antifungal agents | Itraconazole | Phototoxic reaction Photoallergic reaction | Unknown | [123,133] |
| Voriconazole | Phototoxic reaction | UVA | [123,134,135,136] | |
| Ketoconazole | Phototoxic reaction | Unknown | [123,137] | |
| Griseofulvin | Phototoxic reaction | UVA | [123,138] | |
| Retinoids | Etretinate/the major metabolite of etretinate | Phototoxic reaction | UVA/poss. UVB | [139,140] |
| Isotretinoin | Phototoxic reaction | UVA/poss. UVB | [140,141] | |
| Tretinoin | Phototoxic reaction | Unknown | [140] | |
| UV filters | PABA derivatives | Photoallergic reaction | UVA | [142] |
| Benzophenones | Photoallergic reaction | UVA | [142] | |
| Isopropyl dibenzoylmethane | Photoallergic reaction | UVA | [142] | |
| Cinnamates | Photoallergic reaction | UVA | [143] | |
| Camphor derivatives | Photoallergic reaction | UVA | [142] | |
| Avobenzone | Photoallergic reaction | UVA | [142] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kryczyk-Poprawa, A.; Kwiecień, A.; Opoka, W. Photostability of Topical Agents Applied to the Skin: A Review. Pharmaceutics 2020, 12, 10. https://doi.org/10.3390/pharmaceutics12010010
Kryczyk-Poprawa A, Kwiecień A, Opoka W. Photostability of Topical Agents Applied to the Skin: A Review. Pharmaceutics. 2020; 12(1):10. https://doi.org/10.3390/pharmaceutics12010010
Chicago/Turabian StyleKryczyk-Poprawa, Agata, Anna Kwiecień, and Włodzimierz Opoka. 2020. "Photostability of Topical Agents Applied to the Skin: A Review" Pharmaceutics 12, no. 1: 10. https://doi.org/10.3390/pharmaceutics12010010
APA StyleKryczyk-Poprawa, A., Kwiecień, A., & Opoka, W. (2020). Photostability of Topical Agents Applied to the Skin: A Review. Pharmaceutics, 12(1), 10. https://doi.org/10.3390/pharmaceutics12010010