Comprehensive Analysis of the Safety Profile of a Single-Stranded RNA Nano-Structure Adjuvant
Abstract
1. Introduction
2. Materials and Methods
2.1. Adjuvant
2.2. Cell Culture
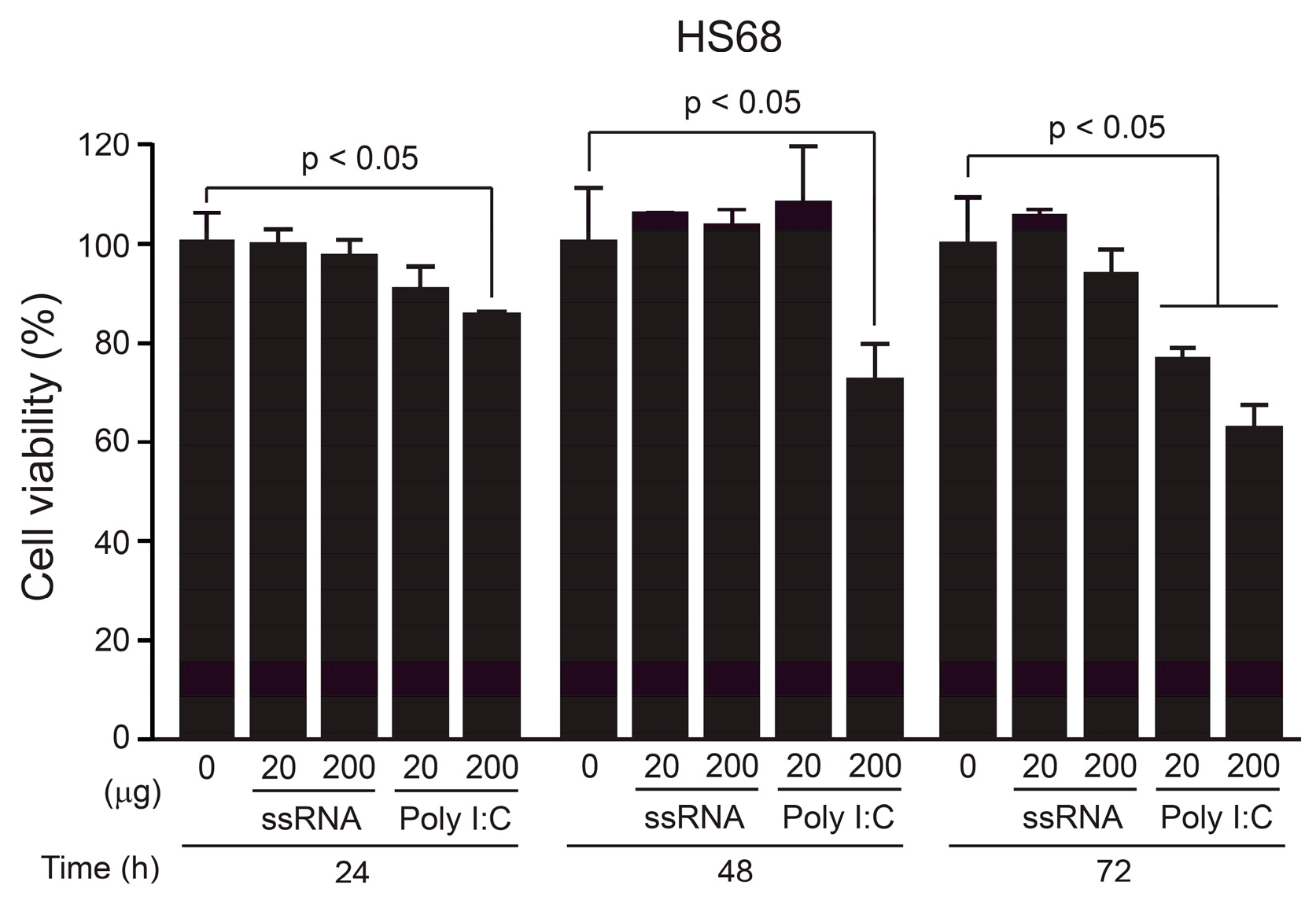
2.3. Cell-Viability Assay
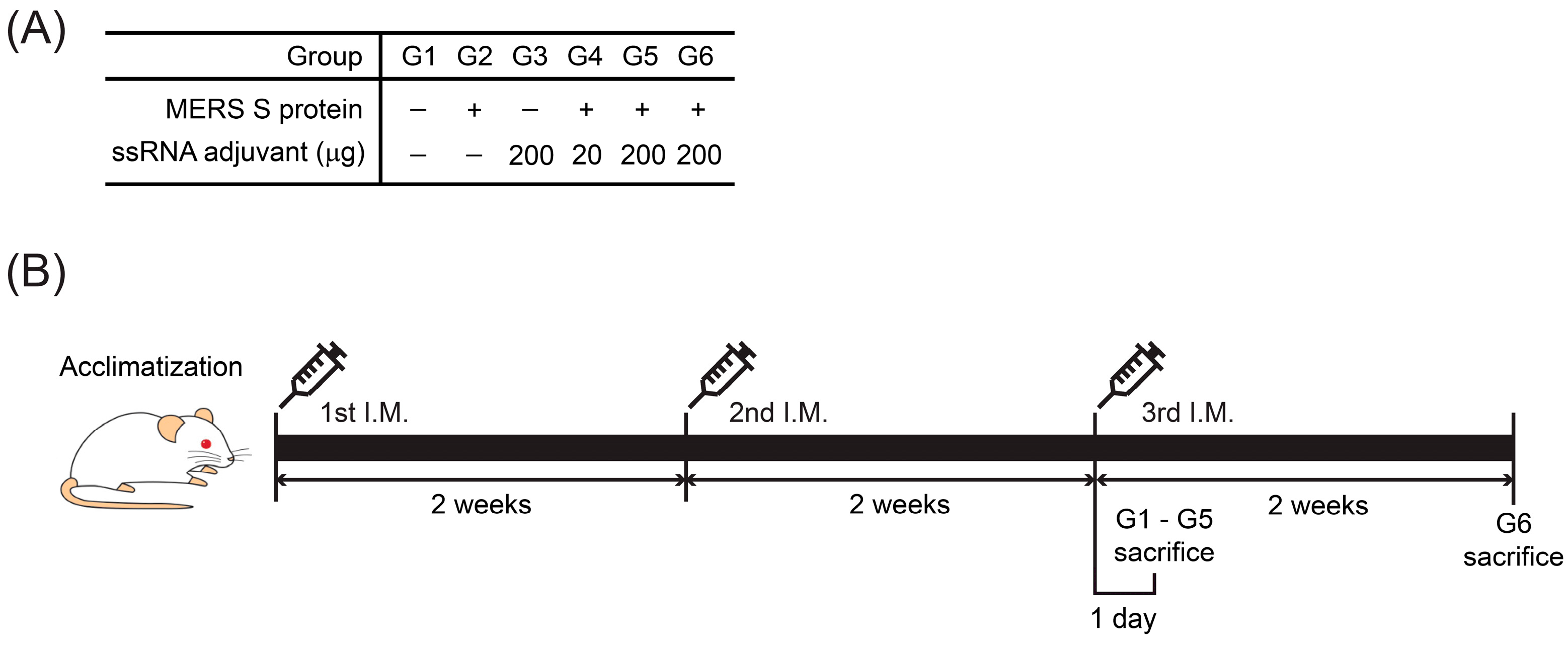
2.4. Mice
2.5. Experimental Design for In Vivo Toxicity Study
2.6. Hematological and Serum Biochemical Analysis In Vivo Toxicity Study
2.7. Gross Findings, Organ Weights, and Histopathological Assessments In Vivo Toxicity Study
2.8. Mouse IgE Mouse Enzyme-Linked Immunosorbent Assays (ELISAs) In Vivo Toxicity Study
2.9. Mouse Anti-Nuclear Antibody ELISAs In Vivo Toxicity Study
2.10. Cytokine Analysis In Vivo Toxicity Study
2.11. Flow Cytometry Analysis In Vivo Toxicity Study
2.12. Statistical Analysis
3. Results
3.1. Cellular Toxicity of the ssRNA Nano-Structure Adjuvant In Vitro
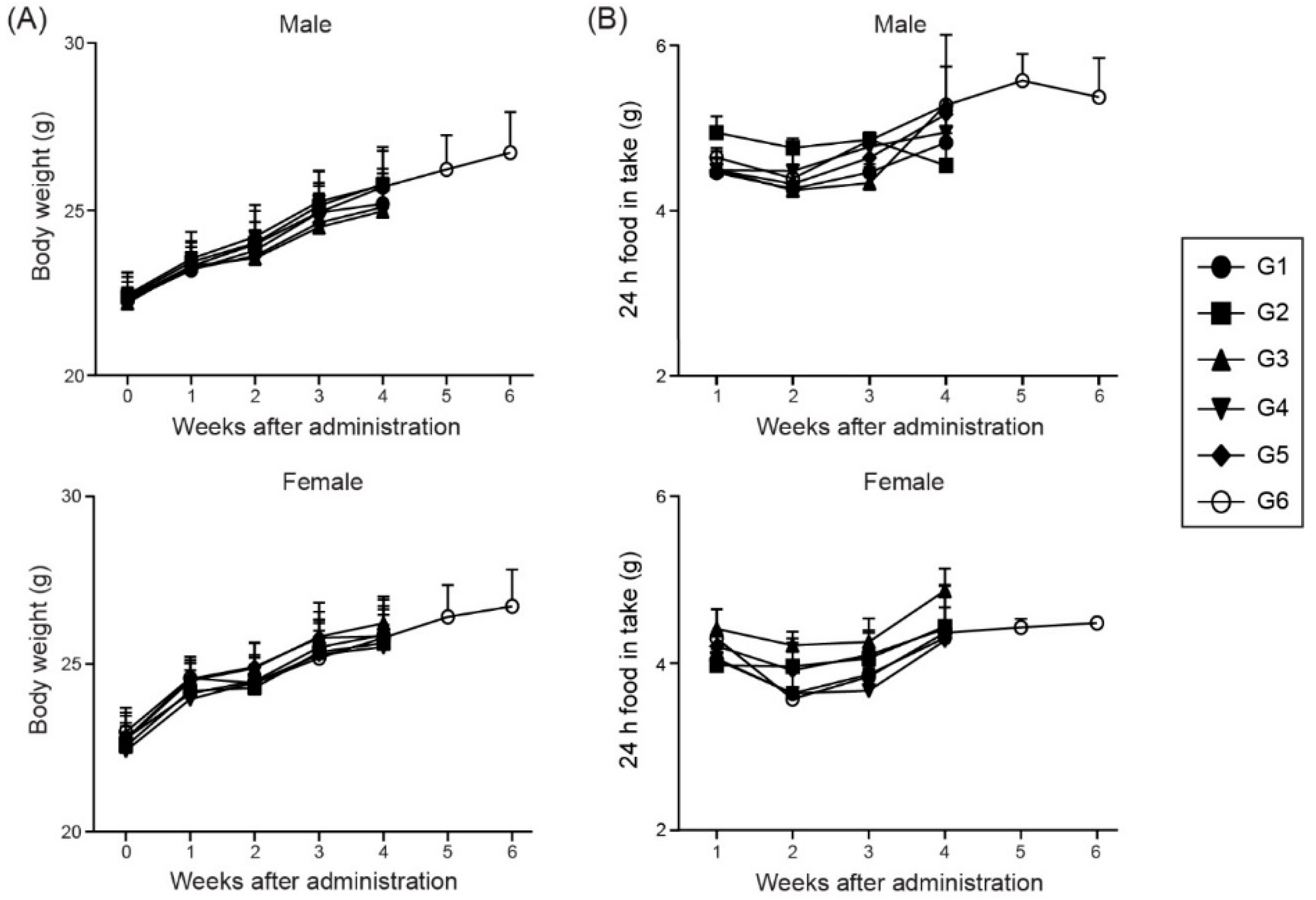
3.2. Changes in Body Weight and Food Intake After Immunization with the ssRNA Nano-Structure Adjuvant
3.3. Hematological and Serum Biochemical Parameters
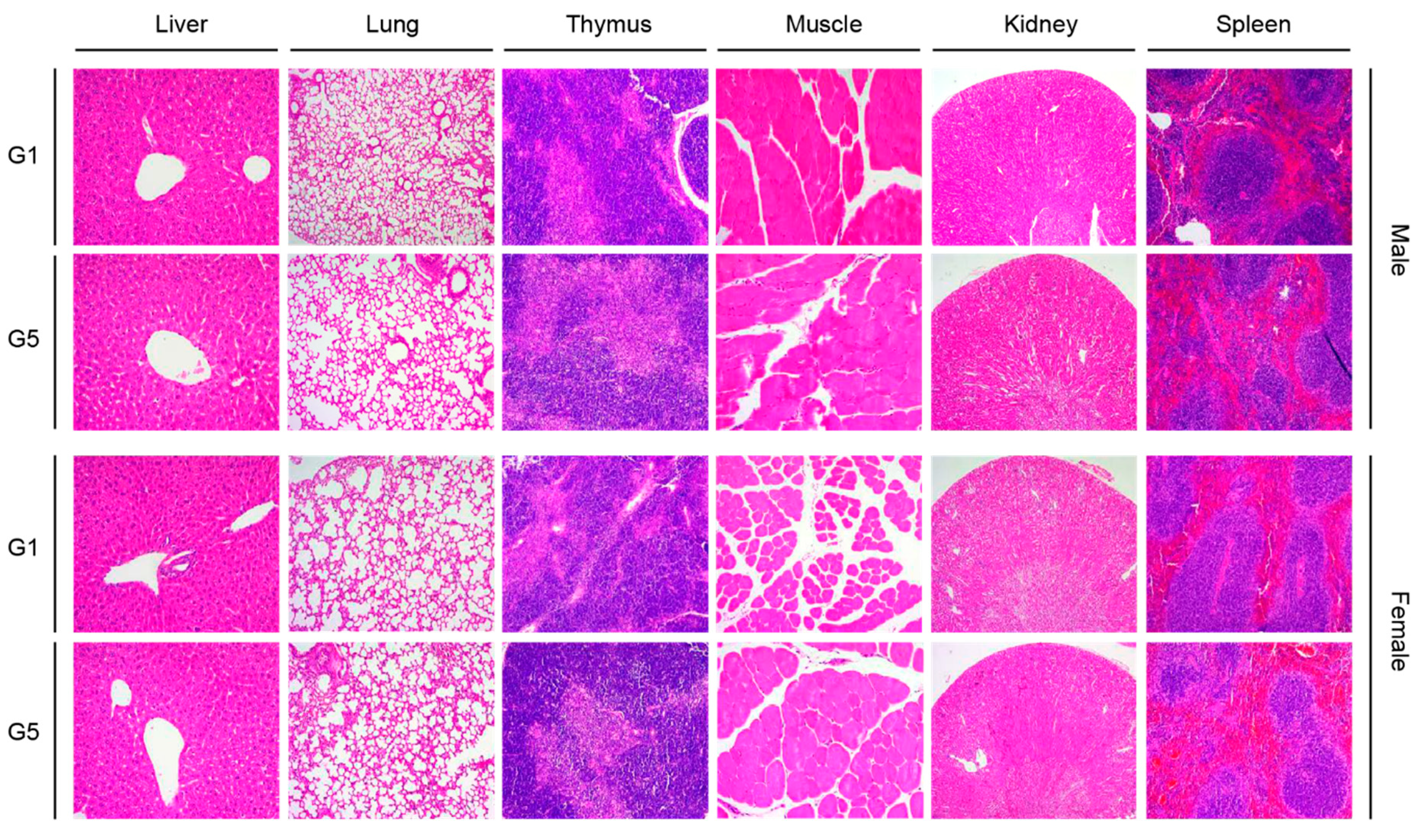
3.4. Organ Weights and Histopathological Analysis
3.5. Autoimmune Disease-Related Data
3.6. Analysis of Inflammatory Cytokines in the Serum
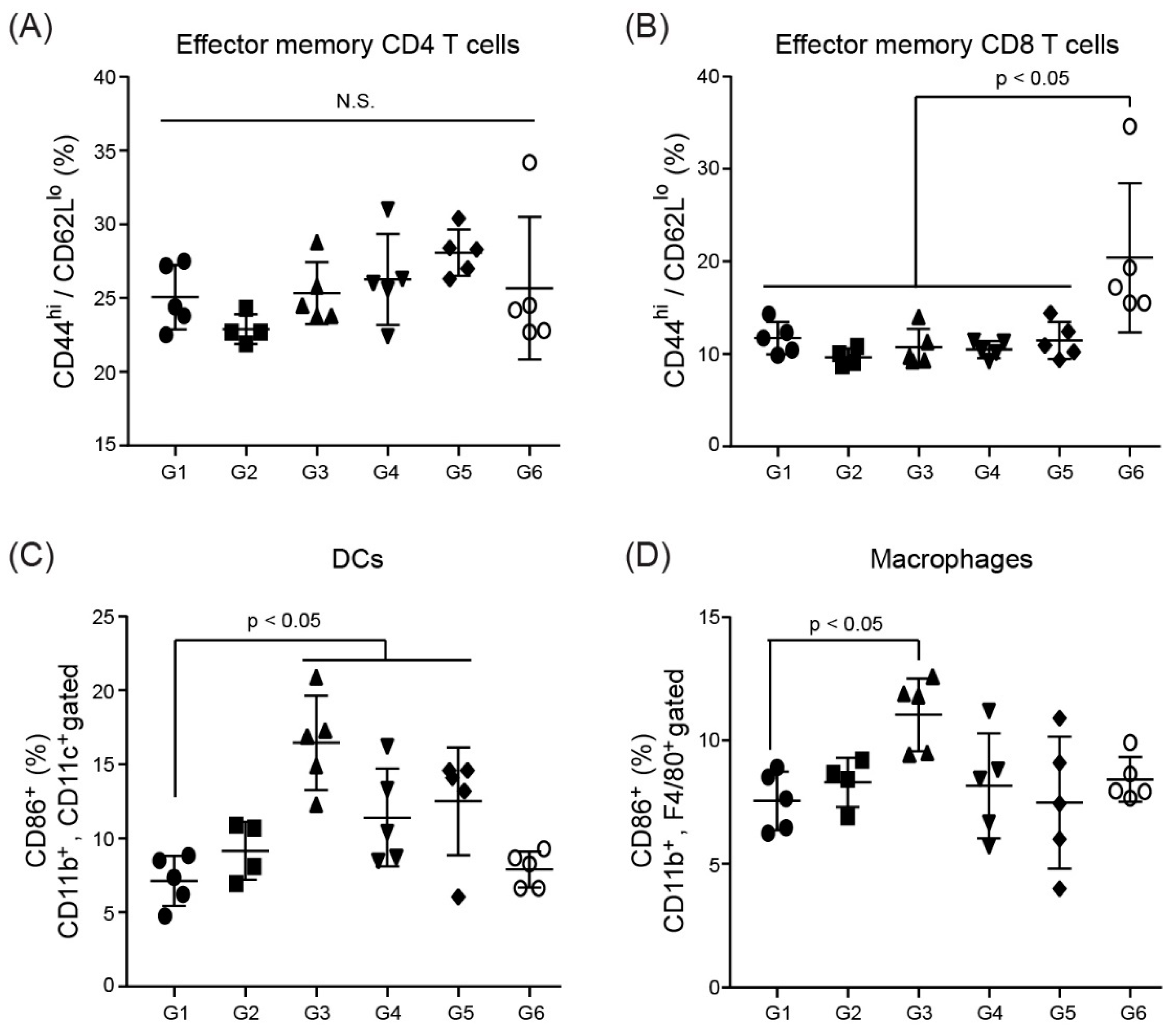
3.7. Immune Cell Activation in Splenocytes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Williamson, E.D.; Titball, R.W. Vaccines against dangerous pathogens. Br. Med. Bull. 2002, 62, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Pati, R.; Shevtsov, M.; Sonawane, A. Nanoparticle vaccines against infectious diseases. Front. Immunol. 2018, 9, 2224. [Google Scholar] [CrossRef] [PubMed]
- Pulendran, B.; Ahmed, R. Immunological mechanisms of vaccination. Nat. Immunol. 2011, 6, 509–517. [Google Scholar] [CrossRef]
- McKee, A.S.; Marrack, P. Old and new adjuvants. Curr. Opin. Immunol. 2017, 47, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Christensen, D. Vaccine adjuvants: Why and how. Hum Vaccines Immunother. 2016, 12, 2709–2711. [Google Scholar] [CrossRef]
- Del Giudice, G.; Rappuoli, R.; Didierlaurent, A.M. Correlates of adjuvanticity: A review on adjuvants in licensed vaccines. Semin. Immunol. 2018, 39, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Nevagi, R.J.; Toth, I.; Skwarczynski, M. Peptide-based vaccines. In Peptide Applications in Biomedicine, Biotechnology and Bioengineering; Koutsopoulos, S., Ed.; Woodhead Publishing: Sawston, UK, 2018; pp. 327–358. [Google Scholar]
- Duthie, M.S.; Windish, H.P.; Fox, C.B.; Reed, S.G. Use of defined TLR ligands as adjuvants within human vaccines. Immunol. Rev. 2011, 239, 178–196. [Google Scholar] [CrossRef] [PubMed]
- Di Pasquale, A.; Preiss, S.; Tavares Da Silva, F.; Garçon, N. Vaccine Adjuvants: From 1920 to 2015 and Beyond. Vaccines 2015, 3, 320–343. [Google Scholar] [CrossRef]
- Kool, M.; Fierens, K.; Lambrecht, B.N. Alum adjuvant: Some of the tricks of the oldest adjuvant. J. Med. Microbiol. 2012, 61, 927–934. [Google Scholar] [CrossRef]
- He, P.; Zou, Y.; Hu, Z. Advances in aluminum hydroxide-based adjuvant research and its mechanism. Hum. Vaccines Immunother. 2015, 11, 477–488. [Google Scholar] [CrossRef]
- Lofano, G.; Mancini, F.; Salvatore, G.; Cantisani, R.; Monaci, E.; Carrisi, C.; Tavarini, S.; Sammicheli, C.; Rossi Paccani, S.; Soldaini, E.; et al. Oil-in-Water Emulsion MF59 Increases Germinal Center B Cell Differentiation and Persistence in Response to Vaccination. J. Immunol. 2015, 195, 1617–1627. [Google Scholar] [CrossRef] [PubMed]
- Perricone, C.; Colafrancesco, S.; Mazor, R.D.; Soriano, A.; Agmon, L.N.; Shoenfeld, Y. Autoimmune/inflammatory syndrome induced by adjuvants (ASIA) 2013: Unveiling the pathogenic, clinical and diagnostic aspects. J. Autoimmun. 2013, 47, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Carlson, B.C.; Jansson, A.M.; Larsson, A.; Bucht, A.; Lorentzen, J.C. The Endogenous Adjuvant Squalene Can Induce a Chronic T-Cell-Mediated Arthritis in Rats. Am. J. Pathol. 2000, 156, 2057–2065. [Google Scholar] [CrossRef]
- Satoh, M.; Kuroda, Y.; Yoshida, H.; Behney, K.M.; Mizutani, A.; Akaogi, J.; Nacionales, D.C.; Lorenson, T.D.; Rosenbauer, R.J.; Reeves, W.H. Induction of lupus autoantibodies by adjuvants. J. Autoimmun. 2003, 21, 1–9. [Google Scholar] [CrossRef]
- Schnare, M.; Barton, G.M.; Takeda, K.; Akira, S.; Medzhitov, R. Toll-like receptors control activation of adaptive immune responses. Nat. Immunol. 2001, 2, 947–950. [Google Scholar] [CrossRef] [PubMed]
- Pulendran, B. Modulating Th1/Th2 responses with microbes, dendritic cells, and pathogen recognition receptors. Immunol. Res. 2004, 29, 187–196. [Google Scholar] [CrossRef]
- Celhar, T.; Pereira-Lopes, S.; Thornhill, S.I.; Lee, H.Y.; Dhillon, M.K.; Poidinger, M.; Connolly, J.E.; Lim, L.H.; Biswas, S.K.; Fairhurst, A.M. TLR7 and TLR9 ligands regulate antigen presentation by macrophages. Int. Immunol. 2016, 28, 223–232. [Google Scholar] [CrossRef]
- Steinhagen, F.; Kinjo, T.; Bode, C.; Klinman, D.M. TLR-based immune adjuvants. Vaccine 2011, 29, 3341–3355. [Google Scholar] [CrossRef]
- Singh, B.; Postic, B. Enhanced resistance of mice to virulent Japanese B encephalitis virus following inactivated vaccine and poly I:C. J. Infect. Dis. 1970, 122, 339–342. [Google Scholar] [CrossRef]
- Hafner, A.M.; Corthésy, B.; Merkle, H.P. Particulate formulations for the delivery of poly(I:C) as vaccine adjuvant. Adv. Drug Deliv. Rev. 2013, 65, 1386–1399. [Google Scholar] [CrossRef]
- Levine, A.S.; Levy, H.B. Phase I-II trials of poly IC stabilized with poly-l-lysine. Cancer Treat. Rep. 1978, 62, 1907–1912. [Google Scholar]
- Antonelli, L.R.; Gigliotti Rothfuchs, A.; Gonçalves, R.; Roffê, E.; Cheever, A.W.; Feng, C.G.; Sher, A. Intranasal Poly-IC treatment exacerbates tuberculosis in mice through the pulmonary recruitment of a pathogen-permissive monocyte/macrophage population. J. Clin. Investig. 2010, 120, 1674–1682. [Google Scholar] [CrossRef] [PubMed]
- Patole, P.S.; Gröne, H.J.; Segerer, S.; Ciubar, R.; Belemezova, E.; Henger, A.; Kretzler, M.; Schlöndorff, D.; Andres, H.J. Viral double-stranded RNA aggravates lupus nephritis through Toll-like receptor 3 on glomerular mesangial cells and antigen-presenting cells. J. Am. Soc. Nephrol. 2005, 16, 1326–1338. [Google Scholar] [CrossRef]
- Mian, M.F.; Ahmed, A.N.; Rad, M.; Babaian, A.; Bowdish, D.; Ashkar, A.A. Length of dsRNA (poly I:C) drives distinct innate immune responses, depending on the cell type. J. Leukoc. Biol. 2013, 94, 1025–1036. [Google Scholar] [CrossRef] [PubMed]
- Diebold, S.S.; Kaisho, T.; Hemmi, H.; Akira, S.; Reis e Sousa, C. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science 2004, 303, 1529–1532. [Google Scholar] [CrossRef]
- Ziegler, A.; Soldner, C.; Lienenklaus, S.; Spanier, J.; Trittel, S.; Riese, P.; Kramps, T.; Weiss, S.; Heidenreich, R.; Jasny, E.; et al. A New RNA-Based Adjuvant Enhances Virus-Specific Vaccine Responses by Locally Triggering TLR- and RLH-Dependent Effects. J. Immunol. 2017, 15, 1595–1605. [Google Scholar] [CrossRef] [PubMed]
- Zagoskin, M.V.; Davis, R.E.; Mukha, D.V. Double Stranded RNA in Human Seminal Plasma. Front. Genet. 2017, 8, 154. [Google Scholar] [CrossRef] [PubMed]
- Pal, A.; Levy, Y. Structure, stability and specificity of the binding of ssDNA and ssRNA with proteins. PLoS Comput. Biol. 2019, 15, e1006768. [Google Scholar] [CrossRef]
- Scheel, B.; Teufel, R.; Probst, J.; Carralot, J.P.; Geginat, J.; Radsak, M.; Jarrossay, D.; Wagner, H.; Jung, G.; Rammensee, H.G.; et al. Toll-like receptor-dependent activation of several human blood cell types by protaminecondensed mRNA. Eur. J. Immunol. 2005, 35, 1557–1566. [Google Scholar] [CrossRef]
- Fotin-Mleczek, M.; Duchardt, K.M.; Lorenz, C.; Pfeiffer, R.; Ojkic’-Zrna, S.; Probst, J.; Kallen, K.J. Messenger RNA-based vaccines with dual activity induce balanced TLR-7 dependent adaptive immune responses and provide antitumor activity. J. Immunother. 2011, 34, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Kallen, K.J.; Heidenreich, R.; Schnee, M.; Petsch, B.; Schlake, T.; Thess, A.; Baumhof, P.; Scheel, B.; Koch, S.D.; Fotin-Mleczek, M. A novel, disruptive vaccination technology: Self-adjuvanted RNActive(®) vaccines. Hum. Vaccines Immunother. 2013, 9, 2263–2276. [Google Scholar] [CrossRef] [PubMed]
- Doener, F.; Hong, H.S.; Meyer, I.; Tadjalli-Mehr, K.; Daehling, A.; Heidenreich, R.; Koch, S.D.; Fotin-Mleczek, M.; Gnad-Vogt, U. RNA-based adjuvant CV8102 enhances the immunogenicity of a licensed rabies vaccine in a first-in-human trial. Vaccine 2019, 37, 1819–1826. [Google Scholar] [CrossRef] [PubMed]
- Lou, B.; De Beuckelaer, A.; Dakwar, G.R.; Remaut, K.; Grooten, J.; Braeckmans, K.; De Geest, B.G.; Mastrobattista, E.; De Koker, S.; Hennink, W.E. Post-PEGylated and crosslinked polymeric ssRNA nanocomplexes as adjuvants targeting lymph nodes with increased cytolytic T cell inducing properties. J. Control. Release 2018, 284, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Edwards, D.K.; Jasny, E.; Yoon, H.; Horscroft, N.; Schanen, B.; Geter, T.; Fotin-Mleczek, M.; Petsch, B.; Wittman, V. Adjuvant effects of a sequence-engineered mRNA vaccine: Translational profiling demonstrates similar human and murine innate response. J. Transl. Med. 2017, 15, 1. [Google Scholar] [CrossRef]
- Heidenreich, R.; Jasny, E.; Kowalczyk, A.; Lutz, J.; Probst, J.; Baumhof, P.; Scheel, B.; Voss, S.; Kallen, K.J.; Fotin-Mleczek, M. A novel RNA-based adjuvant combines strong immunostimulatory capacities with a favorable safety profile. Int. J. Cancer 2015, 137, 372–384. [Google Scholar] [CrossRef] [PubMed]
- Ko, H.L.; Park, H.J.; Kim, J.; Kim, H.; Youn, H.; Nam, J.H. Development of an RNA expression platform controlled by viral internal ribosome entry sites. J. Microbiol. Biotechnol. 2019, 29, 127–140. [Google Scholar] [CrossRef]
- Park, H.J.; Kwak, H.W.; Lee, S.M.; Kim, H.; Nam, J.H. Application of RNA Transcribed from Viral Internal Ribosome Entry Site Control as an Adjuvant: Utilization of Vaccine Development for Middle East Respiratory Syndrome Coronavirus. In Proceedings of the Keystone Symposia on Molecular and Cellular Biology, Banff, AB, Canada, 30 January 2018. [Google Scholar]
- Kwak, H.Y.; Park, H.J.; Ko, H.L.; Park, H.; Cha, M.H.; Lee, S.M.; Kang, K.W.; Kim, R.H.; Ryu, S.R.; Kim, H.J.; et al. Cricket paralysis virus internal ribosome entry site-derived RNA promotes conventional vaccine efficacy by enhancing a balanced Th1/Th2 response. Vaccine 2019, 37, 5191–5202. [Google Scholar] [CrossRef] [PubMed]
- Oh, H.; Kim, G.; Park, H.J.; Hwang, E.H.; Koo, B.S.; Kang, P.; Lee, H.Y.; Jeong, K.J.; Yun, J.W.; Villinger, F.; et al. Cricket paralysis virus internal ribosome entry site-derived RNA for humoral immunity to Middle East respiratory syndrome coronavirus spike protein in non-human primate. In Proceedings of the Korean Society of Veterinary Science (KSVS) Spring Conference, Seoul, Korea, 25–27 April 2019. [Google Scholar]
- Guideline on Nonclinical Evaluation of Biopharmaceuticals. Available online: http://www.nifds.go.kr/brd/m_15/view.do?seq=12606&srchFr=&srchTo=&srchWord=&srchTp=&itm_seq_1=0&itm_seq_2=0&multi_itm_seq=0&company_cd=&company_nm=&page=60 (accessed on 29 July 2019).
- Babu, L.; Uppalapati, S.R.; Sripathy, M.H.; Reddy, P.N. Evaluation of Recombinant Multi-Epitope Outer Membrane Protein-Based Klebsiella pneumoniae Subunit Vaccine in Mouse Model. Front. Microbiol. 2017, 20, 1805. [Google Scholar] [CrossRef]
- Ngoi, S.M.; Tovey, M.G.; Vella, A.T. Targeting poly(I:C) to the TLR3-independent pathway boosts effector CD8 T cell differentiation through IFNα/β. J. Immunol. 2008, 181, 7670–7680. [Google Scholar] [CrossRef]
- Chen, H.; Chuai, X.; Deng, Y.; Wen, B.; Wang, W.; Xiong, S.; Ruan, L.; Tan, W. Optimisation of Prime-Boost Immunization in Mice Using Novel Protein-Based and Recombinant Vaccinia (Tiantan)-Based HBV Vaccine. PLoS ONE 2012, 7, e43730. [Google Scholar] [CrossRef]
- Park, H.J.; Choi, W.S.; Lee, W.Y.; Choi, Y.; Park, C.; Kim, J.H.; Hong, K.H.; Song, H. A novel mouse model of atopic dermatitis that is T helper 2 (Th2)-polarized by an epicutaneous allergen. Environ. Toxicol. Pharmacol. 2018, 58, 122–130. [Google Scholar] [CrossRef]
- Smith, B.T. Cell line A549: A model system for the study of alveolar type II cell function. Am. Rev. Respir. Dis. 1977, 115, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Snopov, S.A.; Teryukova, N.P.; Sakhenberg, E.I.; Teplyashina, V.V.; Nasyrova, R.F. Use of HepG2 cell line for evaluation of toxic and metabolic antipsychotic action. Cell Tissue Biol. 2017, 11, 405–415. [Google Scholar] [CrossRef]
- Hsu, C.C.; Lee, Y.C.; Yeh, S.H.; Chen, C.H.; Wu, C.C.; Wang, T.Y.; Chen, Y.N.; Hung, L.Y.; Liu, Y.W.; Chen, H.K.; et al. 58-kDa microspherule protein (MSP58) is novel Brahma-related gene 1 (BRG1)-associated protein that modulates p53/p21 senescence pathway. J. Biol. Chem. 2012, 287, 22533–22548. [Google Scholar] [CrossRef]
- Santos, E.W.; de Oliveira, D.C.; Hastreiter, A.; da Silava, G.B.; Beltran, J.S.O.; Tsujita, M.; Crisma, A.R.; Neves, S.M.P.; Fock, R.A.; Borelli, P. Hematological and biochemical reference values for C57BL/6, Swiss Webster and BALB/c mice. J. Vet. Res. Anim. Sci. 2016, 53, 138–145. [Google Scholar] [CrossRef]
- Kim, Y.H.; Lee, H.S.; Kim, Y.B.; Im, W.J.; Lim, S.; Kang, B.H. Hematological and Serum Biochemical Values in Specific Pathogen-Free BALB/c and C57BL/6 Mice. Lab. Anim. Res. 2005, 21, 205–211. [Google Scholar]
- Cojocaru, M.; Cojocaru, I.M.; Silosi, I.; Vrabie, C.D. Kidney damage in autoimmune diseases. J. Mol. Biol. 2010, 29, 61–65. [Google Scholar] [CrossRef]
- Sanjuan, M.A.; Sagar, D.; Kolbeck, R. Role of IgE in autoimmunity. J. Allergy Clin. Immunol. 2016, 137, 1651–1661. [Google Scholar] [CrossRef]
- Tan, E.M. Antinuclear antibodies defining autoimmunity pathways. Arthritis Res. Ther. 2014, 16, 104. [Google Scholar] [CrossRef] [PubMed]
- Solomon, D.H.; Kavanaugh, A.J.; Schur, P.H. American College of Rheumatology Ad Hoc Committee on Immunologic Testing Guidelines. Evidence-based guidelines for the use of immunologic tests; antinuclear antibody testing. Arthritis Rheumatol. 2002, 47, 434–444. [Google Scholar] [CrossRef]
- Matsumoto, H.; Ogura, H.; Shimizu, K.; Ikeda, M.; Hirose, T.; Matsuura, H.; Kang, S.; Takahashi, K.; Tanaka, T.; Shimazu, T. The clinical importance of a cytokine network in the acute phase of sepsis. Sci. Rep. 2018, 8, 13995. [Google Scholar] [CrossRef]
- Iyer, S.S.; Cheng, G. Role of Interleukin 10 Transcriptional Regulation in Inflammation and autoimmune disease. Crit. Rev. Immunol. 2012, 32, 23–63. [Google Scholar] [CrossRef]
- Reiser, J.; Banerjee, A. Effector, Memory, and Dysfunctional CD8+ T Cell Fates in the Antitumor Immune Response. J. Immunol. Res. 2016, 2016, 8941260. [Google Scholar] [CrossRef]
- Bovard, D.; Sandoz, A.; Luettich, K.; Frentzel, S.; Iskandar, A.; Marescotti, D.; Trivedi, K.; Guedj, E.; Dutertre, Q.; Peitsch, M.C.; et al. A lung/liver-on-a-chip platform for acute and chronic toxicity studies. Lab Chip 2018, 18, 3814–3829. [Google Scholar] [CrossRef]
- Grant, D.M. Detoxification pathways in the liver. J. Inherit. Metab. Dis. 1991, 14, 421–430. [Google Scholar] [CrossRef]
- Kong, D.Y.; Park, J.H.; Lee, K.W.; Park, H.; Cho, J.A. Comparative analysis of 3 experimental mouse model for blood hematology and chemistry. Biomed. Sci. Lett. 2016, 22, 75–82. [Google Scholar] [CrossRef]
- Batista-Duharte, A.; Martínez, D.T.; Carlos, I.Z. Efficacy and safety of immunological adjuvants. Where is the cut-off? Biomed. Pharmacother. 2018, 105, 616–624. [Google Scholar] [CrossRef]
- Costa, S.; Bevilacqua, D.; Cassatella, M.A.; Scapini, P. Recent advances on the crosstalk between neutrophils and B or T lymphocytes. Immunology 2019, 156, 23–32. [Google Scholar] [CrossRef]
- Angela, S.C. Fundamentals of vaccine immunology. J. Glob. Infect. Dis. 2011, 3, 73–78. [Google Scholar] [CrossRef]
- Hyder, M.A.; Hasan, M.; Mohieldein, A.H. Comparative Levels of ALT, AST, ALP and GGT in Liver associated Diseases. Eur. J. Exp. Biol. 2013, 3, 280–284. [Google Scholar]
- Salazar, J.H. Overview of Urea and Creatinine. Lab. Med. 2014, 45, 19–20. [Google Scholar] [CrossRef]
- Tomizawa, M.; Kawanabe, Y.; Shinozaki, F.; Sato, S.; Motoyoshi, Y.; Sugiyama, T.; Yamamoto, S.; Sueishi, M. Triglyceride is strongly associated with nonalcoholic fatty liver disease among markers of hyperlipidemia and diabetes. Biomed. Rep. 2014, 2, 633–636. [Google Scholar] [CrossRef]
- De Souza Barros, C.; Gomes, M.W.L.; Gomes, R.d.S.P.; Melchiades, V.; Nogueira, C.C.R.; Cirne-Santos, C.C.; Garrido, V.; Pinto, C.E.C.; Teixeira, V.L.; de Palmer Paixão, I.C.N. Acute toxicity evaluation of ethanol extract of red algae, Osmundaria obtusiloba, in BALB/c mice. J. Med. Plants Res. 2018, 12, 217–221. [Google Scholar]
- Yamanishi, R.; Yusa, I.; Bando, N.; Terao, J. Adjuvant Activity of Alum in Inducing Antigen Specific IgE Antibodies in BALB/c Mice: A Reevaluation. Biosci. Biotechnol. Biochem. 2003, 67, 166–169. [Google Scholar] [CrossRef]
- Sur, L.M.; Floca, E.; Sur, D.G.; Colceriu, M.C.; Samasca, G.; Sur, G. Antinucler antibodies: Marker of diagnosis and evolution in autoimmune diseases. Lab. Med. 2018, 49, e62–e73. [Google Scholar] [CrossRef]
- Grygiel-Górniak, B.; Rogacka, N.; Puszczewicz, M. Antinuclear antibodies in healthy people and non-rheumatic diseases—diagnostic and clinical implications. Reumatologia 2018, 56, 243–248. [Google Scholar] [CrossRef]
- Yadav, A.; Saini, V.; Arora, S. MCP-1: Chemoattractant with a role beyond immunity: A review. Clin. Chim. Acta 2010, 411, 1570–1579. [Google Scholar] [CrossRef]
- Baud, V.; Karin, M. Signal transduction by tumor necrosis factor and its relatives. Trends Cell Biol. 2001, 11, 372–377. [Google Scholar] [CrossRef]
- Madej, M.P.; Töpfer, E.; Boraschi, D.; Italiani, P. Different Regulation of Interleukin-1 Production and Activity in Monocytes and Macrophages: Innate Memory as an Endogenous Mechanism of IL-1 Inhibition. Front. Pharmacol. 2017, 8, 335. [Google Scholar] [CrossRef]
- Del Giudice, M.; Gangestad, S.W. Rethinking IL-6 and CRP: Why they are more than inflammatory biomarkers, and why it matters. Brain Behav. Immun. 2018, 70, 61–75. [Google Scholar] [CrossRef]
- Moudgil, K.D.; Choubey, D. Cytokines in autoimmunity: Role in induction, regulation, and treatment. J. Interferon Cytokine Res. 2011, 31, 695–703. [Google Scholar] [CrossRef]
- Sun, L.; He, C.; Nair, L.; Yeung, J.; Egwuagu, C.E. Interleukin 12 (IL-12) family cytokines—Role in immune pathogenesis and treatment of CNS autoimmune disease. Cytokine 2015, 75, 249–255. [Google Scholar] [CrossRef]
- Awate, S.; Babiuk, L.A.; Mutwiri, G. Mechanisms of action of adjuvants. Front. Immunol. 2013, 16, 114. [Google Scholar] [CrossRef]
- Li, X.; Wang, X.; Ito, A. Tailoring inorganic nanoadjuvants towards next-generation vaccines. Chem. Soc. Rev. 2018, 47, 4954–4980. [Google Scholar] [CrossRef]


| Group 1 | Group 2 | Group 3 | Group 4 | Group 5 | Group 6 | |
|---|---|---|---|---|---|---|
| Male | ||||||
| RBC (×106 cells/μL) | 10.3 ± 0.5 | 10.0 ± 0.7 | 10.3 ± 0.6 | 10.2 ± 0.2 | 10.3 ± 0.5 | 10.1 ± 0.3 |
| HGB (g/dL) | 14.8 ± 0.6 | 14.5 ± 0.9 | 14.9 ± 0.9 | 14.6 ± 0.4 | 14.7 ± 0.8 | 14.4 ± 0.4 |
| HCT (%) | 50.6 ± 2.5 | 48.7 ± 3.2 | 51.4 ± 3.2 | 50.5 ± 1.6 | 51.4 ± 3.1 | 48.5 ± 1.7 |
| MCV (fL) | 49.1 ± 0.5 | 48.8 ± 0.3 | 49.8 ± 0.2 * | 49.7 ± 0.7 | 49.9 ± 0.4 * | 48.2 ± 0.5 * |
| MCH (pg) | 14.4 ± 0.1 | 14.6 ± 0.1 * | 14.4 ± 0.1 | 14.4 ± 0.1 | 14.2 ± 0.1 | 14.3 ± 0.1 |
| MCHC (g/dL) | 29.3 ± 0.3 | 29.8 ± 0.2 * | 29.0 ± 0.3 | 28.9 ± 0.2 | 28.6 ± 0.3 * | 29.8 ± 0.2 * |
| PLT (×103 cells/μL) | 923.0 ± 61.0 | 929.0 ± 54.0 | 1128.0 ± 206.0 | 970.0 ± 20.0 | 956.0 ± 76.0 | 899.0 ± 43.0 |
| WBC (×103 cells/μL) | 3.2 ± 1.3 | 3.8 ± 1.2 | 2.2 ± 0.7 | 2.4 ± 0.7 | 2.1 ± 0.6 | 3.3 ± 0.7 |
| Neutrophils (%) | 27.7 ± 7.1 | 26.9 ± 2.2 | 40.5 ± 4.4 * | 30.6 ± 4.2 | 34.0 ± 8.1 | 18.0 ± 2.2 * |
| Lymphocytes (%) | 69.5 ± 7.2 | 70.5 ± 2.7 | 56.9 ± 4.7 * | 65.5 ± 3.8 | 61.3 ± 7.5 | 78.6 ± 1.9 * |
| Monocytes (%) | 0.8 ± 0.3 | 0.7 ± 0.4 | 0.9 ± 0.4 | 0.7 ± 0.3 | 1.3 ± 0.3 * | 0.5 ± 0.4 |
| Eosinophils (%) | 1.9 ± 0.8 | 1.8 ± 0.6 | 1.6 ± 1.1 | 3.0 ± 0.7 | 3.3 ± 1.0 * | 2.9 ± 0.8 |
| Basophils (%) | 0.1 ± 0.2 | 0.1 ± 0.2 | 0.0 ± 0.0 | 0.2 ± 0.2 | 0.1 ± 0.2 | 0.1 ± 0.2 |
| Female | ||||||
| RBC (×106 cells/μL) | 10.3 ± 0.7 | 9.8 ± 0.4 | 9.9 ± 0.3 | 9.8 ± 0.3 | 9.7 ± 0.4 | 9.6 ± 0.3 |
| HGB (g/dL) | 15.4 ± 1.0 | 14.6 ± 0.4 | 14.6 ± 0.5 | 14.4 ± 0.3 | 14.3 ± 0.6 | 14.2 ± 0.3 * |
| HCT (%) | 52.3 ± 4.1 | 50.4 ± 1.1 | 49.6 ± 1.7 | 48.3 ± 1.4 | 48.1 ± 2.3 | 47.7 ± 1.4 * |
| MCV (fL) | 50.7 ± 1.0 | 51.5 ± 0.7 | 50.3 ± 0.4 | 49.3 ± 0.4 * | 49.6 ± 0.8 | 49.5 ± 0.4 |
| MCH (pg) | 14.9 ± 0.2 | 14.9 ± 0.1 | 14.8 ± 0.1 | 14.7 ± 0.2 | 14.8 ± 0.1 | 14.8 ± 0.3 |
| MCHC (g/dL) | 29.4 ± 0.3 | 28.9 ± 0.2 * | 29.4 ± 0.2 | 29.9 ± 0.2 * | 29.8 ± 0.3 | 29.9 ± 0.4 |
| PLT (×103 cells/μL) | 777.0 ± 84.0 | 742.0 ± 145.0 | 857.0 ± 50.0 | 820.0 ± 99.0 | 834.0 ± 42.0 | 780.0 ± 83.0 |
| WBC (×103 cells/μL) | 2.7 ± 0.7 | 3.8 ± 0.5 * | 1.6 ± 0.4 * | 3.0 ± 0.2 | 1.6 ± 0.6 * | 2.3 ± 0.6 |
| Neutrophils (%) | 28.5 ± 4.5 | 26.4 ± 3.6 | 27.6 ± 3.2 | 30.6 ± 6.4 | 37.8 ± 11.8 | 19.9 ± 6.4 * |
| Lymphocytes (%) | 67.5 ± 4.7 | 68.1 ± 3.2 | 66.9 ± 3.4 | 64.5 ± 5.8 | 59.5 ± 10.5 | 75.3 ± 5.9 |
| Monocytes (%) | 0.7 ± 0.2 | 1.3 ± 0.3 * | 1.2 ± 0.3 * | 1.3 ± 0.6 | 1.0 ± 0.4 | 0.8 ± 0.2 |
| Eosinophils (%) | 3.3 ± 1.5 | 4.1 ± 1.7 | 4.1 ± 0.8 | 3.4 ± 0.6 | 1.7 ± 1.1 | 4.0 ± 1.2 |
| Basophils (%) | 0.0 ± 0.0 | 0.1 ± 0.1 | 0.2 ± 0.4 | 0.1 ± 0.2 | 0.0 ± 0.0 | 0.1 ± 0.1 |
| Group 1 | Group 2 | Group 3 | Group 4 | Group 5 | Group 6 | |
|---|---|---|---|---|---|---|
| Male | ||||||
| ALT (U/L) | 53.7 ± 52.5 | 36.0 ± 8.9 | 36.2 ± 3.1 | 32.7 ± 3.5 | 39.1 ± 5.4 | 25.9 ± 4.6 |
| AST (U/L) | 73.0 ± 20.3 | 66.3 ± 9.3 | 67.1 ± 12.6 | 66.5 ± 14.1 | 79.5 ± 15.3 | 51.5 ± 3.6 * |
| BUN (mg/dL) | 31.7 ± 6.4 | 38.5 ± 6.7 | 27.3 ± 3.6 | 32.2 ± 7.1 | 35.3 ± 8.8 | 24.1 ± 1.0 * |
| Creatinine (mg/dL) | 0.2 ± 0.0 | 0.2 ± 0.0 | 0.2 ± 0.1 | 0.2 ± 0.0 | 0.2 ± 0.0 | 0.1 ± 0.0 * |
| TP (g/dL) | 5.8 ± 0.5 | 5.6 ± 0.3 | 5.5 ± 0.3 | 5.5 ± 0.2 | 5.3 ± 0.3 | 5.1 ± 0.5 |
| Albumin (g/dL) | 3.3 ± 0.2 | 3.2 ± 0.3 | 3.2 ± 0.1 | 3.3 ± 0.1 | 3.0 ± 0.1 * | 3.2 ± 0.2 |
| A/G | 1.4 ± 0.2 | 1.3 ± 0.2 | 1.4 ± 0.1 | 1.5 ± 0.1 | 1.4 ± 0.1 | 1.7 ± 0.2 * |
| T-Chol (mg/dL) | 148.0 ± 9.0 | 144.0 ± 18.0 | 154.0 ± 6.0 | 148.0 ± 8.0 | 152.0 ± 16.0 | 146.0 ± 12.0 |
| TG (mg/dL) | 99.0 ± 14.0 | 121.0 ± 33.0 | 125.0 ± 24.0 | 84.0 ± 10.0 | 49.0 ± 21.0 * | 150.0 ± 38.0 * |
| Glucose (mg/dL) | 165.7 ± 21.7 | 200.0 ± 35.5 | 178.2 ± 20.5 | 174.5 ± 22.9 | 122.8 ± 40.3 | 169.2 ± 20.3 |
| HDL (mg/dL) | 113.3 ± 6.2 | 117.1 ± 10.8 | 129.1 ± 7.0 * | 117.3 ± 5.5 | 124.2 ± 13.5 | 106.6 ± 8.2 |
| LDL (mg/dL) | 5.2 ± 1.3 | 5.5 ± 1.0 | 6.2 ± 0.9 | 4.7 ± 0.7 | 6.2 ± 1.1 | 6.2 ± 0.9 |
| Female | ||||||
| ALT (U/L) | 32.6 ± 3.2 | 35.2 ± 5.3 | 29.1 ± 2.6 | 35.5 ± 7.5 | 29.8 ± 2.9 | 35.8 ± 8.5 |
| AST (U/L) | 69.3 ± 6.0 | 76.3 ± 5.9 | 63.9 ± 3.8 | 75.8 ± 11.6 | 63.9 ± 6.4 | 74.3 ± 9.6 |
| BUN (mg/dL) | 26.3 ± 3.4 | 32.6 ± 11.6 | 44.1 ± 6.0 * | 28.8 ± 5.6 | 35.1 ± 4.7 * | 28.0 ± 4.1 |
| Creatinine (mg/dL) | 0.2 ± 0.1 | 0.2 ± 0.0 | 0.2 ± 0.0 | 0.2 ± 0.0 | 0.2 ± 0.0 | 0.1 ± 0.0 * |
| TP (g/dL) | 5.9 ± 0.6 | 6.0 ± 0.5 | 5.0 ± 0.4 * | 5.6 ± 0.2 | 5.0 ± 0.4 * | 5.0 ± 0.3 * |
| Albumin (g/dL) | 3.3 ± 0.3 | 3.3 ± 0.2 | 3.1 ± 0.1 | 3.0 ± 0.6 | 3.1 ± 0.1 | 3.2 ± 0.2 |
| A/G | 1.3 ± 0.4 | 1.3 ± 0.3 | 1.7 ± 0.3 | 1.2 ± 0.4 | 1.8 ± 0.5 | 1.8 ± 0.3 |
| T-Chol (mg/dL) | 109.0 ± 17.0 | 105.0 ± 13.0 | 110.0 ± 7.0 | 101.0 ± 23.0 | 109.0 ± 9.0 | 105.0 ± 13.0 |
| TG (mg/dL) | 148.0 ± 18.0 | 109.0 ± 37.0 | 148.0 ± 36.0 | 100.0 ± 22.0 * | 112.0 ± 7.0 * | 130.0 ± 49.0 |
| Glucose (mg/dL) | 165.2 ± 21.0 | 176.5 ± 50.5 | 190.0 ± 37.0 | 196.6 ± 29.4 | 197.7 ± 31.5 | 216.9 ± 9.3 * |
| HDL (mg/dL) | 92.9 ± 4.2 | 92.2 ± 4.1 | 82.4 ± 5.0 * | 91.1 ± 5.5 | 83.9 ± 11.5 | 80.8 ± 10.8 * |
| LDL (mg/dL) | 9.2 ± 0.6 | 9.7 ± 0.8 | 9.6 ± 1.4 | 9.7 ± 0.6 | 9.3 ± 1.4 | 8.1 ± 0.8 * |
| Group 1 | Group 2 | Group 3 | Group 4 | Group 5 | Group 6 | ||
|---|---|---|---|---|---|---|---|
| Male | |||||||
| Liver | (g) | 1.06 ± 0.11 | 1.08 ± 0.07 | 0.99 ± 0.07 | 0.97 ± 0.04 | 1.02 ± 0.03 | 1.25 ± 0.09 * |
| (%BW) | 4.54 ± 0.45 | 4.31 ± 0.25 | 4.14 ± 0.22 | 4.04 ± 0.05 * | 4.26 ± 0.09 | 4.62 ± 0.16 | |
| Spleen | (g) | 0.09 ± 0.01 | 0.10 ± 0.01 | 0.09 ± 0.01 | 0.09 ± 0.01 | 0.10 ± 0.01 * | 0.09 ± 0.01 |
| (%BW) | 0.38 ± 0.03 | 0.39 ± 0.03 | 0.39 ± 0.05 | 0.39 ± 0.05 | 0.44 ± 0.03 * | 0.35 ± 0.02 | |
| Lung | (g) | 0.16 ± 0.01 | 0.16 ± 0.02 | 0.17 ± 0.01 | 0.15 ± 0.01 | 0.15 ± 0.02 | 0.15 ± 0.01 |
| (%BW) | 0.67 ± 0.07 | 0.63 ± 0.07 | 0.70 ± 0.03 | 0.63 ± 0.05 | 0.63 ± 0.08 | 0.55 ± 0.04 * | |
| Kidney(L) | (g) | 0.18 ± 0.02 | 0.19 ± 0.01 | 0.19 ± 0.02 | 0.18 ± 0.01 | 0.17 ± 0.01 | 0.21 ± 0.01 * |
| (%BW) | 0.79 ± 0.08 | 0.76 ± 0.02 | 0.79 ± 0.06 | 0.75 ± 0.03 | 0.71 ± 0.03 | 0.76 ± 0.03 | |
| Kidney(R) | (g) | 0.19 ± 0.02 | 0.19 ± 0.01 | 0.19 ± 0.01 | 0.18 ± 0.01 | 0.17 ± 0.01 | 0.21 ± 0.01 * |
| (%BW) | 0.80 ± 0.09 | 0.77 ± 0.02 | 0.81 ± 0.04 | 0.74 ± 0.03 | 0.73 ± 0.03 | 0.77 ± 0.04 | |
| Thymus | (g) | 0.06 ± 0.01 | 0.05 ± 0.01 | 0.04 ± 0.01 * | 0.04 ± 0.01 * | 0.03 ± 0.01 * | 0.04 ± 0.01 * |
| (%BW) | 0.24 ± 0.05 | 0.19 ± 0.03 | 0.17 ± 0.04 * | 0.18 ± 0.01 * | 0.14 ± 0.01 * | 0.15 ± 0.02 * | |
| Brain | (g) | 0.43 ± 0.02 | 0.43 ± 0.02 | 0.42 ± 0.01 | 0.43 ± 0.01 | 0.42 ± 0.03 | 0.44 ± 0.02 |
| (%BW) | 1.83 ± 0.08 | 1.70 ± 0.09 * | 1.74 ± 0.06 | 1.78 ± 0.02 | 1.77 ± 0.13 | 1.62 ± 0.04 * | |
| Heart | (g) | 0.13 ± 0.01 | 0.15 ± 0.01 * | 0.14 ± 0.01 | 0.13 ± 0.01 | 0.13 ± 0.01 | 0.15 ± 0.01 * |
| (%BW) | 0.58 ± 0.04 | 0.60 ± 0.04 | 0.57 ± 0.03 | 0.54 ± 0.03 | 0.56 ± 0.02 | 0.54 ± 0.03 | |
| Testis(L) | (g) | 0.10 ± 0.01 | 0.09 ± 0.01 | 0.10 ± 0.01 | 0.10 ± 0.01 | 0.10 ± 0.01 | 0.09 ± 0.04 |
| (%BW) | 0.44 ± 0.04 | 0.37 ± 0.04 * | 0.42 ± 0.04 | 0.41 ± 0.03 | 0.43 ± 0.02 | 0.33 ± 0.14 | |
| Testis(R) | (g) | 0.10 ± 0.01 | 0.11 ± 0.02 | 0.10 ± 0.01 | 0.10 ± 0.01 | 0.10 ± 0.01 | 0.09 ± 0.04 |
| (%BW) | 0.44 ± 0.05 | 0.42 ± 0.05 | 0.44 ± 0.04 | 0.42 ± 0.02 | 0.42 ± 0.02 | 0.33 ± 0.14 | |
| Group 1 | Group 2 | Group 3 | Group 4 | Group 5 | Group 6 | ||
|---|---|---|---|---|---|---|---|
| Female | |||||||
| Liver | (g) | 0.78 ± 0.07 | 0.91 ± 0.11 | 0.89 ± 0.07 * | 0.77 ± 0.04 | 0.74 ± 0.09 | 0.94 ± 0.05 * |
| (%BW) | 3.92 ± 0.23 | 4.39 ± 0.33 * | 4.17 ± 0.19 | 3.80 ± 0.13 | 3.80 ± 0.23 | 4.40 ± 0.09 * | |
| Spleen | (g) | 0.10 ± 0.01 | 0.10 ± 0.01 | 0.12 ± 0.01 * | 0.10 ± 0.01 | 0.10 ± 0.01 | 0.10 ± 0.01 |
| (%BW) | 0.48 ± 0.04 | 0.49 ± 0.03 | 0.55 ± 0.04 * | 0.49 ± 0.02 | 0.50 ± 0.04 | 0.46 ± 0.04 | |
| Lung | (g) | 0.13 ± 0.01 | 0.14 ± 0.01 | 0.15 ± 0.01 * | 0.14 ± 0.01 | 0.14 ± 0.01 | 0.14 ± 0.01 * |
| (%BW) | 0.64 ± 0.03 | 0.66 ± 0.03 | 0.68 ± 0.03 | 0.69 ± 0.06 | 0.74 ± 0.03 * | 0.67 ± 0.03 | |
| Kidney(L) | (g) | 0.12 ± 0.01 | 0.13 ± 0.01 * | 0.13 ± 0.01 | 0.12 ± 0.01 | 0.12 ± 0.01 | 0.13 ± 0.01 * |
| (%BW) | 0.59 ± 0.03 | 0.63 ± 0.03 | 0.62 ± 0.03 | 0.60 ± 0.02 | 0.64 ± 0.05 | 0.61 ± 0.02 | |
| Kidney(R) | (g) | 0.12 ± 0.01 | 0.14 ± 0.01 * | 0.14 ± 0.01 * | 0.12 ± 0.01 | 0.12 ± 0.01 | 0.13 ± 0.01 * |
| (%BW) | 0.61 ± 0.02 | 0.65 ± 0.04 | 0.65 ± 0.02 * | 0.60 ± 0.04 | 0.63 ± 0.03 | 0.63 ± 0.04 | |
| Thymus | (g) | 0.04 ± 0.01 | 0.03 ± 0.01 | 0.04 ± 0.01 | 0.04 ± 0.01 | 0.03 ± 0.01 | 0.04 ± 0.01 |
| (%BW) | 0.20 ± 0.02 | 0.16 ± 0.04 | 0.20 ± 0.02 | 0.20 ± 0.02 | 0.18 ± 0.02 | 0.19 ± 0.02 | |
| Brain | (g) | 0.44 ± 0.02 | 0.44 ± 0.02 | 0.45 ± 0.01 | 0.44 ± 0.01 | 0.43 ± 0.01 | 0.44 ± 0.02 |
| (%BW) | 2.18 ± 0.07 | 2.12 ± 0.10 | 2.11 ± 0.07 | 2.17 ± 0.02 | 2.25 ± 0.18 | 2.06 ± 0.16 | |
| Heart | (g) | 0.11 ± 0.01 | 0.11 ± 0.01 | 0.09 ± 0.05 | 0.11 ± 0.01 | 0.11 ± 0.01 | 0.11 ± 0.01 |
| (%BW) | 0.53 ± 0.02 | 0.55 ± 0.02 | 0.44 ± 0.21 | 0.53 ± 0.03 | 0.58 ± 0.07 | 0.52 ± 0.02 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, H.-J.; Ko, H.L.; Won, D.-H.; Hwang, D.-B.; Shin, Y.-S.; Kwak, H.-W.; Kim, H.-J.; Yun, J.-W.; Nam, J.-H. Comprehensive Analysis of the Safety Profile of a Single-Stranded RNA Nano-Structure Adjuvant. Pharmaceutics 2019, 11, 464. https://doi.org/10.3390/pharmaceutics11090464
Park H-J, Ko HL, Won D-H, Hwang D-B, Shin Y-S, Kwak H-W, Kim H-J, Yun J-W, Nam J-H. Comprehensive Analysis of the Safety Profile of a Single-Stranded RNA Nano-Structure Adjuvant. Pharmaceutics. 2019; 11(9):464. https://doi.org/10.3390/pharmaceutics11090464
Chicago/Turabian StylePark, Hyeong-Jun, Hae Li Ko, Dong-Hoon Won, Da-Bin Hwang, Yoo-Sub Shin, Hye-Won Kwak, Hye-Jung Kim, Jun-Won Yun, and Jae-Hwan Nam. 2019. "Comprehensive Analysis of the Safety Profile of a Single-Stranded RNA Nano-Structure Adjuvant" Pharmaceutics 11, no. 9: 464. https://doi.org/10.3390/pharmaceutics11090464
APA StylePark, H.-J., Ko, H. L., Won, D.-H., Hwang, D.-B., Shin, Y.-S., Kwak, H.-W., Kim, H.-J., Yun, J.-W., & Nam, J.-H. (2019). Comprehensive Analysis of the Safety Profile of a Single-Stranded RNA Nano-Structure Adjuvant. Pharmaceutics, 11(9), 464. https://doi.org/10.3390/pharmaceutics11090464





