Ex-Vivo and In-Vivo Assessment of Cyclamen europaeum Extract After Nasal Administration
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
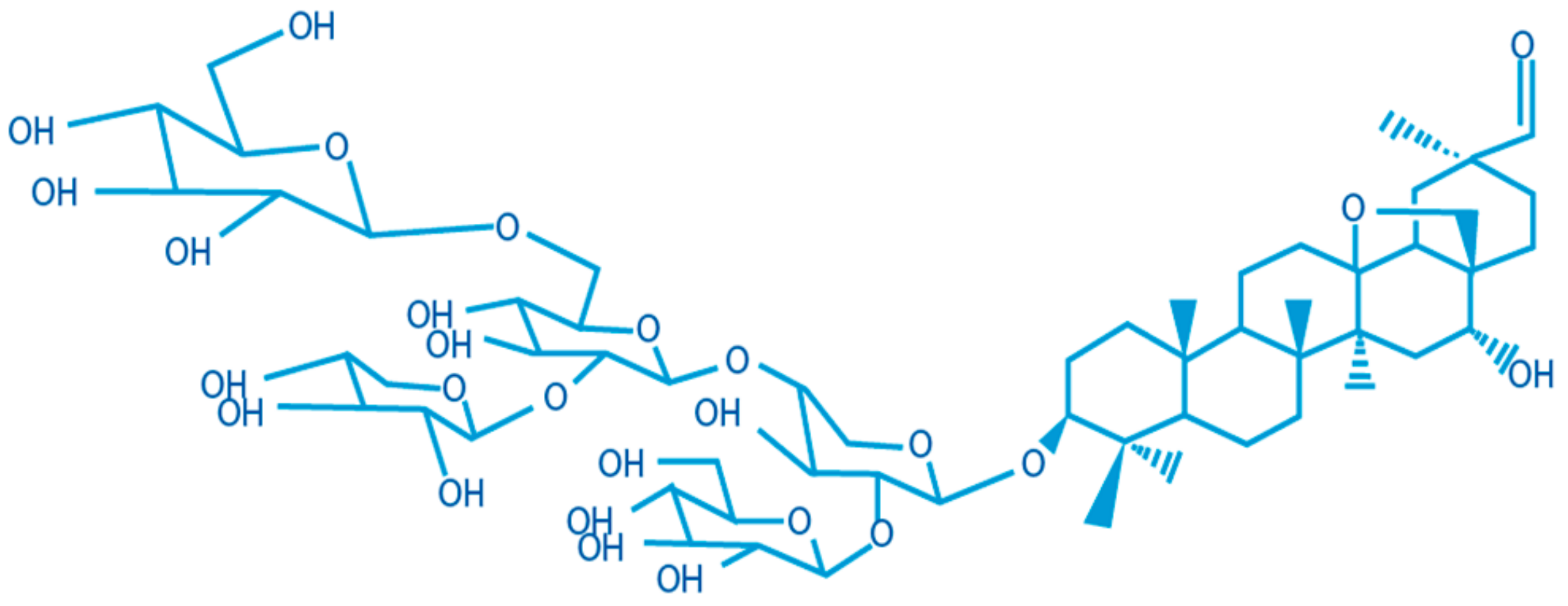
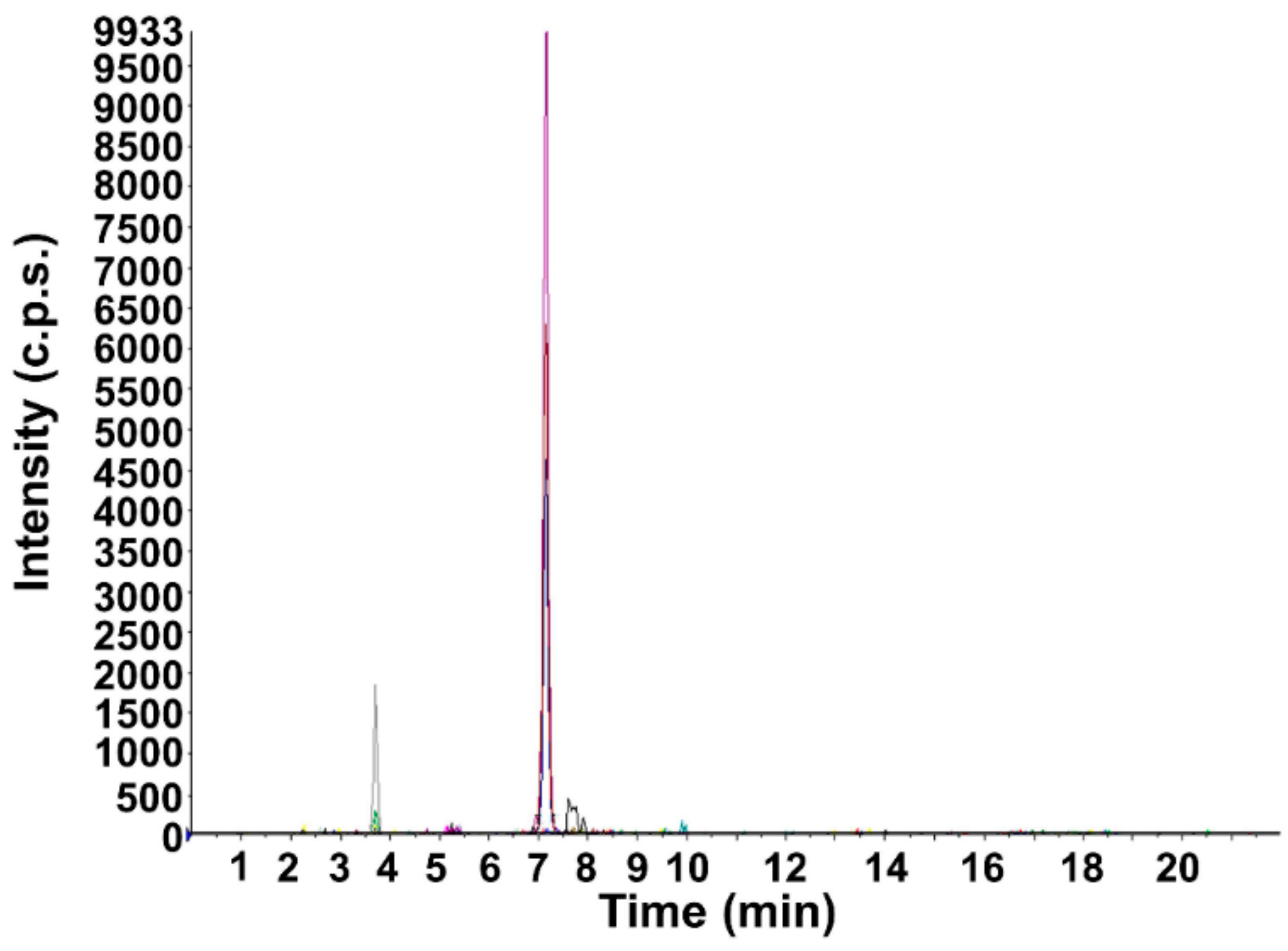
2.2. Drug Analytical Determination
2.3. Nasal Mucosa Permeation Experiment
2.4. Nasal Mucosa Integrity
2.5. In Vivo Assessment Evaluation in Rabbits
2.5.1. Multiple Dose Schemes
2.5.2. Animal Evaluation
3. Results and Discussion
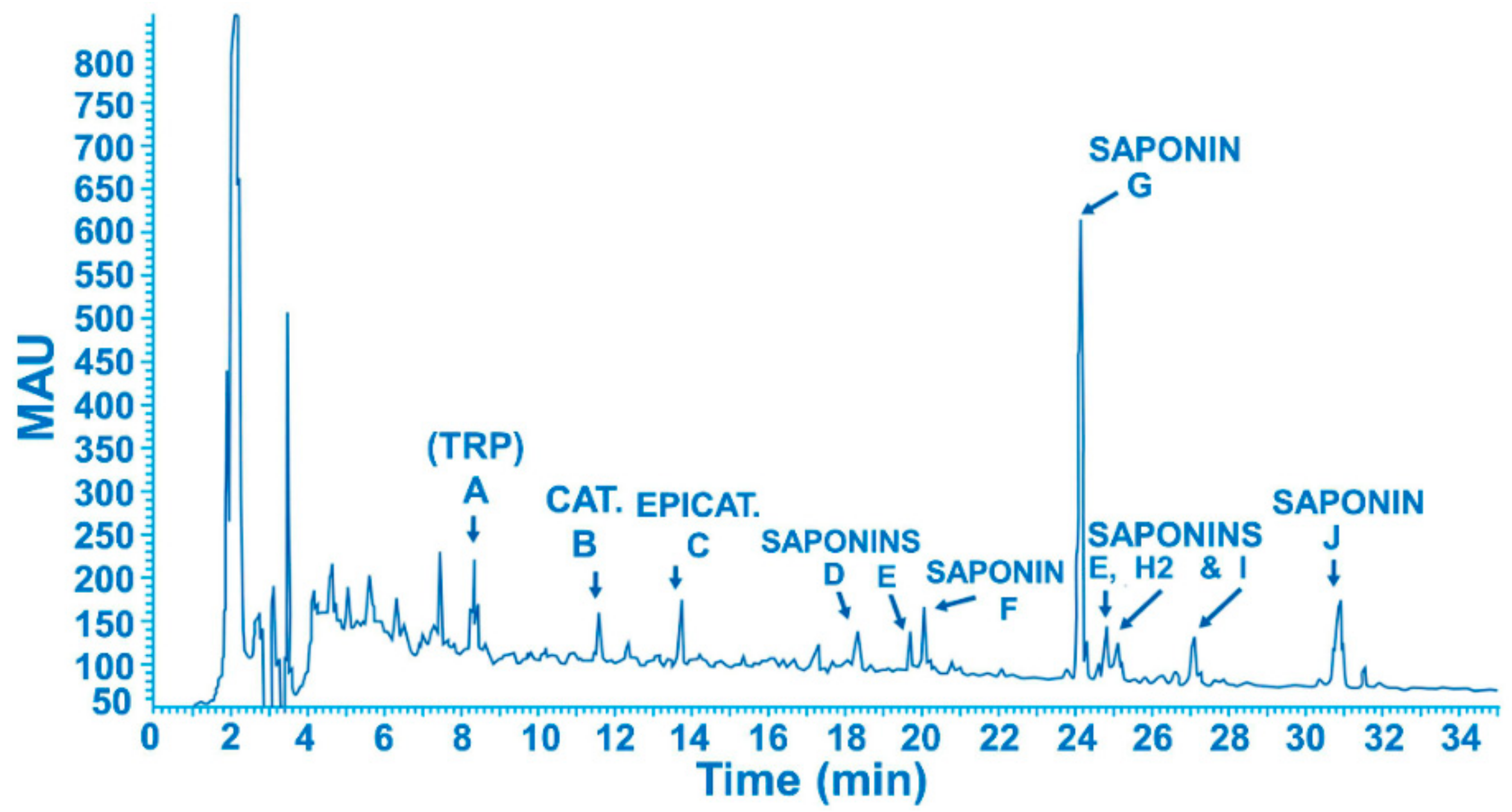
3.1. Analytical Method Development
3.2. Nasal Mucosa Permeation Experiment
3.3. Multiple Dose Schemes
3.3.1. Macroscopical Evaluation
3.3.2. Hematological and Biochemical Examination
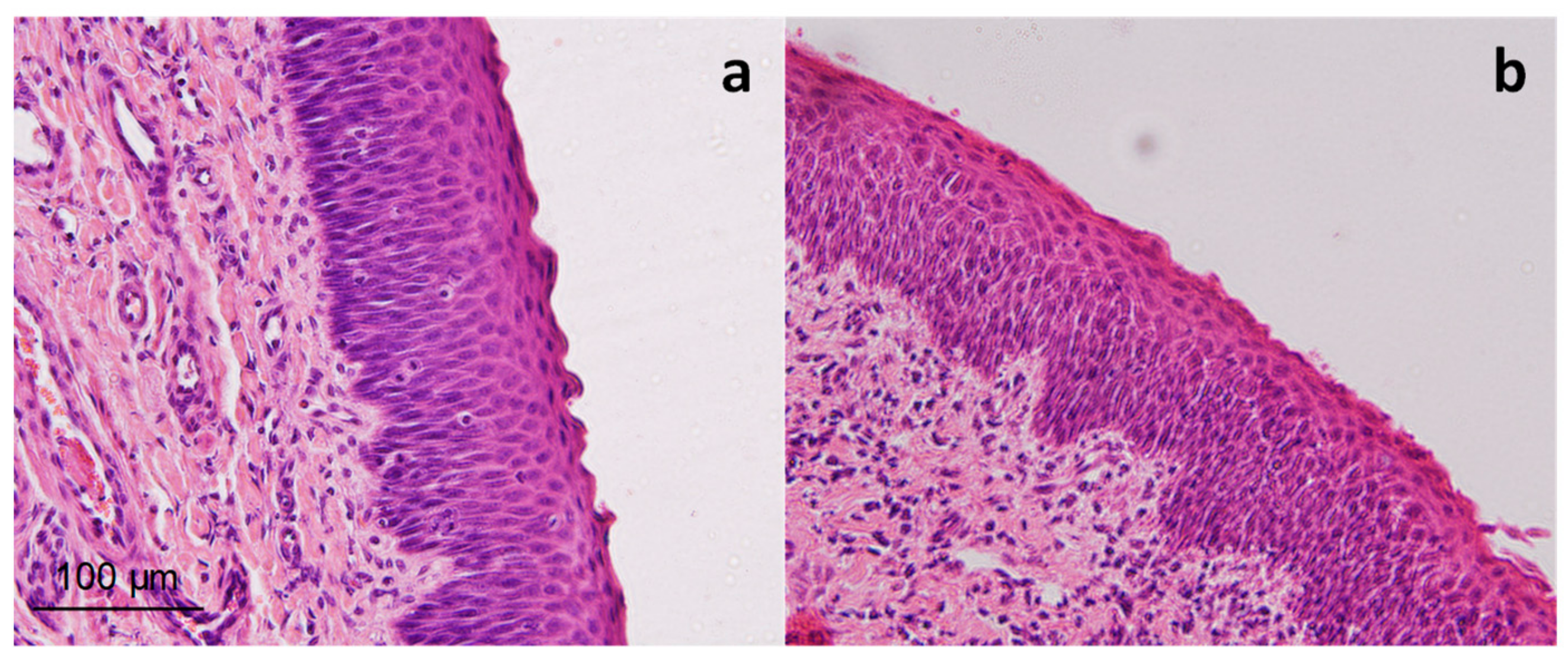
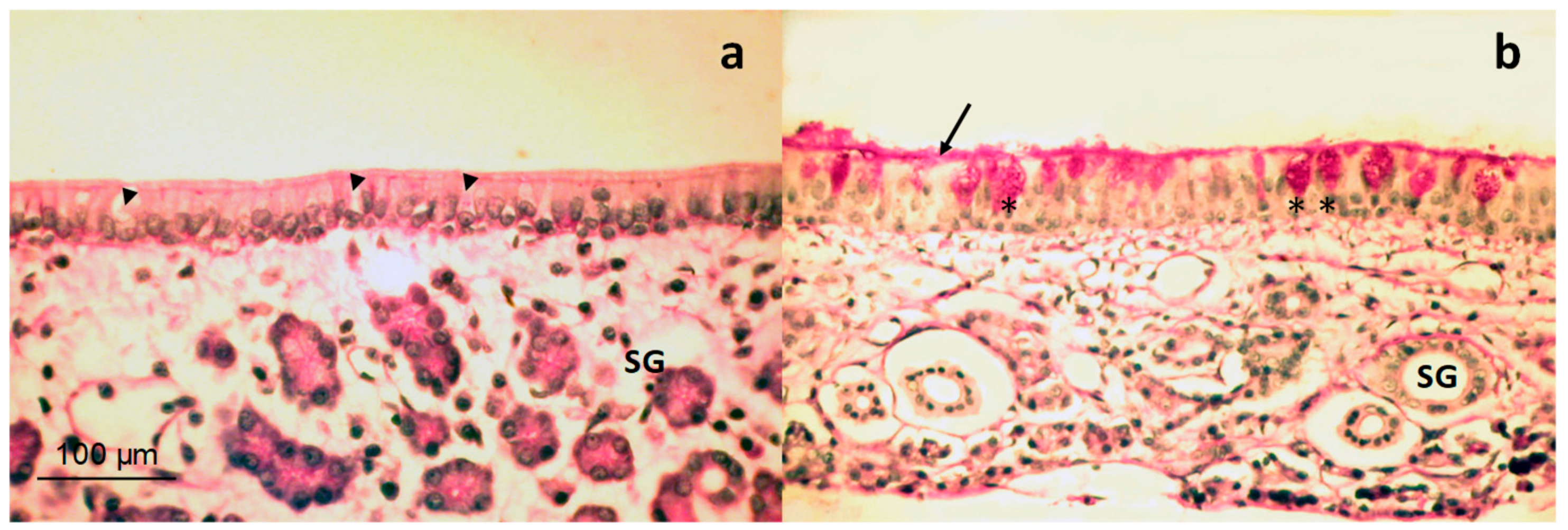
3.3.3. Histological Examination
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mandal, R.; Patel, N.; Ferguson, B.J. Role of Antibiotics in Sinusitis. Curr. Opin. Infect. Dis. 2012, 25, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Marcus, S.; Roland, L.T.; DelGaudio, J.M.; Wise, S.K. The Relationship between Allergy and Chronic Rhinosinusitis. Laryngoscope Investig. Otolaryngol. 2019, 4, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Ebell, M.H.; McKay, B.; Dale, A.; Guilbault, R.; Ermias, Y. Accuracy of Signs and Symptoms for the Diagnosis of Acute Rhinosinusitis and Acute Bacterial Rhinosinusitis. Ann. Fam. Med. 2019, 17, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Zalmanovici Trestioreanu, A.; Barua, A.; Pertzov, B. Cyclamen europaeum extract for acute sinusitis. Cochrane Database Syst. Rev. 2018, 5. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.S.; Yan, Y.; Ong, H.H.; Chow, V.T.K.; Shi, L.; Wang, D.Y. Impact of Respiratory Virus Infections in Exacerbation of Acute and Chronic Rhinosinusitis. Curr. Allergy Asthma Rep. 2017, 17, 24. [Google Scholar] [CrossRef] [PubMed]
- Ponikau, J.U.; Hamilos, D.L.; Barreto, A.; Cecil, J.; Jones, S.W.; Manthei, S.E.; Collins, J. An exploratory trial of Cyclamen europaeum extract for acute rhinosinusitis. Laryngoscope 2012, 122, 1887–1892. [Google Scholar] [CrossRef] [PubMed]
- Dall’Acqua, S.; Castagliuolo, I.; Brun, P.; Ditadi, F.; Palù, G.; Innocenti, G. Triterpene glycosides with in vitro anti-inflammatory activity from Cyclamen repandum tubers. Carbohydr. Res. 2010, 345, 709–714. [Google Scholar] [CrossRef] [PubMed]
- Pfaar, O.; Mullol, J.; Anders, C.; Hörmann, K.; Klimek, L. Cyclamen europaeum nasal spray, a novel phytotherapeutic product for the management of acute rhinosinusitis: A randomized double-blind, placebo-controlled trial. Rhinology 2012, 50, 37–44. [Google Scholar] [CrossRef]
- Altunkeyik, H.; Gülcemal, D.; Masullo, M.; Alankus-Caliskan, O.; Piacente, S.; Karayildirim, T. Triterpene saponins from Cyclamen hederifolium. Phytochemistry 2012, 73, 127–133. [Google Scholar] [CrossRef]
- Gedevanisvili, M.; Gogitidze, N.; Sikharulidze, I. Reflex mechanisms in naso-paranasal secretion. Vestn. Otorinolaringol. 2007, 3, 54–56. [Google Scholar]
- Wine, J. Parasympathetic control of airway mucosal glands: Central reflexes and the airway intrinsic nervous system. Auton. Neurosci. 2007, 133, 35–54. [Google Scholar] [CrossRef] [PubMed]
- Baraniuk, J.N.; Ali, M.; Yuta, A.; Fang, S.Y.; Naranch, K. Hypertonic saline nasal provocation stimulates nociceptive nerves, substance P release, and glandular mucous exocytosis in normal humans. Am. J. Respir. Crit. Care Med. 1999, 160, 655–662. [Google Scholar] [CrossRef] [PubMed]
- Böttger, S.; Hofmann, K.; Melzig, M.F. Saponins can perturb biologic membranes and reduce the surface tension of aqueous solutions: A correlation? Bioorg. Med. Chem. 2012, 20, 2822–2828. [Google Scholar] [CrossRef] [PubMed]
- Mullol, J.; Crespo, C.; Carré, C.; Brosa, M. Pharmacoeconomics of Cyclamen europaeum in the management of acute rhinosinusitis. Laryngoscope 2013, 123, 2620–2625. [Google Scholar] [CrossRef]
- Lopatin, A.S.; Ivanchenko, O.A.; Soshnikov, S.S.; Mullol, J. Cyclamen europaeum improves the effect of oral antibiotics on exacerbations and recurrences of chronic rhinosinusitis: A real-life observational study (CHRONOS). Acta Otorhinolaryngol. Ital. 2018, 38, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Jurkiewicz, D.; Hassmann-Poznańska, E.; Kaźmierczak, H.; Składzień, J.; Pietruszewska, W.; Burduk, P.; Rapiejko, P. Lyophilized Cyclamen europaeum tuber extract in the treatment of rhinosinusitis. Otolaryngol. Pol. 2016, 70, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Bone, K.; Mills, S. Principles of herbal pharmacology. In Principles and Practice of Phytotherapy, 2nd ed.; Bone, K., Mills, S., Eds.; Elsevier Churchill Livingstone: London, UK, 2013; pp. 17–82. [Google Scholar] [CrossRef]
- Singh, D.; Chaudhuri, P.K. Structural characteristics, bioavailability and cardioprotective potential of saponins. Integr. Med. Res. 2018, 7, 33–43. [Google Scholar] [CrossRef] [PubMed]
- El Hosry, L.; Di Giorgio, C.; Birer, C.; Habib, J.; Tueni, M.; Bun, S.S.; Herbette, G.; De Meo, M.; Ollivier, E.; Elias, R. In vitro cytotoxic and anticlastogenic activities of saxifragifolin B and cyclamin isolated from Cyclamen persicum and Cyclamen libanoticum. Pharm. Biol. 2014, 52, 1134–1140. [Google Scholar] [CrossRef] [PubMed]
- Arslan, S.; Ozgun, O. Cyclamen Trochopteranthum: Cytotoxic activity and possible adverse interactions including drugs and carcinogens. Chin. J. Integr. Med. 2012, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Mihci-Gaidi, G.; Pertuit, D.; Miyamoto, T.; Mirjolet, J.F.; Duchamp, O.; Mitaine-Offer, A.C.; Lacaille-Dubois, M.A. Triterpene saponins from Cyclamen persicum. Nat. Prod. Commun. 2010, 5, 1023–1025. [Google Scholar] [CrossRef] [PubMed]
- Richter, T.; Keipert, S. In vitro permeation studies comparing bovine nasal mucosa, porcine cornea and artificial membrane: Androstenedione in microemulsions and their components. Eur. J. Pharm. Biopharm. 2004, 58, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Ghori, M.U.; Mahdi, M.H.; Smith, A.M.; Conway, B.R. Nasal Drug Delivery Systems: An Overview. Am. J. Pharmacol. Sci. 2015, 3, 110–119. [Google Scholar] [CrossRef]
- Shojaei, A.H. Buccal mucosa as a route for systemic drug delivery: A review. J. Pharm. Sci. 1998, 1, 15–30. [Google Scholar]
- Lillie, R.D. Histopathologic Technic and Practical Histochemistry; McGraw-Hill: New York, NY, USA, 1965. [Google Scholar]
- Kolb, V.G.; Kamyshnikov, V.S. Clinical Biochemistry; Izdatelstvo: Minsk, Belarus, 1976; p. 311. [Google Scholar]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 1997, 23, 3–25. [Google Scholar] [CrossRef]
- Merkus, F.W.; Verhoef, J.C.; Schipper, N.G.; Marttin, E. Nasal mucociliary clearance as a factor in nasal drug delivery. Adv. Drug Deliv. Rev. 1998, 29, 13–38. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, M.; Walker, S.; Mains, J.; Wilson, C.G. Drug Delivery across the Nasal Mucosa. In Mucoadhesive Materials and Drug Delivery Systems; Khutoryanskiy, V.V., Ed.; John Wiley & Sons: New York, NY, USA, 2014; pp. 61–76. [Google Scholar]
- Fernandez-Campos, F.; Calpena-Campmany, A.C.; Delgado, G.R.; Serrano, O.L.; Naveros, B.C. Development and characterization of a novel nystatin-loaded nanoemulsion for the buccal treatment of candidosis: Ultrastructural effects and release studies. J. Pharm. Sci. 2012, 101, 3739–3752. [Google Scholar] [CrossRef]
- Cooper, R.A.; Bunn, H.F. Hemolytic anemias. In Harrisons Principles of Internal Medicine, 11th ed.; Kasper, D.L., Fauci, A.S., Longo, D.L., Hauser, S.L., Jameson, J.L., Loscalzo, J., Eds.; McGraw-Hill: New York, NY, USA, 1987; pp. 1505–1518. [Google Scholar]

| Parameter | Unit | C (Control) Group (Mean ± SD) | B (3-Fold Dose Treated) Group (Mean ± SD) |
|---|---|---|---|
| Red blood cells | 1012/L | 5.90 ± 0.14 | 5.28 ± 0.40 |
| Hemoglobin | g/L | 74.40 ± 14.40 | 78.90 ± 2.00 |
| Platelets | 109/L | 319.40 ± 22.30 | 349.40 ± 38.00 |
| ESR * | mm/h | 2.5 ± 0.5 | 2.5 ± 0.5 |
| Total white blood cells | 109/L | 8.70 ± 0.23 | 8.60 ± 0.20 |
| Total bilirubin | mmol/L | 3.92 ± 0.90 | 4.30 ± 0.06 |
| Conjugated bilirubin | mmol/L | 0.94 ± 0.40 | 1.50 ± 0.80 |
| ALT/SGPT ** | U/L | 66.0 ± 2.22 | 54.45 ± 4.78 |
| AST/SGOT *** | U/L | 74.2 ± 2.79 | 61.3 ± 3.71 |
| Total protein | g/L | 52.96 ± 2.00 | 58.44 ± 7.40 |
| Urea | mmol/L | 3.85 ± 2.77 | 5.82 ± 3.00 |
| Creatinine | µmol/L | 184.40 ± 13.00 | 167.60 ± 1.60 |
| Na+ | mmol/L | 172.40 ± 3.63 | 158.60 ± 1.80 |
| K+ | mmol/L | 4.78 ± 0.88 | 4.81 ± 0.64 |
| Glucose | mmol/L | 4.74 ± 1.04 | 6.67 ± 4.00 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Campos, F.; Clares, B.; Rodríguez-Lagunas, M.J.; Jauregui, O.; Casals, I.; Calpena, A.C. Ex-Vivo and In-Vivo Assessment of Cyclamen europaeum Extract After Nasal Administration. Pharmaceutics 2019, 11, 426. https://doi.org/10.3390/pharmaceutics11090426
Fernández-Campos F, Clares B, Rodríguez-Lagunas MJ, Jauregui O, Casals I, Calpena AC. Ex-Vivo and In-Vivo Assessment of Cyclamen europaeum Extract After Nasal Administration. Pharmaceutics. 2019; 11(9):426. https://doi.org/10.3390/pharmaceutics11090426
Chicago/Turabian StyleFernández-Campos, Francisco, Beatriz Clares, María J Rodríguez-Lagunas, Olga Jauregui, Isidre Casals, and Ana C Calpena. 2019. "Ex-Vivo and In-Vivo Assessment of Cyclamen europaeum Extract After Nasal Administration" Pharmaceutics 11, no. 9: 426. https://doi.org/10.3390/pharmaceutics11090426
APA StyleFernández-Campos, F., Clares, B., Rodríguez-Lagunas, M. J., Jauregui, O., Casals, I., & Calpena, A. C. (2019). Ex-Vivo and In-Vivo Assessment of Cyclamen europaeum Extract After Nasal Administration. Pharmaceutics, 11(9), 426. https://doi.org/10.3390/pharmaceutics11090426






