Abstract
Despite advances in intensive care, several neonatal conditions typically due to prematurity affect vital organs and are associated with high mortality and long-term morbidities. Current treatment strategies for these babies are only partially successful or are effective only in selected patients. Regenerative medicine has been shown to be a promising option for these conditions at an experimental level, but still warrants further exploration for the development of optimal treatment. Although stem cell-based therapy has emerged as a treatment option, studies have shown that it is associated with potential risks and hazards, especially in the fragile population of babies. Recently, extracellular vesicles (EVs) have emerged as an attractive therapeutic alternative that holds great regenerative potential and is cell-free. EVs are nanosized particles endogenously produced by cells that mediate intercellular communication through the transfer of their cargo. Currently, EVs are garnering considerable attention as they are the key effectors of stem cell paracrine signaling and can epigenetically regulate target cell genes through the release of RNA species, such as microRNA. Herein, we review the emerging literature on the therapeutic potential of EVs derived from different sources for the treatment of neonatal conditions that affect the brain, retinas, spine, lungs, and intestines and discuss the challenges for the translation of EVs into clinical practice.
1. Introduction
According to the World Health Organization, one of the leading causes of pediatric mortality globally is prematurity [1]. With improvements in neonatal intensive care management over the last decades, the number of premature babies, i.e., babies born before 37 weeks of gestation, has been increasing, with an estimated 15 million premature babies worldwide every year [2]. As more premature babies are surviving, we have observed an increase in acute and chronic conditions that are related partly to organ underdevelopment and partly to the invasiveness of supportive measures. Disorders of premature babies that require medical treatment commonly affect the central nervous system, the cardiorespiratory system, and the gastrointestinal tract. These include conditions such as intraventricular hemorrhage (IVH), cerebral palsy, bronchopulmonary dysplasia (BPD), and necrotizing enterocolitis (NEC) [3]. Efforts to cure these diseases have had modest success, and better therapeutic interventions to improve infant morbidity and mortality are called for. Stem cells have surfaced as promising therapies for some of these neonatal conditions, and there has been a large number of experimental studies that have successfully shown improvements in neonatal disease models [4,5,6,7,8]. For some neonatal conditions, such as BPD, favorable experimental findings have been translated to clinical trials in human babies. However, these clinical trials are still ongoing, and it has become clear that stem cell therapy in neonates is challenging. Recognized risk factors and hazards associated with stem cell-based therapy include rejection, toxicity, unwanted biological effects, tumorigenic potential, and contamination by adventitious agents [9]. To overcome these challenges, researchers have investigated whether stem cell derivatives could hold the same regenerative potential as their parent cells without the mentioned associated risks and whether they could be used as alternative therapeutic agents for neonatal conditions. This is also supported by the knowledge that stem cells mainly exert their effects through a paracrine mechanism, whereby their secreted factors signal to target cells.
Recently, extracellular vesicles (EVs) have been identified as one of the key mediators of stem cell paracrine signaling [10], with an effect that, if concentrated, can be greater than that of parent cells [11,12]. EVs are particles that are naturally released by cells, are delimited by a phospholipid bilayer, and do not replicate, as they do not possess a functional nucleus [13]. EVs play a role in intercellular communication by delivering their cargo contents in the form of proteins, lipids, and nucleic acids [13,14,15]. In particular, EV cargo in the form of RNA species have been shown to epigenetically regulate the genes of target cells [16]. Although EVs were described in the literature more than 50 years ago [17], their therapeutic potential has only recently been recognized. Clinical applications of EVs include, on the one hand, EVs as drug carriers, whereby they are used as pharmacological delivery systems for molecules of interests, and on the other hand, EVs as substitutes for stem cell-based therapy for tissue and organ regeneration. Herein, we review the current literature on the therapeutic potential of EVs that are being tested as alternatives to drugs or stem cells as a therapy for neonates. Moreover, we discuss the challenges related to the translation of EVs as therapeutics into clinical practice for this fragile population of patients.
2. Methods
A review of the literature was performed using a defined strategy. Searching scientific databases (PubMed, Medline, Scopus), we reviewed studies published in the literature (in English) from 1980 to present that reported the effects of EV treatment on models of neonatal diseases (see Section 8).
For this review, we use the generic term “extracellular vesicles”, or EVs, as endorsed by the International Society for Extracellular Vesicles in their 2018 position statement [13]. However, in descriptions of specific studies, we decided to maintain the same terminology used by their authors, which includes terms such as exosomes.
3. Hypoxic Ischemic Encephalopathy
Neonatal hypoxic ischemic encephalopathy (HIE) is a form of brain injury that typically occurs in premature babies and is due to perinatal oxygen deprivation [18,19]. This brain injury leads to damage in cell populations, such as the highly vulnerable immature oligodendrocytes that provide structural support to the brain [20,21]. HIE leads to unfavorable neurodevelopmental outcomes, such as cognitive disorders, in 20–50% of cases, and motor deficits, such as cerebral palsy, in 5–10% of cases [22,23,24,25]. Despite advances that have shown therapeutic benefits using brain cooling, moderate hypothermia is still associated with poor neurodevelopmental outcomes in a proportion of babies, and novel therapeutic strategies are being explored [26]. Researchers initially studied the regenerative potential of stem cell therapies for neuroprotection and repair in experimental models of HIE. Several studies showed that the administration of bone marrow-derived mesenchymal stem cells (BM-MSCs) to neonatal rat pups with hypoxic ischemic injury improved neurologic performance, increased cerebral cell proliferation and differentiation toward neurons and oligodendrocytes, decreased the degree of neuroinflammation, and restored hemispheric volume and axonal connectivity [6,27,28]. Similarly, the administration of MSCs derived from human umbilical cord blood attenuated the severity of brain injury and increased animal survival [29,30]. These results were replicated in an ovine model of hypoxic ischemic injury, where BM-MSCs increased myelination and reduced microglial proliferation, white matter injury, and oligodendrocyte loss [31]. To elucidate the BM-MSC mechanism of action, the same group of researchers tested the therapeutic efficacy of the BM-MSC secretome. This group was the first to report that antenatal administration of EVs derived from BM-MSCs resulted in neuroprotection in an ovine model of hypoxic ischemic injury [32]. This included a reduction in seizure activity and the restoration of subcortical white matter myelination. However, in that study, the authors reported that BM-MSC EVs did not protect against hypoxic ischemic-induced neuroinflammation. In search of another possible mechanism for EV-mediated neuroprotection, the same research group studied whether the administration of BM-MSC EVs could restore blood–brain barrier (BBB) integrity [33]. The BBB is a protective, semipermeable, and highly selective barrier between the brain and systemic circulation. The BBB can be disrupted during HIE, allowing immune cells to enter the central nervous system and induce a neuroinflammatory response [34,35]. Gussenhoven et al. showed that the administration of BM-MSC EVs prevented BBB leakage in fetal ovine brains by targeting the Annexin A1/formyl peptide receptor axis [33]. The BM-MSC EVs were reported to contain Annexin A1, an essential regulator of BBB integrity (Section 8). To prove that the Annexin A1 contained in EVs played a key role, the authors administered purified human Annexin A1 or BM-MSC EVs and confirmed that both improved BBB integrity, which in turn was abolished by the Annexin A1 receptor blocker, the formyl peptide receptor inhibitor [33]. Taken together, these findings suggest the delivery of Annexin A1 via EVs maintains BBB integrity in the immature brain following hypoxic ischemic brain injury. Although the authors did not report the size of these EVs, it is likely the positive effect on the BBB was produced by microvesicles. In fact, it has been recently shown that Annexin A1 is a specific marker of microvesicles shed from the plasma membrane and that Annexin A1-positive vesicles have a size distribution of 150 nm to 1 μm, which is consistent with microvesicles [36].
In 2018, Joerger-Messerli et al. further investigated the neuroprotective effects of another source of MSC-EVs derived from human umbilical cord Wharton’s jelly MSCs (WJ-MSCs) [37]. In this study, the authors reported that the administration of WJ-MSC-EVs to an in vitro model of oxygen–glucose deprivation/reoxygenation in the mouse neuroblastoma cell line neuro2a reduced hypoxic ischemic-induced apoptosis [37]. Joerger-Messerli et al. hypothesized that the effects were mediated by the WJ-MSC EV RNA cargo content, specifically microRNAs (miRNAs), which are noncoding regulatory sequences that regulate target cell gene expression. They showed that fluorescently labeled EV RNA was delivered into neuro2a cells and found that the antiapoptotic effect was likely mediated by the transfer of let-7-5p miRNA from EVs to neuronal cells (Section 8) [37]. Exploring other routes of administration, Sisa et al. tested the intranasal route of BM-MSC EV delivery in experimental hypoxic ischemic injury [26]. In this study, the authors confirmed the beneficial effect of BM-MSC EV administration in reducing microglial activation, cell death, and brain tissue loss.
4. Retinopathy of Prematurity
Retinopathy of prematurity (ROP) is a proliferative retinal vascular disease affecting preterm infants and remains the second leading cause of childhood blindness in the United States [38,39]. In preterm infants, retinal development is incomplete, and the degree of retinal immaturity depends on the degree of prematurity. The greatest risk factors for ROP include low gestational age, low birth weight, and supplemental oxygen use [40]. The pathophysiology of ROP is a two-step process, with the first phase occurring when the preterm infant is born and breathes: The retina becomes hyperoxic and levels of vascular endothelial growth factor (VEGF) and insulin-like growth factor 1 (IGF-1) decrease, leading to the cessation of retinal blood vessel growth. Phase two is characterized by disorganized retinal vascular growth and oxidative damage to endothelial cells [39]. Current treatment options for ROP depend on the severity of disease and include laser treatment, bevacizumab, and scleral buckling and/or vitrectomy [39]. Better treatment for ROP is still needed to target aberrant vasoproliferation, facilitate retinal vascular development, and restore ambulatory vision without harmful side effects for the infant.
Promising results have been obtained with stem cell treatment in models of retinal ischemia/reperfusion injury in rats. Li et al. reported that intravitreal injection of BM-MSCs homed to the inner limiting membrane and integrated into the nerve fiber layer and retinal ganglion cell layer [5]. Two to four weeks after transplantation, BM-MSCs differentiated into cell types expressing neuron-specific markers, such as neuron-specific enolase, neurofilament, and neurotrophic factors. Furthermore, BM-MSCs attenuated the reduction of retinal ganglion cells [5]. Dreixler et al. proved that the effect exerted by BM-MSCs could be replicated by intravitreal administration of BM-MSC-conditioned media (CM) in a rat model of retinal ischemia, which restored retinal function and decreased apoptosis [41]. More recently, Moisseiev et al. showed that intravitreal administration of human BM-MSC exosomes preserved retinal vascular flow, reduced neovascularization, and reduced retinal thinning in a mouse model of oxygen-induced retinopathy simulating ROP [42]. Of note, the exosome administration did not provoke an immune response and was not associated with ocular or systemic adverse effects [42]. Proteomic analysis demonstrated that BM-MSC exosomes were packaged with prosurvival-associated proteins, such as those from the cAMP response element-binding protein (CREB) pathway [42]. Aberrant CREB signaling has been associated with retinal ischemia and alterations to the retinal neurotrophic and inflammatory systems, and CREB signaling proteins are critical to the survival of neurons among other cell types [43]. Therefore, intravitreal administration of human-derived BM-MSC exosomes hold therapeutic potential for the treatment of retinal pathologies such as ischemia and ROP.
Further evidence that EV therapy might be beneficial for retinal conditions derives from a recent study that employed microglial-derived exosomes [44]. The rationale behind the use of these exosomes in ROP is that microglial cells play a role in the innate immune response and normal vascular development of the retina [45,46]. Using an in vivo mouse model of oxygen-induced retinopathy, Xu et al. showed that intravitreal administration of microglial-derived exosomes limited the central avascular area, reduced the area of retinal neovascularization, and decreased the expression of hypoxia-induced VEGF, which is considered the most important factor involved in the retinal neovascularization process [44]. Moreover, electroretinography data established better visual function in microglia-derived exosome-treated retinas [44]. RNA sequencing analysis of the EV cargo showed that miR-24-3p levels were high and that this miRNA was shuttled into photoreceptors. Both exosomes and miR-24-3p inhibited the inositol-requiring enzyme 1a (IRE1a)-X-box binding protein 1 (XBP1) pathway, which plays a role in endoplasmic reticulum stress-mediated hypoxia-induced photoreceptor apoptosis [44]. However, miR-24-3p inhibition dramatically reversed this inhibitory effect. Administration of either exosomes or miR-24-3p reduced photoreceptor apoptosis, suggesting that exosomes decreased hypoxia-induced apoptosis by transferring miR-24-3p to target cells. These novel EV-based studies show that different sources of exosomes might be employed as a cell-free treatment for ROP.
5. Spina Bifida
Neural tube defects are a group of congenital malformations that result from incomplete neurulation during week four of gestation. Spina bifida is one of the most common and severe forms of neural tube defects and is characterized by an incomplete closure of the spinal column [47]. The most severe subtype of spina bifida is myelomeningocele, which is caused by a failure of the lumbosacral spinal neural tube to close and results in exposure of the spinal cord to toxins and shear stress from amniotic fluid [48]. As a result, infants born with spina bifida often suffer from lifelong paralysis, bowel and bladder dysfunction, and hydrocephalus [49]. Years of experimental research have led to a clinical trial that showed the benefit of antenatal fetal surgical repair via skin closure during the second trimester of pregnancy [50]. This fetal surgical technique decreases the need for cerebrospinal fluid shunting; however, there is little improvement in motor function or the damage to the exposed spinal cord in babies with spina bifida who undergo this surgery [51].
Therefore, in search of a regenerative therapy that would be an adjunct to fetal surgical repair for spina bifida, researchers have explored the use of stem cell implantation to allow neuronal regeneration. Li et al. showed that topical administration of BM-MSCs to the spinal column of rats with spina bifida during fetal surgery regenerated neurons and reduced spinal neuron death in the defective spinal cord [7]. For a source of stem cells derived from tissues that are closer to the fetus, the placenta has been shown to be a promising source for human MSCs (P-MSCs) [52]. Studies have demonstrated that P-MSCs are neuroprotective, immunomodulatory, and improve wound healing [53,54,55]. The administration of P-MSCs has been shown to be beneficial in two different models: the first in lambs, where in utero administration of P-MSCs to an ovine model of spina bifida improved limb motor function [56]; and the second in a rat model of spina bifida induced via the administration of retinoic acid, where P-MSC-seeded patches placed in utero had significantly less dense apoptotic cells at the site of injury compared to patches without P-MSCs [51]. These previous studies showed that P-MSCs did not engraft into the host tissue, suggesting they mediate their effects through a paracrine mechanism rather than their direct differentiation [55,57]. To investigate whether P-MSCs mediate their effects via EV release, Kumar et al. reported on a study where they used the staurosporine-induced apoptotic human neuroblastoma cell line, SH-SY5Y [58]. In this study, the authors found that P-MSC exosomes increased the number of neuronal projections, the total number of branch points, circuitry length, and tube length, suggesting they play a role in neuroprotection [58]. To identify the potential mediators of P-MSC-derived EVs, Kumar et al. performed proteomics and an RNA sequencing analysis and found that galectin 1 was highly expressed on the surface of P-MSCs and P-MSC exosomes. Galectin 1 has both immunomodulatory and neuroprotective functions and has been demonstrated to regulate axonal regeneration, cell adhesion, and the proliferation of neural stem cells [59,60]. For further proof that this effect was mediated by galectin 1, Kumar et al. pre-incubated P-MSC exosomes with anti-galectin 1 antibody. Upon administration, a decrease in the total number of branching points and total neurite segments and diminished neuroprotective effects were observed [58]. These encouraging findings initiated the prospect for EV therapeutic application of P-MSC exosomes, either alone or in combination with trophic factors, for the treatment of spina bifida.
6. Bronchopulmonary Dysplasia
Bronchopulmonary dysplasia (BPD) is a lung disease, multifactorial in nature, which almost exclusively affects preterm babies [61]. Lungs affected by BPD are immature, as alveolar development and pulmonary angiogenesis are arrested. Moreover, these lungs are susceptible to inflammation, infection, and injury secondary to intensive care maneuvers, such as mechanical ventilation [3]. BPD is a leading cause of significant mortality and morbidity, including long-term respiratory and neurodevelopmental sequelae that extend beyond childhood [62]. Over the years, there has been extensive research aimed at finding an effective therapy for babies with BPD, and promising results have been obtained with the use of MSCs [63]. Experimental evidence has led to the translation of MSC clinical application, so that at present, there are ongoing clinical trials using MSCs in human babies with BPD [64,65,66]. However, several studies have demonstrated that the administration of MSC CM was not only beneficial in preventing alveolar loss, but also had a greater regenerative potential than parent cells did in BPD [4,67,68,69]. In fact, experimental studies have also shown improvements in alveolar loss in the absence of MSC engraftment in the target lungs, thus confirming a paracrine mechanism of action [70].
With this in mind, and given the concerns that MSC administration might induce tumor formation, researchers have started exploring the role of EV-based therapy in experimental BPD [65,71,72]. Lee et al. were the first to report that intravenously administered exosomes derived from BM-MSCs and WJ-MSCs, but not exosome-depleted CM, were able to suppress pulmonary macrophage influx and inhibit pulmonary vascular remodeling, ameliorating pulmonary hypertension [72]. In this study, the authors used adult mice and modeled hypoxia-induced pulmonary hypertension, a form of lung damage similar to BPD. When they investigated the cargo content of BM-MSC exosomes, they found an upregulation of miR-16, miR-21, and let7b pre-miRNA. The same group of researchers more recently compared the efficacy of exosomes derived from BM-MSCs and WJ-MSCs in a mouse model of BPD and showed that both improved lung structure and function [70]. Specifically, exosomes from both sources improved alveolarization and lung angiogenesis and decreased collagen, fibrosis, arteriole muscularization, and pulmonary hypertension [70]. The authors performed pulmonary function tests on mice and showed that exosomes reduced BPD-related increased lung capacity and emphysema [70]. Moreover, Willis et al. reported that the administration of WJ-MSC exosomes to pulmonary macrophages in vitro modulated their phenotype toward M2-like anti-inflammatory macrophages and suppressed M1-like proinflammatory macrophages [70].
Other research groups have shown similar beneficial effects with MSC exosome administration in experimental BPD. Chaubey et al. reported that CM and exosomes derived from human umbilical cord MSCs (hUC-MSCs) isolated from Wharton’s jelly collected at 25–30 weeks of gestation attenuated the degree of BPD in neonatal mice [73]. The authors concluded that the effect of exosomes was partly mediated by tumor necrosis factor alpha-stimulated gene-6 (TSG-6), an anti-inflammatory factor present in hUC-MSCs. Specifically, hUC-MSC exosome intraperitoneal administration reversed lung inflammation, alveolar injury, alveolar–capillary leak, and pulmonary hypertension [74]. When they examined the brains of BPD pups, they found that hUC-MSC exosome-treated mice had less neuronal apoptosis and restored myelination.
Braun et al. also used the intraperitoneal route to administer BM-MSC exosomes in a rat model of BPD [74]. In this study, the authors showed that BM-MSC exosomes preserved alveolar growth, increased peripheral blood vessel formation, and decreased the degree of pulmonary hypertension in experimental BPD. Moreover, this group showed that in an in vitro tube formation assay using human umbilical vein endothelial cells, BM-MSC exosomes promoted angiogenesis in part through a VEGF-mediated mechanism [75]. Similarly, Ahn et al. showed that VEGF mediated the therapeutic efficacy of MSC-derived EVs in a model of neonatal hyperoxic lung injury [75]. In rats with BPD, the authors compared the intratracheal administration of either hUC-MSC exosomes, hUC-MSCs, or hUC-MSC VEGF knockdown exosomes transfected with siRNA [75]. Ahn et al. found that MSCs and MSC-derived EVs, but not EVs derived from VEGF knockdown MSCs, were able to attenuate the degree of cell death, inflammation, and impaired alveolarization and angiogenesis. Importantly, this study showed that hUC-MSC EVs were as effective as parental MSCs at attenuating neonatal hyperoxic lung injuries and that this effect was mediated primarily by the transfer of VEGF [75]. Conversely, Porzionato et al. reported that intratracheal administration of hUC-MSC EVs was more effective than hUC-MSCs in repairing hyperoxia-induced lung injury [12]. In fact, hUC-MSC EVs had the most significant increase in total number of alveoli, decrease in mean alveolar volume, and decrease in arteriole muscularization compared to the hUC-MSCs from which they were derived [12].
7. Necrotizing Enterocolitis
Necrotizing enterocolitis (NEC) remains one of the most common and severe gastrointestinal emergencies in the neonatal period, primarily affecting premature neonates and extremely low-birth-weight infants (<1000 g) [76,77]. NEC is characterized by an extensive intestinal inflammatory process, ranging from mucosal injury to full-thickness necrosis and perforation that often leads to systemic inflammation affecting distant organs, including the brain [76,78,79]. The etiology is considered multifactorial, with several contributing causes, such as prematurity, formula feeding, hypoxia, and bacterial contamination [77]. Preterm babies who develop NEC have an immature gastrointestinal tract and naïve immune system that predispose them to the development of NEC [80,81]. Moreover, premature babies are at risk of NEC not only because they are formula-fed, which predisposes them to bowel ischemia, but also because they are not breast-fed, which is recognized as being a protective measure against NEC [76,77]. Despite advancements in the medical and surgical treatment of NEC over the last six decades, mortality is still very high and remains at 30–50%.
Several treatment strategies have been tested in experimental models of NEC, and stem cell therapy has emerged as an attractive treatment option. In a neonatal rat model of NEC, intraperitoneal administration of amniotic fluid stem cells (AFSCs) improved survival and morbidity, decreased NEC incidence, improved intestinal damage and function, decreased bowel inflammation, and regenerated the damaged bowel by increasing enterocyte proliferation and reducing apoptosis [8]. The beneficial effect of AFSCs was achieved via the modulation of stromal cells expressing cyclooxygenase 2 in the lamina propria, as shown by survival studies using selective and nonselective cyclooxygenase 2 inhibitors. In that study, compared to AFSCs, BM-MSC administration was not as effective at improving pup survival [8,82]. Interestingly, the beneficial effect exerted by AFSCs on the damaged neonatal intestine was achieved despite a low degree of cell engraftment, thus suggesting a paracrine mechanism of action. This observation has also been reported by other research groups who used different sources of MSCs, such as the bone marrow and the umbilical cord, and concluded that the administered stem cells were releasing factors that could regenerate the damaged bowel [83,84].
Recent studies have shown that stem cell-derived EVs play a crucial role in paracrine signaling, and researchers are now focusing on EVs as a potential cell-free therapy for NEC babies. Rager et al. were the first to report that the biologically active vectors of BM-MSCs administered to neonatal rats with NEC were the exosomes released by the cells [85]. Intraperitoneal administration of BM-MSC exosomes led to a significantly lower incidence of NEC and improvement in the severity of intestinal injury. BM-MSC exosomes proved to restore the intestinal barrier function to levels comparable to BM-MSC treatment alone [85]. Using an in vitro wound healing assay with intestinal epithelial cells (IEC-6), the authors confirmed that the beneficial effects of BM-MSC exosomes were specific, as they were not replicated by exosome-depleted BM-MSC CM [85]. In a rat model of NEC, the same group of researchers demonstrated a reduction of NEC incidence, employing exosomes derived from different types of stem cells (e.g., amniotic fluid-derived MSCs, BM-MSCs, amniotic fluid-derived neural stem cells, and neonatal enteric neural stem cells) [86]. A decrease in the severity of intestinal injury upon a histological analysis was observed with increasing exosome concentrations [86]. This observation is in line with other reports where EV effects were dose-dependent and not influenced by variations in EV size distribution or the method of isolation [87].
Similar protective effects toward bowel damage in experimental NEC were obtained by other groups with the use of milk-derived EVs. As previously mentioned, breastfeeding is associated with a decreased incidence of NEC; however, the protective mediators against NEC present in breastmilk are only partly known [88]. In 2017, Hock et al. were the first to report that in an in vitro model of bowel damage, exosomes derived from rat breastmilk promoted epithelial cell viability, proliferation, and stem cell activity [89]. Similarly, Martin et al. showed that exosomes derived from human breast milk conferred protection to intestinal epithelial cells from H2O2-induced oxidative stress [90]. These results were in line with the observation by Chen et al. that porcine milk-derived exosomes increased the villus height and crypt depth of the murine intestine [91]. Wang et al. compared breastmilk-derived exosomes from mothers who delivered term versus preterm babies [92]. The authors demonstrated that preterm exosomes had an enhanced ability to improve the proliferation of intestinal epithelial cells compared to term exosomes [92]. A proteomic analysis showed that the preterm exosomes contained peptides that participated in metabolic, developmental, and immune system processes; biological adhesion; and cell proliferation [92].
Due to the scarcity of breastmilk availability, Li et al. explored the role of exosomes in widely available bovine milk in a model of human intestinal cells [93]. Exosome administration promoted goblet cell expression, as confirmed by increased mucin production and increased goblet cell-associated markers (trefoil factor 3 and mucin 2). Goblet cells and their product mucin are known to be impaired in the intestine during NEC [94]. Moreover, Li et al. showed that bovine milk-derived exosomes reduced mucosal inflammation and protected against intestinal injury in neonatal mice with NEC [93]. Taken together, all of these studies call for further exploration into the development of EV-based therapies for the prevention and treatment of NEC.
8. Summary of Neonatal Conditions with EV Based Therapy Reported in the Literature
Here we present all reported effects of EV treatment on models of neonatal diseases (Figure 1). Details on EV species, source, isolation technique, and administration route are reported in Table 1. Moreover, we focused on the mediators of EV beneficial effects and the pathways affected by their cargo, which are ported in Table 2.
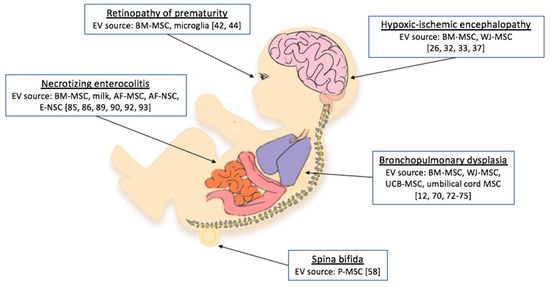
Figure 1.
Neonatal conditions for which studies have tested extracellular vesicle (EV) administration as a treatment option. BM-MSCs, bone marrow mesenchymal stem cells; AF-MSCs, amniotic fluid mesenchymal stem cells; AF-NSCs, amniotic fluid neural stem cells; E-NSCs, neonatal enteric neuronal stem cells; P-MSCs, placental mesenchymal stem cells; WJ-MSCs, umbilical cord Wharton’s jelly mesenchymal stem cells; UCB-MSCs, umbilical cord blood-derived mesenchymal stem cells [12,26,32,33,37,42,44,58,70,72,73,74,75,85,86,89,90,92,93].

Table 1.
Studies reporting the use of EVs as treatment for neonatal conditions.

Table 2.
Reported mediators of EV beneficial effects.
9. Considerations and Challenges in the Therapeutic Application of EVs: Identity, Potency, Purity, Safety, and Quality
The promising results obtained with EVs in experimental models of neonatal conditions reported here are an encouraging foundation for the translation of EV-based therapies into clinical practice. However, there are several challenges that remain to be overcome. The identity of the EVs remains partially unknown, especially the nature of their therapeutically active components. Efforts are being progressively made to understand the bioactive elements of the EV cargo, as shown in Table 2. EVs are known to contain proteins, lipids, and genetic material, yet there is still a lack of knowledge regarding the role of some noncoding RNA species, such as piwi-interacting RNAs (piRNAs), ribosomal RNAs (rRNAs), small nuclear RNAs (snRNAs), small nucleolar RNAs (snoRNAs), long ncRNAs (lncRNAs), long intergenic RNAs (lincRNAs), and circular RNAs. Similarly, the DNA contained in EVs has an undefined role. Only recently, double-stranded DNA (dsDNA) was shown to be present in EVs, and mutations in parent cells were identified in the EV dsDNA [95]. Studies on patients with pancreatic cancer have shown that EVs from human serum samples contain genomic DNA spanning all chromosomes, indicating their use as biomarkers for genomic mutations in cancer patients [96]. Takahashi et al. proposed that the dsDNA fragments contained in EVs represent in part one way that parent cells maintain homeostasis by removing harmful cytoplasmic DNA [97]. Recently, Jeppesen et al. assessed the composition of small EVs (exosomes) through high-resolution density gradient fractionation and direct immunoaffinity capture and challenged the presence of DNA in small EVs [36]. According to that study, dsDNA is present only in larger EVs, i.e., microvesicles. Hence, the quest for the identity of the bioactive components of EVs continues.
Another important aspect to consider in the use of EVs as therapeutics is their potency. There are several methods to quantify EV doses, and the most common are based on the number of parent cells (cell equivalents), EV protein cargo (protein concentration), and EV number and size using specialized quantitative analytical measurements, such as tunable resistive pulse sensing and nanoparticle tracking analysis [98]. In the studies included in our review, dose, frequency, and route of administration varied considerably, and the safe and effective dose to be used in the treatment of such neonatal conditions has yet to be determined. It has been reported that EVs have a short half-life and that their effects could be short-lived [99,100]. Therefore, to maintain their therapeutic potential over time, researchers have administered EVs in repeat doses [12,101]. Sjoqvist et al. observed that the repeated administration of EVs was more important than the dose itself in promoting wound healing in a pig model of esophageal wound repair [100]. Furthermore, repeat doses have been reported to not result in increased toxicity and immunogenicity [102,103].
Another challenge for the use of EVs as therapy relates to the purity of EV preparations [104]. Purity has been expressed as the ratio between protein content and the number of EVs and has been considered to be directly related to the EV isolation technique [104]. For this reason, several studies have assessed different isolation techniques to improve EV purity and to clear preparations of coprecipitates [104,105,106,107]. Isolation methods are recognized to play a role not only in EV purity, but also in EV yield, size distribution, and potential biological effects, and therefore they are considered crucial in obtaining optimal EV preparation. The different methods currently in use to isolate EVs include differential sedimentation (ultracentrifugation), density gradient, size exclusion chromatography, and kit-based isolation [87,108]. Several studies have shown that different isolation methods yield a different amount of EVs [87,107,109,110,111,112]. Variable yield, i.e., the number of isolated EV particles, has been shown to result in variable biological effects, regardless of the method employed to isolate the EVs [87]. Antounians et al. recently reported that when equal volumes of EVs isolated with different methods were used as a treatment in a model of lung epithelial injury, a significant biological variation was observed [87]. This effect appeared to be dose-dependent, as was also reported by other groups, thus highlighting the importance of choosing the right isolation technique for optimal yield [70,113,114]. To reduce the variability due to different isolation techniques, it has been proposed that the efficacious EV dose could be quantified. This could be obtained through surrogate markers for EVs, such as microRNAs, through fingerprinting assays [98].
The safety of EV preparations is also paramount to their translation into clinical practice. To date, a number of clinical trials have demonstrated the safety and feasibility of an EV-based approach [115]. The International Society for Extracellular Vesicles has published a position paper for applying EV-based therapeutics in clinical trials and has highlighted the safety and regulatory requirements that must be considered for pharmaceutical manufacturing and clinical applications [115]. However, to the best of our knowledge, there have been no reported trials to date that have tested the safety of EVs as a therapy in neonates. For this fragile population of patients, similar stringent regulations would apply, and the potential for an immunological response to treatment would need to be addressed.
Alongside safety considerations, the quality control of EV preparations is critical to ensure that a sustainable EV source is maintained and that variations between batches of EVs are minimized. Furthermore, compliance with regulations outlined by regulatory and scientific bodies should safeguard the production of EVs through good manufacturing practices. Lastly, the scalability of EVs for isolation, administration, and storage should be considered [116]. While the regulatory frameworks for manufacturing and clinical trials already exist, specific guidelines for using EVs as therapeutics are currently being established [115,117,118].
10. Final Remarks
Although stem cell-based therapies have emerged as a treatment option for some orphan diseases, in the last few years we have witnessed a paradigm shift toward cell-free therapy. EVs have been shown to be a promising alternative for many different diseases, including those that affect neonates, for whom cell-based therapies might be challenging or hazardous.
As EVs are considered the key mediators of stem cell paracrine signaling, the most common source of EVs used as treatment agents is from stem cells. In particular, the large body of literature on the use of MSCs has helped further the testing of EVs derived from MSCs in a variety of disease models. However, other sources of EVs have recently emerged. For example, milk is a source of EVs that appears to have regenerative potential in neonatal bowel conditions such as NEC.
The explosion of interest in EV-based therapies is still at an experimental stage. Nevertheless, for certain neonatal conditions, such as BPD, there are already a number of preclinical studies from different research groups that are concordantly showing a beneficial effect with EV administration. On the other hand, the use of EVs as treatment for diseases such as ROP and spina bifida is encouraging, but has been limited to a few experimental reports. Further studies are needed to confirm the beneficial effects of EVs in neonatal conditions before these findings can be tested in clinical trials. In addition, little is known about the mechanisms by which EVs exert their effects and the bioactive mediators present in their cargo. Some studies have reported EV effects mediated by proteins, but increasingly the focus has shifted to the RNA species contained inside the EVs and their potential to epigenetically regulate target cells. Lastly, further studies are required to address the possible side effects of EVs and to overcome the challenges related to their therapeutic application. Nonetheless, we believe that innovative EV-based therapies are opening new avenues in neonatal medicine as alternatives to drugs and stem cells.
Author Contributions
Conceptualization, A.C.M., L.A., and A.Z.; writing—original draft preparation, A.C.M.; writing—review and editing, A.C.M., L.A., and A.Z.; supervision, L.A., and A.Z.
Funding
This work was supported by SickKids start-up funds and by the Canadian Institutes of Health Research (CIHR)–SickKids Foundation New Investigator Research Grant (NI18-1270R).
Acknowledgments
The authors would like to thank Sree Gandhi for providing assistance in the graphical component of this manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Liu, L.; Oza, S.; Hogan, D.; Chu, Y.; Perin, J.; Zhu, J.; Lawn, J.E.; Cousens, S.; Mathers, C.; Black, R.E. Global, regional, and national causes of under-5 mortality in 2000-15: An updated systematic analysis with implications for the Sustainable Development Goals. Lancet 2016, 388, 3027–3035. [Google Scholar] [CrossRef]
- World Health Organization. Preterm Birth. Available online: https://www.who.int/news-room/fact-sheets/detail/preterm-birth (accessed on 10 June 2019).
- Glass, H.C.; Costarino, A.T.; Stayer, S.A.; Brett, C.M.; Cladis, F.; Davis, P.J. Outcomes for extremely premature infants. Anesth. Analg. 2015, 120. [Google Scholar] [CrossRef]
- Aslam, M.; Baveja, R.; Liang, O.D.; Fernandez-Gonzalez, A.; Lee, C.; Mitsialis, S.A.; Kourembanas, S. Bone marrow stromal cells attenuate lung injury in a murine model of neonatal chronic lung disease. Am. J. Respir. Crit. Care Med. 2009, 180, 1122–1130. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Li, X.-R.; Yuan, J. Effects of bone-marrow mesenchymal stem cells transplanted into vitreous cavity of rat injured by ischemia/reperfusion. Graefe’s Arch. Clin. Exp. Ophthalmol. 2009, 247, 503–514. [Google Scholar] [CrossRef]
- Lee, J.A.; Kim, B., II; Jo, C.H.; Choi, C.W.; Kim, E.K.; Kim, H.S.; Yoon, K.S.; Choi, J.H. Mesenchymal stem-cell transplantation for hypoxic-ischemic brain injury in neonatal rat model. Pediatr. Res. 2010, 67, 42–46. [Google Scholar] [CrossRef]
- Li, H.; Gao, F.; Ma, L.; Jiang, J.; Miao, J.; Jiang, M.; Fan, Y.; Wang, L.; Wu, D.; Liu, B.; et al. Therapeutic potential of in utero mesenchymal stem cell (MSCs) transplantation in rat foetuses with spina bifida aperta. J. Cell. Mol. Med. 2012, 16, 1606–1617. [Google Scholar] [CrossRef]
- Zani, A.; Cananzi, M.; Fascetti-Leon, F.; Lauriti, G.; Smith, V.V.; Bollini, S.; Ghionzoli, M.; D’Arrigo, A.; Pozzobon, M.; Piccoli, M.; et al. Amniotic fluid stem cells improve survival and enhance repair of damaged intestine in necrotising enterocolitis via a COX-2 dependent mechanism. Gut 2014, 63, 300–309. [Google Scholar] [CrossRef]
- Herberts, C.A.; Kwa, M.S.G.; Hermsen, H.P.H. Risk factors in the development of stem cell therapy. J. Transl. Med. 2011, 9, 29. [Google Scholar] [CrossRef]
- Baraniak, P.R.; McDevitt, T.C. Stem cell paracrine actions and tissue regeneration. Regen. Med. 2010, 5, 121–143. [Google Scholar] [CrossRef]
- Adamiak, M.; Cheng, G.; Bobis-Wozowicz, S.; Zhao, L.; Kedracka-Krok, S.; Samanta, A.; Karnas, E.; Xuan, Y.-T.; Skupien-Rabian, B.; Chen, X.; et al. Induced Pluripotent Stem Cell (iPSC)-Derived Extracellular Vesicles Are Safer and More Effective for Cardiac Repair Than iPSCs. Circ. Res. 2018, 122, 296–309. [Google Scholar] [CrossRef]
- Porzionato, A.; Zaramella, P.; Dedja, A.; Guidolin, D.; Van Wemmel, K.; Macchi, V.; Jurga, M.; Perilongo, G.; De Caro, R.; Baraldi, E.; et al. Intratracheal administration of clinical-grade mesenchymal stem cell-derived extracellular vesicles reduces lung injury in a rat model of bronchopulmonary dysplasia. Am. J. Physiol. Lung Cell. Mol. Physiol. 2019, 316, L6–L19. [Google Scholar] [CrossRef]
- Thery, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef]
- Van Niel, G.; Porto-Carreiro, I.; Simoes, S.; Raposo, G. Exosomes: A common pathway for a specialized function. J. Biochem. 2006, 140, 13–21. [Google Scholar] [CrossRef]
- Kourembanas, S. Exosomes: Vehicles of intercellular signaling, biomarkers, and vectors of cell therapy. Annu. Rev. Physiol. 2015, 77, 13–27. [Google Scholar] [CrossRef]
- Valadi, H.; Ekstrom, K.; Bossios, A.; Sjostrand, M.; Lee, J.J.; Lotvall, J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007, 9, 654–659. [Google Scholar] [CrossRef]
- Wolf, P. The Nature and Significance of Platelet Products in Human Plasma. Br. J. Haematol. 1967, 13, 269–288. [Google Scholar] [CrossRef]
- Hagberg, H.; Ichord, R.; Palmer, C.; Yager, J.Y.; Vannucci, S.J. Animal models of developmental brain injury: Relevance to human disease. A summary of the panel discussion from the Third Hershey Conference on Developmental Cerebral Blood Flow and Metabolism. Dev. Neurosci. 2002, 24, 364–366. [Google Scholar] [CrossRef]
- Mallard, C.; Welin, A.-K.; Peebles, D.; Hagberg, H.; Kjellmer, I. White matter injury following systemic endotoxemia or asphyxia in the fetal sheep. Neurochem. Res. 2003, 28, 215–223. [Google Scholar] [CrossRef]
- Back, S.A.; Luo, N.L.; Borenstein, N.S.; Levine, J.M.; Volpe, J.J.; Kinney, H.C. Late oligodendrocyte progenitors coincide with the developmental window of vulnerability for human perinatal white matter injury. J. Neurosci. 2001, 21, 1302–1312. [Google Scholar] [CrossRef]
- Volpe, J.J.; Kinney, H.C.; Jensen, F.E.; Rosenberg, P.A. The developing oligodendrocyte: Key cellular target in brain injury in the premature infant. Int. J. Dev. Neurosci. 2011, 29, 423–440. [Google Scholar] [CrossRef]
- Robertson, C.; Finer, N. Term infants with hypoxic-ischemic encephalopathy: Outcome at 3.5 years. Dev. Med. Child. Neurol. 1985, 27, 473–484. [Google Scholar] [CrossRef]
- Hack, M.; Breslau, N.; Aram, D.; Weissman, B.; Klein, N.; Borawski-Clark, E. The effect of very low birth weight and social risk on neurocognitive abilities at school age. J. Dev. Behav. Pediatr. 1992, 13, 412–420. [Google Scholar] [CrossRef]
- Allen, K.A.; Brandon, D.H. Hypoxic Ischemic Encephalopathy: Pathophysiology and Experimental Treatments. Newborn Infant Nurs. Rev. 2011, 11, 125–133. [Google Scholar] [CrossRef]
- Lee, A.C.C.; Kozuki, N.; Blencowe, H.; Vos, T.; Bahalim, A.; Darmstadt, G.L.; Niermeyer, S.; Ellis, M.; Robertson, N.J.; Cousens, S.; et al. Intrapartum-related neonatal encephalopathy incidence and impairment at regional and global levels for 2010 with trends from 1990. Pediatr. Res. 2013, 74 (Suppl. S1), 50–72. [Google Scholar] [CrossRef]
- Sisa, C.; Kholia, S.; Naylor, J.; Herrera Sanchez, M.B.; Bruno, S.; Deregibus, M.C.; Camussi, G.; Inal, J.M.; Lange, S.; Hristova, M. Mesenchymal Stromal Cell Derived Extracellular Vesicles Reduce Hypoxia-Ischaemia Induced Perinatal Brain Injury. Front. Physiol. 2019, 10, 1–10. [Google Scholar] [CrossRef]
- Van Velthoven, C.T.J.; Kavelaars, A.; van Bel, F.; Heijnen, C.J. Mesenchymal stem cell treatment after neonatal hypoxic-ischemic brain injury improves behavioral outcome and induces neuronal and oligodendrocyte regeneration. Brain Behav. Immun. 2010, 24, 387–393. [Google Scholar] [CrossRef]
- Van Velthoven, C.T.J.; Van De Looij, Y.; Kavelaars, A.; Zijlstra, J.; Van Bel, F.; Huppi, P.S.; Sizonenko, S.; Heijnen, C.J. Mesenchymal stem cells restore cortical rewiring after neonatal ischemia in mice. Ann. Neurol. 2012, 71, 785–796. [Google Scholar] [CrossRef]
- Xia, G.; Hong, X.; Chen, X.; Lan, F.; Zhang, G.; Liao, L. Intracerebral transplantation of mesenchymal stem cells derived from human umbilical cord blood alleviates hypoxic ischemic brain injury in rat neonates. J. Perinat. Med. 2010, 38, 215–221. [Google Scholar] [CrossRef]
- Kim, E.S.; Ahn, S.Y.; Im, G.H.; Sung, D.K.; Park, Y.R.; Choi, S.H.; Choi, S.J.; Chang, Y.S.; Oh, W.; Lee, J.H.; et al. Human umbilical cord blood-derived mesenchymal stem cell transplantation attenuates severe brain injury by permanent middle cerebral artery occlusion in newborn rats. Pediatr. Res. 2012, 72, 277–284. [Google Scholar] [CrossRef]
- Jellema, R.K.; Wolfs, T.G.A.M.; Lima Passos, V.; Zwanenburg, A.; Ophelders, D.R.M.G.; Kuypers, E.; Hopman, A.H.N.; Dudink, J.; Steinbusch, H.W.; Andriessen, P.; et al. Mesenchymal Stem Cells Induce T-Cell Tolerance and Protect the Preterm Brain after Global Hypoxia-Ischemia. PLoS ONE 2013, 8, 1–15. [Google Scholar] [CrossRef]
- Ophelders, D.R.M.G.; Wolfs, T.G.A.M.; Jellema, R.K.; Zwanenburg, A.; Andriessen, P.; Delhaas, T.; Ludwig, A.-K.; Radtke, S.; Peters, V.; Janssen, L.; et al. Mesenchymal Stromal Cell-Derived Extracellular Vesicles Protect the Fetal Brain After Hypoxia-Ischemia. Stem Cells Transl. Med. 2016, 5, 754–763. [Google Scholar] [CrossRef]
- Gussenhoven, R.; Klein, L.; Ophelders, D.; Habets, D.; Giebel, B.; Kramer, B.; Schurgers, L.; Reutelingsperger, C.; Wolfs, T. Annexin A1 as Neuroprotective Determinant for Blood-Brain Barrier Integrity in Neonatal Hypoxic-Ischemic Encephalopathy. J. Clin. Med. 2019, 8, 137. [Google Scholar] [CrossRef]
- Kumar, A.; Mittal, R.; Khanna, H.D.; Basu, S. Free Radical Injury and Blood-Brain Barrier Permeability in Hypoxic-Ischemic Encephalopathy. Pediatrics 2008, 122, e722–e727. [Google Scholar] [CrossRef]
- Moretti, R.; Pansiot, J.; Bettati, D.; Strazielle, N.; Ghersi-Egea, J.-F.; Damante, G.; Fleiss, B.; Titomanlio, L.; Gressens, P. Blood-brain barrier dysfunction in disorders of the developing brain. Front. Neurosci. 2015, 9, 40. [Google Scholar] [CrossRef]
- Jeppesen, D.K.; Fenix, A.M.; Franklin, J.L.; Higginbotham, J.N.; Zhang, Q.; Zimmerman, L.J.; Liebler, D.C.; Ping, J.; Liu, Q.; Evans, R.; et al. Reassessment of Exosome Composition. Cell 2019, 177, 428–445. [Google Scholar] [CrossRef]
- Joerger-Messerli, M.S.; Oppliger, B.; Spinelli, M.; Thomi, G.; di Salvo, I.; Schneider, P.; Schoeberlein, A. Extracellular Vesicles Derived from Wharton’s Jelly Mesenchymal Stem Cells Prevent and Resolve Programmed Cell Death Mediated by Perinatal Hypoxia-Ischemia in Neuronal Cells. Cell Transpl. 2018, 27, 168–180. [Google Scholar] [CrossRef]
- Sommer, A.; Taylor, H.R.; Ravilla, T.D.; West, S.; Lietman, T.M.; Keenan, J.D.; Chiang, M.F.; Robin, A.L.; Mills, R.P. Challenges of ophthalmic care in the developing world. JAMA Ophthalmol. 2014, 132, 640–644. [Google Scholar] [CrossRef]
- Bashinsky, A. Retinopathy of Prematurity. N. C. Med. J. 2017, 78, 124–128. [Google Scholar] [CrossRef]
- Stenson, B.J.; Tarnow-Mordi, W.O.; Darlow, B.A.; Simes, J.; Juszczak, E.; Askie, L.; Battin, M.; Bowler, U.; Broadbent, R.; Cairns, P.; et al. Oxygen saturation and outcomes in preterm infants. N. Engl. J. Med. 2013, 368, 2094–2104. [Google Scholar] [CrossRef]
- Dreixler, J.C.; Poston, J.N.; Balyasnikova, I.; Shaikh, A.R.; Tupper, K.Y.; Conway, S.; Boddapati, V.; Marcet, M.M.; Lesniak, M.S.; Roth, S. Delayed administration of bone marrow mesenchymal stem cell conditioned medium significantly improves outcome after retinal ischemia in rats. Investig. Ophthalmol. Vis. Sci. 2014, 55, 3785–3796. [Google Scholar] [CrossRef]
- Moisseiev, E.; Anderson, J.D.; Oltjen, S.; Goswami, M.; Zawadzki, R.J.; Nolta, J.A.; Park, S.S. Protective Effect of Intravitreal Administration of Exosomes Derived from Mesenchymal Stem Cells on Retinal Ischemia. Curr. Eye Res. 2017, 42, 1358–1367. [Google Scholar] [CrossRef]
- Guo, X.J.; Tian, X.S.; Ruan, Z.; Chen, Y.T.; Wu, L.; Gong, Q.; Wang, W.; Zhang, H.Y. Dysregulation of neurotrophic and inflammatory systems accompanied by decreased CREB signaling in ischemic rat retina. Exp. Eye Res. 2014, 125, 156–163. [Google Scholar] [CrossRef]
- Xu, W.; Wu, Y.; Hu, Z.; Sun, L.; Dou, G.; Zhang, Z.; Wang, H.; Guo, C.; Wang, Y. Exosomes from microglia attenuate photoreceptor injury and neovascularisation in an animal model of retinopathy of prematurity. Mol. Ther. Nucleic Acids 2019, 16, 778–790. [Google Scholar] [CrossRef]
- Salter, M.W.; Beggs, S. Sublime Microglia: Expanding Roles for the Guardians of the CNS. Cell 2014, 158, 15–24. [Google Scholar] [CrossRef]
- Ebneter, A.; Kokona, D.; Schneider, N.; Zinkernagel, M.S. Microglia Activation and Recruitment of Circulating Macrophages During Ischemic Experimental Branch Retinal Vein Occlusion. Investig. Ophthalmol. Vis. Sci. 2017, 58, 944–953. [Google Scholar] [CrossRef]
- Mitchell, L.E.; Adzick, N.S.; Melchionne, J.; Pasquariello, P.S.; Sutton, L.N.; Whitehead, A.S. Spina bifida. Lancet 2004, 364, 1885–1895. [Google Scholar] [CrossRef]
- Copp, A.J.; Adzick, N.S.; Chitty, L.S.; Fletcher, J.M.; Holmbeck, G.N.; Shaw, G.M. Spina bifida. Nat. Rev. Dis. Prim. 2015, 1, 15007. [Google Scholar] [CrossRef]
- Fichter, M.A.; Dornseifer, U.; Henke, J.; Schneider, K.T.M.; Kovacs, L.; Biemer, E.; Bruner, J.; Adzick, N.S.; Harrison, M.R.; Papadopulos, N.A. Fetal Spina Bifida Repair—Current Trends and Prospects of Intrauterine Neurosurgery. Fetal Diagn. Ther. 2008, 23, 271–286. [Google Scholar] [CrossRef]
- Adzick, N.S.; Thom, E.A.; Spong, C.Y.; Brock, J.W., III; Burrows, P.K.; Johnson, M.P.; Howell, L.J.; Farrell, J.A.; Dabrowiak, M.E.; Sutton, L.N.; et al. A randomized trial of prenatal versus postnatal repair of myelomeningocele. N. Engl. J. Med. 2011, 364, 993–1004. [Google Scholar] [CrossRef]
- Chen, Y.J.; Chung, K.; Pivetti, C.; Lankford, L.; Kabagambe, S.K.; Vanover, M.; Becker, J.; Lee, C.; Tsang, J.; Wang, A.; et al. Fetal surgical repair with placenta-derived mesenchymal stromal cell engineered patch in a rodent model of myelomeningocele. J. Pediatr. Surg. 2017, 53, 183–188. [Google Scholar] [CrossRef]
- Murphy, M.B.; Moncivais, K.; Caplan, A.I. Mesenchymal stem cells: Environmentally responsive therapeutics for regenerative medicine. Exp. Mol. Med. 2013, 45, e54. [Google Scholar] [CrossRef]
- Lee, J.M.; Jung, J.; Lee, H.-J.; Jeong, S.J.; Cho, K.J.; Hwang, S.-G.; Kim, G.J. Comparison of immunomodulatory effects of placenta mesenchymal stem cells with bone marrow and adipose mesenchymal stem cells. Int. Immunopharmacol. 2012, 13, 219–224. [Google Scholar] [CrossRef]
- Jones, G.N.; Moschidou, D.; Puga-Iglesias, T.-I.; Kuleszewicz, K.; Vanleene, M.; Shefelbine, S.J.; Bou-Gharios, G.; Fisk, N.M.; David, A.L.; De Coppi, P.; et al. Ontological Differences in First Compared to Third Trimester Human Fetal Placental Chorionic Stem Cells. PLoS ONE 2012, 7, e43395. [Google Scholar] [CrossRef]
- Calzarossa, C.; Bossolasco, P.; Besana, A.; Manca, M.P.; De Grada, L.; De Coppi, P.; Giardino, D.; Silani, V.; Cova, L. Neurorescue effects and stem properties of chorionic villi and amniotic progenitor cells. Neuroscience 2013, 234, 158–172. [Google Scholar] [CrossRef]
- Kabagambe, S.; Keller, B.; Becker, J.; Goodman, L.; Pivetti, C.; Lankford, L.; Chung, K.; Lee, C.; Chen, Y.J.; Kumar, P.; et al. Placental mesenchymal stromal cells seeded on clinical grade extracellular matrix improve ambulation in ovine myelomeningocele. J. Pediatr. Surg. 2017. [Google Scholar] [CrossRef]
- Wang, A.; Brown, E.G.; Lankford, L.; Keller, B.A.; Pivetti, C.; Sitkin, N.A.; Beattie, M.S.; Bresnahan, J.C.; Farmer, D.L. Placental mesenchymal stromal cells rescue ambulation in ovine myelomeningocele. Stem Cells Transl. Med. 2015, 4, 659–669. [Google Scholar] [CrossRef]
- Kumar, P.; Becker, J.C.; Gao, K.; Carney, R.P.; Lankford, L.; Keller, B.A.; Herout, K.; Lam, K.S.; Farmer, D.L.; Wang, A. Neuroprotective effect of placenta-derived mesenchymal stromal cells: Role of exosomes. FASEB J. 2019, 33, 5836–5849. [Google Scholar] [CrossRef]
- Sakaguchi, M.; Shingo, T.; Shimazaki, T.; Okano, H.J.; Shiwa, M.; Ishibashi, S.; Oguro, H.; Ninomiya, M.; Kadoya, T.; Horie, H.; et al. A carbohydrate-binding protein, Galectin-1, promotes proliferation of adult neural stem cells. Proc. Natl. Acad. Sci. USA 2006, 103, 7112–7117. [Google Scholar] [CrossRef]
- Horie, H.; Inagaki, Y.; Sohma, Y.; Nozawa, R.; Okawa, K.; Hasegawa, M.; Muramatsu, N.; Kawano, H.; Horie, M.; Koyama, H.; et al. Galectin-1 regulates initial axonal growth in peripheral nerves after axotomy. J. Neurosci. 1999, 19, 9964–9974. [Google Scholar] [CrossRef]
- Baraldi, E.; Filippone, M. Chronic lung disease after premature birth. N. Engl. J. Med. 2007, 357, 1946–1955. [Google Scholar] [CrossRef]
- Goldenberg, R.L.; Culhane, J.F.; Iams, J.D.; Romero, R. Epidemiology and causes of preterm birth. Lancet 2008, 371, 75–84. [Google Scholar] [CrossRef]
- Thebaud, B. Mesenchymal Stromal Cell Therapy for Respiratory Complications of Extreme Prematurity. Am. J. Perinatol. 2018, 35, 566–569. [Google Scholar] [CrossRef]
- Chang, Y.S.; Ahn, S.Y.; Yoo, H.S.; Sung, S.I.; Choi, S.J.; Oh, W., II; Park, W.S. Mesenchymal stem cells for bronchopulmonary dysplasia: Phase 1 dose-escalation clinical trial. J. Pediatr. 2014, 164, 966–972. [Google Scholar] [CrossRef]
- Ahn, S.Y.; Chang, Y.S.; Kim, J.H.; Sung, S.I.; Park, W.S. Two-Year Follow-Up Outcomes of Premature Infants Enrolled in the Phase I Trial of Mesenchymal Stem Cells Transplantation for Bronchopulmonary Dysplasia. J. Pediatr. 2017, 185, 49–54. [Google Scholar] [CrossRef]
- Powell, S.B.; Silvestri, J.M. Safety of Intratracheal Administration of Human Umbilical Cord Blood Derived Mesenchymal Stromal Cells in Extremely Low Birth Weight Preterm Infants. J. Pediatr. 2019, 210, 209–213. [Google Scholar] [CrossRef]
- Hansmann, G.; Fernandez-Gonzalez, A.; Aslam, M.; Vitali, S.H.; Martin, T.; Mitsialis, S.A.; Kourembanas, S. Mesenchymal Stem Cell-Mediated Reversal of Bronchopulmonary Dysplasia and Associated Pulmonary Hypertension. Pulm. Circ. 2012, 2, 170–181. [Google Scholar] [CrossRef]
- Curley, G.F.; Hayes, M.; Ansari, B.; Shaw, G.; Ryan, A.; Barry, F.; O’Brien, T.; O’Toole, D.; Laffey, J.G. Mesenchymal stem cells enhance recovery and repair following ventilator-induced lung injury in the rat. Thorax 2012, 67, 496–501. [Google Scholar] [CrossRef]
- Pierro, M.; Ionescu, L.; Montemurro, T.; Vadivel, A.; Weissmann, G.; Oudit, G.; Emery, D.; Bodiga, S.; Eaton, F.; Péault, B.; et al. Short-term, long-term and paracrine effect of human umbilical cord-derived stem cells in lung injury prevention and repair in experimental bronchopulmonary dysplasia. Thorax 2013, 68, 475–484. [Google Scholar] [CrossRef]
- Willis, G.R.; Fernandez-Gonzalez, A.; Anastas, J.; Vitali, S.H.; Liu, X.; Ericsson, M.; Kwong, A.; Mitsialis, S.A.; Kourembanas, S. Mesenchymal stromal cell exosomes ameliorate experimental bronchopulmonary dysplasia and restore lung function through macrophage immunomodulation. Am. J. Respir. Crit. Care Med. 2018, 197, 104–116. [Google Scholar] [CrossRef]
- Prockop, D.J. Defining the probability that a cell therapy will produce a malignancy. Mol. Ther. 2010, 18, 1249–1250. [Google Scholar] [CrossRef]
- Lee, C.; Ph, D.; Mitsialis, S.A.; Aslam, M.; Vitali, S.H.; Vergadi, E.; Konstantinou, G.; Sdrimas, K.; Fernandez-Gonzalez, A.; Kourembanas, S. Exosomes mediate the cytoprotective action of mesenchymal stromal cells on hypoxia-induced pulmonary hypertension. Circulation 2012, 126, 2601–2611. [Google Scholar] [CrossRef]
- Chaubey, S.; Thueson, S.; Ponnalagu, D.; Alam, M.A.; Gheorghe, C.P.; Aghai, Z.; Singh, H.; Bhandari, V. Early gestational mesenchymal stem cell secretome attenuates experimental bronchopulmonary dysplasia in part via exosome-associated factor TSG-6. Stem Cell Res. Ther. 2018, 9, 1–26. [Google Scholar] [CrossRef]
- Braun, R.K.; Chetty, C.; Balasubramaniam, V.; Centanni, R.; Haraldsdottir, K.; Hematti, P.; Eldridge, M.W. Intraperitoneal injection of MSC-derived exosomes prevent experimental bronchopulmonary dysplasia. Biochem. Biophys. Res. Commun. 2018, 503, 2653–2658. [Google Scholar] [CrossRef]
- Ahn, S.Y.; Park, W.S.; Kim, Y.E.; Sung, D.K.; Sung, S.I.; Ahn, J.Y.; Chang, Y.S. Vascular endothelial growth factor mediates the therapeutic efficacy of mesenchymal stem cell-derived extracellular vesicles against neonatal hyperoxic lung injury. Exp. Mol. Med. 2018, 50, 26. [Google Scholar] [CrossRef]
- Neu, J.; Walker, W.A. Necrotizing enterocolitis. N. Engl. J. Med. 2011, 364, 255–264. [Google Scholar] [CrossRef]
- Zani, A.; Pierro, A. Necrotizing enterocolitis: Controversies and challenges. F1000Res 2015, 4. [Google Scholar] [CrossRef]
- Mutanen, A.; Pierro, A.; Zani, A. Perioperative Complications Following Surgery for Necrotizing Enterocolitis. Eur. J. Pediatr. Surg. 2018, 28, 148–151. [Google Scholar] [CrossRef]
- Biouss, G.; Antounians, L.; Li, B.; O’Connell, J.S.; Seo, S.; Catania, V.D.; Guadagno, J.; Rahman, A.; Zani-Ruttenstock, E.; Svergun, N.; et al. Experimental necrotizing enterocolitis induces neuroinflammation in the neonatal brain. J. Neuroinflammation 2019, 16, 97. [Google Scholar] [CrossRef]
- Lee, J.H. An update on necrotizing enterocolitis: Pathogenesis and preventive strategies. Korean J. Pediatr. 2011, 54, 368–372. [Google Scholar] [CrossRef][Green Version]
- Hunter, C.J.; Upperman, J.S.; Ford, H.R.; Camerini, V. Understanding the susceptibility of the premature infant to necrotizing enterocolitis (NEC). Pediatr. Res. 2008, 63, 117–123. [Google Scholar] [CrossRef]
- Eaton, S.; Zani, A.; Pierro, A.; De Coppi, P. Stem cells as a potential therapy for necrotizing enterocolitis. Expert Opin. Biol. Ther. 2013, 13, 1683–1689. [Google Scholar] [CrossRef]
- Yang, J.; Su, Y.; Besner, G.E. Stem cell therapy for necrotizing enterocolitis: Innovative techniques and procedures for pediatric translational research. Methods Mol. Biol. 2014, 1213, 121–137. [Google Scholar] [CrossRef]
- Drucker, N.A.; McCulloh, C.J.; Li, B.; Pierro, A.; Besner, G.E.; Markel, T.A. Stem cell therapy in necrotizing enterocolitis: Current state and future directions. Semin. Pediatr. Surg. 2018, 27, 57–64. [Google Scholar] [CrossRef]
- Rager, T.M.; Olson, J.K.; Zhou, Y.; Wang, Y.; Besner, G.E. Exosomes secreted from bone marrow-derived mesenchymal stem cells protect the intestines from experimental necrotizing enterocolitis. J. Pediatr. Surg. 2016, 51, 942–947. [Google Scholar] [CrossRef]
- McCulloh, C.J.; Olson, J.K.; Wang, Y.; Zhou, Y.; Tengberg, N.H.; Deshpande, S.; Besner, G.E. Treatment of experimental necrotizing enterocolitis with stem cell-derived exosomes. J. Pediatr. Surg. 2018, 53, 1215–1220. [Google Scholar] [CrossRef]
- Antounians, L.; Tzanetakis, A.; Pellerito, O.; Catania, V.D.; Sulistyo, A.; Montalva, L.; McVey, M.J.; Zani, A. The Regenerative Potential of Amniotic Fluid Stem Cell Extracellular Vesicles: Lessons Learned by Comparing Different Isolation Techniques. Sci. Rep. 2019, 9, 1837. [Google Scholar] [CrossRef]
- Quigley, M.; Embleton, N.D.; McGuire, W. Formula versus donor breast milk for feeding preterm or low birth weight infants. Cochrane Database Syst. Rev. 2018, 6, CD002971. [Google Scholar] [CrossRef]
- Hock, A.; Miyake, H.; Li, B.; Lee, C.; Ermini, L.; Koike, Y.; Chen, Y.; Määttänen, P.; Zani, A.; Pierro, A. Breast milk-derived exosomes promote intestinal epithelial cell growth. J. Pediatr. Surg. 2017, 52, 755–759. [Google Scholar] [CrossRef]
- Martin, C.; Patel, M.; Williams, S.; Arora, H.; Sims, B. Human breast milk-derived exosomes attenuate cell death in intestinal epithelial cells. Innate Immun. 2018, 24, 278–284. [Google Scholar] [CrossRef]
- Chen, T.; Xie, M.-Y.; Sun, J.-J.; Ye, R.-S.; Cheng, X.; Sun, R.-P.; Wei, L.-M.; Li, M.; Lin, D.-L.; Jiang, Q.-Y.; et al. Porcine milk-derived exosomes promote proliferation of intestinal epithelial cells. Sci. Rep. 2016, 6, 33862. [Google Scholar] [CrossRef]
- Wang, X.; Yan, X.; Zhang, L.; Cai, J.; Zhou, Y.; Liu, H.; Hu, Y.; Chen, W.; Xu, S.; Liu, P.; et al. Identification and peptidomic profiling of exosomes in preterm human milk: Insights into necrotizing enterocolitis prevention. Mol. Nutr. Food Res. 2019, 63, e1801247. [Google Scholar] [CrossRef]
- Li, B.; Hock, A.; Wu, R.Y.; Minich, A.; Botts, S.R.; Lee, C.; Antounians, L.; Miyake, H.; Koike, Y.; Chen, Y.; et al. Bovine milk-derived exosomes enhance goblet cell activity and prevent the development of experimental necrotizing enterocolitis. PLoS ONE 2019, 14, e0211431. [Google Scholar] [CrossRef]
- Clark, J.A.; Doelle, S.M.; Halpern, M.D.; Saunders, T.A.; Holubec, H.; Dvorak, K.; Boitano, S.A.; Dvorak, B. Intestinal barrier failure during experimental necrotizing enterocolitis: Protective effect of EGF treatment. Am. J. Physiol. Gastrointest Liver Physiol. 2006, 291, G938–G949. [Google Scholar] [CrossRef]
- Thakur, B.K.; Zhang, H.; Becker, A.; Matei, I.; Huang, Y.; Costa-Silva, B.; Zheng, Y.; Hoshino, A.; Brazier, H.; Xiang, J.; et al. Double-stranded DNA in exosomes: A novel biomarker in cancer detection. Cell Res. 2014, 24, 766–769. [Google Scholar] [CrossRef]
- Kahlert, C.; Melo, S.A.; Protopopov, A.; Tang, J.; Seth, S.; Koch, M.; Zhang, J.; Weitz, J.; Chin, L.; Futreal, A.; et al. Identification of double-stranded genomic DNA spanning all chromosomes with mutated KRAS and p53 DNA in the serum exosomes of patients with pancreatic cancer. J. Biol. Chem. 2014, 289, 3869–3875. [Google Scholar] [CrossRef]
- Takahashi, A.; Okada, R.; Nagao, K.; Kawamata, Y.; Hanyu, A.; Yoshimoto, S.; Takasugi, M.; Watanabe, S.; Kanemaki, M.T.; Obuse, C.; et al. Exosomes maintain cellular homeostasis by excreting harmful DNA from cells. Nat. Commun. 2017, 8, 15287. [Google Scholar] [CrossRef]
- Willis, G.R.; Kourembanas, S.; Mitsialis, S.A. Toward Exosome-Based Therapeutics: Isolation, Heterogeneity, and Fit-for-Purpose Potency. Front. Cardiovasc. Med. 2017, 4, 63. [Google Scholar] [CrossRef]
- Takahashi, Y.; Nishikawa, M.; Shinotsuka, H.; Matsui, Y.; Ohara, S.; Imai, T.; Takakura, Y. Visualization and in vivo tracking of the exosomes of murine melanoma B16-BL6 cells in mice after intravenous injection. J. Biotechnol. 2013, 165, 77–84. [Google Scholar] [CrossRef]
- Sjoqvist, S.; Ishikawa, T.; Shimura, D.; Kasai, Y.; Imafuku, A.; Bou-Ghannam, S.; Iwata, T.; Kanai, N. Exosomes derived from clinical-grade oral mucosal epithelial cell sheets promote wound healing. J. Extracell Vesicles 2019, 8, 1565264. [Google Scholar] [CrossRef]
- Aliotta, J.M.; Pereira, M.; Wen, S.; Dooner, M.S.; Del Tatto, M.; Papa, E.; Goldberg, L.R.; Baird, G.L.; Ventetuolo, C.E.; Quesenberry, P.J.; et al. Exosomes induce and reverse monocrotaline-induced pulmonary hypertension in mice. Cardiovasc. Res. 2016, 110, 319–330. [Google Scholar] [CrossRef]
- Zhu, X.; Badawi, M.; Pomeroy, S.; Sutaria, D.S.; Xie, Z.; Baek, A.; Jiang, J.; Elgamal, O.A.; Mo, X.; Perle, K.; et al. Comprehensive toxicity and immunogenicity studies reveal minimal effects in mice following sustained dosing of extracellular vesicles derived from HEK293T cells. J. Extracell Vesicles 2017, 6, 1324730. [Google Scholar] [CrossRef]
- Mendt, M.; Kamerkar, S.; Sugimoto, H.; McAndrews, K.M.; Wu, C.-C.; Gagea, M.; Yang, S.; Blanko, E.V.R.; Peng, Q.; Ma, X.; et al. Generation and testing of clinical-grade exosomes for pancreatic cancer. JCI Insight 2018, 3, 99263. [Google Scholar] [CrossRef]
- Webber, J.; Clayton, A. How pure are your vesicles? J. Extracell Vesicles 2013, 2. [Google Scholar] [CrossRef]
- Abramowicz, A.; Widlak, P.; Pietrowska, M. Proteomic analysis of exosomal cargo: The challenge of high purity vesicle isolation. Mol. Biosyst. 2016, 12, 1407–1419. [Google Scholar] [CrossRef]
- Takov, K.; Yellon, D.M.; Davidson, S.M. Comparison of small extracellular vesicles isolated from plasma by ultracentrifugation or size-exclusion chromatography: Yield, purity and functional potential. J. Extracell Vesicles 2019, 8, 1560809. [Google Scholar] [CrossRef]
- Gheinani, A.H.; Vogeli, M.; Baumgartner, U.; Vassella, E.; Draeger, A.; Burkhard, F.C.; Monastyrskaya, K. Improved isolation strategies to increase the yield and purity of human urinary exosomes for biomarker discovery. Sci. Rep. 2018, 8, 3945. [Google Scholar] [CrossRef]
- Li, P.; Kaslan, M.; Lee, S.H.; Yao, J.; Gao, Z. Progress in Exosome Isolation Techniques. Theranostics 2017, 7, 789–804. [Google Scholar] [CrossRef]
- Lobb, R.J.; Becker, M.; Wen, S.W.; Wong, C.S.F.; Wiegmans, A.P.; Leimgruber, A.; Moller, A. Optimized exosome isolation protocol for cell culture supernatant and human plasma. J. Extracell Vesicles 2015, 4, 27031. [Google Scholar] [CrossRef]
- Helwa, I.; Cai, J.; Drewry, M.D.; Zimmerman, A.; Dinkins, M.B.; Khaled, M.L.; Seremwe, M.; Dismuke, W.M.; Bieberich, E.; Stamer, W.D.; et al. A Comparative Study of Serum Exosome Isolation Using Differential Ultracentrifugation and Three Commercial Reagents. PLoS ONE 2017, 12, e0170628. [Google Scholar] [CrossRef]
- Konoshenko, M.Y.; Lekchnov, E.A.; Vlassov, A.V.; Laktionov, P.P. Isolation of Extracellular Vesicles: General Methodologies and Latest Trends. Biomed. Res. Int. 2018, 2018, 8545347. [Google Scholar] [CrossRef]
- Patel, G.K.; Khan, M.A.; Zubair, H.; Srivastava, S.K.; Khushman, M.; Singh, S.; Singh, A.P. Comparative analysis of exosome isolation methods using culture supernatant for optimum yield, purity and downstream applications. Sci. Rep. 2019, 9, 5335. [Google Scholar] [CrossRef]
- Tassew, N.G.; Charish, J.; Shabanzadeh, A.P.; Luga, V.; Harada, H.; Farhani, N.; D’Onofrio, P.; Choi, B.; Ellabban, A.; Nickerson, P.E.B.; et al. Exosomes Mediate Mobilization of Autocrine Wnt10b to Promote Axonal Regeneration in the Injured CNS. Cell Rep. 2017, 20, 99–111. [Google Scholar] [CrossRef]
- Tabak, S.; Schreiber-Avissar, S.; Beit-Yannai, E. Extracellular vesicles have variable dose-dependent effects on cultured draining cells in the eye. J. Cell. Mol. Med. 2018, 22, 1992–2000. [Google Scholar] [CrossRef]
- Lener, T.; Gimona, M.; Aigner, L.; Borger, V.; Buzas, E.; Camussi, G.; Chaput, N.; Chatterjee, D.; Court, F.A.; Del Portillo, H.A.; et al. Applying extracellular vesicles based therapeutics in clinical trials—An ISEV position paper. J. Extracell Vesicles 2015, 4, 30087. [Google Scholar] [CrossRef]
- Rohde, E.; Pachler, K.; Gimona, M. Manufacturing and characterization of extracellular vesicles from umbilical cord-derived mesenchymal stromal cells for clinical testing. Cytotherapy 2019, 21, 581–592. [Google Scholar] [CrossRef]
- Gimona, M.; Pachler, K.; Laner-Plamberger, S.; Schallmoser, K.; Rohde, E. Manufacturing of Human Extracellular Vesicle-Based Therapeutics for Clinical Use. Int. J. Mol. Sci. 2017, 18, 1190. [Google Scholar] [CrossRef]
- Ayers, L.; Pink, R.; Carter, D.R.F.; Nieuwland, R. Clinical requirements for extracellular vesicle assays. J. Extracell Vesicles 2019, 8, 1593755. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).