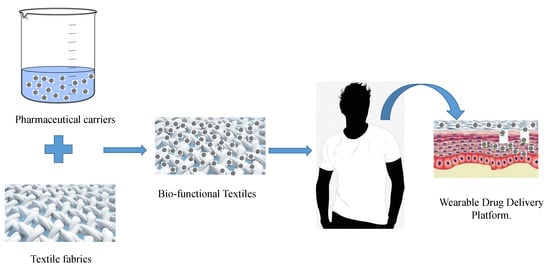
Bio-Functional Textiles: Combining Pharmaceutical Nanocarriers with Fibrous Materials for Innovative Dermatological Therapies
Abstract
1. Introduction
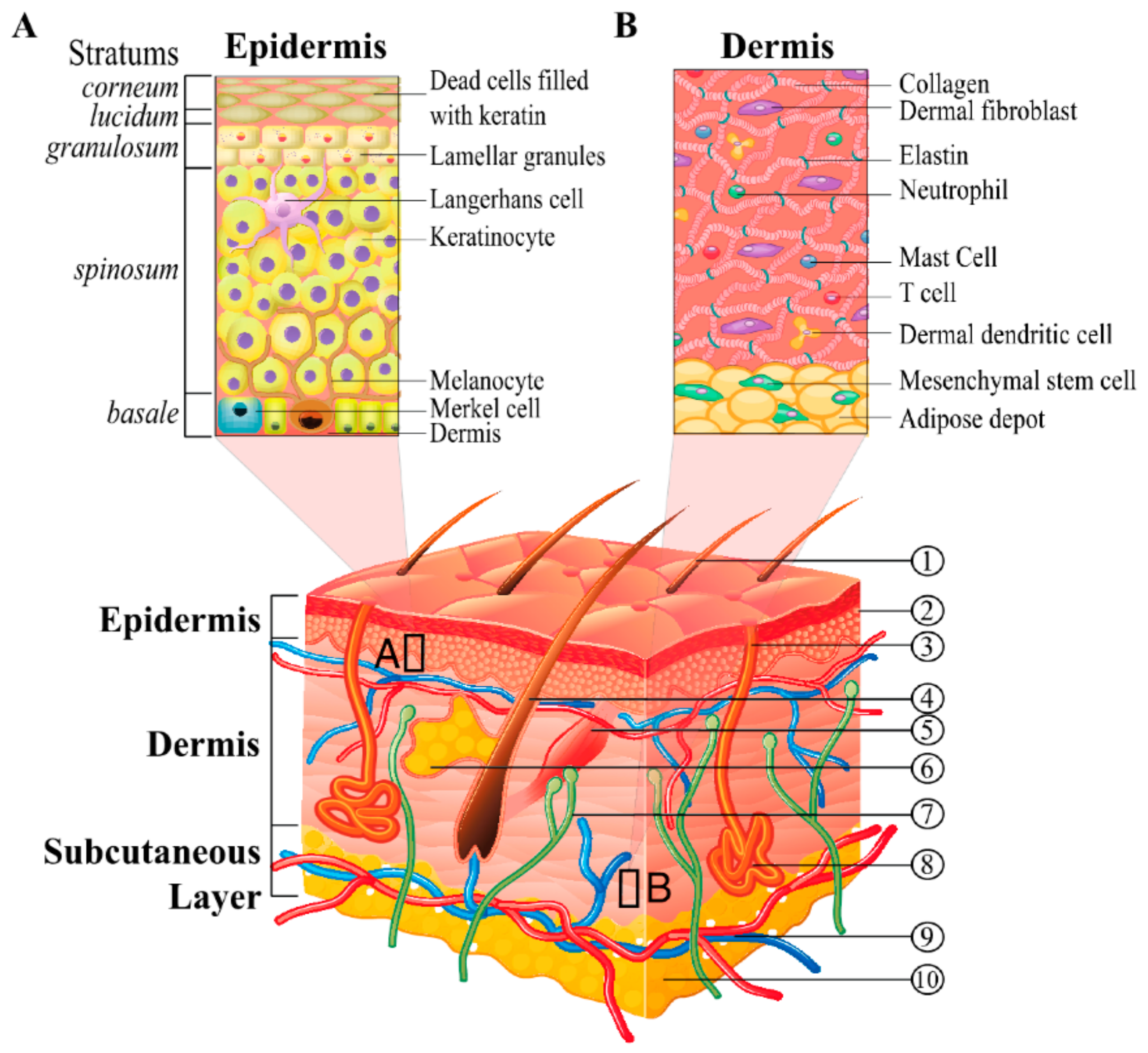
2. Nature and Physiology of the Skin
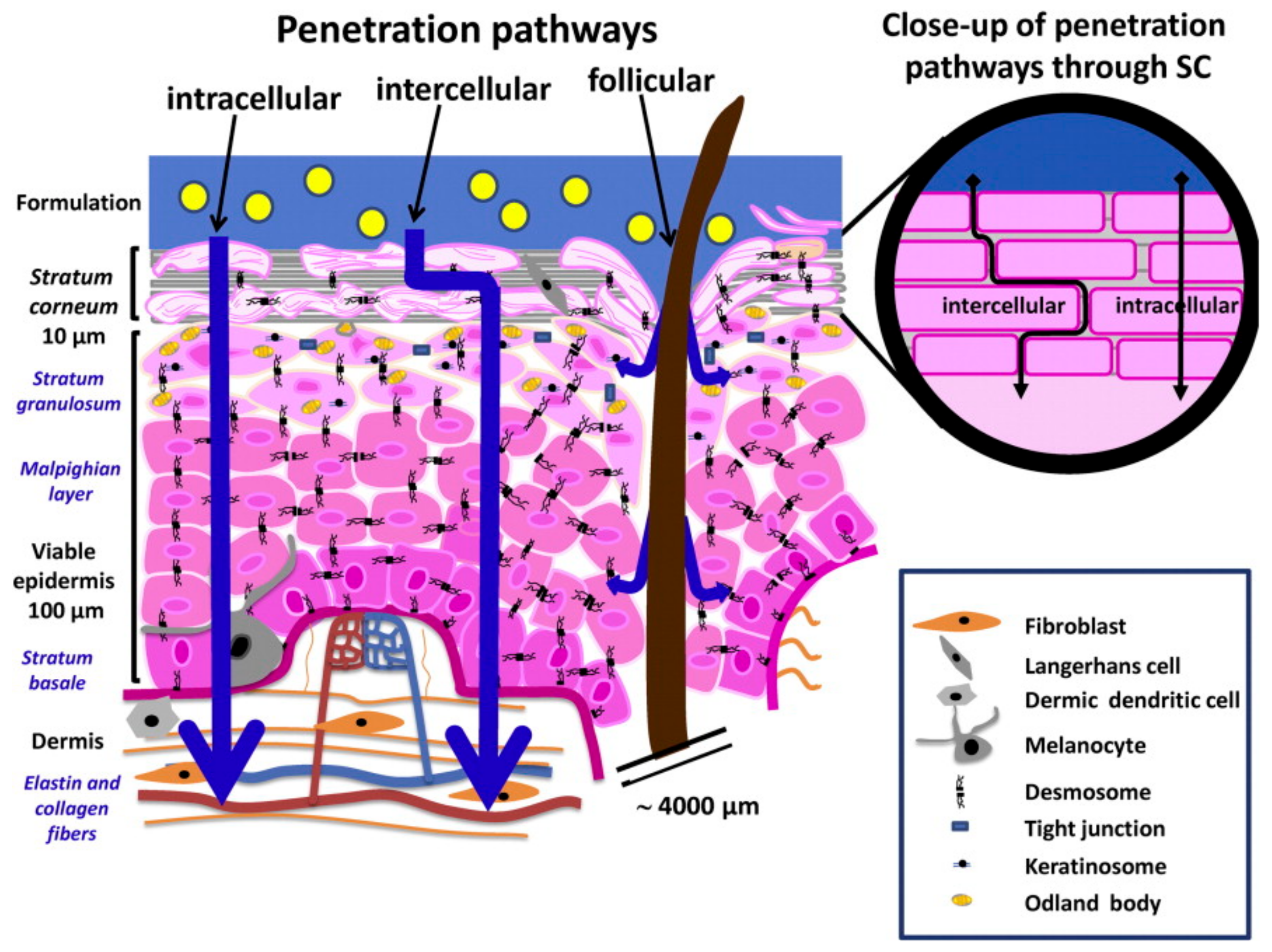
3. Skin Interaction with Drugs and Current Challenges in Dermatological Delivery
4. Pharmaceutical Nanocarriers for Dermatological Applications
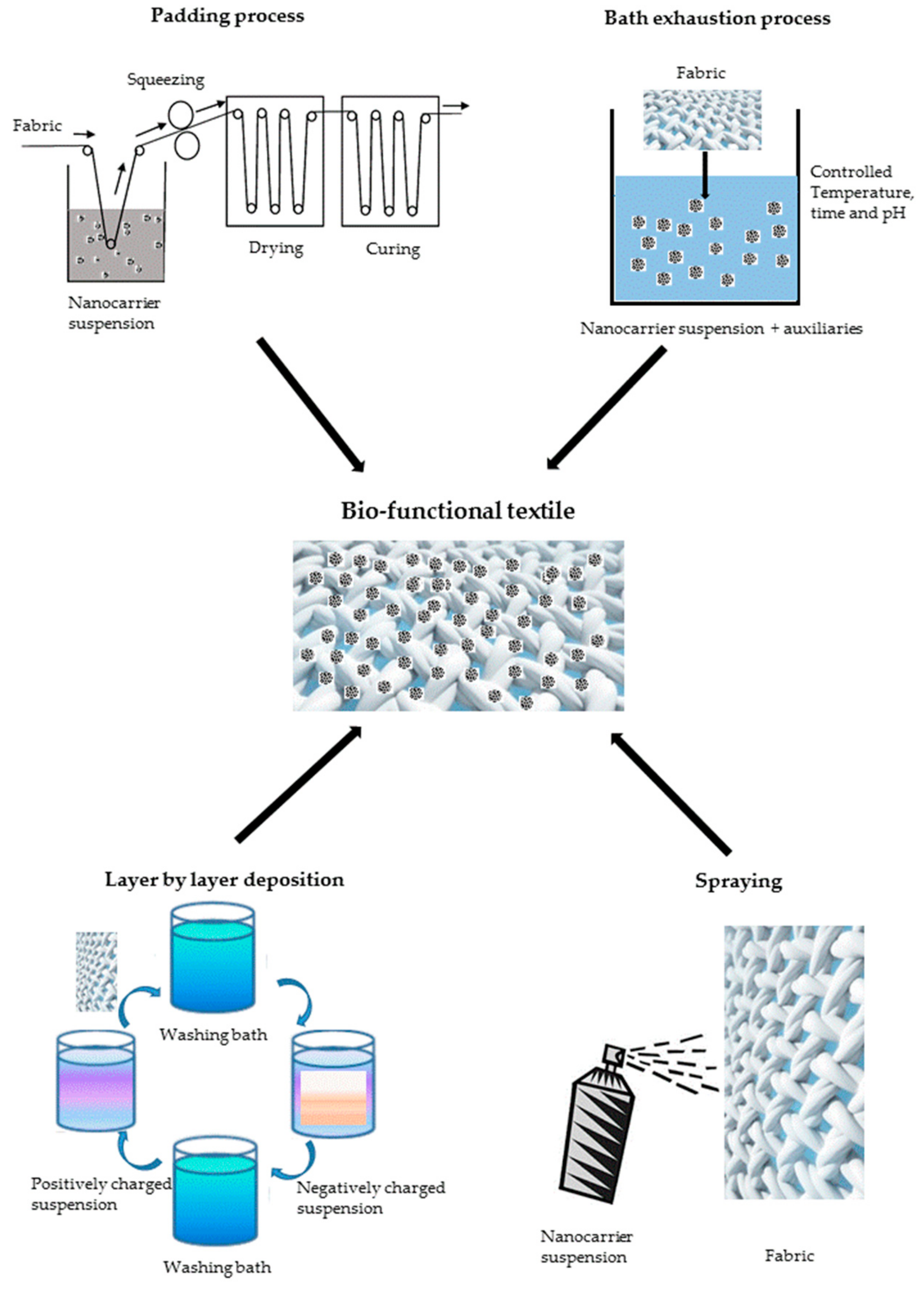
5. Bio-Functional Textiles
6. Bio-Functional Textiles and Other Dermatological Delivery Technologies
7. Regulatory Status
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rawal, S.; Patel, M.M. Threatening cancer with nanoparticle aided combination oncotherapy. J. Control. Release 2019, 301, 76–109. [Google Scholar] [CrossRef] [PubMed]
- Bruneau, M.; Bennici, S.; Brendle, J.; Dutournie, P.; Limousy, L.; Pluchon, S. Systems for stimuli-controlled release: Materials and applications. J. Control. Release 2019, 294, 355–371. [Google Scholar] [CrossRef] [PubMed]
- Leone, F.; Cavalli, R. Drug nanosuspensions: A ZIP tool between traditional and innovative pharmaceutical formulations. Expert Opin. Drug Deliv. 2015, 12, 1607–1625. [Google Scholar] [CrossRef] [PubMed]
- Donalisio, M.; Leone, F.; Civra, A.; Spagnolo, R.; Ozer, O.; Lembo, D.; Cavalli, R. Acyclovir-loaded chitosan nanospheres from nano-emulsion templating for the topical treatment of herpesviruses infections. Pharmaceutics 2018, 10, 46. [Google Scholar] [CrossRef] [PubMed]
- Bento da Silva, P.; Fioramonti Calixto, G.; Oshiro, J., Jr.; Bombardelli, R.; Fonseca-Santos, B.; Rodero, C.; Chorilli, M. Structural features and the anti-inflammatory effect of green tea extract-loaded liquid crystalline systems intended for skin delivery. Polymers 2017, 9, 30. [Google Scholar] [CrossRef] [PubMed]
- Alkilani, A.; McCrudden, M.T.; Donnelly, R. Transdermal Drug Delivery: Innovative pharmaceutical developments based on disruption of the barrier properties of the stratum corneum. Pharmaceutics 2015, 7, 438–470. [Google Scholar] [CrossRef] [PubMed]
- Goyal, R.; Macri, L.K.; Kaplan, H.M.; Kohn, J. Nanoparticles and nanofibers for topical drug delivery. J. Control. Release 2016, 240, 77–92. [Google Scholar] [CrossRef] [PubMed]
- Prow, T.W.; Grice, J.E.; Lin, L.L.; Faye, R.; Butler, M.; Becker, W.; Wurm, E.M.T.; Yoong, C.; Robertson, T.A.; Soyer, H.P.; et al. Nanoparticles and microparticles for skin drug delivery. Adv. Drug Deliv. Rev. 2011, 63, 470–491. [Google Scholar] [CrossRef] [PubMed]
- Sgorbini, B.; Cagliero, C.; Argenziano, M.; Cavalli, R.; Bicchi, C.; Rubiolo, P. In vitro release and permeation kinetics of Melaleuca alternifolia (tea tree) essential oil bioactive compounds from topical formulations. Flavour Fragr. J. 2017, 32, 354–361. [Google Scholar] [CrossRef]
- Roberts, M.; Mohammed, Y.; Pastore, M.; Namjoshi, S.; Yousef, S.; Alinaghi, A.; Haridass, I.; Abd, E.; Leite-Silva, V.; Benson, H.; et al. Topical and cutaneous delivery using nanosystems. J. Control. Release 2017, 247, 86–105. [Google Scholar] [CrossRef] [PubMed]
- Al-Hashimi, N.; Begg, N.; Alany, R.; Hassanin, H.; Elshaer, A. Oral modified release multiple-unit particulate systems: Compressed pellets, microparticles and nanoparticles. Pharmaceutics 2018, 10, 176. [Google Scholar] [CrossRef] [PubMed]
- Rigon, R.; Fachinetti, N.; Severino, P.; Santana, M.; Chorilli, M. Skin delivery and in vitro biological evaluation of trans-resveratrol-loaded solid lipid nanoparticles for skin disorder therapies. Molecules 2016, 21, 116. [Google Scholar] [CrossRef] [PubMed]
- Palmer, B.; DeLouise, L. Nanoparticle-enabled transdermal drug delivery systems for enhanced dose control and tissue targeting. Molecules 2016, 21, 1719. [Google Scholar] [CrossRef] [PubMed]
- Chuang, S.-Y.; Lin, Y.-K.; Lin, C.-F.; Wang, P.-W.; Chen, E.-L.; Fang, J.-Y. Elucidating the skin delivery of aglycone and glycoside flavonoids: How the structures affect cutaneous absorption. Nutrients 2017, 9, 1304. [Google Scholar] [CrossRef] [PubMed]
- Calatayud-Pascual, M.; Sebastian-Morelló, M.; Balaguer-Fernández, C.; Delgado-Charro, M.; López-Castellano, A.; Merino, V. Influence of chemical enhancers and iontophoresis on the in vitro transdermal permeation of propranolol: Evaluation by dermatopharmacokinetics. Pharmaceutics 2018, 10, 265. [Google Scholar] [CrossRef] [PubMed]
- Sidat, Z.; Marimuthu, T.; Kumar, P.; du Toit, L.C.; Kondiah, P.P.D.; Choonara, Y.E.; Pillay, V. Ionic liquids as potential and synergistic permeation enhancers for transdermal drug delivery. Pharmaceutics 2019, 11, 96. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.; Banga, A. Electrically and ultrasonically enhanced transdermal delivery of methotrexate. Pharmaceutics 2018, 10, 117. [Google Scholar] [CrossRef]
- Nisbet, S.J. Absence of human skin irritation and allergenic potential after repeated patch applications of a lamellar moisturizer. J. Cosmet. Derm. 2019, 18, 377–382. [Google Scholar] [CrossRef]
- Kulkarni, M.; Hastak, V.; Jadhav, S. Development and characterization of transdermal delivery system of doxazosin mesylate. Int. J. Appl. Pharm. 2019, 11, 43–48. [Google Scholar] [CrossRef]
- Abd, E.; Benson, H.; Roberts, M.; Grice, J. Minoxidil skin delivery from nanoemulsion formulations containing eucalyptol or oleic acid: Enhanced diffusivity and follicular targeting. Pharmaceutics 2018, 10, 19. [Google Scholar] [CrossRef]
- Nigro, A.; Pellegrino, M.; Greco, M.; Comandè, A.; Sisci, D.; Pasqua, L.; Leggio, A.; Morelli, C. Dealing with skin and blood-brain barriers: The unconventional challenges of mesoporous silica nanoparticles. Pharmaceutics 2018, 10, 250. [Google Scholar] [CrossRef] [PubMed]
- Hipler, U.-C.; Elsner, P. Current Problems in Dermatology. In Biofunctional Textiles and the Skin; Karger AG: Basel, Switzerland, 2006; Volume 33, ISBN 978-3-8055-8121-9. [Google Scholar]
- Zallmann, M.; Smith, P.; Tang, M.; Spelman, L.; Cahill, J.; Wortmann, G.; Katelaris, C.; Allen, K.; Su, J. Debunking the myth of wool allergy: Reviewing the evidence for immune and non-immune cutaneous reactions. Acta Derm. Venereol. 2017, 97, 906–915. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.; Schleusener, J.; Knorr, F.; Kraft, M.; Thiede, G.; Richter, H.; Darvin, M.E.; Schanzer, S.; Gallinger, S.; Wegener, U.; et al. Influence of polyester spacer fabric, cotton, chloroprene rubber, and silicone on microclimatic and morphologic physiologic skin parameters in vivo. Skin Res. Technol. 2019, 25, 389–398. [Google Scholar] [CrossRef]
- McCarthy, B.J. An overview of the technical textiles sector. In Handbook of Technical Textiles; Elsevier: Amsterdam, The Netherlands, 2016; pp. 1–20. ISBN 978-1-78242-458-1. [Google Scholar]
- Mather, R.; Wilson, J. Fabrication of photovoltaic textiles. Coatings 2017, 7, 63. [Google Scholar] [CrossRef]
- Fernández-Caramés, T.; Fraga-Lamas, P. Towards the internet-of-smart-clothing: A review on IOT wearables and garments for creating intelligent connected E-Textiles. Electronics 2018, 7, 405. [Google Scholar] [CrossRef]
- Roudjane, M.; Khalil, M.; Miled, A.; Messaddeq, Y. New generation wearable antenna based on multimaterial fiber for wireless communication and real-time breath detection. Photonics 2018, 5, 33. [Google Scholar] [CrossRef]
- Lavagna, L.; Massella, D.; Pantano, M.F.; Bosia, F.; Pugno, N.M.; Pavese, M. Grafting carbon nanotubes onto carbon fibres doubles their effective strength and the toughness of the composite. Compos. Sci. Technol. 2018, 146, 140–149. [Google Scholar] [CrossRef]
- Islam, M.S.; Deng, Y.; Tong, L.; Faisal, S.N.; Roy, A.K.; Minett, A.I.; Gomes, V.G. Grafting carbon nanotubes directly onto carbon fibers for superior mechanical stability: Towards next generation aerospace composites and energy storage applications. Carbon 2016, 96, 701–710. [Google Scholar] [CrossRef]
- Lavagna, L.; Massella, D.; Pavese, M. Preparation of hierarchical material by chemical grafting of carbon nanotubes onto carbon fibers. Diam. Relat. Mater. 2017, 80, 118–124. [Google Scholar] [CrossRef]
- Lavagna, L.; Musso, S.; Ferro, G.; Pavese, M. Cement-based composites containing functionalized carbon fibers. Cem. Concr. Compos. 2018, 88, 165–171. [Google Scholar] [CrossRef]
- Cui, H.; Jin, Z.; Zheng, D.; Tang, W.; Li, Y.; Yun, Y.; Lo, T.Y.; Xing, F. Effect of carbon fibers grafted with carbon nanotubes on mechanical properties of cement-based composites. Constr. Build. Mater. 2018, 181, 713–720. [Google Scholar] [CrossRef]
- Malucelli, G. Surface-engineered fire protective coatings for fabrics through sol-gel and layer-by-layer methods: An overview. Coatings 2016, 6, 33. [Google Scholar] [CrossRef]
- Abtew, M.A.; Bruniaux, P.; Boussu, F.; Loghin, C.; Cristian, I.; Chen, Y.; Wang, L. A systematic pattern generation system for manufacturing customized seamless multi-layer female soft body armour through dome-formation (moulding) techniques using 3D warp interlock fabrics. J. Manuf. Syst. 2018, 49, 61–74. [Google Scholar] [CrossRef]
- Self-sterilizing sputtered films for applications in hospital facilities. Molecules 2017, 22, 1074. [CrossRef] [PubMed]
- Functionalized textile based therapy for the treatment of atopic dermatitis. Coatings 2017, 7, 82. [CrossRef]
- Morais, D.; Guedes, R.; Lopes, M. Antimicrobial approaches for textiles: From research to market. Materials 2016, 9, 498. [Google Scholar] [CrossRef] [PubMed]
- Fiedot-Toboła, M.; Ciesielska, M.; Maliszewska, I.; Rac-Rumijowska, O.; Suchorska-Woźniak, P.; Teterycz, H.; Bryjak, M. Deposition of zinc oxide on different polymer textiles and their antibacterial properties. Materials 2018, 11, 707. [Google Scholar] [CrossRef]
- Bezerra, F.M.; Carmona, O.G.; Carmona, C.G.; Lis, M.J.; de Moraes, F.F. Controlled release of microencapsulated citronella essential oil on cotton and polyester matrices. Cellulose 2016, 23, 1459–1470. [Google Scholar] [CrossRef]
- Peila, R.; Scordino, P.; Shanko, D.B.; Caldera, F.; Trotta, F.; Ferri, A. Synthesis and characterization of β-cyclodextrin nanosponges for N,N-diethyl-meta-toluamide complexation and their application on polyester fabrics. React. Funct. Polym. 2017, 119, 87–94. [Google Scholar] [CrossRef]
- Alonso, C.; Martí, M.; Martínez, V.; Rubio, L.; Parra, J.L.; Coderch, L. Antioxidant cosmeto-textiles: Skin assessment. Eur. J. Pharm. Biopharm. 2013, 84, 192–199. [Google Scholar] [CrossRef]
- Zemljič, L.F.; Peršin, Z.; Šauperl, O.; Rudolf, A.; Kostić, M. Medical textiles based on viscose rayon fabrics coated with chitosan-encapsulated iodine: Antibacterial and antioxidant properties. Text. Res. J. 2017, 004051751772511. [Google Scholar] [CrossRef]
- Alonso, C.; Martí, M.; Barba, C.; Lis, M.; Rubio, L.; Coderch, L. Skin penetration and antioxidant effect of cosmeto-textiles with gallic acid. J. Photochem. Photobiol. 2016, 156, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Martí, M.; Martínez, V.; Lis, M.J.; Valldeperas, J.; de la Maza, A.; Parra, J.L.; Coderch, L. Gallic acid vehiculized through liposomes or mixed micelles in biofunctional textiles. J. Text. Inst. 2014, 105, 175–186. [Google Scholar] [CrossRef]
- Amjadi, M.; Sheykhansari, S.; Nelson, B.J.; Sitti, M. Recent advances in wearable transdermal delivery systems. Adv. Mater. 2018, 30, 1704530. [Google Scholar] [CrossRef] [PubMed]
- Lis Arias, M.; Coderch, L.; Martí, M.; Alonso, C.; García Carmona, O.; García Carmona, C.; Maesta, F. Vehiculation of active principles as a way to create smart and biofunctional textiles. Materials 2018, 11, 2152. [Google Scholar] [CrossRef] [PubMed]
- Pinho, E.; Soares, G. Functionalization of cotton cellulose for improved wound healing. J. Mater. Chem. B 2018, 6, 1887–1898. [Google Scholar] [CrossRef]
- Wysocki, A.B. Skin anatomy, physiology, and pathophysiology. Nurs. Clin. N. Am. 1999, 34, 777–797. [Google Scholar]
- Zhang, Z.; Michniak-Kohn, B.B. Tissue engineered human skin equivalents. Pharmaceutics 2012, 4, 26–41. [Google Scholar] [CrossRef]
- Suñer-Carbó, J.; Calpena-Campmany, A.; Halbaut-Bellowa, L.; Clares-Naveros, B.; Rodriguez-Lagunas, M.; Barbolini, E.; Zamarbide-Losada, J.; Boix-Montañés, A. Biopharmaceutical development of a bifonazole multiple emulsion for enhanced epidermal delivery. Pharmaceutics 2019, 11, 66. [Google Scholar] [CrossRef]
- Rincón, M.; Calpena, A.; Fabrega, M.-J.; Garduño-Ramírez, M.; Espina, M.; Rodríguez-Lagunas, M.; García, M.; Abrego, G. Development of pranoprofen loaded nanostructured lipid carriers to improve its release and therapeutic efficacy in skin inflammatory disorders. Nanomaterials 2018, 8, 1022. [Google Scholar] [CrossRef]
- Eckhart, L.; Lippens, S.; Tschachler, E.; Declercq, W. Cell death by cornification. Biochim. Biophys. Acta 2013, 1833, 3471–3480. [Google Scholar] [CrossRef] [PubMed]
- Van Smeden, J.; Janssens, M.; Gooris, G.S.; Bouwstra, J.A. The important role of stratum corneum lipids for the cutaneous barrier function. Biochim. Biophys. Acta 2014, 1841, 295–313. [Google Scholar] [CrossRef] [PubMed]
- Briggaman, R.A.; Wheeler, C.E. The epidermal-dermal junction. J. Investig. Derm. 1975, 65, 71–84. [Google Scholar] [CrossRef] [PubMed]
- Freeman, S.C.; Sonthalia, S. Histology, Keratohyalin granules. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2019; Available online: https://www.ncbi.nlm.nih.gov/books/NBK537049/ (accessed on 7 August 2019).
- Yousef, H.; Sharma, S. Anatomy, skin (integument), epidermis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2019; Available online: https://www.ncbi.nlm.nih.gov/books/NBK470464/ (accessed on 7 August 2019).
- Tolleson, W.H. Human melanocyte biology, toxicology, and pathology. J. Environ. Sci. Health C Environ. Carcinog. Ecotoxicol. Rev. 2005, 23, 105–161. [Google Scholar] [CrossRef] [PubMed]
- Jackson, C.J.; Tønseth, K.A.; Utheim, T.P. Cultured epidermal stem cells in regenerative medicine. Stem Cell Res. Ther. 2017, 8, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Abraham, J.; Mathew, S. Merkel Cells: A collective review of current concepts. Int. J. Appl. Basic Med. Res. 2019, 9, 9–13. [Google Scholar] [PubMed]
- Goletz, S.; Zillikens, D.; Schmidt, E. Structural proteins of the dermal-epidermal junction targeted by autoantibodies in pemphigoid diseases. Exp. Derm. 2017, 26, 1154–1162. [Google Scholar] [CrossRef] [PubMed]
- Bonifant, H.; Holloway, S. A review of the effects of ageing on skin integrity and wound healing. Br. J. Community Nurs. 2019, 24, S28–S33. [Google Scholar] [CrossRef]
- Shimizu, H.; Ishiko, A.; Masunaga, T.; Kurihara, Y.; Sato, M.; Bruckner-Tuderman, L.; Nishikawa, T. Most anchoring fibrils in human skin originate and terminate in the lamina densa. Lab. Investig. 1997, 76, 753–763. [Google Scholar]
- Arda, O.; Göksügür, N.; Tüzün, Y. Basic histological structure and functions of facial skin. Clin. Derm. 2014, 32, 3–13. [Google Scholar] [CrossRef]
- Yu, J.R.; Navarro, J.; Coburn, J.C.; Mahadik, B.; Molnar, J.; Holmes, J.H.; Nam, A.J.; Fisher, J.P. Current and future perspectives on skin tissue engineering: Key features of biomedical research, translational assessment, and clinical application. Adv. Healthc. Mater. 2019, 8, 1801471–1801490. [Google Scholar] [CrossRef] [PubMed]
- Nastiti, C.; Ponto, T.; Abd, E.; Grice, J.; Benson, H.; Roberts, M. Topical nano and microemulsions for skin delivery. Pharmaceutics 2017, 9, 37. [Google Scholar] [CrossRef] [PubMed]
- Gaur, M.; Dobke, M.; Lunyak, V. Mesenchymal stem cells from adipose tissue in clinical applications for dermatological indications and skin aging. Int. J. Mol. Sci. 2017, 18, 208. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, F.; Alves, A.C.; Nunes, C.; Sarmento, B.; Amaral, M.H.; Reis, S.; Oliveira, M.B.P.P. Permeation of topically applied caffeine from a food by—Product in cosmetic formulations: Is nanoscale in vitro approach an option? Int. J. Pharm. 2016, 513, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Leppert, W.; Malec–Milewska, M.; Zajaczkowska, R.; Wordliczek, J. Transdermal and topical drug administration in the treatment of pain. Molecules 2018, 23, 681. [Google Scholar] [CrossRef] [PubMed]
- Marto, J.; Duarte, A.; Simões, S.; Gonçalves, L.; Gouveia, L.; Almeida, A.; Ribeiro, H. Starch-based pickering emulsions as platforms for topical antibiotic delivery: In vitro and in vivo studies. Polymers 2019, 11, 108. [Google Scholar] [CrossRef]
- Tatke, A.; Dudhipala, N.; Janga, K.; Balguri, S.; Avula, B.; Jablonski, M.; Majumdar, S. In situ gel of triamcinolone acetonide-loaded solid lipid nanoparticles for improved topical ocular delivery: Tear kinetics and ocular disposition studies. Nanomaterials 2018, 9, 33. [Google Scholar] [CrossRef] [PubMed]
- Nagula, R.L.; Wairkar, S. Recent advances in topical delivery of flavonoids: A review. J. Control. Release 2019, 296, 190–201. [Google Scholar] [CrossRef]
- Simitzis, P. Agro-industrial by-products and their bioactive compounds—An ally against oxidative stress and skin aging. Cosmetics 2018, 5, 58. [Google Scholar] [CrossRef]
- Rendon, A.; Schäkel, K. Psoriasis pathogenesis and treatment. Int. J. Mol. Sci. 2019, 20, 1475. [Google Scholar] [CrossRef]
- Castellana, E.; Cavalli, R.; Mussatto, C.; Leone, F.; Crosasso, P.; Chiappetta, M.R.; Cattel, F. Sviluppo ed ottimizzazione di una emulsione topica in ambito dermatologico, il caso di una malattia rara: L’ittiosi congenita. G. Ital. Farm. Clin. 2018, 32, 108–114. [Google Scholar]
- Cantin-Warren, L.; Guignard, R.; Cortez Ghio, S.; Larouche, D.; Auger, F.; Germain, L. Specialized living wound dressing based on the self-assembly approach of tissue engineering. J. Funct. Biomater. 2018, 9, 53. [Google Scholar] [CrossRef] [PubMed]
- Johnson, T.; Gómez, B.; McIntyre, M.; Dubick, M.; Christy, R.; Nicholson, S.; Burmeister, D. The cutaneous microbiome and wounds: New molecular targets to promote wound healing. Int. J. Mol. Sci. 2018, 19, 2699. [Google Scholar] [CrossRef] [PubMed]
- Bazbouz, M.; Tronci, G. Two-layer electrospun system enabling wound exudate management and visual infection response. Sensors 2019, 19, 991. [Google Scholar] [CrossRef] [PubMed]
- Mostafalu, P.; Kiaee, G.; Giatsidis, G.; Khalilpour, A.; Nabavinia, M.; Dokmeci, M.R.; Sonkusale, S.; Orgill, D.P.; Tamayol, A.; Khademhosseini, A. A textile dressing for temporal and dosage controlled drug delivery. Adv. Funct. Mater. 2017, 27, 1702399. [Google Scholar] [CrossRef]
- Pandey, P.; Shukla, S.; Skoog, S.; Boehm, R.; Narayan, R. Current advancements in transdermal biosensing and targeted drug delivery. Sensors 2019, 19, 1028. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Kalluri, H.; Banga, A.K. Effects of chemical and physical enhancement techniques on transdermal delivery of cyanocobalamin (vitamin B12) in vitro. Pharmaceutics 2011, 3, 474–484. [Google Scholar] [CrossRef] [PubMed]
- Carreras, N.; Alonso, C.; Martí, M.; Lis, M.J. Mass transport model through the skin by microencapsulation system. J. Microencapsul. 2015, 32, 358–363. [Google Scholar] [CrossRef]
- Hadgraft, J.; Lane, M.E. Drug crystallization—Implications for topical and transdermal delivery. Expert Opin. Drug. Deliv. 2016, 13, 817–830. [Google Scholar] [CrossRef]
- Alonso, C.; Carrer, V.; Barba, C.; Coderch, L. Caffeine delivery in porcine skin: A confocal Raman study. Arch. Derm. Res. 2018, 310, 657–664. [Google Scholar] [CrossRef]
- Lademann, J.; Knorr, F.; Richter, H.; Blume-Peytavi, U.; Vogt, A.; Antoniou, C.; Sterry, W.; Patzelt, A. Hair follicles an efficient storage and penetration pathway for topically applied substances. Skin Pharm. Physiol. 2008, 21, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Otberg, N.; Richter, H.; Schaefer, H.; Blume-Peytavi, U.; Sterry, W.; Lademann, J. Variations of hair follicle size and distribution in different body sites. J. Investig. Derm. 2004, 122, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Bolzinger, M.-A.; Briançon, S.; Pelletier, J.; Chevalier, Y. Penetration of drugs through skin, a complex rate-controlling membrane. Curr. Opin. Colloid Interface Sci. 2012, 17, 156–165. [Google Scholar] [CrossRef]
- Simovic, L.; Skundric, P.; Pajic-Lijakovic, I.; Ristic, K.; Medovic, A.; Tasić, G. Mathematical model of gentamicin sulfate release from a bioactive textile material as a transdermal system under in vitro conditions. J. Appl. Polym. Sci. 2010, 117, 1424–1430. [Google Scholar]
- Simovic, L.; Skundric, P.; Baralic, A.M.; Pajic-Lijakovic, I.; Milutinovic-Nikolic, A. Characterization and behavior of anesthetic bioactive textile complex in vitro condition. J. Biomed. Mater. Res. Part. A 2012, 100A, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Amjadi, M.; Mostaghaci, B.; Sitti, M. Recent advances in skin penetration enhancers for transdermal gene and drug delivery. Curr. Gene Ther. 2017, 17, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.; Ghosh, P.; Li, S.K.; Newman, B.; Kasting, G.B.; Raney, S.G. Heat effects on drug delivery across human skin. Expert Opin. Drug Deliv. 2016, 13, 755–768. [Google Scholar] [CrossRef] [PubMed]
- Schwöbel, J.A.H.; Klamt, A. Mechanistic skin penetration model by the COSMOperm method: Routes of permeation, vehicle effects and skin variations in the healthy and compromised skin. Comput. Toxicol. 2019, 11, 50–64. [Google Scholar] [CrossRef]
- Cintra, G.; Pinto, L.; Calixto, G.; Soares, C.; Von Zuben, E.; Scarpa, M.; Gremião, M.; Chorilli, M. Bioadhesive surfactant systems for methotrexate skin delivery. Molecules 2016, 21, 231. [Google Scholar] [CrossRef] [PubMed]
- Şenyiğit, T.; Sonvico, F.; Rossi, A.; Tekmen, I.; Santi, P.; Colombo, P.; Nicoli, S.; Özer, Ö. In vivo assessment of clobetasol propionate-loaded lecithin-chitosan nanoparticles for skin delivery. Int. J. Mol. Sci. 2016, 18, 32. [Google Scholar] [CrossRef] [PubMed]
- Vitellaro-Zuccarello, L.; Cappelletti, S.; Rossi, V.D.P.; Sari-Gorla, M. Stereological analysis of collagen and elastic fibers in the normal human dermis: Variability with age, sex, and body region. Anat. Rec. 1994, 238, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Darlenski, R.; Fluhr, J.W. Influence of skin type, race, sex, and anatomic location on epidermal barrier function. Clin. Derm. 2012, 30, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Mottaleb, M.M.A.; Moulari, B.; Beduneau, A.; Pellequer, Y.; Lamprecht, A. Surface-charge-dependent nanoparticles accumulation in inflamed skin. J. Pharm. Sci. 2012, 101, 4231–4239. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xia, Q.; Li, Y.; He, Z.; Li, Z.; Guo, T.; Wu, Z.; Feng, N. CD44 Assists the topical anti-psoriatic efficacy of curcumin-loaded hyaluronan-modified ethosomes: A new strategy for clustering drug in inflammatory skin. Theranostics 2019, 9, 48–64. [Google Scholar] [CrossRef] [PubMed]
- Ita, K. Perspectives on transdermal electroporation. Pharmaceutics 2016, 8, 9. [Google Scholar] [CrossRef] [PubMed]
- Karpiński, T. Selected medicines used in iontophoresis. Pharmaceutics 2018, 10, 204. [Google Scholar] [CrossRef] [PubMed]
- Seah, B.C.-Q.; Teo, B.M. Recent advances in ultrasound-based transdermal drug delivery. Int. J. Nanomed. 2018, 13, 7749–7763. [Google Scholar] [CrossRef]
- Haj-Ahmad, R.; Khan, H.; Arshad, M.; Rasekh, M.; Hussain, A.; Walsh, S.; Li, X.; Chang, M.-W.; Ahmad, Z. Microneedle coating techniques for transdermal drug delivery. Pharmaceutics 2015, 7, 486–502. [Google Scholar] [CrossRef]
- McConville, A.; Hegarty, C.; Davis, J. Mini-review: Assessing the potential impact of microneedle technologies on home healthcare applications. Medicines 2018, 5, 50. [Google Scholar] [CrossRef]
- Kapoor, M.S.; GuhaSarkar, S.; Banerjee, R. Stratum corneum modulation by chemical enhancers and lipid nanostructures: Implications for transdermal drug delivery. Ther. Deliv. 2017, 8, 701–718. [Google Scholar] [CrossRef]
- Czajkowska-Kośnik, A.; Szekalska, M.; Winnicka, K. Nanostructured lipid carriers: A potential use for skin drug delivery systems. Pharm. Rep. 2019, 71, 156–166. [Google Scholar] [CrossRef] [PubMed]
- Nam, E.; Yoo, S.; Kim, H.-Y.; Kim, Y.-R.; Heo, Y.J. Transdermal water-in-oil nanocarriers of nitric oxide for triggering penile erection. Sci. Rep. 2018, 8, 7312–7320. [Google Scholar] [CrossRef] [PubMed]
- Salaün, F. Microencapsulation technology for smart textile coatings. In Active Coatings for Smart Textiles; Elsevier: Amsterdam, The Netherlands, 2016; pp. 179–220. ISBN 978-0-08-100263-6. [Google Scholar]
- Massella, D.; Giraud, S.; Guan, J.; Ferri, A.; Salaün, F. Manufacture techniques of chitosan-based microcapsules to enhance functional properties of textiles. In Sustainable Agriculture Reviews 35; Crini, G., Lichtfouse, E., Eds.; Springer International Publishing: Cham, Switzerland, 2019; Volume 35, pp. 303–336. ISBN 978-3-030-16537-6. [Google Scholar]
- Artusio, F.; Bazzano, M.; Pisano, R.; Coulon, P.-E.; Rizza, G.; Schiller, T.; Sangermano, M. Polymeric nanocapsules via interfacial cationic photopolymerization in miniemulsion. Polymer 2018, 139, 155–162. [Google Scholar] [CrossRef]
- Parisi, O.I.; Scrivano, L.; Sinicropi, M.S.; Puoci, F. Polymeric nanoparticle constructs as devices for antibacterial therapy. Curr. Opin. Pharm. 2017, 36, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Ancona, A.; Dumontel, B.; Garino, N.; Demarco, B.; Chatzitheodoridou, D.; Fazzini, W.; Engelke, H.; Cauda, V. Lipid-coated zinc oxide nanoparticles as innovative ros-generators for photodynamic therapy in cancer cells. Nanomaterials 2018, 8, 143. [Google Scholar] [CrossRef]
- Chen, Y.-Y.; Lu, Y.-H.; Ma, C.-H.; Tao, W.-W.; Zhu, J.-J.; Zhang, X. A novel elastic liposome for skin delivery of papain and its application on hypertrophic scar. Biomed. Pharm. 2017, 87, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.K.; Thareja, S. In vitro and in vivo characterization of pharmaceutical nanocarriers used for drug delivery. Artif. Cells Nanomed. Biotechnol. 2019, 47, 524–539. [Google Scholar] [CrossRef]
- Kumari, A.; Yadav, S.K.; Yadav, S.C. Biodegradable polymeric nanoparticles based drug delivery systems. Colloids Surf. B Biointerfaces 2010, 75, 1–18. [Google Scholar] [CrossRef]
- Bansal, M.; Jamil, S. Micellar microparticles: A novel approach to topical drug delivery system. Int. J. Appl. Pharm. 2018, 10, 1–5. [Google Scholar] [CrossRef]
- Lam, P.-L.; Lee, K.K.-H.; Wong, R.S.-M.; Cheng, G.Y.M.; Cheng, S.Y.; Yuen, M.C.-W.; Lam, K.-H.; Gambari, R.; Kok, S.H.-L.; Chui, C.-H. Development of hydrocortisone succinic acid/and 5-fluorouracil/chitosan microcapsules for oral and topical drug deliveries. Bioorg. Med. Chem. Lett. 2012, 22, 3213–3218. [Google Scholar] [CrossRef]
- AbdElhady, M.M. Preparation and characterization of chitosan/zinc oxide nanoparticles for imparting antimicrobial and UV protection to cotton fabric. Int. J. Carbohydr. Chem. 2012, 2012, 840591. [Google Scholar] [CrossRef]
- Garino, N.; Limongi, T.; Dumontel, B.; Canta, M.; Racca, L.; Laurenti, M.; Castellino, M.; Casu, A.; Falqui, A.; Cauda, V. A microwave-assisted synthesis of zinc oxide nanocrystals finely tuned for biological applications. Nanomaterials 2019, 9, 212. [Google Scholar] [CrossRef] [PubMed]
- Lalloz, A.; Bolzinger, M.-A.; Faivre, J.; Latreille, P.-L.; Garcia Ac, A.; Rakotovao, C.; Rabanel, J.-M.; Hildgen, P.; Banquy, X.; Briançon, S. Effect of surface chemistry of polymeric nanoparticles on cutaneous penetration of cholecalciferol. Int. J. Pharm. 2018, 553, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Lavino, A.D.; Di Pasquale, N.; Carbone, P.; Marchisio, D.L. A novel multiscale model for the simulation of polymer flash nano-precipitation. Chem. Eng. Sci. 2017, 171, 485–494. [Google Scholar] [CrossRef]
- Massella, D.; Ancona, A.; Garino, N.; Cauda, V.; Guan, J.; Salaun, F.; Barresi, A.A.; Ferri, A. Preparation of bio-functional textiles by surface functionalization of cellulose fabrics with caffeine loaded nanoparticles. IOP Conf. Ser. Mater. Sci. Eng. 2018, 460, 012044. [Google Scholar] [CrossRef]
- Massella, D.; Celasco, E.; Salaün, F.; Ferri, A.; Barresi, A. Overcoming the limits of flash nanoprecipitation: Effective loading of hydrophilic drug into polymeric nanoparticles with controlled structure. Polymers 2018, 10, 1092. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.L.; Leites, F.I.; Paese, K.; Sponchiado, R.M.; Michalowski, C.B.; Guterres, S.S.; Schapoval, E.E.S. Hydrogel containing adapalene- and dapsone-loaded lipid-core nanocapsules for cutaneous application: Development, characterization, in vitro irritation and permeation studies. Drug Dev. Ind. Pharm. 2016, 42, 2001–2008. [Google Scholar] [CrossRef]
- Marto, J.; Ruivo, E.; Lucas, S.D.; Gonçalves, L.M.; Simões, S.; Gouveia, L.F.; Felix, R.; Moreira, R.; Ribeiro, H.M.; Almeida, A.J. Starch nanocapsules containing a novel neutrophil elastase inhibitor with improved pharmaceutical performance. Eur. J. Pharm. Biopharm. 2018, 127, 1–11. [Google Scholar] [CrossRef]
- Mathes, C.; Melero, A.; Conrad, P.; Vogt, T.; Rigo, L.; Selzer, D.; Prado, W.A.; De Rossi, C.; Garrigues, T.M.; Hansen, S.; et al. Nanocarriers for optimizing the balance between interfollicular permeation and follicular uptake of topically applied clobetasol to minimize adverse effects. J. Control. Release 2016, 223, 207–214. [Google Scholar] [CrossRef]
- Cavalli, R.; Soster, M.; Argenziano, M. Nanobubbles: A promising efficienft tool for therapeutic delivery. Ther. Deliv. 2016, 7, 117–138. [Google Scholar] [CrossRef]
- Hsiao, K.-H.; Huang, C.-M.; Lee, Y.-H. Development of rifampicin-indocyanine green-loaded perfluorocarbon nanodroplets for photo-chemo-probiotic antimicrobial therapy. Front. Pharm. 2018, 9, 1254. [Google Scholar] [CrossRef] [PubMed]
- Argenziano, M.; Banche, G.; Luganini, A.; Finesso, N.; Allizond, V.; Gulino, G.R.; Khadjavi, A.; Spagnolo, R.; Tullio, V.; Giribaldi, G.; et al. Vancomycin-loaded nanobubbles: A new platform for controlled antibiotic delivery against methicillin-resistant Staphylococcus aureus infections. Int. J. Pharm. 2017, 523, 176–188. [Google Scholar] [CrossRef] [PubMed]
- Sayadi, L.R.; Banyard, D.A.; Ziegler, M.E.; Obagi, Z.; Prussak, J.; Klopfer, M.J.; Evans, G.R.; Widgerow, A.D. Topical oxygen therapy & micro/nanobubbles: A new modality for tissue oxygen delivery. Int. Wound J. 2018, 15, 363–374. [Google Scholar] [PubMed]
- Basilico, N.; Magnetto, C.; D’Alessandro, S.; Panariti, A.; Rivolta, I.; Genova, T.; Khadjavi, A.; Gulino, G.R.; Argenziano, M.; Soster, M.; et al. Dextran-shelled oxygen-loaded nanodroplets reestablish a normoxia-like pro-angiogenic phenotype and behavior in hypoxic human dermal microvascular endothelium. Toxicol. Appl. Pharm. 2015, 288, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Banche, G.; Prato, M.; Magnetto, C.; Allizond, V.; Giribaldi, G.; Argenziano, M.; Khadjavi, A.; Gulino, G.R.; Finesso, N.; Mandras, N.; et al. Antimicrobial chitosan nanodroplets: New insights for ultrasound-mediated adjuvant treatment of skin infection. Future Microbiol. 2015, 10, 929–939. [Google Scholar] [CrossRef] [PubMed]
- Gulino, G.R.; Magnetto, C.; Khadjavi, A.; Panariti, A.; Rivolta, I.; Soster, M.; Argenziano, M.; Cavalli, R.; Giribaldi, G.; Guiot, C.; et al. Oxygen-loaded nanodroplets effectively abrogate hypoxia dysregulating effects on secretion of mmp-9 and timp-1 by human monocytes. Mediat. Inflamm. 2015, 2015, 1–11. [Google Scholar] [CrossRef]
- Khadjavi, A.; Magnetto, C.; Panariti, A.; Argenziano, M.; Gulino, G.R.; Rivolta, I.; Cavalli, R.; Giribaldi, G.; Guiot, C.; Prato, M. Chitosan-shelled oxygen-loaded nanodroplets abrogate hypoxia dysregulation of human keratinocyte gelatinases and inhibitors: New insights for chronic wound healing. Toxicol. Appl. Pharm. 2015, 286, 198–206. [Google Scholar] [CrossRef]
- Prato, M.; Magnetto, C.; Jose, J.; Khadjavi, A.; Cavallo, F.; Quaglino, E.; Panariti, A.; Rivolta, I.; Benintende, E.; Varetto, G.; et al. 2H,3H-Decafluoropentane-based nanodroplets: New perspectives for oxygen delivery to hypoxic cutaneous tissues. PLoS ONE 2015, 10, e0119769. [Google Scholar] [CrossRef]
- Carneiro, S.; Costa Duarte, F.; Heimfarth, L.; Siqueira Quintans, J.; Quintans-Júnior, L.; Veiga Júnior, V.; Neves de Lima, Á. Cyclodextrin–drug inclusion complexes: In vivo and in vitro approaches. Int. J. Mol. Sci. 2019, 20, 642. [Google Scholar] [CrossRef]
- Ikuta, D.; Hirata, Y.; Wakamori, S.; Shimada, H.; Tomabechi, Y.; Kawasaki, Y.; Ikeuchi, K.; Hagimori, T.; Matsumoto, S.; Yamada, H. Conformationally supple glucose monomers enable synthesis of the smallest cyclodextrins. Science 2019, 364, 674–677. [Google Scholar] [CrossRef]
- Jug, M.; Mura, P. Grinding as solvent-free green chemistry approach for cyclodextrin inclusion complex preparation in the solid state. Pharmaceutics 2018, 10, 189. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Liu, W.; Huang, J.; Qiu, S.; Zhong, H.; Liu, D.; Liu, J. Cyclodextrin-based metal-organic frameworks (cd-mofs) in pharmaceutics and biomedicine. Pharmaceutics 2018, 10, 271. [Google Scholar] [CrossRef] [PubMed]
- Trotta, F.; Zanetti, M.; Cavalli, R. Cyclodextrin-based nanosponges as drug carriers. Beilstein J. Org. Chem. 2012, 8, 2091–2099. [Google Scholar] [CrossRef] [PubMed]
- Chilajwar, S.V.; Pednekar, P.P.; Jadhav, K.R.; Gupta, G.J.; Kadam, V.J. Cyclodextrin-based nanosponges: A propitious platform for enhancing drug delivery. Expert Opin. Drug Deliv. 2014, 11, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Conte, C.; Caldera, F.; Catanzano, O.; D’Angelo, I.; Ungaro, F.; Miro, A.; Pellosi, D.S.; Trotta, F.; Quaglia, F. β-Cyclodextrin nanosponges as multifunctional ingredient in water-containing semisolid formulations for skin delivery. J. Pharm. Sci. 2014, 103, 3941–3949. [Google Scholar] [CrossRef] [PubMed]
- Bastiancich, C.; Scutera, S.; Alotto, D.; Cambieri, I.; Fumagalli, M.; Casarin, S.; Rossi, S.; Trotta, F.; Stella, M.; Cavalli, R.; et al. Cyclodextrin-based nanosponges as a nanotechnology strategy for imiquimod delivery in pathological scarring prevention and treatment. J. Nanopharm. Drug Deliv. 2014, 2, 311–324. [Google Scholar] [CrossRef]
- Argenziano, M.; Haimhoffer, A.; Bastiancich, C.; Jicsinszky, L.; Caldera, F.; Trotta, F.; Scutera, S.; Alotto, D.; Fumagalli, M.; Musso, T.; et al. In vitro enhanced skin permeation and retention of imiquimod loaded in β-cyclodextrin nanosponge hydrogel. Pharmaceutics 2019, 11, 138. [Google Scholar] [CrossRef]
- Ansari, K.A.; Vavia, P.R.; Trotta, F.; Cavalli, R. Cyclodextrin-based nanosponges for delivery of resveratrol: In vitro characterisation, stability, cytotoxicity and permeation study. AAPS PharmSciTech 2011, 12, 279–286. [Google Scholar] [CrossRef]
- Sharma, R.; Pathak, K. Polymeric nanosponges as an alternative carrier for improved retention of econazole nitrate onto the skin through topical hydrogel formulation. Pharm. Dev. Technol. 2011, 16, 367–376. [Google Scholar] [CrossRef]
- Badr-Eldin, S.M.; Aldawsari, H.; Labib, G.; El-Kamel, A. Design and formulation of a topical hydrogel integrating lemongrass-loaded nanosponges with an enhanced antifungal effect: In vitro/in vivo evaluation. Int. J. Nanomed. 2015, 893–902. [Google Scholar] [CrossRef]
- Mahrhauser, D.-S.; Reznicek, G.; Gehrig, S.; Geyer, A.; Ogris, M.; Kieweler, R.; Valenta, C. Simultaneous determination of active component and vehicle penetration from F-DPPC liposomes into porcine skin layers. Eur. J. Pharm. Biopharm. 2015, 97, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Haque, M.W.; Singh, S.K.; Ahmed, F.J. Optimized permeation enhancer for topical delivery of 5-fluorouracil-loaded elastic liposome using Design Expert: Part II. Drug Deliv. 2016, 23, 1242–1253. [Google Scholar] [CrossRef] [PubMed]
- Hong, I.K.; Ha, J.H.; Han, S.; Kang, H.; Park, S.N. The effect of alkyl chain number in sucrose surfactant on the physical properties of quercetin-loaded deformable nanoliposome and its effect on in vitro human skin penetration. Nanomaterials 2018, 8, 622. [Google Scholar] [CrossRef] [PubMed]
- Zorec, B.; Zupančič, Š.; Kristl, J.; Pavšelj, N. Combinations of nanovesicles and physical methods for enhanced transdermal delivery of a model hydrophilic drug. Eur. J. Pharm. Biopharm. 2018, 127, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Pradhan, M.; Nag, M.; Singh, M.R. Vesicular system: Versatile carrier for transdermal delivery of bioactives. Artif. Cell Nanomed. Biotechnol. 2015, 43, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Das, S.K.; Chakraborty, S.; Roy, C.; Rajabalaya, R.; Mohaimin, A.W.; Khanam, J.; Nanda, A.; David, S.R. Ethosomes as novel vesicular carrier: An overview of the principle, preparation and its applications. Curr. Drug Deliv. 2018, 15, 795–817. [Google Scholar] [CrossRef] [PubMed]
- Abdulbaqi, I.M.; Darwis, Y.; Abdul Karim Khan, N.; Abou Assi, R.; Ali Khan, A. Ethosomal nanocarriers: The impact of constituents and formulation techniques on ethosomal properties, in vivo studies, and clinical trials. Int. J. Nanomed. 2016, 11, 2279–2304. [Google Scholar]
- Jondhalekar, T.M.; Aher, S.S.; Saudagar, R.B. Transethosome: Novel vesicular carrier for enhanced transdermal drug delivery system. Res. J. Pharm. Technol. 2017, 10, 1816–1819. [Google Scholar] [CrossRef]
- Chatterjee, S.; Hui, P.; Kan, C. Thermoresponsive hydrogels and their biomedical applications: Special insight into their applications in textile based transdermal therapy. Polymers 2018, 10, 480. [Google Scholar] [CrossRef]
- Jeong, H.J.; Nam, S.J.; Song, J.Y.; Park, S.N. Synthesis and physicochemical properties of pH-sensitive hydrogel based on carboxymethyl chitosan/2-hydroxyethyl acrylate for transdermal delivery of nobiletin. J. Drug Deliv. Sci. Technol. 2019, 51, 194–203. [Google Scholar] [CrossRef]
- Taktak, F.; Bütün, V.; Tuncer, C.; Demirel, H.H. Production of LMWH-conjugated core/shell hydrogels encapsulating paclitaxel for transdermal delivery: In vitro and in vivo assessment. Int. J. Biol. Macromol. 2019, 128, 610–620. [Google Scholar] [CrossRef] [PubMed]
- Baino, F.; Hamzehlou, S.; Kargozar, S. Bioactive glasses: Where are we and where are we going? J. Funct. Biomater. 2018, 9, 25. [Google Scholar] [CrossRef] [PubMed]
- Limongi, T.; Canta, M.; Racca, L.; Ancona, A.; Tritta, S.; Vighetto, V.; Cauda, V. Improving dispersal of therapeutic nanoparticles in the human body. Nanomedicine 2019, 14, 797–801. [Google Scholar] [CrossRef] [PubMed]
- Leone, F.; Cataldo, R.; Mohamed, S.; Manna, L.; Banchero, M.; Ronchetti, S.; Mandras, N.; Tullio, V.; Cavalli, R.; Onida, B. Nanostructured ZnO as multifunctional carrier for a green antibacterial drug delivery system—A feasibility study. Nanomaterials 2019, 9, 407. [Google Scholar] [CrossRef] [PubMed]
- Leone, F.; Gignone, A.; Ronchetti, S.; Cavalli, R.; Manna, L.; Banchero, M.; Onida, B. A green organic-solvent-free route to prepare nanostructured zinc oxide carriers of clotrimazole for pharmaceutical applications. J. Clean. Prod. 2018, 172, 1433–1439. [Google Scholar] [CrossRef]
- Anirudhan, T.S.; Nair, A.S. Temperature and ultrasound sensitive gatekeepers for the controlled release of chemotherapeutic drugs from mesoporous silica nanoparticles. J. Mater. Chem. B 2018, 6, 428–439. [Google Scholar] [CrossRef]
- Xu, B.; Cao, Q.; Zhang, Y.; Yu, W.; Zhu, J.; Liu, D.; Jiang, G. Microneedles integrated with ZnO quantum-dot-capped mesoporous bioactive glasses for glucose-mediated insulin delivery. ACS Biomater. Sci. Eng. 2018, 4, 2473–2483. [Google Scholar] [CrossRef]
- Massella, D.; Leone, F.; Peila, R.; Barresi, A.; Ferri, A. Functionalization of cotton fabrics with polycaprolactone nanoparticles for transdermal release of melatonin. J. Funct. Biomater. 2017, 9, 1. [Google Scholar] [CrossRef]
- Yip, J.; Luk, M.Y.A. Microencapsulation technologies for antimicrobial textiles. In Antimicrobial Textiles; Elsevier: Amsterdam, The Netherlands, 2016; pp. 19–46. ISBN 978-0-08-100576-7. [Google Scholar]
- Zhang, S.; Campagne, C.; Salaün, F. Influence of solvent selection in the electrospraying process of polycaprolactone. Appl. Sci. 2019, 9, 402. [Google Scholar] [CrossRef]
- Malucelli, G. Layer-by-layer nanostructured assemblies for the fire protection of fabrics. Mater. Lett. 2016, 166, 339–342. [Google Scholar] [CrossRef]
- Martí, M.; Rodríguez, R.; Carreras, N.; Lis, M.; Valldeperas, J.; Coderch, L.; Parra, J.L. Monitoring of the microcapsule/liposome application on textile fabrics. J. Text. Inst. 2012, 103, 19–27. [Google Scholar] [CrossRef]
- Hu, J.; Xiao, Z.-B.; Zhou, R.-J.; Ma, S.-S.; Li, Z.; Wang, M.-X. Comparison of compounded fragrance and chitosan nanoparticles loaded with fragrance applied in cotton fabrics. Text. Res. J. 2011, 81, 2056–2064. [Google Scholar] [CrossRef]
- Hassan, M.M.; Sunderland, M. Antimicrobial and insect-resist wool fabrics by coating with microencapsulated antimicrobial and insect-resist agents. Prog. Org. Coat. 2015, 85, 221–229. [Google Scholar] [CrossRef]
- Ali, S.W.; Rajendran, S.; Joshi, M. Synthesis and characterization of chitosan and silver loaded chitosan nanoparticles for bioactive polyester. Carbohydr. Polym. 2011, 83, 438–446. [Google Scholar] [CrossRef]
- Alonso, D.; Gimeno, M.; Sepúlveda-Sánchez, J.D.; Shirai, K. Chitosan-based microcapsules containing grapefruit seed extract grafted onto cellulose fibers by a non-toxic procedure. Carbohydr. Res. 2010, 345, 854–859. [Google Scholar] [CrossRef] [PubMed]
- Nada, A.; Al-Moghazy, M.; Soliman, A.A.F.; Rashwan, G.M.T.; Eldawy, T.H.A.; Hassan, A.A.E.; Sayed, G.H. Pyrazole-based compounds in chitosan liposomal emulsion for antimicrobial cotton fabrics. Int. J. Biol. Macromol. 2018, 107, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Souza, J.M.; Caldas, A.L.; Tohidi, S.D.; Molina, J.; Souto, A.P.; Fangueiro, R.; Zille, A. Properties and controlled release of chitosan microencapsulated limonene oil. Rev. Bras. Farm. 2014, 24, 691–698. [Google Scholar] [CrossRef]
- Sharkawy, A.; Fernandes, I.P.; Barreiro, M.F.; Rodrigues, A.E.; Shoeib, T. Aroma-loaded microcapsules with antibacterial activity for eco-friendly textile application: Synthesis, characterization, release, and green grafting. Ind. Eng. Chem. Res. 2017, 56, 5516–5526. [Google Scholar] [CrossRef]
- De Falco, F.; Guarino, V.; Gentile, G.; Cocca, M.; Ambrogi, V.; Ambrosio, L.; Avella, M. Design of functional textile coatings via non-conventional electrofluidodynamic processes. J. Colloid Interface Sci. 2019, 541, 367–375. [Google Scholar] [CrossRef]
- Zhang, S.; Campagne, C.; Salaün, F. preparation of electrosprayed poly(caprolactone) microparticles based on green solvents and related investigations on the effects of solution properties as well as operating parameters. Coatings 2019, 9, 84. [Google Scholar] [CrossRef]
- Carosio, F.; Cuttica, F.; Di Blasio, A.; Alongi, J.; Malucelli, G. Layer by layer assembly of flame retardant thin films on closed cell PET foams: Efficiency of ammonium polyphosphate versus DNA. Polym. Degrad. Stab. 2015, 113, 189–196. [Google Scholar] [CrossRef]
- Alongi, J.; Di Blasio, A.; Carosio, F.; Malucelli, G. UV-cured hybrid organic–inorganic layer by layer assemblies: Effect on the flame retardancy of polycarbonate films. Polym. Degrad. Stab. 2014, 107, 74–81. [Google Scholar] [CrossRef]
- Lam, P.L.; Li, L.; Yuen, C.W.M.; Gambari, R.; Wong, R.S.M.; Chui, C.H.; Lam, K.H. Effects of multiple washing on cotton fabrics containing berberine microcapsules with anti- Staphylococcus aureus activity. J. Microencapsul. 2013, 30, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Wijesirigunawardana, P.B.; Perera, B.G.K. Development of a cotton smart textile with medicinal properties using lime oil microcapsules. Acta Chim. Slov. 2018, 65, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Chien, Y.W.; Valia, K.H. Development of a dynamic skin permeation system for long-term permeation studies. Drug Dev. Ind. Pharm. 1984, 10, 575–599. [Google Scholar] [CrossRef]
- Salamanca, C.; Barrera-Ocampo, A.; Lasso, J.; Camacho, N.; Yarce, C. Franz diffusion cell approach for pre-formulation characterisation of ketoprofen semi-solid dosage forms. Pharmaceutics 2018, 10, 148. [Google Scholar] [CrossRef] [PubMed]
- Carrer, V.; Guzmán, B.; Martí, M.; Alonso, C.; Coderch, L. Lanolin-based synthetic membranes as percutaneous absorption models for transdermal drug delivery. Pharmaceutics 2018, 10, 73. [Google Scholar] [CrossRef] [PubMed]
- Todo, H. Transdermal permeation of drugs in various animal species. Pharmaceutics 2017, 9, 33. [Google Scholar] [CrossRef] [PubMed]
- Hui, P.C.-L.; Wang, W.-Y.; Kan, C.-W.; Ng, F.S.-F.; Zhou, C.-E.; Wat, E.; Zhang, V.X.; Chan, C.-L.; Lau, C.B.-S.; Leung, P.-C. Preparation and characterization of chitosan/sodium alginate (CSA) microcapsule containing Cortex Moutan. Colloids Surf. A 2013, 434, 95–101. [Google Scholar] [CrossRef]
- Hui, P.C.-L.; Wang, W.-Y.; Kan, C.-W.; Ng, F.S.-F.; Wat, E.; Zhang, V.X.; Chan, C.-L.; Lau, C.B.-S.; Leung, P.-C. Microencapsulation of traditional Chinese herbs—Pentaherbs extracts and potential application in healthcare textiles. Colloids Surf. B 2013, 111, 156–161. [Google Scholar] [CrossRef]
- Junthip, J.; Tabary, N.; Chai, F.; Leclercq, L.; Maton, M.; Cazaux, F.; Neut, C.; Paccou, L.; Guinet, Y.; Staelens, J.-N.; et al. Layer-by-layer coating of textile with two oppositely charged cyclodextrin polyelectrolytes for extended drug delivery: Intrisic antibacterial activity of multilayer assemblies. J. Biomed. Mater. Res. A 2016, 104, 1408–1424. [Google Scholar] [CrossRef] [PubMed]
- Mossotti, R.; Ferri, A.; Innocenti, R.; Zelenková, T.; Dotti, F.; Marchisio, D.L.; Barresi, A.A. Cotton fabric functionalisation with menthol/PCL micro- and nano-capsules for comfort improvement. J. Microencapsul. 2015, 32, 650–660. [Google Scholar] [CrossRef] [PubMed]
- Ferri, A.; Kumari, N.; Peila, R.; Barresi, A.A. Production of menthol-loaded nanoparticles by solvent displacement. Can. J. Chem. Eng. 2017, 95, 1690–1706. [Google Scholar] [CrossRef]
- Butstraen, C.; Salaün, F.; Devaux, E.; Giraud, S.; Vroman, P. Application of flame-retardant double-layered shell microcapsules to nonwoven polyester. Polymers 2016, 8, 267. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zeng, Z.; Xiao, Z.; Ji, H. Preparation and controllable release of chitosan/vanillin microcapsules and their application to cotton fabric: Controllable release of vanillin. Flavour Fragr. J. 2014, 29, 114–120. [Google Scholar] [CrossRef]
- Ghayempour, S.; Montazer, M. Tragacanth nanocapsules containing chamomile extract prepared through sono-assisted W/O/W microemulsion and UV cured on cotton fabric. Carbohydr. Polym. 2017, 170, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Rubio, L.; Alonso, C.; Coderch, L.; Parra, J.L.; Martí, M.; Cebrián, J.; Navarro, J.A.; Lis, M.; Valldeperas, J. Skin delivery of caffeine contained in biofunctional textiles. Text. Res. J. 2010, 80, 1214–1221. [Google Scholar] [CrossRef]
- Martí, M.; Martínez, V.; Rubio, L.; Coderch, L.; Parra, J.L. Biofunctional textiles prepared with liposomes: In vivo and in vitro assessment. J. Microencapsul. 2011, 28, 799–806. [Google Scholar] [CrossRef]
- Junthip, J.; Tabary, N.; Leclercq, L.; Martel, B. Cationic β-cyclodextrin polymer applied to a dual cyclodextrin polyelectrolyte multilayer system. Carbohydr. Polym. 2015, 126, 156–167. [Google Scholar] [CrossRef]
- Mihailiasa, M.; Caldera, F.; Li, J.; Peila, R.; Ferri, A.; Trotta, F. Preparation of functionalized cotton fabrics by means of melatonin loaded β-cyclodextrin nanosponges. Carbohydr. Polym. 2016, 142, 24–30. [Google Scholar] [CrossRef]
- Maestá Bezerra, F.; García Carmona, Ó.; García Carmona, C.; Souza Plath, A.M.; Lis, M. Biofunctional wool using β-cyclodextrins as vehiculizer of citronella oil. Process. Biochem. 2018, 77, 151–158. [Google Scholar] [CrossRef]
- Hassabo, A.; Mohamed, A.; Nada, A.; Zeid, N. Controlled release of drugs from cellulosic wound bandage using silica microsphere as drug encapsulator module. J. Appl. Pharm. Sci. 2015, 5, 67–73. [Google Scholar] [CrossRef]
- Perelshtein, I.; Ruderman, E.; Perkas, N.; Tzanov, T.; Beddow, J.; Joyce, E.; Mason, T.J.; Blanes, M.; Mollá, K.; Patlolla, A.; et al. Chitosan and chitosan–ZnO-based complex nanoparticles: Formation, characterization, and antibacterial activity. J. Mater. Chem. B 2013, 1, 1968–1977. [Google Scholar] [CrossRef]
- Petrusic, S.; Jovancic, P.; Lewandowski, M.; Giraud, S.; Bugarski, B.; Djonlagic, J.; Koncar, V. Synthesis, characterization and drug release properties of thermosensitive poly(N-isopropylacrylamide) microgels. J. Polym. Res. 2012, 19, 9979. [Google Scholar] [CrossRef]
- Petrusic, S.; Jovancic, P.; Lewandowski, M.; Giraud, S.; Grujic, S.; Ostojic, S.; Bugarski, B.; Koncar, V. Properties and drug release profile of poly(N-isopropylacrylamide) microgels functionalized with maleic anhydride and alginate. J. Mater. Sci. 2013, 48, 7935–7948. [Google Scholar] [CrossRef]
- Li, D.; Xia, Y. Electrospinning of nanofibers: Reinventing the wheel? Adv. Mater. 2004, 16, 1151–1170. [Google Scholar] [CrossRef]
- Vineis, C.; Varesano, A. Natural polymer-based electrospun fibers for antibacterial uses. In Electrofluidodynamic Technologies (EFDTs) for Biomaterials and Medical Devices; Elsevier: Amsterdam, The Netherlands, 2018; pp. 275–294. ISBN 978-0-08-101745-6. [Google Scholar]
- Yoon, J.; Yang, H.-S.; Lee, B.-S.; Yu, W.-R. Recent progress in coaxial electrospinning: New parameters, various structures, and wide applications. Adv. Mater. 2018, 30, 1704765. [Google Scholar] [CrossRef]
- Shah Hosseini, N.; Simon, B.; Messaoud, T.; Khenoussi, N.; Schacher, L.; Adolphe, D. Quantitative approaches of nanofibers organization for biomedical patterned nanofibrous scaffold by image analysis: Quantitative approaches of nanofibers organization for biomedical patterned nanofibrous scaffold by image analysis. J. Biomed. Mater. Res. A 2018, 106, 2963–2972. [Google Scholar] [CrossRef]
- Wróblewska-Krepsztul, J.; Rydzkowski, T.; Michalska-Pożoga, I.; Thakur, V.K. Biopolymers for biomedical and pharmaceutical applications: Recent advances and overview of alginate electrospinning. Nanomaterials 2019, 9, 404. [Google Scholar] [CrossRef]
- Ding, J.; Zhang, J.; Li, J.; Li, D.; Xiao, C.; Xiao, H.; Yang, H.; Zhuang, X.; Chen, X. Electrospun polymer biomaterials. Prog. Polym. Sci. 2019, 90, 1–34. [Google Scholar] [CrossRef]
- Fereydouni, N.; Darroudi, M.; Movaffagh, J.; Shahroodi, A.; Butler, A.E.; Ganjali, S.; Sahebkar, A. Curcumin nanofibers for the purpose of wound healing. J. Cell Physiol. Suppl. 2018, 5, 5537–5554. [Google Scholar] [CrossRef] [PubMed]
- Dharadhar, S.; Majumdar, A.; Dhoble, S.; Patravale, V. Microneedles for transdermal drug delivery: A systematic review. Drug Dev. Ind. Pharm. 2019, 45, 188–201. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, X.; van der Linde, P.; Homburg, E.; van Breemen, L.; de Jong, A.; Luttge, R. Insertion process of ceramic nanoporous microneedles by means of a novel mechanical applicator design. Pharmaceutics 2015, 7, 503–522. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Hu, L.; Xu, C. Recent advances in the design of polymeric microneedles for transdermal drug delivery and biosensing. Lab Chip 2017, 17, 1373–1387. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Zhu, D.D.; Chen, B.Z.; Ashfaq, M.; Guo, X.D. Insulin delivery systems combined with microneedle technology. Adv. Drug Deliv. Rev. 2018, 127, 119–137. [Google Scholar] [CrossRef] [PubMed]
- Lan, X.; She, J.; Lin, D.; Xu, Y.; Li, X.; Yang, W.; Lui, V.W.Y.; Jin, L.; Xie, X.; Su, Y. Microneedle-mediated delivery of lipid-coated cisplatin nanoparticles for efficient and safe cancer therapy. ACS Appl. Mater. Interface 2018, 10, 33060–33069. [Google Scholar] [CrossRef]
- Mutoh, M.; Ueda, H.; Nakamura, Y.; Hirayama, K.; Atobe, M.; Kobayashi, D.; Morimoto, Y. Characterization of transdermal solute transport induced by low-frequency ultrasound in the hairless rat skin. J. Control. Release 2003, 92, 137–146. [Google Scholar] [CrossRef]
- Polat, B.E.; Hart, D.; Langer, R.; Blankschtein, D. Ultrasound-mediated transdermal drug delivery: Mechanisms, scope, and emerging trends. J. Control. Release 2011, 152, 330–348. [Google Scholar] [CrossRef]
- Park, D.; Park, H.; Seo, J.; Lee, S. Sonophoresis in transdermal drug deliverys. Ultrasonics 2014, 54, 56–65. [Google Scholar] [CrossRef]
- Katikaneni, S.; Li, G.; Badkar, A.; Banga, A.K. Transdermal delivery of a approximately 13 kDa protein—An in vivo comparison of physical enhancement methods. J. Drug Target. 2010, 18, 141–147. [Google Scholar] [CrossRef]
- Mitragotri, S.; Blankschtein, D.; Langer, R. An explanation for the variation of the sonophoretic transdermal transport enhancement from drug to drug. J. Pharm. Sci. 1997, 86, 1190–1192. [Google Scholar] [CrossRef] [PubMed]
- Ita, K. Transdermal iontophoretic drug delivery: Advances and challenges. J. Drug Target. 2016, 24, 386–391. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, I.; Takeshita, T.; Suzuki, T.; Makino, K. Iontophoretic transdermal delivery using chitosan-coated PLGA nanoparticles for positively charged drugs. Colloids Surf. B 2017, 160, 520–526. [Google Scholar] [CrossRef] [PubMed]
- Musazzi, U.M.; Marini, V.; Casiraghi, A.; Minghetti, P. Is the European regulatory framework sufficient to assure the safety of citizens using health products containing nanomaterials? Drug Discov. Today 2017, 22, 870–882. [Google Scholar] [CrossRef] [PubMed]
- Rauscher, H.; Rasmussen, K.; Sokull-Klüttgen, B. Regulatory aspects of nanomaterials in the EU. Chem. Ing. Tech. 2017, 89, 224–231. [Google Scholar] [CrossRef]
- Nanotechnology Task Force Report. 2007. Available online: www.fda.gov/ScienceResearch/SpecialTopics/Nanotechnology/NanotechnologyTaskForceReport2007/default.html (accessed on 29 July 2019).
- U.S. Food and Drug Administration. Guidance for Industry. Considering Whether an FDA-Regulated Product Involves the Application of Nanotechnology (issued 2013). Available online: www.fda.gov/RegulatoryInformation/Guidances/ucm257698.html (accessed on 29 July 2019).
- Hamburg, M.A. FDA’s approach to regulation of products of nanotechnology. Science 2012, 336, 299–300. [Google Scholar] [CrossRef] [PubMed]
- SCENIHR (Scientific Committee on Emerging and Newly Identified Health Risks). Final Opinion on the Guidance on the Determination of Potential Health Effects of Nanomaterials Used in Medical Devices. 2015. Available online: https://ec.europa.eu/health/scientific_committees/emerging/docs/scenihr_o_045.pdf (accessed on 29 July 2019).
- ISO. International Standard ISO 10993. Biological Evaluation of Medical Devices—Part. 1: Evaluation and Testing within a Risk Management Process, 5th ed.; 2018-08. Reference number ISO 10993-1:2018 (E); ISO: Geneva, Switzerland, 2018. [Google Scholar]
- Jones, A.A.D., III; Mi, G.; Webster, T.J. A status report on FDA approval of medical devices containing nanostructured materials. Trends Biotechnol. 2019, 37, 117–120. [Google Scholar] [CrossRef]
- Lee Ventola, C. The nanomedicine revolution, Part 3: Regulatory and safety challenges. Pharm. Ther. 2012, 37, 635–639. [Google Scholar]
- European Parliament and Council. Regulation (EC) No 1223/2009 of the of 30 November 2009 on cosmetic products. Off. J. Eur. Union 2009, 342, 59–209. [Google Scholar]
- U.S. Food and Drug Administration. Guidance for Industry: Safety of Nanomaterials in Cosmetic Products (Issued 2014); U.S. Food and Drug Administration: Silver Spring, MD, USA, 2014. Available online: http://www.fda.gov/Cosmetics/GuidanceRegulation/GuidanceDocuments/ucm300886.html (accessed on 29 July 2019).
| Drug | Hydrophilicity | Carrier | Therapeutic Indication | Experimentation | Ref. |
|---|---|---|---|---|---|
| Vitamin D | Hydrophobic | Nanospheres | Supplement administration | Healthy and damaged porcine skin | [119] |
| Caffeine | Hydrophilic | Nanospheres | Antioxidant and anti-cellulite | Artificial Membrane | [122] |
| Adapalene and adapsone | Hydrophobic and hydrophilic | Nanocapsules | Dermatitis treatment | Porcine skin | [123] |
| HNE inhibitor | Hydrophobic | Nanocapsules | Psoriasis | In vitro and in vivo (rats) | [124] |
| Clobetasol propionate | Hydrophobic | Nanospheres and nanocapsules | Alopecia treatment | Ex vivo pig and human skin | [125] |
| Vancomycin | Hydrophilic | Nanobubbles | Skin infection | Porcine skin | [128] |
| Rifampicin | Hydrophobic | Nanobubbles | Acne treatment | In vitro studies | [127] |
| Imiquimod | Hydrophobic | Nanosponges | Aberrant wounds | Porcine skin | [143] |
| Resveratrol | Hydrophobic | Nanosponges | Antioxidant | Porcine skin | [144] |
| Econazole nitrate | Hydrophobic | Nanosponges | Fungal infection | In vitro studies | [145] |
| Sodium Fluorescein | Hydrophilic | Liposomes | Model system | Porcine skin | [147] |
| Quercitin | Slightly hydrophilic | Liposomes | Antioxidant | Human excised skin | [149] |
| Nobiletin | Hydrophobic | Hydrogel | Acne treatment | Porcine skin | [156] |
| Heparin and Paclitaxel | Hydrophilic and hydrophobic | Hydrogel | Transdermal cancer therapy | In vitro and in vivo | [157] |
| 5-fluroracil | Hydrophilic | Silica nanoparticles | Cancer therapy | Rat skin | [162] |
| Insulin | Hydrophobic | Silica nanoparticles and ZnO quantum dots | Transdermal diabetes therapy | In vivo in rats | [163] |
| Carrier | Active Substance | Textile | Carrier | Finishing Technique | Application | Reference |
|---|---|---|---|---|---|---|
| Poly-ε-caprolactone (PCL) nanospheres | Melatonin | Cotton | PCL nanospheres | Imbibition | Transdermal delivery | [164] |
| PCL nanospheres | Menthol | Cotton | PCL nanospheres | Imbibition | Thermal regulation | [189,190] |
| PCL nanospheres | Caffeine | Cotton/Micromodal | PCL nanospheres | Imbibition | Antioxidant activity | [121,122] |
| Chitosan microcapsules | Vanillin | Cotton | Chitosan microcapsules | Bath Exhaustion/Crosslinking | Antibacterial and aroma release | [192] |
| Chitosan microcapsules | Chamomile extracts | Cotton | Chitosan microcapsules | Resin finishing/UV curing | Topical antibacterial | [193] |
| Liposomes and microcapsules | Sunscreen | Cotton, PA, PAC, PES. | Liposomes and microcapsules | Foulard | UV protection | [168] |
| Liposomes | Sunscreen | Cotton | Liposomes | Bath Exhaustion | UV Protection | [195] |
| Liposomes | Caffeine | Cotton | Liposomes | Imbibition | Transdermal administration | [194] |
| Cyclodextrins | 4-tert-butylbenzoic acid (TBBA) | polyester (PES) | Cyclodextrins | Layer by layer deposition | Topical infections treatment | [188,196] |
| Cyclodextrin nanosponges | Melatonin | Cotton | Cyclodextrin nanosponges | Bath Exhaustion | Transdermal release | [197] |
| Cyclodextrin | Citronella oil | Wool | Cyclodextrin | Padding | Insect repellency | [198] |
| Silica nanoparticles | Diclofenac | Cotton | Silica nanoparticles | Spray | Topical treatment | [199] |
| Chitosan hydrogel | Chinese Herbal extract | Cotton | Chitosan hydrogel | Pad-dry curing | Topical treatment | [186,187] |
| Bio-Functional Textiles | Microneedles | Sonophoresis | Iontophoresis | |
|---|---|---|---|---|
| Drug applicability | Drugs deliverable by nanocarrier system | Most of the drugs | Small substances | Charged and polar drugs |
| Penetration mechanism | Complex: release from textile + transdermal penetration | Simple: direct release in the epidermis | Transdermal penetration + cavitation | Electro osmosis and electrophoresis |
| Control of dosage | Lower due to complex release | Very Good | Fair | Fair |
| Patient usability | Simple to wear | Simple patch application | Ultrasound device needed | Electrical current to be applied |
| Administration required | Few | Few | Several | Several |
| Possible side effect | None reported | Skin piercing and irritation | Stratum Corneum (SC) disrupted | Surface damages |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Massella, D.; Argenziano, M.; Ferri, A.; Guan, J.; Giraud, S.; Cavalli, R.; Barresi, A.A.; Salaün, F. Bio-Functional Textiles: Combining Pharmaceutical Nanocarriers with Fibrous Materials for Innovative Dermatological Therapies. Pharmaceutics 2019, 11, 403. https://doi.org/10.3390/pharmaceutics11080403
Massella D, Argenziano M, Ferri A, Guan J, Giraud S, Cavalli R, Barresi AA, Salaün F. Bio-Functional Textiles: Combining Pharmaceutical Nanocarriers with Fibrous Materials for Innovative Dermatological Therapies. Pharmaceutics. 2019; 11(8):403. https://doi.org/10.3390/pharmaceutics11080403
Chicago/Turabian StyleMassella, Daniele, Monica Argenziano, Ada Ferri, Jinping Guan, Stéphane Giraud, Roberta Cavalli, Antonello A. Barresi, and Fabien Salaün. 2019. "Bio-Functional Textiles: Combining Pharmaceutical Nanocarriers with Fibrous Materials for Innovative Dermatological Therapies" Pharmaceutics 11, no. 8: 403. https://doi.org/10.3390/pharmaceutics11080403
APA StyleMassella, D., Argenziano, M., Ferri, A., Guan, J., Giraud, S., Cavalli, R., Barresi, A. A., & Salaün, F. (2019). Bio-Functional Textiles: Combining Pharmaceutical Nanocarriers with Fibrous Materials for Innovative Dermatological Therapies. Pharmaceutics, 11(8), 403. https://doi.org/10.3390/pharmaceutics11080403