BMP-2 Gene Delivery-Based Bone Regeneration in Dentistry
Abstract
1. Introduction
2. BMPs in Bone Tissue Healing
3. rhBMP-2 Delivery in Protein Form
4. BMP-2 Gene Delivery
4.1. Ex Vivo BMP-2 Gene Delivery
4.1.1. Cells for Ex Vivo BMP-2 Gene Delivery
4.1.2. Viral Vectors for Ex Vivo BMP-2 Gene Delivery
4.1.3. Non-Viral Vectors for Ex Vivo BMP-2 Gene Delivery
4.1.4. Bone Regeneration via Ex Vivo BMP-2 Gene Delivery
4.1.5. Dental Application via Ex Vivo BMP-2 Gene Delivery
4.2. In Vivo BMP-2 Gene Delivery
4.2.1. Viral Vectors for In Vivo BMP-2 Gene Delivery
4.2.2. Non-Viral Vectors for In Vivo BMP-2 Gene Delivery
4.2.3. Bone Regeneration through In Vivo BMP-2 Gene Delivery
4.3. Complementary Strategies of In Vivo and Ex Vivo BMP-2 Gene Delivery
4.4. Combined Approaches Improving Bone Regeneration
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Urist, M.R. Bone: Formation by Autoinduction. Science 1965, 150, 893–899. [Google Scholar] [CrossRef] [PubMed]
- Kelly, M.P.; Vaughn, O.L.A.; Anderson, P.A. Systematic Review and Meta-Analysis of Recombinant Human Bone Morphogenetic Protein-2 in Localized Alveolar Ridge and Maxillary Sinus Augmentation. J. Oral Maxillofac. Surg 2016, 74, 928–939. [Google Scholar] [CrossRef] [PubMed]
- Araújo, M.G.; Silva, C.O.; Misawa, M.; Sukekava, F. Alveolar Socket Healing: What Can We Learn? Periodontol. 2000 2015, 68, 122–134. [Google Scholar] [CrossRef] [PubMed]
- Araújo, M.G.; Lindhe, J. Dimensional Ridge Alterations Following Tooth Extraction. An Experimental Study in the Dog. J. Clin. Periodontol. 2005, 32, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Hollinger, J.O.; Hart, C.E.; Hirsch, S.N.; Lynch, S.; Friedlaender, G.E. Recombinant Human Platelet-Derived Growth Factor: Biology and Clinical Applications. J. Bone Joint Surg. Am. 2008, 90, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Kanczler, J.; Oreffo, R. Osteogenesis and Angiogenesis: The Potential for Engineering Bone. J. Eur. Cell Mater. 2008, 15, 100–114. [Google Scholar] [CrossRef]
- Sigurdsson, T.J.; Lee, M.B.; Kubota, K.; Turek, T.J.; Wozney, J.M.; Wikesjö, U.M. Periodontal Repair in Dogs: Recombinant Human Bone Morphogenetic Protein-2 Significantly Enhances Periodontal Regeneration. J. Periodontol. 1995, 66, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Wikesjo, U.M.; Sorensen, R.G.; Wozney, J.M. Augmentation of Alveolar Bone and Dental Implant Osseointegration: Clinical Implications of Studies with rhBMP-2. J. Bone Joint Surg. Am. 2001, 83, S136–S145. [Google Scholar] [CrossRef]
- Butura, C.C.; Galindo, D.F. Implant Placement in Alveolar Composite Defects Regenerated with Rhbmp-2, Anorganic Bovine Bone, and Titanium Mesh: A Report of Eight Reconstructed Sites. Int J. Oral Maxillofac Implants 2014, 29, e139–e146. [Google Scholar] [CrossRef]
- Jovanovic, S.A.; Hunt, D.R.; Bernard, G.W.; Spiekermann, H.; Wozney, J.M.; Wikesjo, U.M. Bone Reconstruction Following Implantation of rhBMP-2 and Guided Bone Regeneration in Canine Alveolar Ridge Defects. Clin. Oral Implant. Res. 2007, 18, 224–230. [Google Scholar] [CrossRef]
- Howell, T.H.; Fiorellini, J.; Jones, A.; Alder, M.; Nummikoski, P.; Lazaro, M.; Lilly, L.; Cochran, D. A Feasibility Study Evaluating rhBMP-2/Absorbable Collagen Sponge Device for Local Alveolar Ridge Preservation or Augmentation. Int. J. Periodontics Restor. Dent. 1997, 17, 124–139. [Google Scholar]
- Boyne, P.J.; Lilly, L.C.; Marx, R.E.; Moy, P.K.; Nevins, M.; Spagnoli, D.B.; Triplett, R.G. De Novo Bone Induction by Recombinant Human Bone Morphogenetic Protein-2 (rhBMP-2) in Maxillary Sinus Floor Augmentation. J. Oral Maxillofac. Surg. 2005, 63, 1693–1707. [Google Scholar] [CrossRef] [PubMed]
- Hanisch, O.; Tatakis, D.N.; Boskovic, M.M.; Rohrer, M.D.; Wikesjö, U.M. Bone Formation and Reosseointegration in Peri-Implantitis Defects Following Surgical Implantation of rhBMP-2. Int. J. Oral Maxillofac. Implant. 1997, 12, 604–610. [Google Scholar]
- Jung, R.E.; Glauser, R.; Schärer, P.; Hämmerle, C.H.; Sailer, H.F.; Weber, F.E. Effect of rhBMP-2 on Guided Bone Regeneration in Humans: A Randomized, Controlled Clinical and Histomorphometric Study. Clin. Oral Implant. Res. 2003, 14, 556–568. [Google Scholar] [CrossRef]
- Viljanen, V.; Gao, T.; Marttinen, A.S.; Lindholm, T. Partial Purification and Characterization of Bone Morphogenetic Protein from Bone Matrix of the Premature Moose (Alces alces): Degradation of Bone-Inducing Activity during Storage. Eur. Surg. Res. 1996, 28, 447–460. [Google Scholar] [CrossRef] [PubMed]
- Wozney, J.; Rosen, V.; Celeste, A.; Mitsock, L.; Whitters, M.; Kriz, R.; Hewick, R.; Wang, E. Novel Regulators of Bone Formation: Molecular Clones and Activities. Science 1988, 242, 1528–1534. [Google Scholar] [CrossRef] [PubMed]
- Israel, D.I.; Nove, J.; Kerns, K.M.; Moutsatsos, I.K.; Kaufman, R.J. Expression and Characterization of Bone Morphogenetic Protein-2 in Chinese Hamster Ovary Cells. Growth Factors 1992, 7, 139–150. [Google Scholar] [CrossRef]
- Vallejo, L.F.; Brokelmann, M.; Marten, S.; Trappe, S.; Cabrera-Crespo, J.; Hoffmann, A.; Gross, G.; Weich, H.A.; Rinas, U. Renaturation and Purification of Bone Morphogenetic Protein-2 Produced as Inclusion Bodies in High-Cell-Density Cultures of Recombinant Escherichia Coli. J. Biotechnol. 2002, 94, 185–194. [Google Scholar] [CrossRef]
- Cho, J.H.; Lee, J.H.; Yeom, J.S.; Chang, B.-S.; Yang, J.J.; Koo, K.H.; Hwang, C.J.; Lee, K.B.; Kim, H.-J.; Lee, C.-K.; et al. Efficacy of Escherichia Coli-Derived Recombinant Human Bone Morphogenetic Protein-2 in Posterolateral Lumbar Fusion: An Open, Active-Controlled, Randomized, Multicenter Trial. Spine J. 2017, 17, 1866–1874. [Google Scholar] [CrossRef]
- Lee, J.-H.; Kim, C.-S.; Choi, K.-H.; Jung, U.-W.; Yun, J.-H.; Choi, S.-H.; Cho, K.-S. The Induction of Bone Formation in Rat Calvarial Defects and Subcutaneous Tissues by Recombinant Human BMP-2, Produced in Escherichia Coli. Biomaterials 2010, 31, 3512–3519. [Google Scholar] [CrossRef]
- Kim, H.J.; Chung, J.H.; Shin, S.Y.; Shin, S.I.; Kye, S.B.; Kim, N.K.; Kwon, T.G.; Paeng, J.Y.; Kim, J.W.; Oh, O.H.; et al. Efficacy of rhBMP-2/Hydroxyapatite on Sinus Floor Augmentation: A Multicenter, Randomized Controlled Clinical Trial. J. Dent. Res. 2015, 94, 158S–165S. [Google Scholar] [CrossRef] [PubMed]
- Govender, S.; Csimma, C.; Genant, H.K.; Valentin-Opran, A.; Amit, Y.; Arbel, R.; Aro, H.; Atar, D.; Bishay, M.; Borner, M.G.; et al. Recombinant Human Bone Morphogenetic Protein-2 for Treatment of Open Tibial Fractures: A Prospective, Controlled, Randomized Study of Four Hundred and Fifty Patients. J. Bone Joint Surg. Am. 2002, 84, 2123–2134. [Google Scholar] [CrossRef] [PubMed]
- Leknes, K.N.; Yang, J.; Qahash, M.; Polimeni, G.; Susin, C.; Wikesjo, U.M. Alveolar Ridge Augmentation Using Implants Coated with Recombinant Human Bone Morphogenetic Protein-2: Radiographic Observations. Clin. Oral Implant. Res. 2008, 19, 1027–1033. [Google Scholar] [CrossRef] [PubMed]
- Carragee, E.J.; Hurwitz, E.L.; Weiner, B.K. A Critical Review of Recombinant Human Bone Morphogenetic Protein-2 Trials in Spinal Surgery: Emerging Safety Concerns and Lessons Learned. Spine J. 2011, 11, 471–491. [Google Scholar] [CrossRef] [PubMed]
- Wikesjo, U.M.; Huang, Y.H.; Xiropaidis, A.V.; Sorensen, R.G.; Rohrer, M.D.; Prasad, H.S.; Wozney, J.M.; Hall, J. Bone Formation at Recombinant Human Bone Morphogenetic Protein-2-Coated Titanium Implants in the Posterior Maxilla (Type IV Bone) in Non-Human Primates. J. Clin. Periodontol. 2008, 35, 992–1000. [Google Scholar] [CrossRef] [PubMed]
- Poynton, A.R.; Lane, J.M. Safety Profile for the Clinical Use of Bone Morphogenetic Proteins in the Spine. Spine 2002, 27, S40–S48. [Google Scholar] [CrossRef] [PubMed]
- Virk, M.S.; Conduah, A.; Park, S.H.; Liu, N.; Sugiyama, O.; Cuomo, A.; Kang, C.; Lieberman, J.R. Influence of Short-Term Adenoviral Vector and Prolonged Lentiviral Vector Mediated Bone Morphogenetic Protein-2 Expression on the Quality of Bone Repair in a Rat Femoral Defect Model. Bone 2008, 42, 921–931. [Google Scholar] [CrossRef]
- Zhang, W.; Tsurushima, H.; Oyane, A.; Yazaki, Y.; Sogo, Y.; Ito, A.; Matsumura, A. BMP-2 Gene-Fibronectin-Apatite Composite Layer Enhances Bone Formation. J. Biomed. Sci. 2011, 18, 62. [Google Scholar] [CrossRef]
- Moutsatsos, I.K.; Turgeman, G.; Zhou, S.; Kurkalli, B.G.; Pelled, G.; Tzur, L.; Kelley, P.; Stumm, N.; Mi, S.; Müller, R.; et al. Exogenously Regulated Stem Cell-Mediated Gene Therapy for Bone Regeneration. Mol. Ther. 2001, 3, 449–461. [Google Scholar] [CrossRef]
- Ye, J.-H.; Xu, Y.-J.; Gao, J.; Yan, S.-G.; Zhao, J.; Tu, Q.; Zhang, J.; Duan, X.-J.; Sommer, C.A.; Mostoslavsky, G.; et al. Critical-Size Calvarial Bone Defects Healing in a Mouse Model with Silk Scaffolds and SATB2-Modified iPSCs. Biomaterials 2011, 32, 5065–5076. [Google Scholar] [CrossRef]
- Musgrave, D.S.; Bosch, P.; Lee, J.Y.; Pelinkovic, D.; Ghivizzani, S.C.; Whalen, J.; Niyibizi, C.; Huard, J. Ex Vivo Gene Therapy to Produce Bone Using Different Cell Types. Clin. Orthop. Res. 2000, 378, 290–305. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Kim, K.H.; Park, C.H.; Shin, S.Y.; Rhyu, I.C.; Lee, Y.M.; Seol, Y.J. Enhanced Bone Regeneration by Diabetic Cell-Based Adenoviral BMP-2 Gene Therapy in Diabetic Animals. Tissue Eng. Part. A 2018, 24, 930–942. [Google Scholar] [CrossRef] [PubMed]
- Worgall, S.; Crystal, R.G. Chapter 34—Gene Therapy. In Principles of Tissue Engineering, 4th ed.; Lanza, R., Langer, R., Vacanti, J., Eds.; Academic Press: Boston, MA, USA, 2014; pp. 657–686. [Google Scholar]
- Moghimi, S.M.; Symonds, P.; Murray, J.C.; Hunter, A.C.; Debska, G.; Szewczyk, A. A Two-Stage Poly(Ethylenimine)-Mediated Cytotoxicity: Implications for Gene Transfer/Therapy. Mol. Ther. 2005, 11, 990–995. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Ries, J.; Gelse, K.; Kloss, F.; von der Mark, K.; Wiltfang, J.; Neukam, F.W.; Schneider, H. Bone Regeneration in Critical Size Defects by Cell-Mediated BMP-2 Gene Transfer: A Comparison of Adenoviral Vectors and Liposomes. Gene Ther. 2003, 10, 1089–1098. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Musgrave, D.; Pelinkovic, D.; Fukushima, K.; Cummins, J.; Usas, A.; Robbins, P.; Fu, F.H.; Huard, J. Effect of Bone Morphogenetic Protein-2-Expressing Muscle-Derived Cells On Healing of Critical-Sized Bone Defects in Mice. J. Bone Joint Surg. Am. 2001, 83, 1032–1039. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Peng, H.; Usas, A.; Musgrave, D.; Cummins, J.; Pelinkovic, D.; Jankowski, R.; Ziran, B.; Robbins, P.; Huard, J. Enhancement of Bone Healing Based On Ex Vivo Gene Therapy Using Human Muscle-Derived Cells Expressing Bone Morphogenetic Protein 2. Hum. Gene Ther. 2002, 13, 1201–1211. [Google Scholar] [CrossRef]
- Xu, L.; Sun, X.; Bai, J.; Jiang, L.; Wang, S.; Zhao, J.; Xia, L.; Zhang, X.; Wen, J.; Li, G.; et al. Reosseointegration Following Regenerative Therapy of Tissue-Engineered Bone in a Canine Model of Experimental Peri-Implantitis. Clin. Implant. Dent. Relat Res. 2016, 18, 379–391. [Google Scholar] [CrossRef]
- Park, S.Y.; Kim, K.H.; Gwak, E.H.; Rhee, S.H.; Lee, J.C.; Shin, S.Y.; Koo, K.T.; Lee, Y.M.; Seol, Y.J. Ex Vivo Bone Morphogenetic Protein 2 Gene Delivery Using Periodontal Ligament Stem Cells for Enhanced Re-Osseointegration in The Regenerative Treatment of Peri-Implantitis. J. Biomed. Mater. Res. Part. A 2015, 103, 38–47. [Google Scholar] [CrossRef]
- Lu, C.H.; Chang, Y.H.; Lin, S.Y.; Li, K.C.; Hu, Y.C. Recent Progresses in Gene Delivery-Based Bone Tissue Engineering. Biotechnol. Adv. 2013, 31, 1695–1706. [Google Scholar] [CrossRef]
- Loozen, L.D.; Kruyt, M.C.; Vandersteen, A.; Kragten, A.H.M.; Croes, M.; Oner, F.C.; Alblas, J. Osteoinduction by Ex Vivo Nonviral Bone Morphogenetic Protein Gene Delivery is Independent of Cell Type. Tissue Eng. Part. A 2018, 24, 1423–1431. [Google Scholar] [CrossRef]
- Gafni, Y.; Turgeman, G.; Liebergal, M.; Pelled, G.; Gazit, Z.; Gazit, D. Stem Cells As Vehicles for Orthopedic Gene Therapy. Gene Ther. 2004, 11, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Steinhardt, Y.; Aslan, H.; Regev, E.; Zilberman, Y.; Kallai, I.; Gazit, D.; Gazit, Z. Maxillofacial-Derived Stem Cells Regenerate Critical Mandibular Bone Defect. Tissue Eng. Part. A 2008, 14, 1763–1773. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.C.; Lin, T.M.; Chung, H.Y.; Chen, P.K.; Lin, F.H.; Lou, J.; Jeng, L.B. Large-Scale Bicortical Skull Bone Regeneration Using Ex Vivo Replication-Defective Adenoviral-Mediated Bone Morphogenetic Protein-2 Gene-Transferred Bone Marrow Stromal Cells and Composite Biomaterials. Neurosurgery 2009, 65, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Kim, K.H.; Shin, S.Y.; Koo, K.T.; Lee, Y.M.; Seol, Y.J. Dual Delivery of rhPDGF-BB and Bone Marrow Mesenchymal Stromal Cells Expressing BMP-2 Gene Enhance Bone Formation in a Critical-Sized Defect Model. Tissue Eng. Part. A 2013, 19, 2495–2505. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.Y.; Chang, Y.H.; Kao, C.Y.; Lu, C.H.; Sung, L.Y.; Yen, T.C.; Lin, K.J.; Hu, Y.C. Augmented Healing of Critical-Size Calvarial Defects by Baculovirus-Engineered MSCs That Persistently Express Growth Factors. Biomaterials 2012, 33, 3682–3692. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.H.; Chang, Y.H.; Sung, L.Y.; Li, K.C.; Yeh, C.L.; Yen, T.C.; Hwang, S.M.; Lin, K.J.; Hu, Y.C. Osteogenic Differentiation of Adipose-Derived Stem Cells and Calvarial Defect Repair Using Baculovirus-Mediated Co-Expression of BMP-2 and miR-148b. Biomaterials 2014, 35, 4901–4910. [Google Scholar] [CrossRef] [PubMed]
- Requicha, J.F.; Viegas, C.A.; Albuquerque, C.M.; Azevedo, J.M.; Reis, R.L.; Gomes, M.E. Effect of Anatomical Origin and Cell Passage Number on the Stemness and Osteogenic Differentiation Potential of Canine Adipose-Derived Stem Cells. Stem Cell Rev. 2012, 8, 1211–1222. [Google Scholar] [CrossRef] [PubMed]
- Bougioukli, S.; Sugiyama, O.; Pannell, W.; Ortega, B.; Tan, M.H.; Tang, A.H.; Yoho, R.; Oakes, D.A.; Lieberman, J.R. Gene Therapy for Bone Repair Using Human Cells: Superior Osteogenic Potential of Bone Morphogenetic Protein 2-Transduced Mesenchymal Stem Cells Derived from Adipose Tissue Compared to Bone Marrow. Hum. Gene Ther. 2018, 29, 507–519. [Google Scholar] [CrossRef]
- Vakhshori, V.; Bougioukli, S.; Sugiyama, O.; Tang, A.; Yoho, R.; Lieberman, J.R. Cryopreservation of Human Adipose-Derived Stem Cells for Use in Ex Vivo Regional Gene Therapy for Bone Repair. Hum. Gene Ther. Methods 2018. [CrossRef]
- Huang, G.T.; Gronthos, S.; Shi, S. Mesenchymal Stem Cells Derived from Dental Tissues vs. Those from Other Sources: Their Biology and Role in Regenerative Medicine. J. Dent. Res. 2009, 88, 792–806. [Google Scholar] [CrossRef]
- Yi, T.; Jun, C.M.; Kim, S.J.; Yun, J.H. Evaluation of In Vivo Osteogenic Potential of Bone Morphogenetic Protein 2-Overexpressing Human Periodontal Ligament Stem Cells Combined with Biphasic Calcium Phosphate Block Scaffolds in a Critical-Size Bone Defect Model. Tissue Eng Part. A 2016, 22, 501–512. [Google Scholar] [CrossRef] [PubMed]
- Hirata, K.; Tsukazaki, T.; Kadowaki, A.; Furukawa, K.; Shibata, Y.; Moriishi, T.; Okubo, Y.; Bessho, K.; Komori, T.; Mizuno, A.; et al. Transplantation of Skin Fibroblasts Expressing BMP-2 Promotes Bone Repair More Effectively Than Those Expressing Runx2. Bone 2003, 32, 502–512. [Google Scholar] [CrossRef]
- Keeney, M.; Chung, M.T.; Zielins, E.R.; Paik, K.J.; McArdle, A.; Morrison, S.D.; Ransom, R.C.; Barbhaiya, N.; Atashroo, D.; Jacobson, G.; et al. Scaffold-Mediated BMP-2 Minicircle DNA Delivery Accelerated Bone Repair in a Mouse Critical-Size Calvarial Defect Model. J. Biomed. Mater. Res. Part. A 2016, 104, 2099–2107. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.H.; Kim, K.H.; Kim, S.H.; Koo, K.T.; Kim, T.I.; Seol, Y.J.; Ku, Y.; Rhyu, I.C.; Chung, C.P.; Lee, Y.M. Ex Vivo Bone Morphogenetic Protein-2 Gene Delivery using Gingival Fibroblasts Promotes Bone Regeneration in Rats. J. Clin. Periodontol. 2010, 37, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Nayak, S.; Herzog, R.W. Progress and Prospects: Immune Responses to Viral Vectors. Gene Ther. 2009, 17, 295. [Google Scholar] [CrossRef] [PubMed]
- Kofron, M.D.; Laurencin, C.T. Bone Tissue Engineering by Gene Delivery. Adv. Drug Deliv. Rev. 2006, 58, 555–576. [Google Scholar] [CrossRef] [PubMed]
- Davé, U.P.; Jenkins, N.A.; Copeland, N.G. Gene Therapy Insertional Mutagenesis Insights. Science 2004, 303, 333. [Google Scholar] [CrossRef]
- Pensak, M.J.; Lieberman, J.R. Gene Therapy for Bone Regeneration. Curr. Pharm. Des. 2013, 19, 3466–3473. [Google Scholar] [CrossRef]
- Kojaoghlanian, T.; Flomenberg, P.; Horwitz, M.S. The Impact of Adenovirus Infection on the Immunocompromised Host. Rev. Med. Virol 2003, 13, 155–171. [Google Scholar] [CrossRef]
- Evans, C.H.; Huard, J. Gene Therapy Approaches to Regenerating the Musculoskeletal System. Nat. Rev. Rheumatol. 2015, 11, 234–242. [Google Scholar] [CrossRef]
- Phillips, J.E.; Gersbach, C.A.; García, A.J. Virus-Based Gene Therapy Strategies for Bone Regeneration. Biomaterials 2007, 28, 211–229. [Google Scholar] [CrossRef] [PubMed]
- Gafni, Y.; Pelled, G.; Zilberman, Y.; Turgeman, G.; Apparailly, F.; Yotvat, H.; Galun, E.; Gazit, Z.; Jorgensen, C.; Gazit, D. Gene Therapy Platform for Bone Regeneration using an Exogenously Regulated, AAV-2-Based Gene Expression System. Mol. Ther. 2004, 9, 587–595. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.W.; Wang, Z.; Hollister, S.J.; Krebsbach, P.H. Localized Viral Vector Delivery to Enhance In Situ Regenerative Gene Therapy. Gen. Ther. 2007, 14, 891–901. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chuang, C.K.; Lin, K.J.; Lin, C.Y.; Chang, Y.H.; Yen, T.C.; Hwang, S.M.; Sung, L.Y.; Chen, H.C.; Hu, Y.C. Xenotransplantation of Human Mesenchymal Stem Cells into Immunocompetent Rats for Calvarial Bone Repair. Tissue Eng. Part. A 2010, 16, 479–488. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.Y.; Shiah, H.C.; Su, H.J.; Chen, C.Y.; Chuang, Y.J.; Lo, W.H.; Huang, J.L.; Chuang, C.K.; Hwang, S.M.; Hu, Y.C. Baculovirus Transduction of Mesenchymal Stem Cells Triggers the Toll-Like Receptor 3 Pathway. J. Virol. 2009, 83, 10548–10556. [Google Scholar] [CrossRef]
- Chuang, C.K.; Wong, T.H.; Hwang, S.M.; Chang, Y.H.; Chen, G.Y.; Chiu, Y.C.; Huang, S.F.; Hu, Y.C. Baculovirus Transduction of Mesenchymal Stem Cells: In Vitro Responses and In Vivo Immune Responses after Cell Transplantation. Mol. Ther. 2009, 17, 889–896. [Google Scholar] [CrossRef]
- Blum, J.S.; Barry, M.A.; Mikos, A.G.; Jansen, J.A. In Vivo Evaluation of Gene Therapy Vectors in Ex Vivo-Derived Marrow Stromal Cells for Bone Regeneration in a Rat Critical-Size Calvarial Defect Model. Hum. Gene Ther. 2003, 14, 1689–1701. [Google Scholar] [CrossRef]
- Wang, R.; Zou, Y.; Yuan, Z.; Wang, Y.; Chen, Y.; Mao, Y.; Zhu, Z.A.; Li, H.; Tang, X.; Lu, J.; et al. Autografts and Xenografts of Skin Fibroblasts Delivering BMP-2 Effectively Promote Orthotopic and Ectopic Osteogenesis. Anat. Rec. 2009, 292, 777–786. [Google Scholar] [CrossRef]
- Bougioukli, S.; Saitta, B.; Sugiyama, O.; Tang, A.H.; Elphingstone, J.; Evseenko, D.; Lieberman, J.R. Lentiviral Gene Therapy for Bone Repair using Human Umbilical Cord Blood-Derived Mesenchymal Stem Cells. Hum. Gene Ther. 2019. [Google Scholar] [CrossRef]
- Alluri, R.; Song, X.; Bougioukli, S.; Pannell, W.; Vakhshori, V.; Sugiyama, O.; Tang, A.; Park, S.H.; Chen, Y.; Lieberman, J.R. Regional Gene Therapy with 3D Printed Scaffolds to Heal Critical Sized Bone Defects in a Rat Model. J. Biomed. Mater. Res. Part. A 2019. [Google Scholar] [CrossRef]
- Alaee, F.; Sugiyama, O.; Virk, M.S.; Tang, H.; Drissi, H.; Lichtler, A.C.; Lieberman, J.R. Suicide Gene Approach using a Dual-Expression Lentiviral Vector to Enhance the Safety of Ex Vivo Gene Therapy for Bone Repair. Gene Ther. 2014, 21, 139–147. [Google Scholar] [CrossRef]
- Jones, C.H.; Chen, C.K.; Ravikrishnan, A.; Rane, S.; Pfeifer, B.A. Overcoming Nonviral Gene Delivery Barriers: Perspective and Future. Mol. Pharm. 2013, 10, 4082–4098. [Google Scholar] [CrossRef]
- Gao, X.; Huang, L. Cationic Liposome-Mediated Gene Transfer. Gene Ther. 1995, 2, 710–722. [Google Scholar]
- Tang, Y.; Tang, W.; Lin, Y.; Long, J.; Wang, H.; Liu, L.; Tian, W. Combination of Bone Tissue Engineering and BMP-2 Gene Transfection Promotes Bone Healing in Osteoporotic Rats. Cell Biol. Int. 2008, 32, 1150–1157. [Google Scholar] [CrossRef]
- Koh, J.T.; Zhao, Z.; Wang, Z.; Lewis, I.S.; Krebsbach, P.H.; Franceschi, R.T. Combinatorial Gene Therapy with BMP-2/7 Enhances Cranial Bone Regeneration. J. Dent. Res. 2008, 87, 845–849. [Google Scholar] [CrossRef]
- Kroczek, A.; Park, J.; Birkholz, T.; Neukam, F.W.; Wiltfang, J.; Kessler, P. Effects of Osteoinduction on Bone Regeneration in Distraction: Results of a Pilot Study. J. Craniomaxillofac Surg. 2010, 38, 334–344. [Google Scholar] [CrossRef]
- Xia, L.; Xu, Y.; Chang, Q.; Sun, X.; Zeng, D.; Zhang, W.; Zhang, X.; Zhang, Z.; Jiang, X. Maxillary Sinus Floor Elevation Using BMP-2 and Nell-1 Gene-Modified Bone Marrow Stromal Cells and TCP in Rabbits. Calcif. Tissue Int. 2011, 89, 53–64. [Google Scholar] [CrossRef]
- Jhin, M.J.; Kim, K.H.; Kim, S.H.; Kim, Y.S.; Kim, S.T.; Koo, K.T.; Kim, T.I.; Seol, Y.J.; Ku, Y.; Rhyu, I.C.; et al. Ex Vivo Bone Morphogenetic Protein-2 Gene Delivery using Bone Marrow Stem Cells in Rabbit Maxillary Sinus Augmentation in Conjunction with Implant Placement. J. Periodontol. 2013, 84, 985–994. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, K.H.; Seo, B.M.; Koo, K.T.; Kim, T.I.; Seol, Y.J.; Ku, Y.; Rhyu, I.C.; Chung, C.P.; Lee, Y.M. Alveolar Bone Regeneration by Transplantation of Periodontal Ligament Stem Cells and Bone Marrow Stem Cells in a Canine Peri-Implant Defect Model: A Pilot Study. J. Periodontol. 2009, 80, 1815–1823. [Google Scholar] [CrossRef]
- Heitz-Mayfield, L.J.; Lang, N.P. Comparative Biology of Chronic and Aggressive Periodontitis Vs. Peri-Implantitis. Periodontol 2000 2010, 53, 167–181. [Google Scholar] [CrossRef]
- He, X.; Dziak, R.; Yuan, X.; Mao, K.; Genco, R.; Swihart, M.; Sarkar, D.; Li, C.; Wang, C.; Lu, L.; et al. BMP-2 Genetically Engineered MSCs and EPCs Promote Vascularized Bone Regeneration in Rat Critical-Sized Calvarial Bone Defects. PLoS ONE 2013, 8, e60473. [Google Scholar] [CrossRef]
- Jin, H.; Zhang, K.; Qiao, C.; Yuan, A.; Li, D.; Zhao, L.; Shi, C.; Xu, X.; Ni, S.; Zheng, C.; et al. Efficiently Engineered Cell Sheet Using A Complex of Polyethylenimine-Alginate Nanocomposites Plus Bone Morphogenetic Protein 2 Gene to Promote New Bone Formation. Int. J. Nanomed. 2014, 9, 2179–2190. [Google Scholar] [CrossRef]
- Vural, A.C.; Odabas, S.; Korkusuz, P.; Yar Saglam, A.S.; Bilgic, E.; Cavusoglu, T.; Piskin, E.; Vargel, I. Cranial Bone Regeneration via BMP-2 Encoding Mesenchymal Stem Cells. Artif. Cells Nanomed. Biotechnol. 2017, 45, 544–550. [Google Scholar] [CrossRef]
- Zhou, Z.; Liu, X.; Zhu, D.; Wang, Y.; Zhang, Z.; Zhou, X.; Qiu, N.; Chen, X.; Shen, Y. Nonviral Cancer Gene Therapy: Delivery Cascade and Vector Nanoproperty Integration. Adv. Drug Deliv. Rev. 2017, 115, 115–154. [Google Scholar] [CrossRef]
- Zhang, Y.; Satterlee, A.; Huang, L. In Vivo Gene Delivery by Nonviral Vectors: Overcoming Hurdles? Mol. Ther. 2012, 20, 1298–1304. [Google Scholar] [CrossRef]
- Alden, T.D.; Beres, E.J.; Laurent, J.S.; Engh, J.A.; Das, S.; London, S.D.; Jane, J.A., Jr.; Hudson, S.B.; Helm, G.A. The Use of Bone Morphogenetic Protein Gene Therapy in Craniofacial Bone Repair. J. Craniofacial Surg. 2000, 11, 24–30. [Google Scholar] [CrossRef]
- Ashinoff, R.L.; Cetrulo, C.L., Jr.; Galiano, R.D.; Dobryansky, M.; Bhatt, K.A.; Ceradini, D.J.; Michaels, J.T.; Mccarthy, J.G.; Gurtner, G.C. Bone Morphogenic Protein-2 Gene Therapy for Mandibular Distraction Osteogenesis. Ann. Plast. Surg. 2004, 52, 585–590. [Google Scholar] [CrossRef]
- Ben Arav, A.; Pelled, G.; Zilberman, Y.; Kimelman-Bleich, N.; Gazit, Z.; Schwarz, E.M.; Gazit, D. Adeno-Associated Virus-Coated Allografts: A Novel Approach for Cranioplasty. J. Tissue Eng. Reg. Med. 2012, 6, E43–E50. [Google Scholar] [CrossRef]
- Ito, H.; Koefoed, M.; Tiyapatanaputi, P.; Gromov, K.; Goater, J.J.; Carmouche, J.; Zhang, X.; Rubery, P.T.; Rabinowitz, J.; Samulski, R.J.; et al. Remodeling of Cortical Bone Allografts Mediated by Adherent Raav-RANKL and VEGF Gene Therapy. Nat. Med. 2005, 11, 291–297. [Google Scholar] [CrossRef]
- Hamm, A.; Krott, N.; Breibach, I.; Blindt, R.; Bosserhoff, A.K. Efficient Transfection Method for Primary Cells. Tissue Eng. 2002, 8, 235–245. [Google Scholar] [CrossRef]
- Aslan, H.; Zilberman, Y.; Arbeli, V.; Sheyn, D.; Matan, Y.; Liebergall, M.; Li, J.Z.; Helm, G.A.; Gazit, D.; Gazit, Z. Nucleofection-Based Ex Vivo Nonviral Gene Delivery to Human Stem Cells as a Platform for Tissue Regeneration. Tissue Eng. 2006, 12, 877–889. [Google Scholar] [CrossRef]
- Wu, G.P.; He, X.C.; Hu, C.B.; Li, D.P.; Yang, Z.H.; Guo, L. Effect of Electroporation-Mediated Transfecting Recombinant Plasmid pIRES-hBMP-2-hVEGF165 on Mandibular Distraction Osteogenesis. Ann. Plast. Surg. 2012, 69, 316–325. [Google Scholar] [CrossRef]
- Kawai, M.; Kataoka, Y.H.; Sonobe, J.; Yamamoto, H.; Inubushi, M.; Ishimoto, T.; Nakano, T.; Maruyama, H.; Miyazaki, J.I.; Yamamoto, T.; et al. Non-Surgical Model for Alveolar Bone Regeneration by Bone Morphogenetic Protein-2/7 Gene Therapy. J. Periodontol. 2018, 89, 85–92. [Google Scholar] [CrossRef]
- Tsuchiya, S.; Chiba, M.; Kishimoto, K.N.; Nakamura, H.; Tsuchiya, M.; Hayashi, H. Transfer of the Bone Morphogenetic Protein 4 Gene into Rat Periodontal Ligament by In Vivo Electroporation. Arch. Oral Biol. 2017, 74, 123–132. [Google Scholar] [CrossRef]
- Saranya, N.; Moorthi, A.; Saravanan, S.; Devi, M.P.; Selvamurugan, N. Chitosan and its Derivatives for Gene Delivery. Int. J. Biol. Macromol. 2011, 48, 234–238. [Google Scholar] [CrossRef]
- Chew, S.A.; Kretlow, J.D.; Spicer, P.P.; Edwards, A.W.; Baggett, L.S.; Tabata, Y.; Kasper, F.K.; Mikos, A.G. Delivery of Plasmid DNA Encoding Bone Morphogenetic Protein-2 with a Biodegradable Branched Polycationic Polymer in a Critical-Size Rat Cranial Defect Model. Tissue Eng. Part. A 2011, 17, 751–763. [Google Scholar] [CrossRef]
- Li, H.; Ji, Q.; Chen, X.; Sun, Y.; Xu, Q.; Deng, P.; Hu, F.; Yang, J. Accelerated Bony Defect Healing Based on Chitosan Thermosensitive Hydrogel Scaffolds Embedded with Chitosan Nanoparticles for the Delivery of BMP-2 Plasmid DNA. J. Biomed. Mater. Res. Part. A 2017, 105, 265–273. [Google Scholar] [CrossRef]
- Kichler, A.; Leborgne, C.; Coeytaux, E.; Danos, O. Polyethylenimine-Mediated Gene Delivery: A Mechanistic Study. J. Gene Med. 2001, 3, 135–144. [Google Scholar] [CrossRef]
- Qiao, C.; Zhang, K.; Jin, H.; Miao, L.; Shi, C.; Liu, X.; Yuan, A.; Liu, J.; Li, D.; Zheng, C.; et al. Using Poly(Lactic-Co-Glycolic Acid) Microspheres to Encapsulate Plasmid of Bone Morphogenetic Protein 2/Polyethylenimine Nanoparticles to Promote Bone Formation In Vitro and In Vivo. Int. J. Nanomed. 2013, 8, 2985–2995. [Google Scholar] [CrossRef]
- Xie, M.K.; Hu, C.B.; Zhou, B.; Wu, G.P. Effect of Gene Transfection Timing on TGF-beta1 Expression in Rabbit Mandibular Distraction Gap. Genet. Mol. Res. 2017, 16. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, D.; Zhang, K.; Jiang, L.; Shi, C.; Fangteng, J.; Zheng, C.; Yang, B.; Sun, H. Novel Synthesized Nanofibrous Scaffold Efficiently Delivered Hbmp-2 Encoded in Adenoviral Vector to Promote Bone Regeneration. J. Biomed. Nanotechnol. 2017, 13, 437–446. [Google Scholar] [CrossRef]
- Kolk, A.; Tischer, T.; Koch, C.; Vogt, S.; Haller, B.; Smeets, R.; Kreutzer, K.; Plank, C.; Bissinger, O. A Novel Nonviral Gene Delivery Tool of Bmp-2 for the Reconstitution of Critical-Size Bone Defects in Rats. J. Biomed. Mater. Res. Part. A 2016, 104, 2441–2455. [Google Scholar] [CrossRef]
- Liu, F.; Porter, R.M.; Wells, J.; Glatt, V.; Pilapil, C.; Evans, C.H. Evaluation of BMP-2 Gene-Activated Muscle Grafts for Cranial Defect Repair. J. Orthop. Res. 2012, 30, 1095–1102. [Google Scholar] [CrossRef]
- Ren, B.; Betz, V.M.; Thirion, C.; Salomon, M.; Jansson, V.; Muller, P.E.; Betz, O.B. Gene-Activated Tissue Grafts for Sustained Bone Morphogenetic Protein-2 Delivery and Bone Engineering: Is Muscle with Fascia Superior to Muscle and Fat? J. Tissue Eng. Reg. Med. 2018, 12, 1002–1011. [Google Scholar] [CrossRef]
- Betz, O.B.; Betz, V.M.; Abdulazim, A.; Penzkofer, R.; Schmitt, B.; Schroder, C.; Augat, P.; Jansson, V.; Muller, P.E. Healing of Large Segmental Bone Defects Induced by Expedited Bone Morphogenetic Protein-2 Gene-Activated, Syngeneic Muscle Grafts. Hum. Gene Ther. 2009, 20, 1589–1596. [Google Scholar] [CrossRef]
- Ren, B.; Betz, V.M.; Thirion, C.; Salomon, M.; Klar, R.M.; Jansson, V.; Muller, P.E.; Betz, O.B. Gene Activated Adipose Tissue Fragments as Advanced Autologous Biomaterials for Bone Regeneration: Osteogenic Differentiation with in the Tissue and Implications for Clinical Translation. Sci. Rep. 2019, 9, 224. [Google Scholar] [CrossRef]
- Virk, M.S.; Sugiyama, O.; Park, S.H.; Gambhir, S.S.; Adams, D.J.; Drissi, H.; Lieberman, J.R. “Same Day” Ex-Vivo Regional Gene Therapy: A Novel Strategy To Enhance Bone Repair. Mol. Ther. 2011, 19, 960–968. [Google Scholar] [CrossRef]
- Koons, G.L.; Mikos, A.G. Progress in Three-Dimensional Printing with Growth Factors. J. Control. Release 2019, 295, 50–59. [Google Scholar] [CrossRef]
- Evans, C.H.; Ghivizzani, S.C.; Robbins, P.D. Arthritis Gene Therapy is Becoming a Reality. Nat. Rev. Rheumatol. 2018, 14, 381–382. [Google Scholar] [CrossRef]
- Ginn, S.L.; Amaya, A.K.; Alexander, I.E.; Edelstein, M.; Abedi, M.R. Gene Therapy Clinical Trials Worldwide to 2017: An Update. J. Gene Med. 2018, 20, E3015. [Google Scholar] [CrossRef]
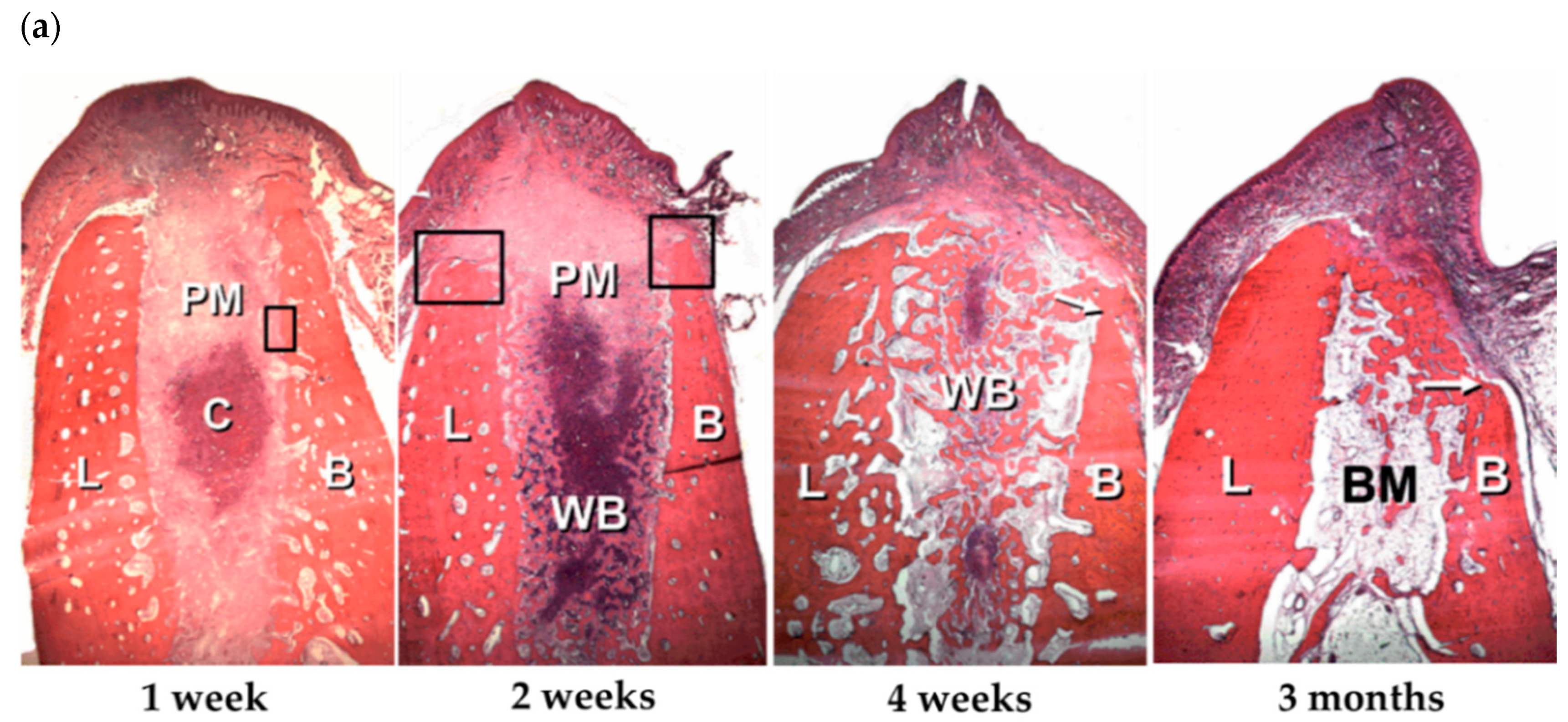
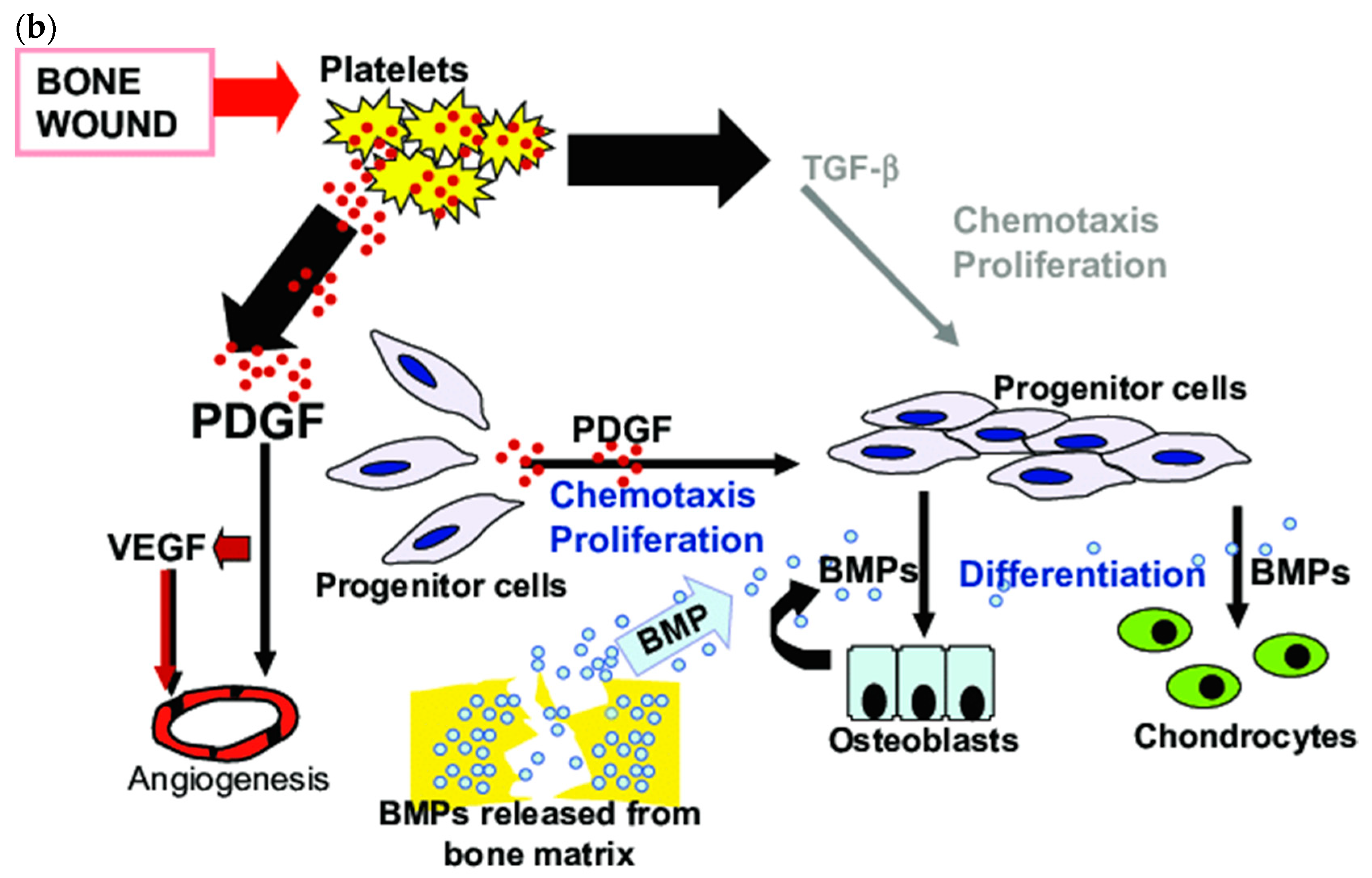
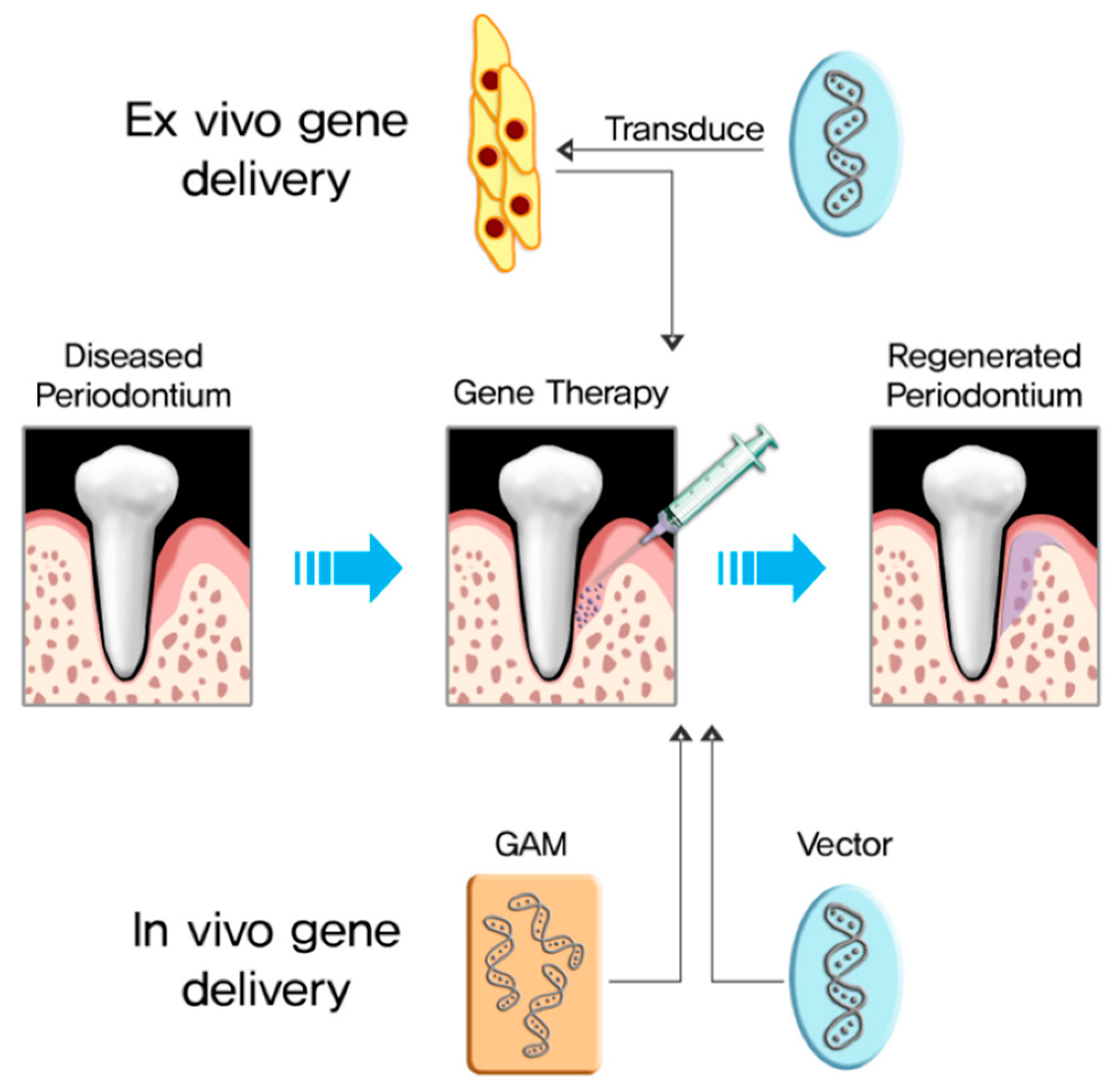
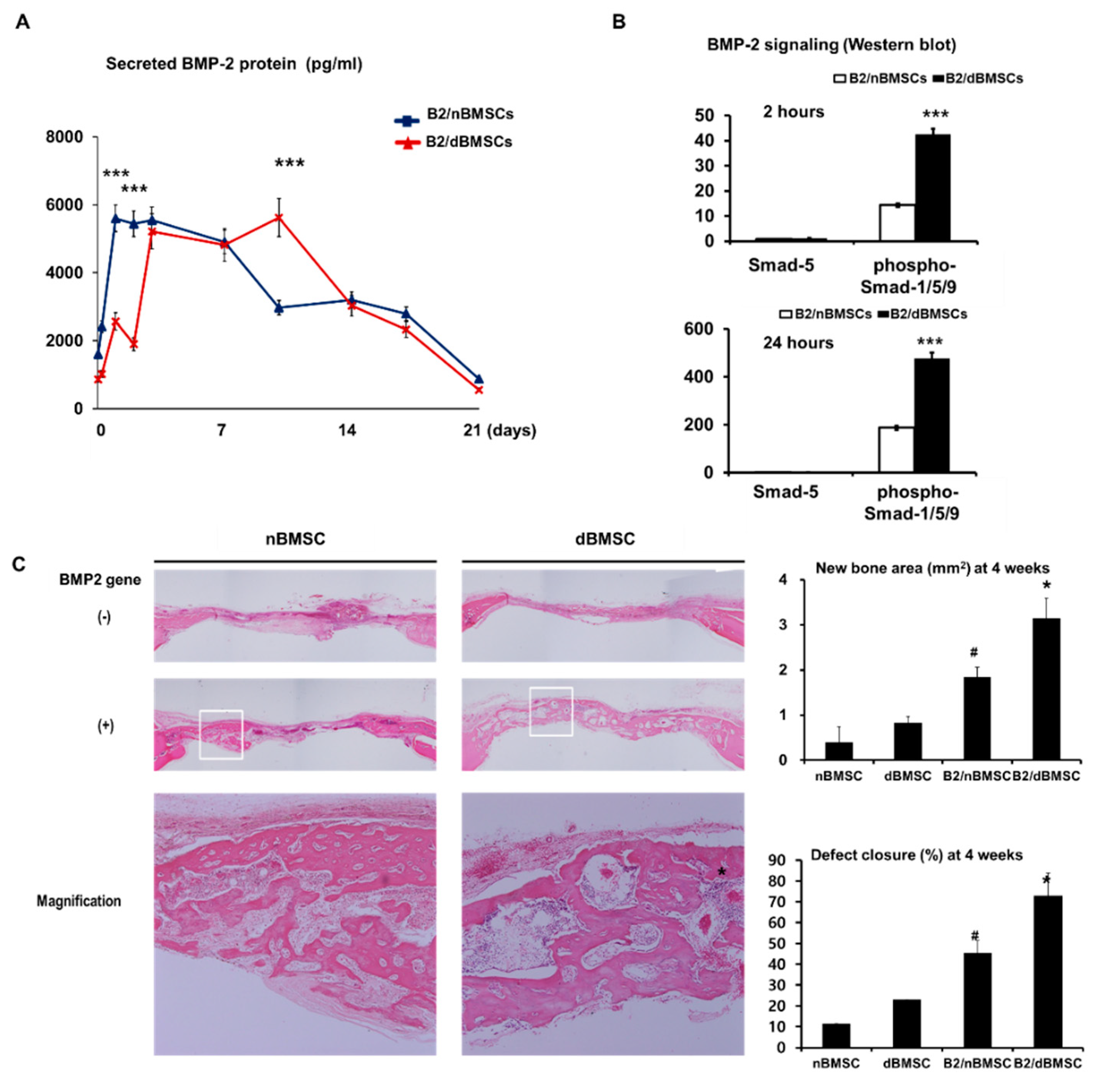
| Delivery Type | Advantages | Disadvantages |
|---|---|---|
| ex vivo | Gene transfer is limited to the target cell population and not to other cells or tissues | Expensive and time-consuming process |
| Can use gene transfer to genetically modify stem cells, e.g., embryonic stem cells and iPSCs [29,30] | Complicated manipulation including cell harvesting, cell expansion and transfection | |
| High efficacy | The outcome can be influenced by the carrier cells [31,32] | |
| Low quantity of vectors is necessary for desired therapeutic effects | ||
| Minimal immune recognition of the gene vectors [33] | ||
| in vivo | Simple process via direct injection into the site or intravenous administration | Low efficacy |
| Avoids complicated process related to cells | High quantity of vectors is necessary for desired therapeutic effects | |
| Relatively low cost | Induction of immune reaction due to direct exposure of vectors | |
| Difficult to target the cell population of interest | ||
| Vector system is potentially toxic [34] |
| Type of Vectors | Advantages | Disadvantages |
|---|---|---|
| Viral vectors | High gene transduction efficiency | Difficult to manufacture, produced in low virus titers |
| Transgene expression can be controlled by virus (transient expression or persistent expression) | Immune reactions to virus [56] | |
| Can target specific cell types such as dividing cells or non-dividing cells [33,57] | Limitation in packaging capacity e.g., 4.5 kb for AAV vectors [33,57] | |
| Safety concerns e.g., insertion mutagenesis [58] | ||
| Non-viral vectors | Simple manufacturing | Low in vivo gene transduction efficiency [57] |
| Low cost | High quantity for therapeutic effects | |
| Low immunogenicity | Cannot target specific cell types | |
| High packaging capacity | Toxicity related to materials [34] |
| References | Cells | Vectors | Transgene | Carrier | Model | Results |
|---|---|---|---|---|---|---|
| Lee et al. 2001,2002 [36,37] | Muscle-derived cells | Adenovirus | BMP-2 | Collagen sponge | Mouse calvarial defect | Mouse calvarial defects treated with BMP-2-producing muscle cells had >85% closure within two weeks and 95–100% closure within four weeks. |
| Blum et al. 2003 [68] | MSCs | Adenovirus Retrovirus Cationic lipid | BMP-2 | Titanium mesh scaffold | Rat calvarial defect | All viral and non-viral vectors carrying the BMP-2 gene were effective in bone regeneration. However, adenoviral vectors resulted in slightly significantly increased amounts of newly formed bone compared to those achieved with other vectors and the control group. |
| Hirata et al. 2003 [51] | Skin fibroblasts | Adenovirus | BMP-2 or Runx2 | PDLLGA /gelatin sponge | Rat calvarial defect | AdBMP-2-transplanted skin fibroblasts were effective on new bone formation. However, cells with AdRunx2 were insufficient in inducing bone repair. |
| Park et al. 2003 [35] | BMSCs | Adenovirus Liposome | BMP-2 | Collagen sponge | Rat mandibular defect | Both liposome-mediated and adenoviral BMP-2 gene transfer to BMSCs successfully achieved the healing of critical-size bone defects in rats. |
| Gafni et al. 2004 [63] | MSCs | AAV | BMP-2 | Collagen sponge | Mouse calvarial defect | AAV-BMP-2 with a tetracycline-sensitive promotor was effective in regulation of bone formation by gene therapy. |
| Hu et al. 2007 [40] | Fibroblasts | Adenovirus | BMP-2 | Gelatin sponge, HA disc | Rat calvarial defect | Lyophilized AdBMP-2 in a gelatin sponge was more effective than the free form of adBMP-2 in rat calvarial defects. |
| Koh et al. 2008 [76] | Fibroblasts | Adenovirus | BMP-2/7 | Gelatin sponge | Mouse calvarial defect | AdBMP-2/7-transduced cells were more effective in healing cranial defects than were cells individually transduced with AdBMP-2 or BMP7. |
| Tang et al. 2008 [74] | BMSCs | Liposome/Plasmid | BMP-2 | Coral hydroxyapatite matrix | Rat mandibular defect osteoporotic model | Autogenous cells transfected with pBMP-2 promoted bone formation in osteoporotic rats. |
| Steinhardt et al. 2008 [43] | BMSCs | Adenovirus | BMP-2 | Collagen sponge | Mouse mandibular defect | Application of genetically engineered BMP-2-producing BMSCs into a mandibular defect led to tissue regeneration at the defect site. |
| Wang et al. 2009 [69] | Skin fibroblasts | Retrovirus | BMP-2 | Gelatin sponge | Rat calvarial defect | Autologous BMP-2-modified skin fibroblasts successfully led to bone regeneration in rat calvarial defects. Fibroblasts could be effectively used in ex vivo gene therapy for local bone repair. |
| Chang et al. 2009 [44] | BMSCs | Adenovirus | BMP-2 | Gelatin/ tricalcium phosphate ceramic/ glutaraldehyde biopolymer | Rat calvarial defect | AdBMP-2-transfected cells with the gelatin/tricalcium phosphate ceramic/glutaraldehyde biopolymer strongly enhanced the bone healing of critical-size bicortical craniofacial defects. |
| Shin et al. 2010 [55] | Human gingival fibroblasts (HGF) | Adenovirus | BMP-2 | Collagen matrix | Rat calvarial defect | AdBMP-2-transfected HGF promoted osseous healing of calvarial defects compared with that achieved in the other groups. |
| Chuang et al. 2010 [64] | Human MSCs | Baculovirus | BMP-2 | PLGA scaffolds | Rat calvarial defect | Although a baculovirus was effective for BMP-2 gene transfer into cells, the use of hMSCs could not overcome the immunological barrier in rats. |
| Kroczek et al. 2010 [77] | BMSCs | Plasmid-liposome | BMP-2 | Direct injection with an aqueous solution of osteoinductive substances | Minipig distraction osteogenesis | BMP-2 expression was maximal in the pBMP-2 group although bone regeneration was not significantly enhanced in the pBMP-2 group compared to that in the rhBMP-2 and rhBMP-7 groups. |
| Xia et al. 2011 [78] | BMSCs | Adenovirus | BMP-2 Nell-1 | Beta-tricalcium phosphate | Rabbit maxillary sinus graft | BMP-2 and Nell-1 genes showed a synergistic effect on osteogenic differentiation of BMSCs and promoted new bone formation and maturation in a rabbit maxillary sinus model. |
| Lin et al. 2012 [46] | BMSCs | Baculovirus | BMP-2 VEGF | Disc-shaped PLGA scaffolds | Rabbit calvarial defect | Baculoviral vectors were effective in BMSCs for sustained BMP-2/VEGF expression and the repair of critical-size calvarial defects. |
| He et al. 2013 [82] | MSCs EPCs | Adenovirus | BMP-2 | Injectable and porous nano calcium sulfate/alginate | Rat calvarial defect | The combination of BMP-2 gene-modified MSCs and EPCs in injectable scaffolds increased new bone and vascular formation. |
| Park et al. 2013 [45] | BMSCs | Adenovirus | BMP-2 | Collagen gel | Rat calvarial defect | Dual delivery of autologous AdBMP-2-transfected BMSCs and rhPDGF-BB enhanced both the quality and quantity of new bone formation. |
| Jhin et al. 2013 [79] | BMSCs | Adenovirus | BMP-2 | Deproteinized bovine bone mineral | Rabbit maxillary sinus Dental implant placement | BMSCs with AdBMP-2 transfection resulted in earlier bone healing with increased amounts in the maxillary sinus defects when dental implants were simultaneously placed. |
| Jin et al. 2014 [83] | BMSCs | PEI-alginate/Plasmid | BMP-2 | Cell sheet | Rat calvarial defect | PEI-al nanocomposites as a carrier for pBMP-2 gene delivery to BMSCs was effective. Bone regeneration was slightly enhanced by BMP-2- producing BMSCs compared to that in the control group. |
| Liao et al. 2014 [47] | ASCs | Baculovirus | BMP-2/miR-148b | Disc-shaped poly (L-lactide-co-glycolide) (PLGA) scaffolds | Mouse calvarial defect | Co-transduction of hASCs with BMP-2/miR-148b via baculovirus vectors enhanced and prolonged BMP-2 expression and synergistically promoted bone regeneration. |
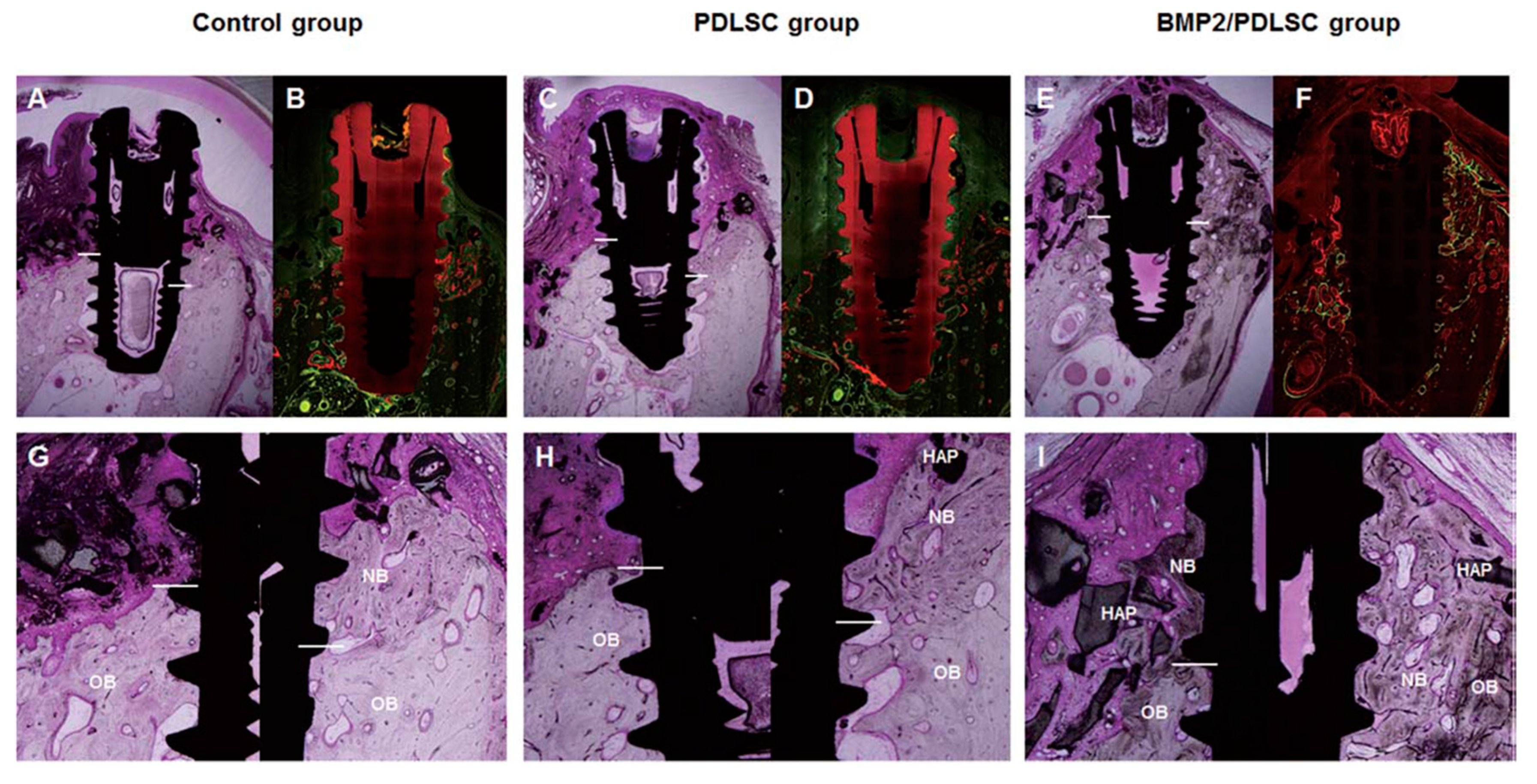
| Park et al. 2015 [39] | PDLSCs | Adenovirus | BMP-2 | HA particle with collagen gel | Beagle peri-implantitis defect | PDLSCs transfected by AdBMP-2 produced significantly greater amounts of new bone in peri-implantitis defects than those produced in other groups. |
| Keeney et al. 2016 [54] | Skull-derived osteoblasts | Cationic amine polymer/Plasmid | BMP-2 | PLGA | Mouse calvarial defect | Skull-derived osteoblasts transfected by pBMP-2 led to substantially accelerated bone repair as early as two weeks, which continued to progress over 12 weeks. |
| Yi et al. 2016 [52] | Human PDLSCs | Adenovirus | BMP-2 | Block-type biphasic calcium phosphate | Rat calvarial defect | hPDLSCs showed an inhibitory action on BMP-2-induced osteogenic differentiation. hPDLSCs-transfected AdBMP-2 produced lower amounts of newly formed bone than did hPDLSCs with rhBMP-2 protein. |
| Xu et al. 2016 [38] | ASCs | Adenovirus | BMP-2 | Beta-tricalcium phosphate | Beagle peri-implantitis defect | ASCs transfected by adenoviral BMP-2 produced significant amounts of new bone formation and re-osseointegration compared to those in control groups. |
| Vural et al. 2017 [84] | BMSCs | Liposome/Plasmid | BMP-2 | Gelatin sponge | Rat calvarial defect | pBMP-2 gene delivery in BMSCs effectively led to bone regeneration in rat calvarial defects. |
| Park et al. 2018 [32] | BMSCs | Adenovirus | BMP-2 | Collagen gel | Rat calvarial defect Diabetic model | In diabetic animals, BMP-2 gene therapy using diabetic cells was more effective in new bone formation than was BMP-2 gene therapy using non-diabetic cells. |
| References | Administration | Vector | Transgene | Model | Results |
|---|---|---|---|---|---|
| Alden et al. 2000 [87] | Direct injection | Adenovirus | BMP-2 BMP9 | Rat mandible defect | Significant bone healing was observed in the BMP gene transfer group. |
| Ashinoff et al. 2004 [100] | Direct injection | Adenovirus | BMP-2 | Rat distraction osteogenesis | Local injection of AdBMP-2 increased bone regeneration during distraction osteogenesis. |
| Chew et al. 2011 [97] | Gelatin microparticle | Triacrylate/ amine polymer/ Plasmid | BMP-2 | Rat calvarial defect | Triacrylate/amine gelatin effectively slowed the degradation rate compared to that of naked pDNA. |
| Zhang et al. 2011 [28] | Fibronectin/apatite | Plasmid | BMP-2 | Rat calvarial defect | Bone formation in the pBMP-2 with fibronectin/hydroxyapatite group was enhanced compared to that in the control group. |
| Wu et al. 2012 [93] | Electroporation | Plasmid | BMP-2 BMP-2/ VEGF | Rabbit distraction osteogenesis | pBMP-2/VEGF gene transfer with electroporation was effective for bone regeneration relative to the control. |
| Liu et al. 2012 [104] | Muscle tissue | Adenovirus | BMP-2 | Rat calvarial defect | The amount of new bone in muscle tissue transduced with AdBMP-2 was more than twice that in the control. |
| Ben Arav et al. 2012 [89] | Bone allograft | AAV | BMP-2 | Mouse calvarial defect | Self-complementary-rAAV-BMP-2-coated allografts were more effective for bone regeneration than were single strand-rAAV-BMP-2-coated allografts, the effects of which were not significantly different from those of autografts or uncoated allografts. |
| Qiao et al. 2013 [100] | PLGA nanoparticle | PEI nanoparticles encapsulated by PLGA/plasmid | BMP-2 | Rat calvarial defect | pBMP-2 gene delivery using a PLGA nanoparticle delivery system was effective for producing BMP-2 cDNA and new bone formation. |
| Kolk et al. 2016 [102] | Poly(d, l-lactide) (PDLLA)-coated titanium disc | PEI/plasmid | BMP-2 | Rat mandibular defect | pBMP-2 gene delivery using a copolymer was successful for controlling new bone formation with an inverse dose dependency. |
| Xie et al. 2017 [88] | Direct injection | Plasmid | BMP-2/VEGF | Rabbit distraction osteogenesis | The direct injection of pBMP-2/VEGF promoted bone formation in the distraction gap with the upregulation of TGF-β1 expression. |
| Li et al. 2017 [98] | Injectable thermosensitive hydrogel scaffold | Chitosan/plasmid | BMP-2 | Rat calvarial defect Dog mandibular defect | An injectable chitosan-based thermosensitive hydrogel scaffold (CS/CSn-GP) enhanced new bone formation in rat calvarial defects and bony defect healing in beagle dogs. |
| Zhu et al. 2017 [103] | Electrospun PLGA nanofibrous scaffold | Adenovirus | BMP-2 | Rat calvarial defect | A lyophilized PLGA nanofibrous scaffold efficiently released functional AdBMP-2 to transduce local cells, resulting in hBMP-2 secretion and promoting new bone formation in vivo. |
| Kawai et al. 2017 [94] | Electroporation | Plasmid | BMP-2/7 | Rat periodontal tissue | The mineral apposition rate of the alveolar bone following BMP-2/7 gene transfer was significantly higher than that in the control group. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.-Y.; Kim, K.-H.; Kim, S.; Lee, Y.-M.; Seol, Y.-J. BMP-2 Gene Delivery-Based Bone Regeneration in Dentistry. Pharmaceutics 2019, 11, 393. https://doi.org/10.3390/pharmaceutics11080393
Park S-Y, Kim K-H, Kim S, Lee Y-M, Seol Y-J. BMP-2 Gene Delivery-Based Bone Regeneration in Dentistry. Pharmaceutics. 2019; 11(8):393. https://doi.org/10.3390/pharmaceutics11080393
Chicago/Turabian StylePark, Shin-Young, Kyoung-Hwa Kim, Sungtae Kim, Yong-Moo Lee, and Yang-Jo Seol. 2019. "BMP-2 Gene Delivery-Based Bone Regeneration in Dentistry" Pharmaceutics 11, no. 8: 393. https://doi.org/10.3390/pharmaceutics11080393
APA StylePark, S.-Y., Kim, K.-H., Kim, S., Lee, Y.-M., & Seol, Y.-J. (2019). BMP-2 Gene Delivery-Based Bone Regeneration in Dentistry. Pharmaceutics, 11(8), 393. https://doi.org/10.3390/pharmaceutics11080393





