Design of a Novel Oxygen Therapeutic Using Polymeric Hydrogel Microcapsules Mimicking Red Blood Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Hydrogel Microcapsules
2.3. Optimization of Hydrogel Microcapsule Preparation Process
2.4. Determination of Hemoglobin Encapsulation Efficiency
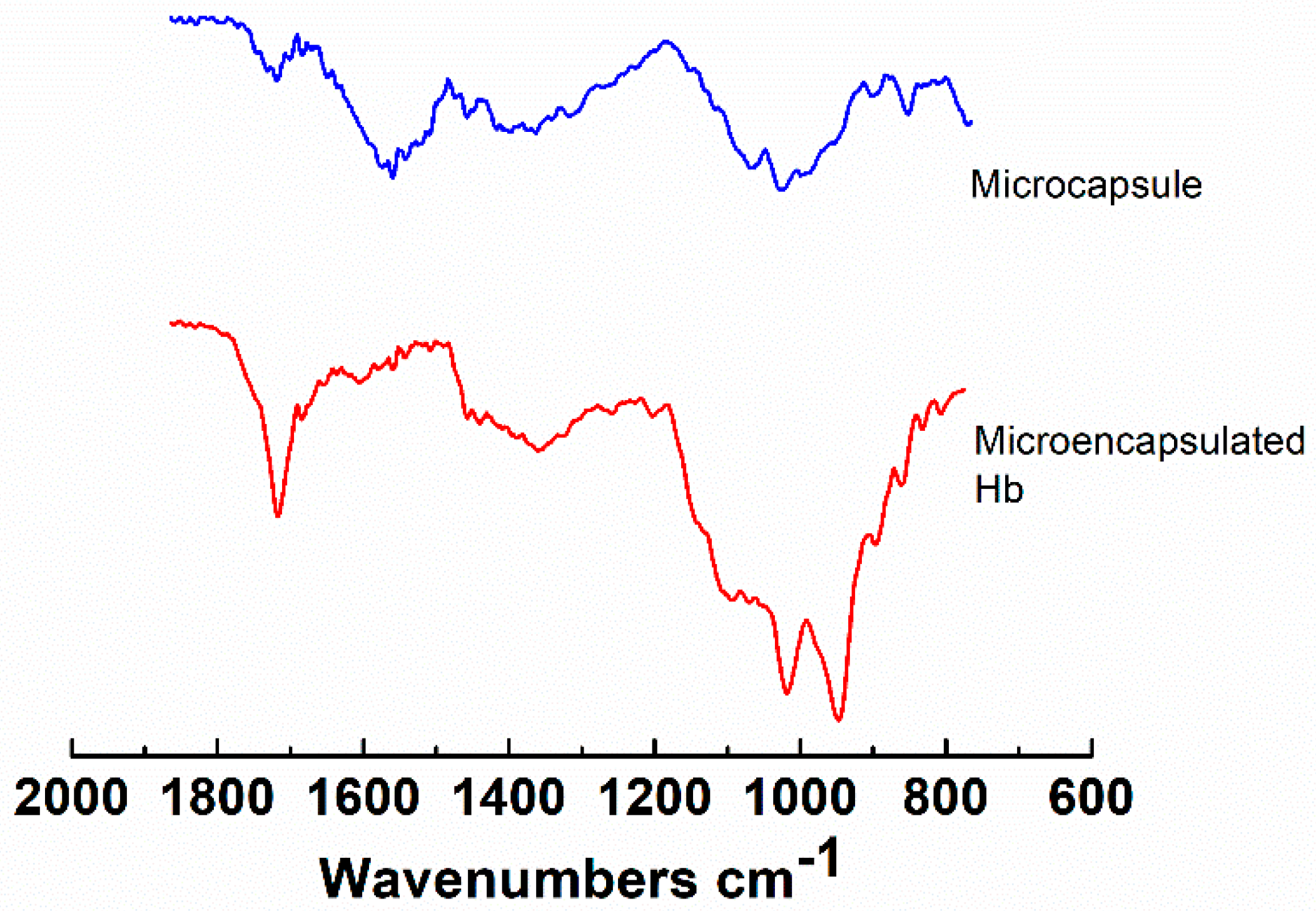
2.5. Characterization of the Hydrogel Microcapsules
3. Results and Discussion
3.1. Formulation of Hydrogel Microcapsules
3.2. Hydrogel Microcapsule Preparation Process Optimization
3.3. Hemoglobin Encapsulation Efficiency
4. Conclusions
5. Patent
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Facts about Blood Supply In The U.S. Available online: https://www.redcrossblood.org/donate-blood/how-to-donate/how-blood-donations-help/blood-needs-blood-supply.html (accessed on 14 August 2019).
- World Health Organization. Blood Donor Selection: Guidelines on Assessing Donor Suitability for Blood Donation; World Health Organization: Geneva, Switzerland, 2012; Available online: https://apps.who.int/iris/handle/10665/76724 (accessed on 14 August 2019).
- Chen, J.Y.; Scerbo, M.; Kramer, G. A review of blood substitutes: Examining the history, clinical trial results, and ethics of hemoglobin-based oxygen carriers. Clinics (Sao Paulo) 2009, 64, 803–813. [Google Scholar] [CrossRef]
- Alayash, A.I. Blood substitutes: Why haven’t we been more successful? Trends Biotechnol 2014, 32, 177–185. [Google Scholar] [CrossRef]
- Zhang, W.; Bissen, M.J.; Savela, E.S.; Clausen, J.N.; Fredricks, S.J.; Guo, X.; Paquin, Z.R.; Dohn, R.P.; Pavelich, I.J.; Polovchak, A.L.; et al. Design of Artificial Red Blood Cells using Polymeric Hydrogel Microcapsules: Hydrogel Stability Improvement and Polymer Selection. Int. J. Artif. Organs 2016, 39, 518–523. [Google Scholar] [CrossRef]
- Zhang, H.; Barralet, J.E. Mimicking oxygen delivery and waste removal functions of blood. Adv. Drug Deliv. Rev. 2017, 122, 84–104. [Google Scholar] [CrossRef]
- Kawaguchi, A.T.; Okamoto, Y.; Kise, Y.; Takekoshi, S.; Murayama, C.; Makuuchi, H. Effects of liposome-encapsulated hemoglobin on gastric wound healing in the rat. Artif. Organs 2014, 38, 641–649. [Google Scholar] [CrossRef] [PubMed]
- Murayama, C.; Kawaguchi, A.T.; Kamijo, A.; Naito, K.; Iwao, K.; Tsukamoto, H.; Yasuda, K.; Nagato, Y. Liposome-encapsulated hemoglobin enhances chemotherapy to suppress metastasis in mice. Artif. Organs 2014, 38, 656–661. [Google Scholar] [CrossRef]
- She, S.; Li, Q.; Shan, B.; Tong, W.; Gao, C. Fabrication of red-blood-cell-like polyelectrolyte microcapsules and their deformation and recovery behavior through a microcapillary. Adv. Mater. 2013, 25, 5814–5818. [Google Scholar] [CrossRef] [PubMed]
- Yadav, V.R.; Nag, O.; Awasthi, V. Biological evaluation of liposome-encapsulated hemoglobin surface-modified with a novel PEGylated nonphospholipid amphiphile. Artif. Organs 2014, 38, 625–633. [Google Scholar] [CrossRef]
- Tao, Z.; Ghoroghchian, P.P. Microparticle, nanoparticle, and stem cell-based oxygen carriers as advanced blood substitutes. Trends Biotechnol. 2014, 32, 466–473. [Google Scholar] [CrossRef] [PubMed]
- Crouse, J.Z.; Mahuta, K.M.; Mikulski, B.A.; Harvestine, J.N.; Guo, X.; Lee, J.C.; Kaltchev, M.G.; Midelfort, K.S.; Tritt, C.S.; Chen, J.; et al. Development of a Microscale Red Blood Cell-Shaped Pectin-Oligochitosan Hydrogel System Using an Electrospray-Vibration Method: Preparation and Characterization. J. Appl. Biomater. Funct. Mater. 2015, 13, 326–331. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; He, X. Encapsulation of Living Cells in Small (∼100 μm) Alginate Microcapsules by Electrostatic Spraying: A Parametric Study. J. Biomech. Eng. 2009, 131, 074515. [Google Scholar] [CrossRef] [PubMed]
- Merkel, T.J.; Jones, S.W.; Herlihy, K.P.; Kersey, F.R.; Shields, A.R.; Napier, M.; Luft, J.C.; Wu, H.; Zamboni, W.C.; Wang, A.Z.; et al. Using mechanobiological mimicry of red blood cells to extend circulation times of hydrogel microparticles. Proc. Natl. Acad. Sci. USA 2011, 108, 586. [Google Scholar] [CrossRef]
- López-Rubio, A.; Sanchez, E.; Wilkanowicz, S.; Sanz, Y.; Lagaron, J.M. Electrospinning as a useful technique for the encapsulation of living bifidobacteria in food hydrocolloids. Food Hydrocoll. 2012, 28, 159–167. [Google Scholar] [CrossRef]
- Mai, Z.; Chen, J.; He, T.; Hu, Y.; Dong, X.; Zhang, H.; Huang, W.; Ko, F.; Zhou, W. Electrospray biodegradable microcapsules loaded with curcumin for drug delivery systems with high bioactivity. RSC Adv. 2017, 7, 1724–1734. [Google Scholar] [CrossRef]
- Hayashi, K.; Ono, K.; Suzuki, H.; Sawada, M.; Moriya, M.; Sakamoto, W.; Yogo, T. Electrosprayed synthesis of red-blood-cell-like particles with dual modality for magnetic resonance and fluorescence imaging. Small 2010, 6, 2384–2391. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Yamada, S.; Hayashi, H.; Sakamoto, W.; Yogo, T. Red blood cell-like particles with the ability to avoid lung and spleen accumulation for the treatment of liver fibrosis. Biomaterials 2018, 156, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Soares, R.M.D.; Siqueira, N.M.; Prabhakaram, M.P.; Ramakrishna, S. Electrospinning and electrospray of bio-based and natural polymers for biomaterials development. Mater. Sci. Eng. C 2018, 92, 969–982. [Google Scholar] [CrossRef]
- Lai, W.-F.; Susha, A.S.; Rogach, A.L.; Wang, G.; Huang, M.; Hu, W.; Wong, W.-T. Electrospray-mediated preparation of compositionally homogeneous core–shell hydrogel microspheres for sustained drug release. RSC Adv. 2017, 7, 44482–44491. [Google Scholar] [CrossRef]
- Moghaddam, M.K.; Mortazavi, S.M.; Khayamian, T. Preparation of calcium alginate microcapsules containing n-nonadecane by a melt coaxial electrospray method. J. Electrost. 2015, 73, 56–64. [Google Scholar] [CrossRef]
- Bock, N.; Woodruff, M.A.; Hutmacher, D.W.; Dargaville, T.R. Electrospraying, a Reproducible Method for Production of Polymeric Microspheres for Biomedical Applications. Polymers 2011, 3, 131–149. [Google Scholar] [CrossRef] [Green Version]
- Pathak, S.; Gupta, B.; Poudel, B.K.; Tran, T.H.; Regmi, S.; Pham, T.T.; Thapa, R.K.; Kim, M.-S.; Yong, C.S.; Kim, J.O.; et al. Preparation of High-Payload, Prolonged-Release Biodegradable Poly(lactic-co-glycolic acid)-Based Tacrolimus Microspheres Using the Single-Jet Electrospray Method. Chem. Pharm. Bull. 2016, 64, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Sha, B.; Zou, W.; Liang, X.; Meng, X.; Xu, H.; Tang, J.; Wu, D.; Xu, L.; Zhang, H. Cationic amylose-encapsulated bovine hemoglobin as a nanosized oxygen carrier. Biomaterials 2011, 32, 9425–9433. [Google Scholar] [CrossRef] [PubMed]
- Kuenstner, J.T.; Norris, K.H. Spectrophotometry of Human Hemoglobin in the near Infrared Region from 1000 to 2500 nm. J. Near Infrared Spectrosc. 1994, 2, 59–65. [Google Scholar] [CrossRef]
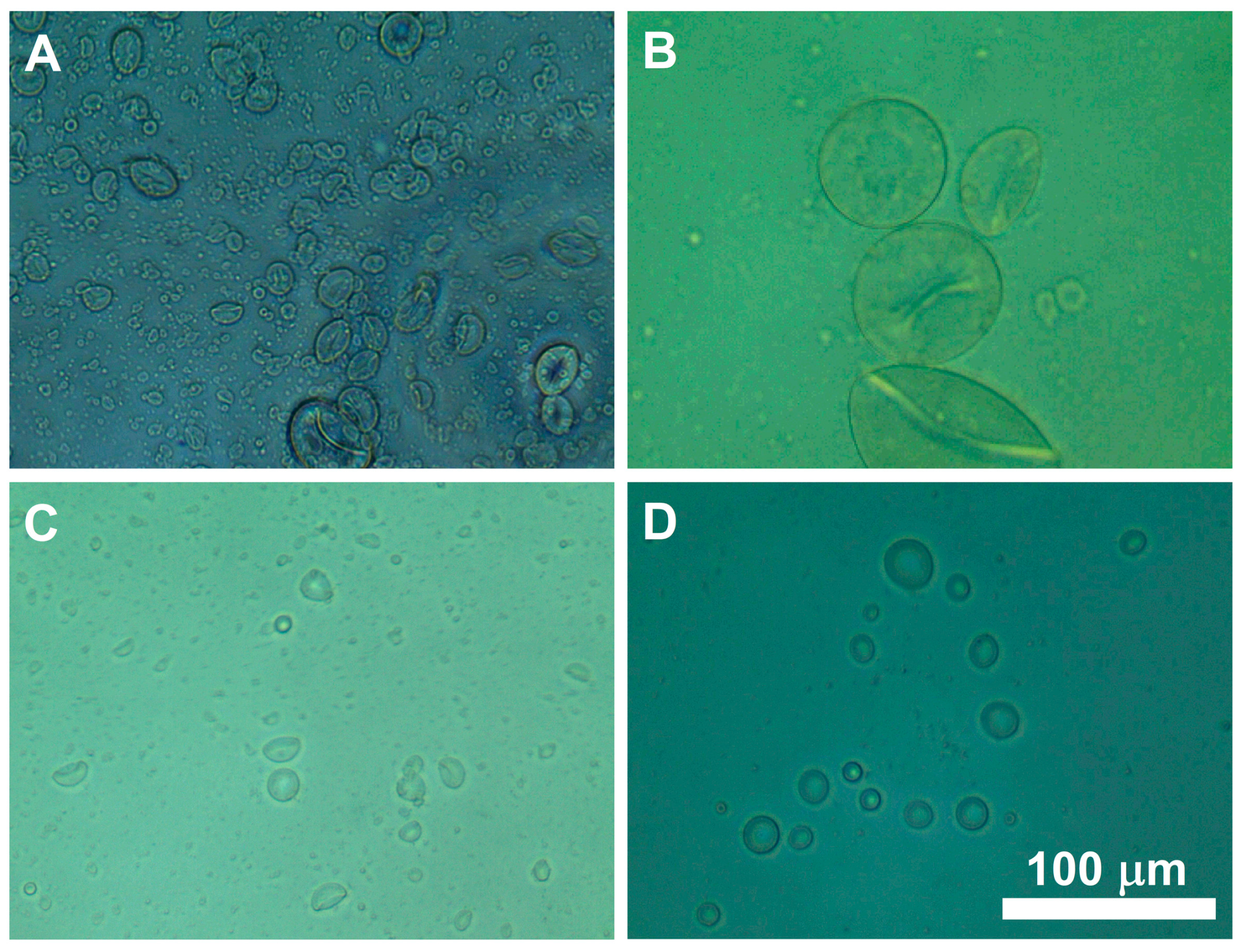
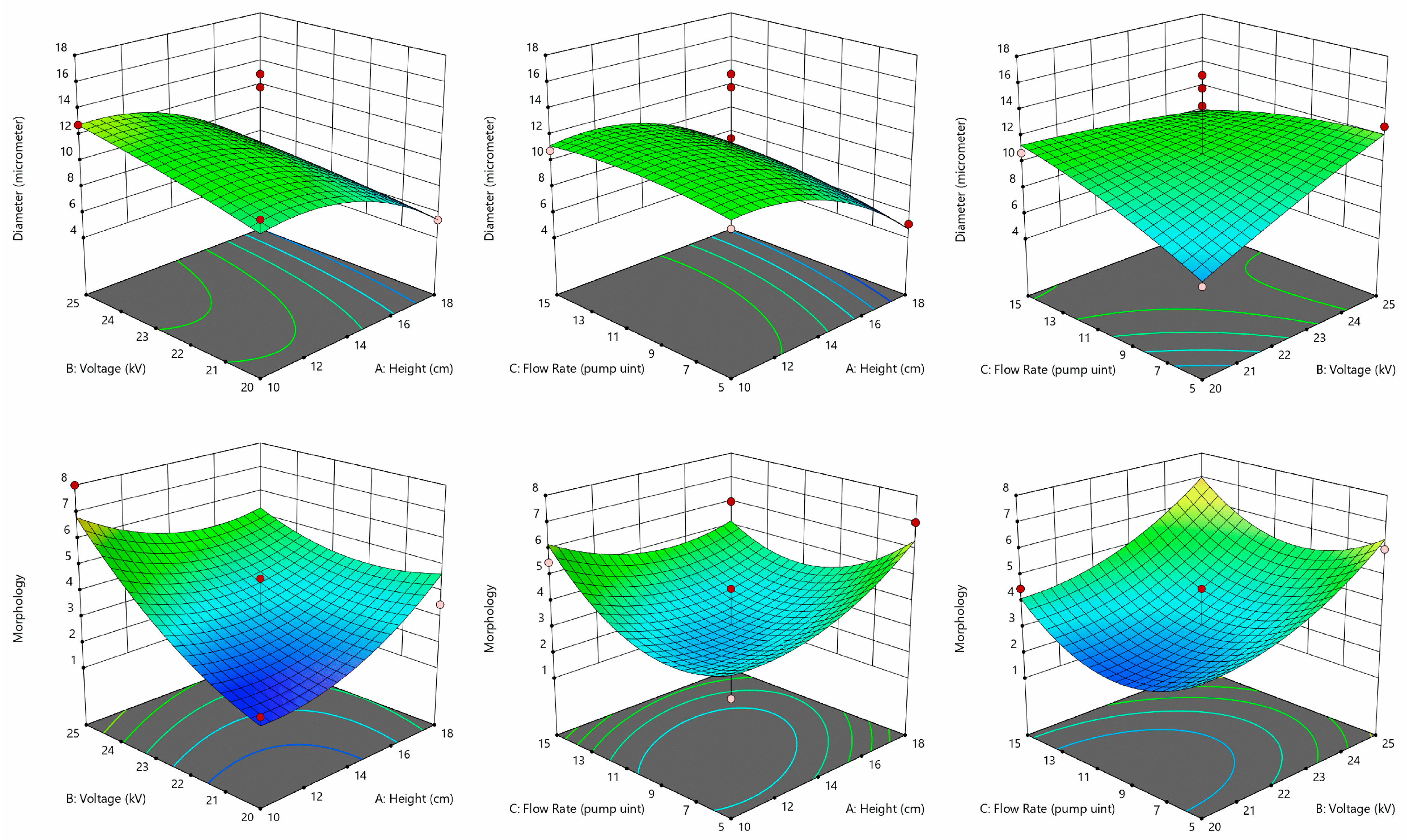
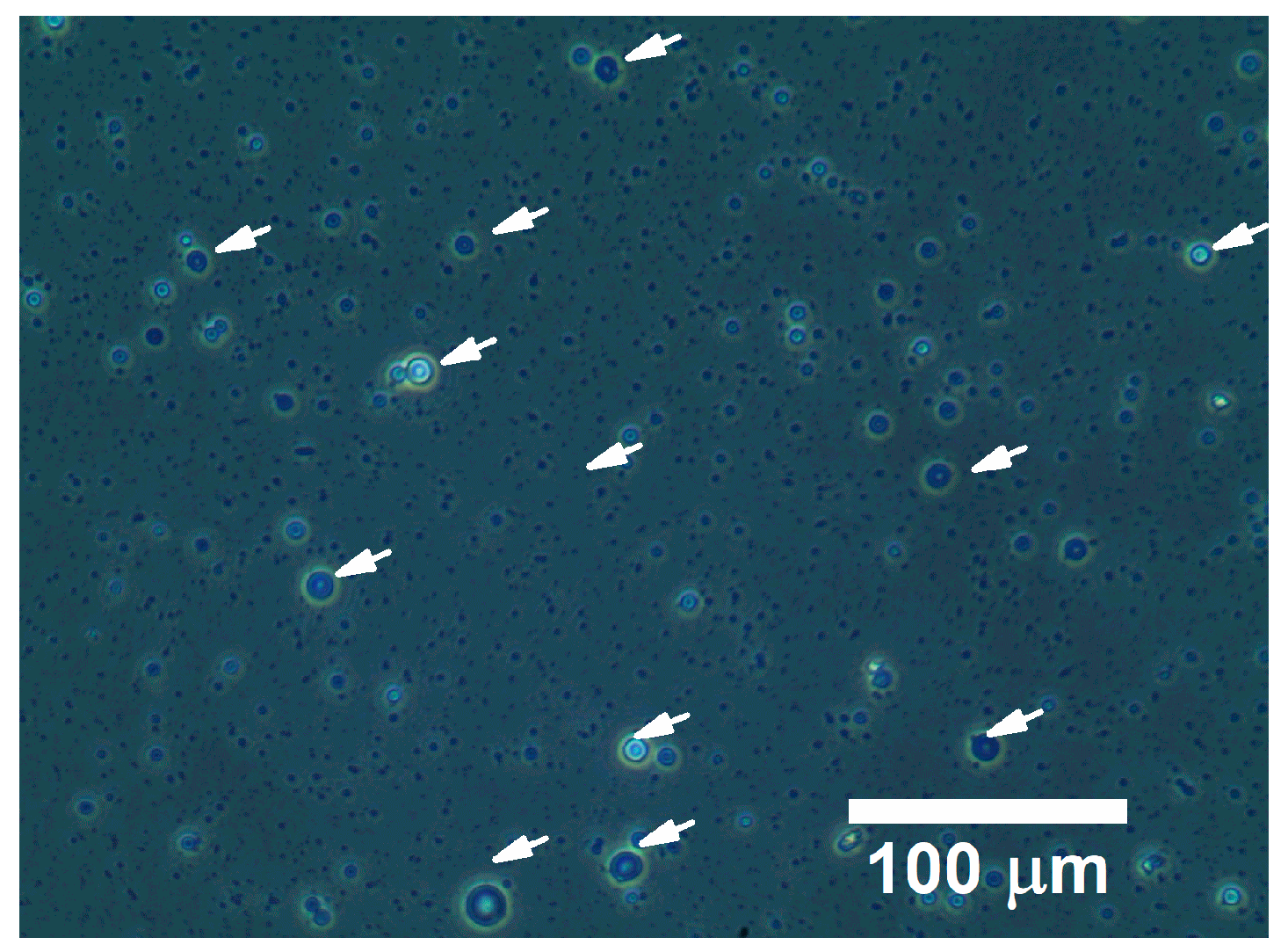
| Parameter | Range | Optimized Value |
|---|---|---|
| Voltage (kV) | 20–25 | 25 |
| Flow Rate 1 | 5–15 | 15 |
| Height (cm) | 10–18 | 13 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cherwin, A.; Namen, S.; Rapacz, J.; Kusik, G.; Anderson, A.; Wang, Y.; Kaltchev, M.; Schroeder, R.; O’Connell, K.; Stephens, S.; et al. Design of a Novel Oxygen Therapeutic Using Polymeric Hydrogel Microcapsules Mimicking Red Blood Cells. Pharmaceutics 2019, 11, 583. https://doi.org/10.3390/pharmaceutics11110583
Cherwin A, Namen S, Rapacz J, Kusik G, Anderson A, Wang Y, Kaltchev M, Schroeder R, O’Connell K, Stephens S, et al. Design of a Novel Oxygen Therapeutic Using Polymeric Hydrogel Microcapsules Mimicking Red Blood Cells. Pharmaceutics. 2019; 11(11):583. https://doi.org/10.3390/pharmaceutics11110583
Chicago/Turabian StyleCherwin, Amanda, Shelby Namen, Justyna Rapacz, Grace Kusik, Alexa Anderson, Yale Wang, Matey Kaltchev, Rebecca Schroeder, Kellen O’Connell, Sydney Stephens, and et al. 2019. "Design of a Novel Oxygen Therapeutic Using Polymeric Hydrogel Microcapsules Mimicking Red Blood Cells" Pharmaceutics 11, no. 11: 583. https://doi.org/10.3390/pharmaceutics11110583
APA StyleCherwin, A., Namen, S., Rapacz, J., Kusik, G., Anderson, A., Wang, Y., Kaltchev, M., Schroeder, R., O’Connell, K., Stephens, S., Chen, J., & Zhang, W. (2019). Design of a Novel Oxygen Therapeutic Using Polymeric Hydrogel Microcapsules Mimicking Red Blood Cells. Pharmaceutics, 11(11), 583. https://doi.org/10.3390/pharmaceutics11110583







