Doxorubicin-Loaded Delta Inulin Conjugates for Controlled and Targeted Drug Delivery: Development, Characterization, and In Vitro Evaluation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
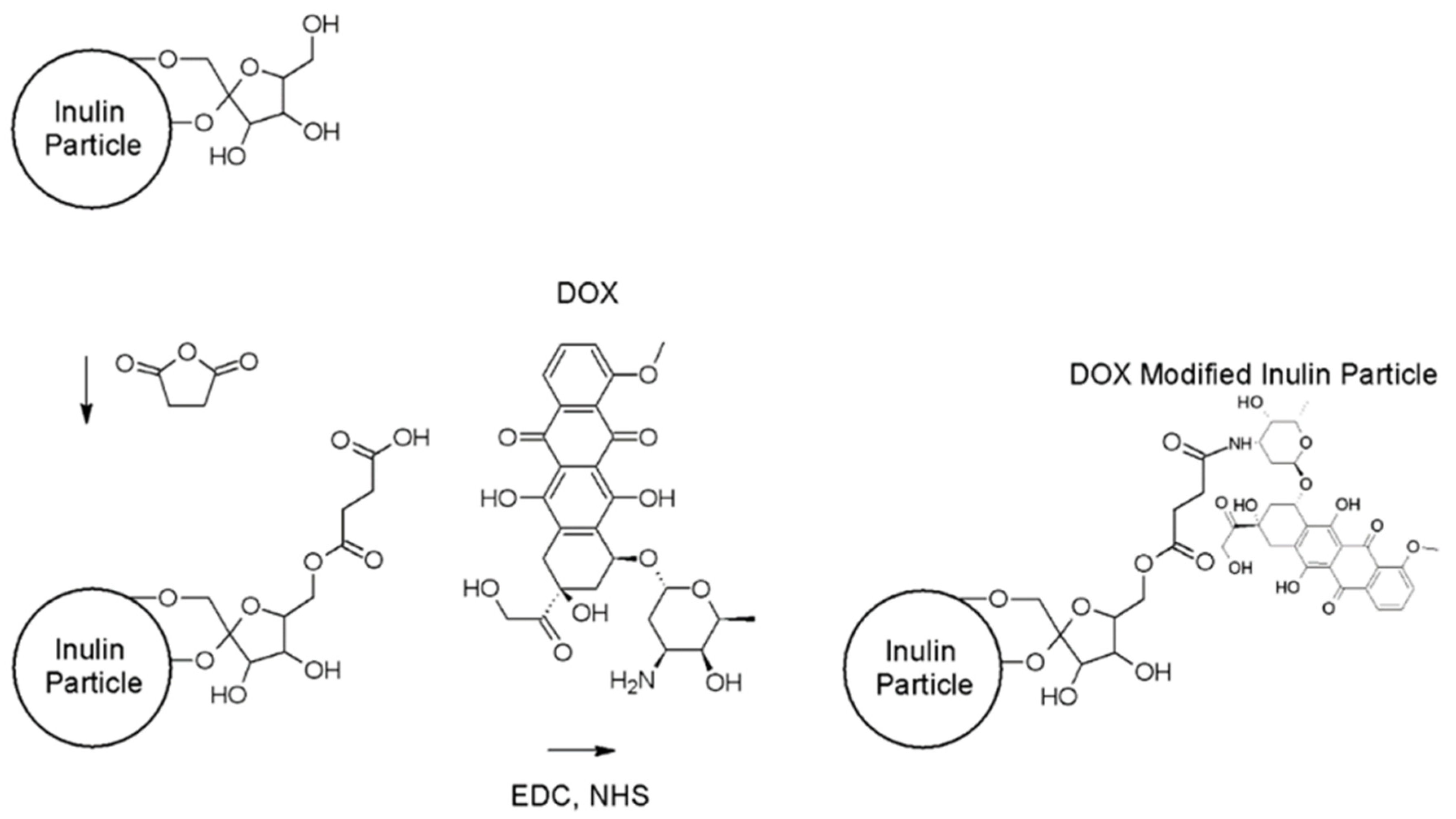
2.2.1. Synthesis of Doxorubicin-Loaded Microparticulate Inulin (MPI)
Modification of MPI Particles with Succinic Anhydride
Doxorubicin Attachment to MPI-Succinate Particles
2.2.2. Physicochemical Characterization of Doxorubicin-Loaded MPI
FTIR
NMR
SEM
2.2.3. Determination of Doxorubicin Loading
2.2.4. In Vitro Cleavage of MPI
Preparation of Artificial Lysosomal Fluid (ALF) and Simulated Body Fluid (SBF)
Cleavage of MPI
2.2.5. Study of Doxorubicin Release from MPI
Validation of the Analytical Method
Sample Preparation and Data Analysis
2.2.6. Cell Culture Conditions
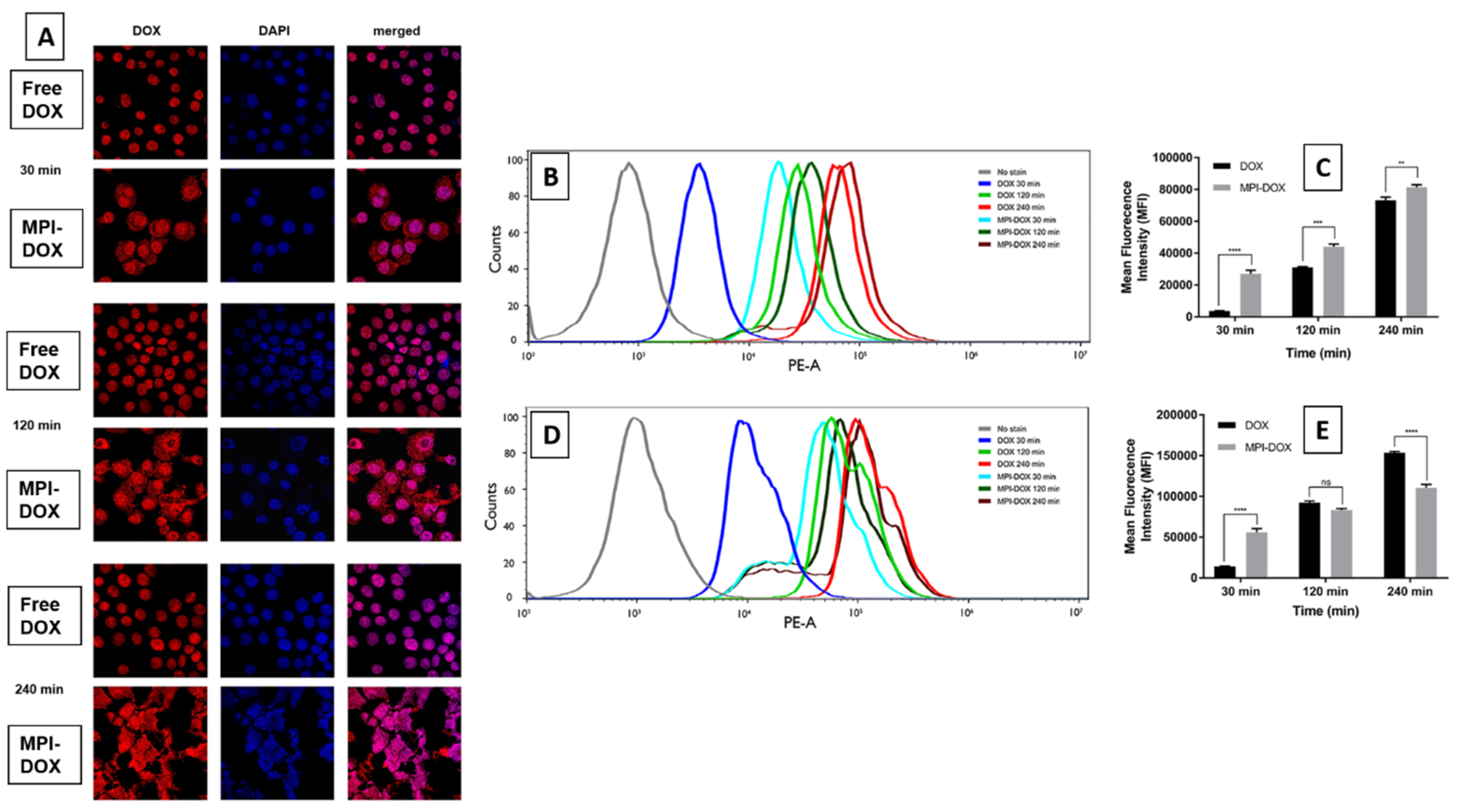
2.2.7. Cellular Uptake of Doxorubicin
Cell Internalization Observations
Flow Cytometry Measurements
2.2.8. In Vitro Cytotoxicity Assay
3. Results/Discussion
3.1. Synthesis and Characterization of MPI-Doxorubicin
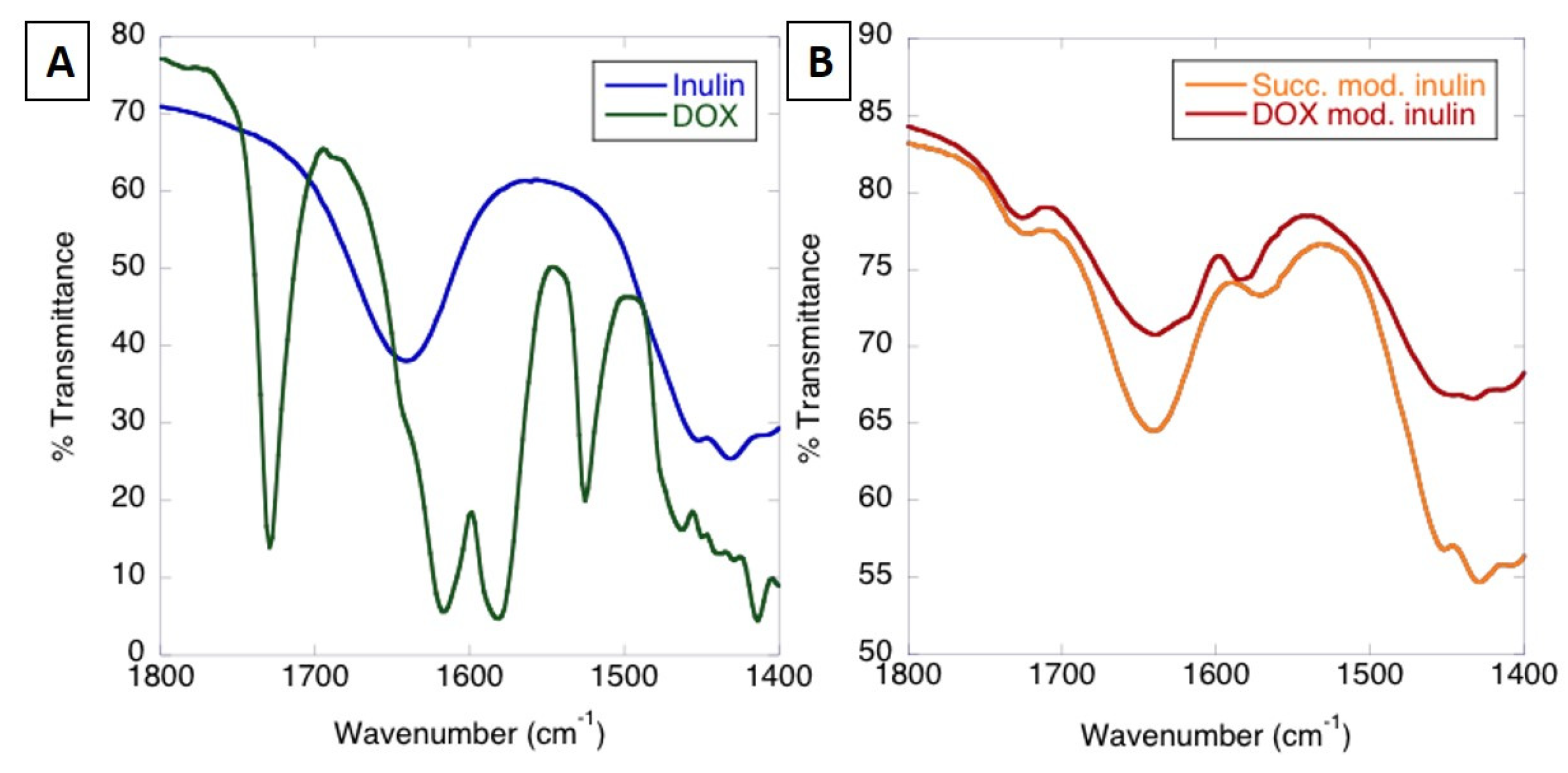
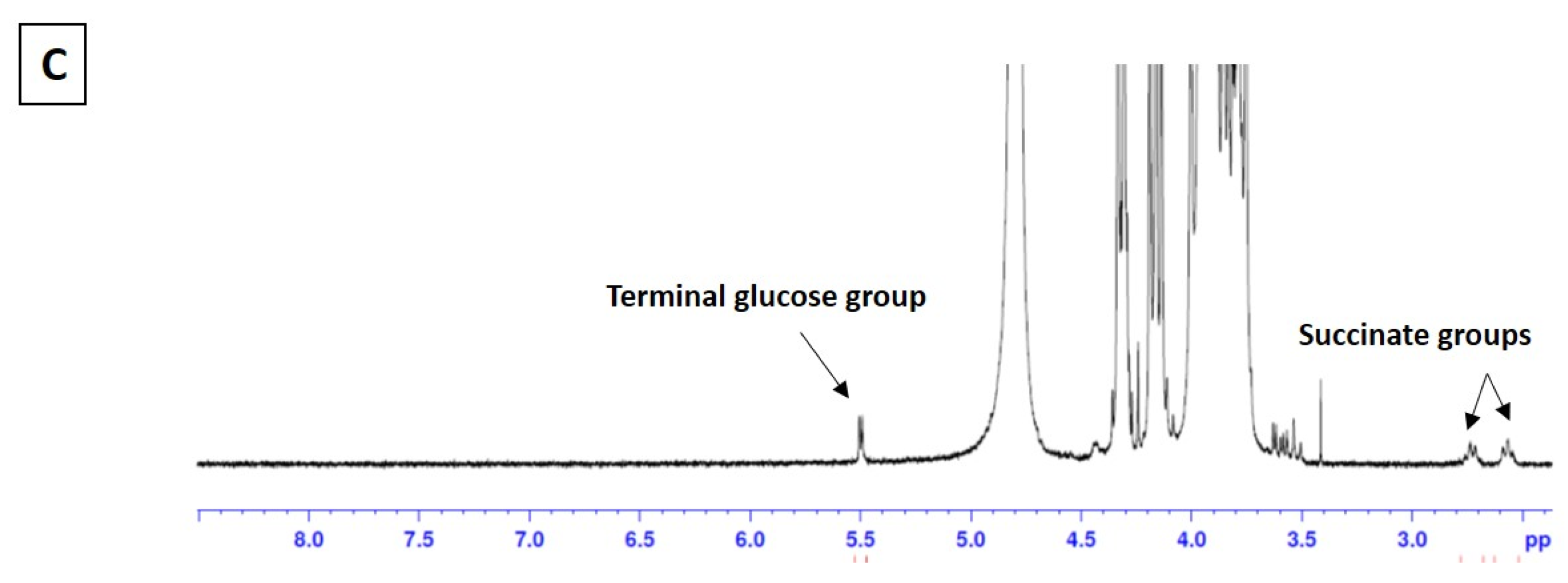
3.1.1. Analysis of MPI-Doxorubicin Using FTIR and NMR
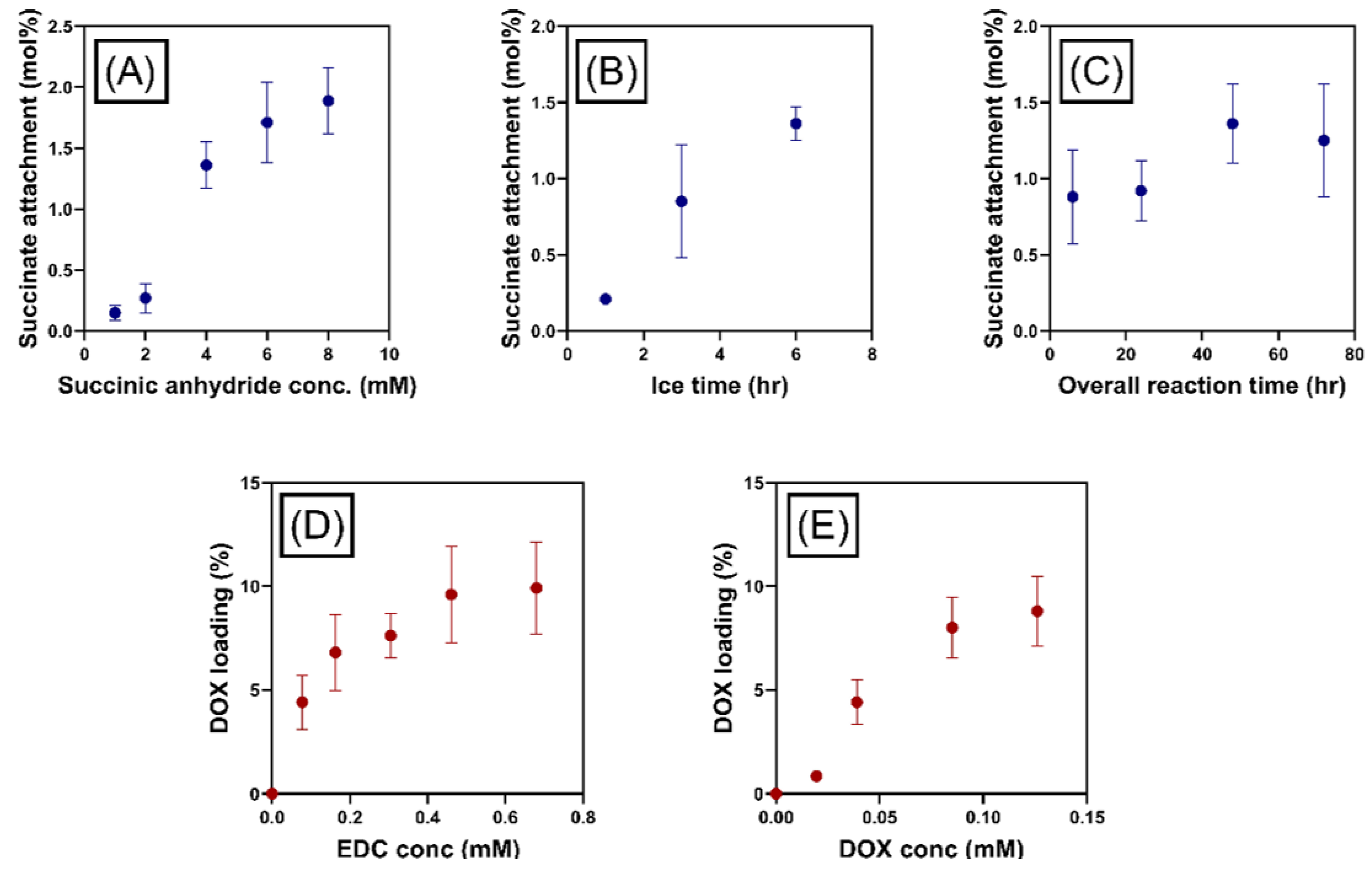
Optimization of Doxorubicin Attachment to MPI
3.1.2. Size and Morphology by SEM
3.2. In Vitro Hydrolysis of MPI
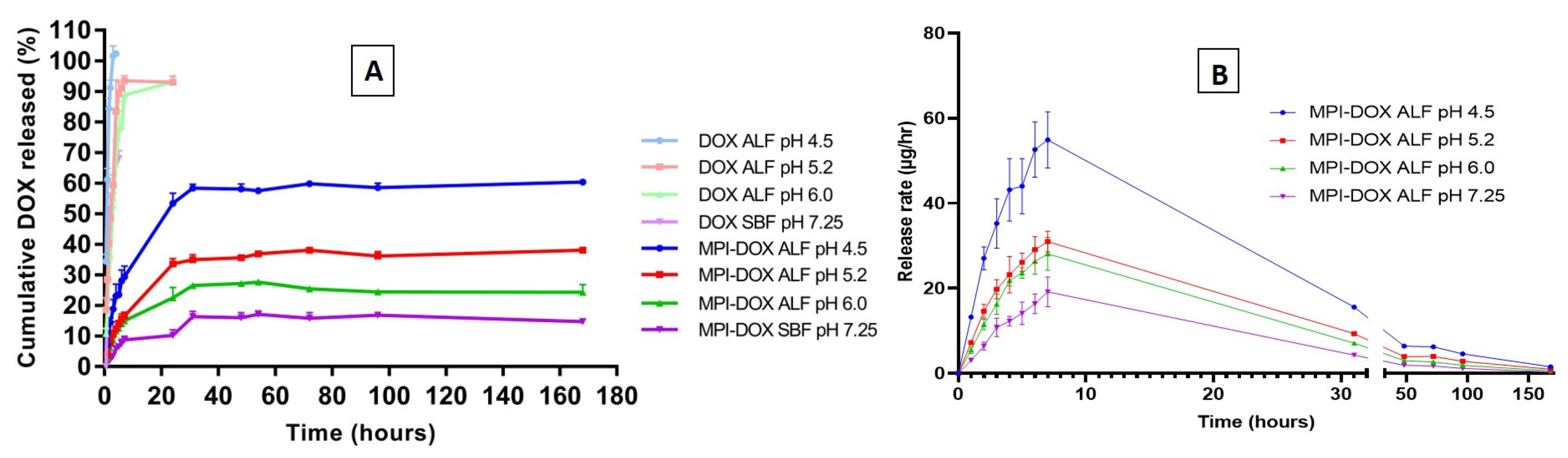
3.3. Doxorubicin Loading and Release Evaluations
3.3.1. Analytical Method Development and Validation
3.3.2. Percentage of Drug Loading Determination
3.3.3. Release Profiles of Doxorubicin-Loaded MPI in the Different Release Medium
3.4. Cellular Uptake of MPI-Doxorubicin by Monocytes Cells
3.5. In Vitro Cytotoxicity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Needham, L.A.; Davidson, A.H.; Bawden, L.J.; Belfield, A.; Bone, E.A.; Brotherton, D.H.; Bryant, S.; Charlton, M.H.; Clark, V.L.; Davies, S.J.; et al. Drug Targeting to Monocytes and Macrophages Using Esterase-Sensitive Chemical Motifs. J. Pharmacol. Exp. Ther. 2011, 339, 132–142. [Google Scholar] [CrossRef] [Green Version]
- Gilboa, E.; Berezhnoy, A.; Schrand, B. Reducing Toxicity of Immune Therapy Using Aptamer-Targeted Drug Delivery. Cancer Immunol. Res. 2015, 3, 1195–1200. [Google Scholar] [CrossRef] [PubMed]
- Emmetiere, F.; Irwin, C.; Viola-Villegas, N.T.; Longo, V.; Cheal, S.M.; Zanzonico, P.; Pillarsetty, N.V.K.; Weber, W.A.; Lewis, J.S.; Reiner, T. 18F-Labeled-Bioorthogonal Liposomes for In Vivo Targeting. Bioconj. Chem. 2013, 24, 1784–1789. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Goins, B.; Zhang, L.; Bao, A. Novel Multifunctional Theranostic Liposome Drug Delivery System: Construction, Characterization, and Multimodality MR, Near-Infrared Fluorescent, and Nuclear Imaging. Bioconj. Chem. 2012, 23, 1322–1332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, C.; Liu, Z.; Wang, S.; Zheng, B.; Guo, W.; Yang, W.; Gong, X.; Wu, X.; Wang, H.; Chang, J. A Protein–Polymer Bioconjugate-Coated Upconversion Nanosystem for Simultaneous Tumor Cell Imaging, Photodynamic Therapy, and Chemotherapy. ACS Appl. Mater. Interfaces 2016, 8, 32688–32698. [Google Scholar] [CrossRef] [PubMed]
- Chakrabortty, S.; Agrawalla, B.K.; Stumper, A.; Vegi, N.M.; Fischer, S.; Reichardt, C.; Kögler, M.; Dietzek, B.; Feuring-Buske, M.; Buske, C.; et al. Mitochondria Targeted Protein-Ruthenium Photosensitizer for Efficient Photodynamic Applications. J. Am. Chem. Soc. 2017, 139, 2512–2519. [Google Scholar] [CrossRef] [Green Version]
- Luo, Y.; Ziebell, M.R.; Prestwich, G.D. A Hyaluronic Acid−Taxol Antitumor Bioconjugate Targeted to Cancer Cells. Biomacromolecules 2000, 1, 208–218. [Google Scholar] [CrossRef]
- Kim, D.; Jeong, Y.Y.; Jon, S. A Drug-Loaded Aptamer−Gold Nanoparticle Bioconjugate for Combined CT Imaging and Therapy of Prostate Cancer. ACS Nano 2010, 4, 3689–3696. [Google Scholar] [CrossRef]
- Tkachenko, A.G.; Xie, H.; Coleman, D.; Glomm, W.; Ryan, J.; Anderson, M.F.; Franzen, S.; Feldheim, D.L. Multifunctional Gold Nanoparticle−Peptide Complexes for Nuclear Targeting. J. Am. Chem. Soc. 2003, 125, 4700–4701. [Google Scholar] [CrossRef]
- Larson, N.; Ghandehari, H. Polymeric Conjugates for Drug Delivery. Chem. Mater. 2012, 24, 840–853. [Google Scholar] [CrossRef] [Green Version]
- Zhang, N.; Wardwell, P.R.; Bader, R.A. Polysaccharide-Based Micelles for Drug Delivery. Pharmaceutics 2013, 5, 329–352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saravanakumar, G.; Jo, D.-G.; Park, J.H. Polysaccharide-based nanoparticles: A versatile platform for drug delivery and biomedical imaging. Curr. Med. Chem. 2012, 19, 3212–3229. [Google Scholar] [CrossRef] [PubMed]
- Goodarzi, N.; Varshochian, R.; Kamalinia, G.; Atyabi, F.; Dinarvand, R. A review of polysaccharide cytotoxic drug conjugates for cancer therapy. Carbohydr. Polym. 2013, 92, 1280–1293. [Google Scholar] [CrossRef] [PubMed]
- Ehrenfreund-Kleinman, T.; Golenser, J.; Domb, A.J. Conjugation of amino-containing drugs to polysaccharides by tosylation: Amphotericin B–arabinogalactan conjugates. Biomaterials 2004, 25, 3049–3057. [Google Scholar] [CrossRef] [PubMed]
- Barclay, T.; Ginic-Markovic, M.; Cooper, P.; Petrovsky, N. Inulin—A versatile polysaccharide: Use as food chemical and pharmaceutical agent. J. Excip. Food Chem. 2010, 1, 27–50. [Google Scholar]
- Barclay, T.G.; Day, C.M.; Petrovsky, N.; Garg, S. Review of polysaccharide particle-based functional drug delivery. Carbohydr. Polym. 2019, 221, 94–112. [Google Scholar] [CrossRef]
- Matthews, S.E.; Pouton, C.W.; Threadgill, M.D. Macromolecular systems for chemotherapy and magnetic resonance imaging. Adv. Drug Deliv. Rev. 1996, 18, 219–267. [Google Scholar] [CrossRef]
- Silva, D.G.; Cooper, P.D.; Petrovsky, N. Inulin-derived adjuvants efficiently promote both Th1 and Th2 immune responses. Immunol. Cell Boil. 2004, 82, 611–616. [Google Scholar] [CrossRef]
- Cooper, P.D.; Petrovsky, N. Delta inulin: A novel, immunologically active, stable packing structure comprising β-d-[2→1] poly(fructo-furanosyl) α-d-glucose polymers. Glycobiology 2011, 21, 595–606. [Google Scholar] [CrossRef]
- Gordon, D.L.; Sajkov, D.; Woodman, R.J.; Honda-Okubo, Y.; Cox, M.M.J.; Heinzel, S.; Petrovsky, N. Randomized clinical trial of immunogenicity and safety of a recombinant H1N1/2009 pandemic influenza vaccine containing Advax™ polysaccharide adjuvant. Vaccine 2012, 30, 5407–5416. [Google Scholar] [CrossRef]
- Lobigs, M.; Pavy, M.; Hall, R.A.; Lobigs, P.; Cooper, P.; Komiya, T.; Toriniwa, H.; Petrovsky, N. An inactivated Vero cell-grown Japanese encephalitis vaccine formulated with Advax, a novel inulin-based adjuvant, induces protective neutralizing antibody against homologous and heterologous flaviviruses. J. Gen. Virol. 2010, 91, 1407–1417. [Google Scholar] [CrossRef] [PubMed]
- Honda-Okubo, Y.; Ong, C.H.; Petrovsky, N. Advax delta inulin adjuvant overcomes immune immaturity in neonatal mice thereby allowing single-dose influenza vaccine protection. Vaccine 2015, 33, 4892–4900. [Google Scholar] [CrossRef] [PubMed]
- Honda-Okubo, Y.; Saade, F.; Petrovsky, N. Advax™, a polysaccharide adjuvant derived from delta inulin, provides improved influenza vaccine protection through broad-based enhancement of adaptive immune responses. Vaccine 2012, 30, 5373–5381. [Google Scholar] [CrossRef] [PubMed]
- Saade, F.; Honda-Okubo, Y.; Trec, S.; Petrovsky, N. A novel hepatitis B vaccine containing Advax™, a polysaccharide adjuvant derived from delta inulin, induces robust humoral and cellular immunity with minimal reactogenicity in preclinical testing. Vaccine 2013, 31, 1999–2007. [Google Scholar] [CrossRef] [PubMed]
- Cooper, P.D.; Carter, M. The anti-melanoma activity of inulin in mice. Mol. Immunol. 1986, 23, 903–908. [Google Scholar] [CrossRef]
- Korbelik, M.; Cooper, P. Potentiation of photodynamic therapy of cancer by complement: The effect of γ-inulin. Br. J. Cancer 2007, 96, 67–72. [Google Scholar] [CrossRef]
- Schoener, C.; Carillo-Conde, B.; Hutson, H.; Peppas, N. An inulin and doxorubicin conjugate for improving cancer therapy. J. Drug Deliv. Sci. Technol. 2013, 23, 111–118. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Barclay, T.; Song, Y.; Joyce, P.; Sakala, I.G.; Petrovsky, N.; Garg, S. Investigation of the biodistribution, breakdown and excretion of delta inulin adjuvant. Vaccine 2017, 35, 4382–4388. [Google Scholar] [CrossRef]
- Afinjuomo, F.; Barclay, T.G.; Parikh, A.; Song, Y.; Chung, R.; Wang, L.; Liu, L.; Hayball, J.D.; Petrovsky, N.; Garg, S. Design and Characterization of Inulin Conjugate for Improved Intracellular and Targeted Delivery of Pyrazinoic Acid to Monocytes. Pharmaceutics 2019, 11, 243. [Google Scholar] [CrossRef]
- Koshkaryev, A.; Sawant, R.; Deshpande, M.; Torchilin, V. Immunoconjugates and long circulating systems: Origins, current state of the art and future directions. Adv. Drug Deliv. Rev. 2013, 65, 24–35. [Google Scholar] [CrossRef]
- Tamura, A.; Nishida, K.; Yui, N. Lysosomal pH-inducible supramolecular dissociation of polyrotaxanes possessing acid-labile N-triphenylmethyl end groups and their therapeutic potential for Niemann-Pick type C disease. Sci. Technol. Adv. Mater. 2016, 17, 361–374. [Google Scholar] [CrossRef] [PubMed]
- Allen, T.M.; Cullis, P.R. Liposomal drug delivery systems: From concept to clinical applications. Adv. Drug Deliv. Rev. 2013, 65, 36–48. [Google Scholar] [CrossRef] [PubMed]
- Parikh, A.; Kathawala, K.; Tan, C.C.; Garg, S.; Zhou, X.-F. Development of a novel oral delivery system of edaravone for enhancing bioavailability. Int. J. Pharm. 2016, 515, 490–500. [Google Scholar] [CrossRef] [PubMed]
- Marques, M.R.C.; Loebenberg, R.; Almukainzi, M. Simulated Biological Fluids with Possible Application in Dissolution Testing. Dissolut. Technol. 2011, 18, 15–28. [Google Scholar] [CrossRef]
- Parikh, A.; Kathawala, K.; Li, J.; Chen, C.; Shan, Z.; Cao, X.; Wang, Y.-J.; Garg, S.; Zhou, X.-F. Self-nanomicellizing solid dispersion of edaravone: Part II: In vivo assessment of efficacy against behavior deficits and safety in Alzheimer’s disease model. Drug Des. Dev. Ther. 2018, 12, 2111–2128. [Google Scholar] [CrossRef] [PubMed]
- Parikh, A.; Kathawala, K.; Li, J.; Chen, C.; Shan, Z.; Cao, X.; Zhou, X.-F.; Garg, S. Curcumin-loaded self-nanomicellizing solid dispersion system: Part II: In vivo safety and efficacy assessment against behavior deficit in Alzheimer disease. Drug Deliv. Transl. Res. 2018, 8, 1406–1420. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, I.; Longenecker, M.; Samuel, J.; Allen, T.M. Antibody-targeted delivery of doxorubicin entrapped in sterically stabilized liposomes can eradicate lung cancer in mice. Cancer Res. 1993, 53, 1484–1488. [Google Scholar]
- Cunha, A.C.; Pereira, L.O.R.; De Souza, M.C.B.V.; Ferreira, V.F. Use of Protecting Groups in Carbohydrate Chemistry: An Advanced Organic Synthesis Experiment. J. Chem. Educ. 1999, 76, 79. [Google Scholar] [CrossRef]
- Mandracchia, D.; Denora, N.; Franco, M.; Pitarresi, G.; Giammona, G.; Trapani, G. New Biodegradable Hydrogels Based on Inulin and alpha,beta-Polyaspartylhydrazide Designed for Colonic Drug Delivery: In Vitro Release of Glutathione and Oxytocin. J. Biomater. Sci. Polym. Ed. 2011, 22, 313–328. [Google Scholar] [CrossRef]
- Pitarresi, G.; Tripodo, G.; Cavallaro, G.; Palumbo, F.S.; Giammona, G. Inulin–iron complexes: A potential treatment of iron deficiency anaemia. Eur. J. Pharm. Biopharm. 2008, 68, 267–276. [Google Scholar] [CrossRef]
- Wu, X.Y.; Lee, P.I. Preparation and characterization of inulin ester microspheres as drug carriers. J. Appl. Polym. Sci. 2000, 77, 833–840. [Google Scholar] [CrossRef]
- Kokubun, S.; Ratcliffe, I.; Williams, P.A. Synthesis, Characterization and Self-Assembly of Biosurfactants Based on Hydrophobically Modified Inulins. Biomacromolecules 2013, 14, 2830–2836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castelli, F.; Sarpietro, M.G.; Micieli, D.; Ottimo, S.; Pitarresi, G.; Tripodo, G.; Carlisi, B.; Giammona, G. Differential scanning calorimetry study on drug release from an inulin-based hydrogel and its interaction with a biomembrane model: pH and loading effect. Eur. J. Pharm. Sci. 2008, 35, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Kodad, H.; Mokhlisse, R.; Davin, E.; Mille, G. Etude IRTF Par Reflexion Totale Attenuee (ATR) De Sucres en Solution Aqueuse. Can. J. Appl. Spectrosc. 1994, 39, 107–112. [Google Scholar]
- Yousefpour, P.; Atyabi, F.; Farahani, E.V.; Sakhtianchi, R.; Dinarvand, R. Polyanionic carbohydrate doxorubicin–dextran nanocomplex as a delivery system for anticancer drugs: In vitro analysis and evaluations. Int. J. Nanomed. 2011, 6, 1487–1496. [Google Scholar]
- Crews, P.; Rodriguez, J.; Jaspars, M. Organic Structure Analysis; Oxford University Press: New York, NY, USA, 1998. [Google Scholar]
- Vermeersch, J.; Schacht, E. Synthesis and characterization of inulin monosuccinates. Die Makromol. Chem. 1986, 187, 125–131. [Google Scholar] [CrossRef]
- Tripodo, G.; Pitarresi, G.; Palumbo, F.S.; Craparo, E.F.; Giammona, G. UV-Photocrosslinking of Inulin Derivatives to Produce Hydrogels for Drug Delivery Application. Macromol. Biosci. 2005, 5, 1074–1084. [Google Scholar] [CrossRef]
- Cooper, P.D.; Barclay, T.G.; Ginic-Markovic, M.; Gerson, A.R.; Petrovsky, N. Inulin isoforms differ by repeated additions of one crystal unit cell. Carbohydr. Polym. 2014, 103, 392–397. [Google Scholar] [CrossRef]
- Barclay, T.; Ginic-Markovic, M.; Johnston, M.R.; Cooper, P.D.; Petrovsky, N. Analysis of the hydrolysis of inulin using real time 1H NMR spectroscopy. Carbohydr. Res. 2012, 352, 117–125. [Google Scholar] [CrossRef] [Green Version]
- Cooper, P.D.; Rajapaksha, K.H.; Barclay, T.G.; Ginic-Markovic, M.; Gerson, A.R.; Petrovsky, N. Inulin crystal initiation via a glucose-fructose cross-link of adjacent polymer chains: Atomic force microscopy and static molecular modelling. Carbohydr. Polym. 2015, 117, 964–972. [Google Scholar] [CrossRef]
- Barclay, T.G.; Rajapaksha, H.; Thilagam, A.; Qian, G.; Ginic-Markovic, M.; Cooper, P.D.; Gerson, A.; Petrovsky, N. Physical characterization and in silico modeling of inulin polymer conformation during vaccine adjuvant particle formation. Carbohydr. Polym. 2016, 143, 108–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Komarov, I.V.; Ishchenko, A.Y.; Hovtvianitsa, A.; Stepanenko, V.; Kharchenko, S.; Bond, A.D.; Kirby, A.J. Fast Amide Bond Cleavage Assisted by a Secondary Amino and a Carboxyl Group—A Model for yet Unknown Peptidases? Molecules 2019, 24, 572. [Google Scholar] [CrossRef] [PubMed]
- Gewirtz, D. A critical evaluation of the mechanisms of action proposed for the antitumor effects of the anthracycline antibiotics adriamycin and daunorubicin. Biochem. Pharmacol. 1999, 57, 727–741. [Google Scholar] [CrossRef]
- Tewey, K.; Rowe, T.; Yang, L.; Halligan, B.; Liu, L. Adriamycin-induced DNA damage mediated by mammalian DNA topoisomerase II. Science 1984, 226, 466–468. [Google Scholar] [CrossRef] [PubMed]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Song, Y.; Parikh, A.; Joyce, P.; Chung, R.; Liu, L.; Afinjuomo, F.; Hayball, J.D.; Petrovsky, N.; Barclay, T.G.; et al. Doxorubicin-Loaded Delta Inulin Conjugates for Controlled and Targeted Drug Delivery: Development, Characterization, and In Vitro Evaluation. Pharmaceutics 2019, 11, 581. https://doi.org/10.3390/pharmaceutics11110581
Wang L, Song Y, Parikh A, Joyce P, Chung R, Liu L, Afinjuomo F, Hayball JD, Petrovsky N, Barclay TG, et al. Doxorubicin-Loaded Delta Inulin Conjugates for Controlled and Targeted Drug Delivery: Development, Characterization, and In Vitro Evaluation. Pharmaceutics. 2019; 11(11):581. https://doi.org/10.3390/pharmaceutics11110581
Chicago/Turabian StyleWang, Lixin, Yunmei Song, Ankit Parikh, Paul Joyce, Rosa Chung, Liang Liu, Franklin Afinjuomo, John D. Hayball, Nikolai Petrovsky, Thomas G. Barclay, and et al. 2019. "Doxorubicin-Loaded Delta Inulin Conjugates for Controlled and Targeted Drug Delivery: Development, Characterization, and In Vitro Evaluation" Pharmaceutics 11, no. 11: 581. https://doi.org/10.3390/pharmaceutics11110581
APA StyleWang, L., Song, Y., Parikh, A., Joyce, P., Chung, R., Liu, L., Afinjuomo, F., Hayball, J. D., Petrovsky, N., Barclay, T. G., & Garg, S. (2019). Doxorubicin-Loaded Delta Inulin Conjugates for Controlled and Targeted Drug Delivery: Development, Characterization, and In Vitro Evaluation. Pharmaceutics, 11(11), 581. https://doi.org/10.3390/pharmaceutics11110581






