Production and Application of Multicistronic Constructs for Various Human Disease Therapies
Abstract
:1. Introduction
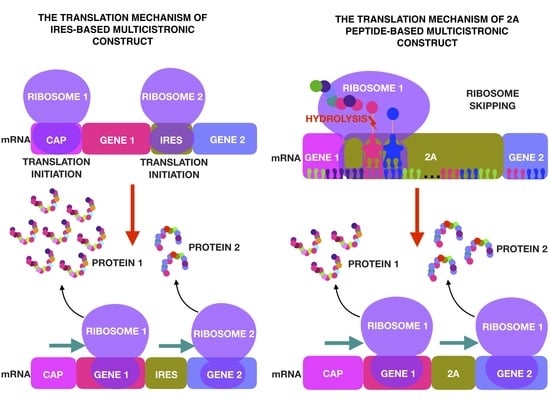
2. IRES
3. Self-Cleaving 2A Peptides
4. Multicistronic Vectors for Neurodegenerative Disease Therapy
5. Multicistronic Vectors for Metabolic Disease Therapy
6. Multicistronic Vectors for the Treatment of Autoimmune Diseases
7. Multicistronic Vectors for Cardiovascular Disease Therapy
8. Multicistronic Vectors for Cancer Therapy
9. Multicistronic Vectors for the Prevention of Viral and Bacterial Infections
10. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Goncalves, G.A.R.; Paiva, R.M.A. Gene therapy: Advances, challenges and perspectives. Einstein 2017, 15, 369–375. [Google Scholar] [CrossRef]
- Raper, S.E.; Chirmule, N.; Lee, F.S.; Wivel, N.A.; Bagg, A.; Gao, G.P.; Wilson, J.M.; Batshaw, M.L. Fatal systemic inflammatory response syndrome in a ornithine transcarbamylase deficient patient following adenoviral gene transfer. Mol. Genet. Metab. 2003, 80, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Chulpanova, D.S.; Solovyeva, V.V.; Kitaeva, K.V.; Dunham, S.P.; Khaiboullina, S.F.; Rizvanov, A.A. Recombinant viruses for cancer therapy. Biomedicines 2018, 6, 94. [Google Scholar] [CrossRef] [PubMed]
- MacLaren, R.E.; Groppe, M.; Barnard, A.R.; Cottriall, C.L.; Tolmachova, T.; Seymour, L.; Clark, K.R.; During, M.J.; Cremers, F.P.; Black, G.C.; et al. Retinal gene therapy in patients with choroideremia: Initial findings from a phase 1/2 clinical trial. Lancet 2014, 383, 1129–1137. [Google Scholar] [CrossRef]
- Kuo, C.Y.; Kohn, D.B. Gene therapy for the treatment of primary immune deficiencies. Curr. Allergy Asthma Rep. 2016, 16, 39. [Google Scholar] [CrossRef] [PubMed]
- Clinical Review Report: Nusinersen (Spinraza): (Biogen Canada Inc.): Indication: Treatment of Patients with 5q SMA; Canadian Agency for Drugs and Technologies in Health: Ottawa, ON, Canada, 2018.
- Goswami, R.; Subramanian, G.; Silayeva, L.; Newkirk, I.; Doctor, D.; Chawla, K.; Chattopadhyay, S.; Chandra, D.; Chilukuri, N.; Betapudi, V. Gene therapy leaves a vicious cycle. Front. Oncol. 2019, 9, 297. [Google Scholar] [CrossRef]
- Lundstrom, K. Viral vectors in gene therapy. Diseases 2018, 6, 42. [Google Scholar] [CrossRef]
- Ramamoorth, M.; Narvekar, A. Non viral vectors in gene therapy—An overview. J. Clin. Diagn. Res. 2015, 9, GE01–GE06. [Google Scholar] [CrossRef]
- Solovieva, V.V.; Kudryashova, N.V.; Rizvanov, A.A. Transfer of recombinant nucleic acids into cells (transfection) by means of histones and other nuclear proteins. Cell. Transpl. Tissue Eng. 2011, 6, 29–40. [Google Scholar]
- Rai, R.; Alwani, S.; Badea, I. Polymeric nanoparticles in gene therapy: New avenues of design and optimization for delivery applications. Polymers 2019, 11, 745. [Google Scholar] [CrossRef]
- Luke, G.A.; Ryan, M.D. Using the 2a protein coexpression system: Multicistronic 2a vectors expressing gene(s) of interest and reporter proteins. Methods Mol. Biol. 2018, 1755, 31–48. [Google Scholar] [PubMed]
- Woodley, E.; Osmon, K.J.L.; Thompson, P.; Richmond, C.; Chen, Z.; Gray, S.J.; Walia, J.S. Efficacy of a bicistronic vector for correction of sandhoff disease in a mouse model. Mol. Ther. Methods Clin. Dev. 2019, 12, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Selleck, W.; Tan, S. Recombinant protein complex expression in E. coli. Curr. Protoc. Protein Sci. 2008, 52, 5–21. [Google Scholar] [CrossRef] [PubMed]
- Efimova, V.S.; Isaeva, L.V.; Labudina, A.A.; Tashlitsky, V.N.; Rubtsov, M.A.; Novikova, L.A. Polycistronic expression of the mitochondrial steroidogenic p450scc system in the hek293t cell line. J. Cell. Biochem. 2019, 120, 3124–3136. [Google Scholar] [CrossRef]
- De Giorgi, M.; Cinti, A.; Pelikant-Malecka, I.; Chisci, E.; Lavitrano, M.; Giovannoni, R.; Smolenski, R.T. Co-expression of functional human heme oxygenase 1, ecto-5′-nucleotidase and ecto-nucleoside triphosphate diphosphohydrolase-1 by “self-cleaving” 2a peptide system. Plasmid 2015, 79, 22–29. [Google Scholar] [CrossRef]
- Luo, H.; Chen, R.; Yang, R.; Liu, Y.; Chen, Y.; Shu, Y.; Chen, H. Reprogramming of mice primary hepatocytes into insulin-producing cells by transfection with multicistronic vectors. J. Diabetes Res. 2014, 2014, 716163. [Google Scholar] [CrossRef]
- Mathison, M.; Singh, V.P.; Gersch, R.P.; Ramirez, M.O.; Cooney, A.; Kaminsky, S.M.; Chiuchiolo, M.J.; Nasser, A.; Yang, J.; Crystal, R.G.; et al. “Triplet” polycistronic vectors encoding gata4, mef2c, and tbx5 enhances postinfarct ventricular functional improvement compared with singlet vectors. J. Thorac. Cardiovasc. Surg. 2014, 148, 1656–1664.e2. [Google Scholar] [CrossRef]
- Solovyeva, V.V.; Salafutdinov, I.I.; Tazetdinova, L.G.; Khaiboullina, S.F.; Masgutov, R.F.; Rizvanov, A.A. Genetic modification of adipose derived stem cells with recombinant plasmid DNA pbud-vegf-fgf2 results in increased of il-8 and mcp-1 secretion. J. Pure Appl. Microbiol. 2014, 8, 523–528. [Google Scholar]
- Solovyeva, V.V.; Chulpanova, D.S.; Tazetdinova, L.G.; Salafutdinov, I.I.; Bozo, I.Y.; Isaev, A.A.; Deev, R.V.; Rizvanov, A.A. In vitro angiogenic properties of plasmid DNA encoding sdf-1alpha and vegf165 genes. Appl. Biochem. Biotechnol. 2019. [Google Scholar] [CrossRef]
- Islamov, R.R.; Rizvanov, A.A.; Mukhamedyarov, M.A.; Salafutdinov, I.I.; Garanina, E.E.; Fedotova, V.Y.; Solovyeva, V.V.; Mukhamedshina, Y.O.; Safiullov, Z.Z.; Izmailov, A.A.; et al. Symptomatic improvement, increased life-span and sustained cell homing in amyotrophic lateral sclerosis after transplantation of human umbilical cord blood cells genetically modified with adeno-viral vectors expressing a neuro-protective factor and a neural cell adhesion molecule. Curr. Gene Ther. 2015, 15, 266–276. [Google Scholar]
- Guseva, D.; Rizvanov, A.A.; Salafutdinov, I.I.; Kudryashova, N.V.; Palotas, A.; Islamov, R.R. Over-expression of oct4 and sox2 transcription factors enhances differentiation of human umbilical cord blood cells in vivo. Biochem. Biophys. Res. Commun. 2014, 451, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Kovac, M.; Litvin, Y.A.; Aliev, R.O.; Zakirova, E.Y.; Rutland, C.S.; Kiyasov, A.P.; Rizvanov, A.A. Gene therapy using plasmid DNA encoding vegf164 and fgf2 genes: A novel treatment of naturally occurring tendinitis and desmitis in horses. Front. Pharm. 2018, 9, 978. [Google Scholar] [CrossRef] [PubMed]
- Mukhamedshina, Y.O.; Gilazieva, Z.E.; Arkhipova, S.S.; Galieva, L.R.; Garanina, E.E.; Shulman, A.A.; Yafarova, G.G.; Chelyshev, Y.A.; Shamsutdinova, N.V.; Rizvanov, A.A. Electrophysiological, morphological, and ultrastructural features of the injured spinal cord tissue after transplantation of human umbilical cord blood mononuclear cells genetically modified with the vegf and gdnf genes. Neural Plast. 2017, 2017, 9857918. [Google Scholar] [CrossRef] [PubMed]
- Islamov, R.R.; Rizvanov, A.A.; Fedotova, V.Y.; Izmailov, A.A.; Safiullov, Z.Z.; Garanina, E.E.; Salafutdinov, I.I.; Sokolov, M.E.; Mukhamedyarov, M.A.; Palotas, A. Tandem delivery of multiple therapeutic genes using umbilical cord blood cells improves symptomatic outcomes in als. Mol. Neurobiol. 2017, 54, 4756–4763. [Google Scholar] [CrossRef]
- Lee, S.E.; Hyun, H.; Park, M.R.; Choi, Y.; Son, Y.J.; Park, Y.G.; Jeong, S.G.; Shin, M.Y.; Ha, H.J.; Hong, H.S.; et al. Production of transgenic pig as an alzheimer’s disease model using a multi-cistronic vector system. PLoS ONE 2017, 12, e0177933. [Google Scholar] [CrossRef]
- Trichas, G.; Begbie, J.; Srinivas, S. Use of the viral 2a peptide for bicistronic expression in transgenic mice. BMC Biol. 2008, 6, 40. [Google Scholar] [CrossRef]
- Curtin, J.A.; Dane, A.P.; Swanson, A.; Alexander, I.E.; Ginn, S.L. Bidirectional promoter interference between two widely used internal heterologous promoters in a late-generation lentiviral construct. Gene Ther. 2008, 15, 384–390. [Google Scholar] [CrossRef] [Green Version]
- Fallot, S.; Ben Naya, R.; Hieblot, C.; Mondon, P.; Lacazette, E.; Bouayadi, K.; Kharrat, A.; Touriol, C.; Prats, H. Alternative-splicing-based bicistronic vectors for ratio-controlled protein expression and application to recombinant antibody production. Nucleic Acids Res. 2009, 37, e134. [Google Scholar] [CrossRef]
- Koller, W.; Vetere-Overfield, B.; Gray, C.; Dubinsky, R. Failure of fixed-dose, fixed muscle injection of botulinum toxin in torticollis. Clin. Neuropharmacol. 1990, 13, 355–358. [Google Scholar] [CrossRef]
- Sequeira, A.F.; Turchetto, J.; Saez, N.J.; Peysson, F.; Ramond, L.; Duhoo, Y.; Blemont, M.; Fernandes, V.O.; Gama, L.T.; Ferreira, L.M.; et al. Gene design, fusion technology and tev cleavage conditions influence the purification of oxidized disulphide-rich venom peptides in Escherichia coli. Microb. Cell Factories 2017, 16, 4. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, O.; Wall, J.B.J.; Zheng, M.; Zhou, Y.; Wang, L.; Ruth Vaseghi, H.; Qian, L.; Liu, J. Systematic comparison of 2a peptides for cloning multi-genes in a polycistronic vector. Sci. Rep. 2017, 7, 2193. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.J.; Kim, H.E.; Lee, K.H.; Han, W.; Yi, M.J.; Jeong, J.; Oh, B.H. Two-promoter vector is highly efficient for overproduction of protein complexes. Protein Sci. A Publ. Protein Soc. 2004, 13, 1698–1703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, J.; Andreadis, S.T. Independent and high-level dual-gene expression in adult stem-progenitor cells from a single lentiviral vector. Gene Ther. 2009, 16, 874–884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szymczak, A.L.; Vignali, D.A. Development of 2a peptide-based strategies in the design of multicistronic vectors. Expert Opin. Biol. Ther. 2005, 5, 627–638. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Feuer, G.; Day, S.L.; Wrzesinski, S.; Planelles, V. Multigene lentiviral vectors based on differential splicing and translational control. Mol. Ther. 2001, 4, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Hong, N.; Lu, W.; Zeng, H.; Song, J.; Hong, Y. Fusion gene vectors allowing for simultaneous drug selection, cell labeling, and reporter assay in vitro and in vivo. Anal. Chem. 2012, 84, 987–993. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Phadtare, S.; Inouye, M. The design and development of tar-envz chimeric receptors. Methods Enzym. 2007, 423, 166–183. [Google Scholar]
- Shirokikh, N.E.; Preiss, T. Translation initiation by cap-dependent ribosome recruitment: Recent insights and open questions. Wiley Interdiscip. Rev. RNA 2018, 9, e1473. [Google Scholar] [CrossRef]
- Merrick, W.C. Cap-dependent and cap-independent translation in eukaryotic systems. Gene 2004, 332, 1–11. [Google Scholar] [CrossRef]
- Pelletier, J.; Sonenberg, N. Internal initiation of translation of eukaryotic mrna directed by a sequence derived from poliovirus rna. Nature 1988, 334, 320–325. [Google Scholar] [CrossRef]
- Lee, K.M.; Chen, C.J.; Shih, S.R. Regulation mechanisms of viral ires-driven translation. Trends Microbiol. 2017, 25, 546–561. [Google Scholar] [CrossRef] [PubMed]
- Komar, A.A.; Hatzoglou, M. Internal ribosome entry sites in cellular mrnas: Mystery of their existence. J. Biol. Chem. 2005, 280, 23425–23428. [Google Scholar] [CrossRef] [PubMed]
- Barrett, L.W.; Fletcher, S.; Wilton, S.D. Regulation of eukaryotic gene expression by the untranslated gene regions and other non-coding elements. Cell. Mol. Life Sci. CMLS 2012, 69, 3613–3634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kieft, J.S. Viral ires rna structures and ribosome interactions. Trends Biochem. Sci. 2008, 33, 274–283. [Google Scholar] [CrossRef] [PubMed]
- Filbin, M.E.; Kieft, J.S. Toward a structural understanding of ires rna function. Curr. Opin. Struct. Biol. 2009, 19, 267–276. [Google Scholar] [CrossRef]
- Perard, J.; Leyrat, C.; Baudin, F.; Drouet, E.; Jamin, M. Structure of the full-length hcv ires in solution. Nat. Commun. 2013, 4, 1612. [Google Scholar] [CrossRef]
- Mizuguchi, H.; Xu, Z.; Ishii-Watabe, A.; Uchida, E.; Hayakawa, T. Ires-dependent second gene expression is significantly lower than cap-dependent first gene expression in a bicistronic vector. Mol. Ther. 2000, 1, 376–382. [Google Scholar] [CrossRef]
- Bouabe, H.; Fassler, R.; Heesemann, J. Improvement of reporter activity by ires-mediated polycistronic reporter system. Nucleic Acids Res. 2008, 36, e28. [Google Scholar] [CrossRef]
- Ebadat, S.; Ahmadi, S.; Ahmadi, M.; Nematpour, F.; Barkhordari, F.; Mahdian, R.; Davami, F.; Mahboudi, F. Evaluating the efficiency of chef and cmv promoter with ires and furin/2a linker sequences for monoclonal antibody expression in cho cells. PLoS ONE 2017, 12, e0185967. [Google Scholar] [CrossRef]
- Garanina, E.E.; Mukhamedshina, Y.O.; Salafutdinov, I.I.; Kiyasov, A.P.; Lima, L.M.; Reis, H.J.; Palotas, A.; Islamov, R.R.; Rizvanov, A.A. Construction of recombinant adenovirus containing picorna-viral 2a-peptide sequence for the co-expression of neuro-protective growth factors in human umbilical cord blood cells. Spinal Cord 2016, 54, 423–430. [Google Scholar] [CrossRef]
- Ryan, M.D.; King, A.M.; Thomas, G.P. Cleavage of foot-and-mouth disease virus polyprotein is mediated by residues located within a 19 amino acid sequence. J. Gen. Virol. 1991, 72 Pt 11, 2727–2732. [Google Scholar] [CrossRef]
- Donnelly, M.L.; Luke, G.; Mehrotra, A.; Li, X.; Hughes, L.E.; Gani, D.; Ryan, M.D. Analysis of the aphthovirus 2a/2b polyprotein ’cleavage’ mechanism indicates not a proteolytic reaction, but a novel translational effect: A putative ribosomal ‘skip’. J. Gen. Virol. 2001, 82, 1013–1025. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Lee, S.R.; Li, L.H.; Park, H.J.; Park, J.H.; Lee, K.Y.; Kim, M.K.; Shin, B.A.; Choi, S.Y. High cleavage efficiency of a 2a peptide derived from porcine teschovirus-1 in human cell lines, zebrafish and mice. PLoS ONE 2011, 6, e18556. [Google Scholar] [CrossRef] [PubMed]
- De Felipe, P.; Hughes, L.E.; Ryan, M.D.; Brown, J.D. Co-translational, intraribosomal cleavage of polypeptides by the foot-and-mouth disease virus 2a peptide. J. Biol. Chem. 2003, 278, 11441–11448. [Google Scholar] [CrossRef] [PubMed]
- de Felipe, P.; Luke, G.A.; Brown, J.D.; Ryan, M.D. Inhibition of 2a-mediated ‘cleavage’ of certain artificial polyproteins bearing n-terminal signal sequences. Biotechnol. J. 2010, 5, 213–223. [Google Scholar] [CrossRef]
- Yang, S.; Cohen, C.J.; Peng, P.D.; Zhao, Y.; Cassard, L.; Yu, Z.; Zheng, Z.; Jones, S.; Restifo, N.P.; Rosenberg, S.A.; et al. Development of optimal bicistronic lentiviral vectors facilitates high-level tcr gene expression and robust tumor cell recognition. Gene Ther. 2008, 15, 1411–1423. [Google Scholar] [CrossRef]
- Chng, J.; Wang, T.; Nian, R.; Lau, A.; Hoi, K.M.; Ho, S.C.; Gagnon, P.; Bi, X.; Yang, Y. Cleavage efficient 2a peptides for high level monoclonal antibody expression in cho cells. mAbs 2015, 7, 403–412. [Google Scholar] [CrossRef]
- Hofacre, A.; Yagiz, K.; Mendoza, D.; Lopez Espinoza, F.; Munday, A.W.; Burrascano, C.; Singer, O.; Gruber, H.E.; Jolly, D.J.; Lin, A.H. Efficient therapeutic protein expression using retroviral replicating vector with 2a peptide in cancer models. Hum. Gene Ther. 2018, 29, 437–451. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, F.; Wang, R.; Zhao, P.; Xia, Q. 2a self-cleaving peptide-based multi-gene expression system in the silkworm bombyx mori. Sci. Rep. 2015, 5, 16273. [Google Scholar] [CrossRef]
- Daniels, R.W.; Rossano, A.J.; Macleod, G.T.; Ganetzky, B. Expression of multiple transgenes from a single construct using viral 2a peptides in drosophila. PLoS ONE 2014, 9, e100637. [Google Scholar] [CrossRef]
- Lee, S.; Kim, J.A.; Kim, H.D.; Chung, S.; Kim, K.; Choe, H.K. Real-time temporal dynamics of bicistronic expression mediated by internal ribosome entry site and 2a cleaving sequence. Mol. Cells 2019, 42, 418–425. [Google Scholar] [PubMed]
- Katsnelson, A.; De Strooper, B.; Zoghbi, H.Y. Neurodegeneration: From cellular concepts to clinical applications. Sci. Transl. Med. 2016, 8, 364ps318. [Google Scholar] [CrossRef] [PubMed]
- Schneider, R.B.; Iourinets, J.; Richard, I.H. Parkinson’s disease psychosis: Presentation, diagnosis and management. Neurodegener. Dis. Manag. 2017, 7, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Azzouz, M.; Martin-Rendon, E.; Barber, R.D.; Mitrophanous, K.A.; Carter, E.E.; Rohll, J.B.; Kingsman, S.M.; Kingsman, A.J.; Mazarakis, N.D. Multicistronic lentiviral vector-mediated striatal gene transfer of aromatic l-amino acid decarboxylase, tyrosine hydroxylase, and gtp cyclohydrolase i induces sustained transgene expression, dopamine production, and functional improvement in a rat model of parkinson’s disease. J. Neurosci. 2002, 22, 10302–10312. [Google Scholar] [PubMed]
- Schwarz, E.J.; Reger, R.L.; Alexander, G.M.; Class, R.; Azizi, S.A.; Prockop, D.J. Rat marrow stromal cells rapidly transduced with a self-inactivating retrovirus synthesize l-dopa in vitro. Gene Ther. 2001, 8, 1214–1223. [Google Scholar] [CrossRef] [PubMed]
- Palfi, S.; Gurruchaga, J.M.; Lepetit, H.; Howard, K.; Ralph, G.S.; Mason, S.; Gouello, G.; Domenech, P.; Buttery, P.C.; Hantraye, P.; et al. Long-term follow-up of a phase i/ii study of prosavin, a lentiviral vector gene therapy for parkinson’s disease. Hum. Gene Ther. Clin. Dev. 2018, 29, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Klein, R.L.; Lewis, M.H.; Muzyczka, N.; Meyer, E.M. Prevention of 6-hydroxydopamine-induced rotational behavior by bdnf somatic gene transfer. Brain Res. 1999, 847, 314–320. [Google Scholar] [CrossRef]
- Villaflores, O.B.; Chen, Y.J.; Chen, C.P.; Yeh, J.M.; Wu, T.Y. Effects of curcumin and demethoxycurcumin on amyloid-beta precursor and tau proteins through the internal ribosome entry sites: A potential therapeutic for alzheimer’s disease. Taiwan J. Obstet. Gynecol. 2012, 51, 554–564. [Google Scholar] [CrossRef]
- Tasi, Y.C.; Chin, T.Y.; Chen, Y.J.; Huang, C.C.; Lee, S.L.; Wu, T.Y. Potential natural products for alzheimer’s disease: Targeted search using the internal ribosome entry site of tau and amyloid-beta precursor protein. Int. J. Mol. Sci. 2015, 16, 8789–8810. [Google Scholar] [CrossRef]
- Stafeev, Y.S.; Menshikov, M.Y.; Parfyonova, Y.V. Gene therapy of type 2 diabetes mellitus: State of art. Ter. Arkhiv 2019, 91, 149–152. [Google Scholar] [CrossRef]
- Wang, L.; Huang, Y.; Guo, Q.; Fan, X.; Lu, Y.; Zhu, S.; Wang, Y.; Bo, X.; Chang, X.; Zhu, M.; et al. Differentiation of ipscs into insulin-producing cells via adenoviral transfection of pdx-1, neurod1 and mafa. Diabetes Res. Clin. Pract. 2014, 104, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Kay, M.A.; Woo, S.L. Gene therapy for metabolic disorders. Trends Genet 1994, 10, 253–257. [Google Scholar] [CrossRef]
- Solovyeva, V.V.; Shaimardanova, A.A.; Chulpanova, D.S.; Kitaeva, K.V.; Chakrabarti, L.; Rizvanov, A.A. New approaches to tay-sachs disease therapy. Front. Physiol. 2018, 9, 1663. [Google Scholar] [CrossRef]
- Sugimoto, Y.; Aksentijevich, I.; Murray, G.J.; Brady, R.O.; Pastan, I.; Gottesman, M.M. Retroviral coexpression of a multidrug resistance gene (mdr1) and human alpha-galactosidase a for gene therapy of fabry disease. Hum. Gene Ther. 1995, 6, 905–915. [Google Scholar] [CrossRef] [PubMed]
- Tardieu, M.; Zerah, M.; Husson, B.; de Bournonville, S.; Deiva, K.; Adamsbaum, C.; Vincent, F.; Hocquemiller, M.; Broissand, C.; Furlan, V.; et al. Intracerebral administration of adeno-associated viral vector serotype rh.10 carrying human sgsh and sumf1 cdnas in children with mucopolysaccharidosis type iiia disease: Results of a phase i/ii trial. Hum. Gene Ther. 2014, 25, 506–516. [Google Scholar] [CrossRef] [PubMed]
- Prud’homme, G.J. Gene therapy of autoimmune diseases with vectors encoding regulatory cytokines or inflammatory cytokine inhibitors. J. Gene Med. 2000, 2, 222–232. [Google Scholar] [CrossRef]
- Hajizadeh-Sikaroodi, S.; Hosseini, A.; Fallah, A.; Estiri, H.; Noormohammadi, Z.; Salehi, M.; Ghaderian, S.M.; Akhavan Niaki, H.; Soleimani, M.; Kazemi, B. Lentiviral mediating genetic engineered mesenchymal stem cells for releasing il-27 as a gene therapy approach for autoimmune diseases. Cell J. 2014, 16, 255–262. [Google Scholar]
- Hosseini, A.; Estiri, H.; Akhavan Niaki, H.; Alizadeh, A.; Abdolhossein Zadeh, B.; Ghaderian, S.M.H.; Farjadfar, A.; Fallah, A. Multiple sclerosis gene therapy with recombinant viral vectors: Overexpression of il-4, leukemia inhibitory factor, and il-10 in wharton’s jelly stem cells used in eae mice model. Cell J. 2017, 19, 361–374. [Google Scholar] [PubMed]
- Biedermann, T.; Rocken, M. Pro- and anti-inflammatory effects of il-4: From studies in mice to therapy of autoimmune diseases in humans. In Animal Models of T Cell-Mediated Skin Diseases; Springer: Berlin/Heidelberg, Germany, 2005; pp. 235–242. [Google Scholar]
- Cao, W.; Yang, Y.; Wang, Z.; Liu, A.; Fang, L.; Wu, F.; Hong, J.; Shi, Y.; Leung, S.; Dong, C.; et al. Leukemia inhibitory factor inhibits t helper 17 cell differentiation and confers treatment effects of neural progenitor cell therapy in autoimmune disease. Immunity 2011, 35, 273–284. [Google Scholar] [CrossRef]
- Furlan, R.; Pluchino, S.; Martino, G. The therapeutic use of gene therapy in inflammatory demyelinating diseases of the central nervous system. Curr. Opin. Neurol. 2003, 16, 385–392. [Google Scholar] [CrossRef]
- Azodi, S.; Jacobson, S. Cytokine therapies in neurological disease. Neurother. J. Am. Soc. Exp. Neurother. 2016, 13, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Rayssac, A.; Neveu, C.; Pucelle, M.; Van den Berghe, L.; Prado-Lourenco, L.; Arnal, J.F.; Chaufour, X.; Prats, A.C. Ires-based vector coexpressing fgf2 and cyr61 provides synergistic and safe therapeutics of lower limb ischemia. Mol. Ther. 2009, 17, 2010–2019. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wang, K.Z.; Qiang, H.; Tang, Y.L.; Li, Q.; Li, M.; Dang, X.Q. Angiopoiesis and bone regeneration via co-expression of the hvegf and hbmp genes from an adeno-associated viral vector in vitro and in vivo. Acta Pharmacol. Sin. 2010, 31, 821–830. [Google Scholar] [CrossRef] [PubMed]
- Jazwa, A.; Tomczyk, M.; Taha, H.M.; Hytonen, E.; Stoszko, M.; Zentilin, L.; Giacca, M.; Yla-Herttuala, S.; Emanueli, C.; Jozkowicz, A.; et al. Arteriogenic therapy based on simultaneous delivery of vegf-a and fgf4 genes improves the recovery from acute limb ischemia. Vasc. Cell 2013, 5, 13. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.J.; Feng, Y.G.; Lu, W.P.; Li, H.T.; Xie, H.W.; Li, S.F. Effect of combined vegf165/ sdf-1 gene therapy on vascular remodeling and blood perfusion in cerebral ischemia. J. Neurosurg. 2017, 127, 670–678. [Google Scholar] [CrossRef]
- Kukula, K.; Chojnowska, L.; Dabrowski, M.; Witkowski, A.; Chmielak, Z.; Skwarek, M.; Kadziela, J.; Teresinska, A.; Malecki, M.; Janik, P.; et al. Intramyocardial plasmid-encoding human vascular endothelial growth factor a165/basic fibroblast growth factor therapy using percutaneous transcatheter approach in patients with refractory coronary artery disease (vif-cad). Am. Heart J. 2011, 161, 581–589. [Google Scholar] [CrossRef]
- Zitvogel, L.; Tahara, H.; Cai, Q.; Storkus, W.J.; Muller, G.; Wolf, S.F.; Gately, M.; Robbins, P.D.; Lotze, M.T. Construction and characterization of retroviral vectors expressing biologically active human interleukin-12. Hum. Gene Ther. 1994, 5, 1493–1506. [Google Scholar] [CrossRef]
- Havunen, R.; Siurala, M.; Sorsa, S.; Gronberg-Vaha-Koskela, S.; Behr, M.; Tahtinen, S.; Santos, J.M.; Karell, P.; Rusanen, J.; Nettelbeck, D.M.; et al. Oncolytic adenoviruses armed with tumor necrosis factor alpha and interleukin-2 enable successful adoptive cell therapy. Mol. Ther. Oncolytics 2017, 4, 77–86. [Google Scholar] [CrossRef]
- Alekseenko, I.V.; Snezhkov, E.V.; Chernov, I.P.; Pleshkan, V.V.; Potapov, V.K.; Sass, A.V.; Monastyrskaya, G.S.; Kopantzev, E.P.; Vinogradova, T.V.; Khramtsov, Y.V.; et al. Therapeutic properties of a vector carrying the hsv thymidine kinase and gm-csf genes and delivered as a complex with a cationic copolymer. J. Transl. Med. 2015, 13, 78. [Google Scholar] [CrossRef]
- Santos, J.M.; Cervera-Carrascon, V.; Havunen, R.; Zafar, S.; Siurala, M.; Sorsa, S.; Anttila, M.; Kanerva, A.; Hemminki, A. Adenovirus coding for interleukin-2 and tumor necrosis factor alpha replaces lymphodepleting chemotherapy in adoptive t cell therapy. Mol. Ther. 2018, 26, 2243–2254. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, H.; Yao, C.; Su, F.; Guan, W.; Yan, S.; Ni, Z. Antitumor activity of combined endostatin and thymidine kinase gene therapy in c6 glioma models. Cancer Med. 2016, 5, 2477–2486. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.H.; Wan, M.X.; Yuan, J.Y.; Pan, B.R. Do there exist synergistic antitumor effects by coexpression of herpes simplex virus thymidine kinase with cytokine genes on human gastric cancer cell line sgc7901? World J. Gastroenterol. 2004, 10, 147–151. [Google Scholar] [CrossRef]
- Prats, A.C.; Van den Berghe, L.; Rayssac, A.; Ainaoui, N.; Morfoisse, F.; Pujol, F.; Legonidec, S.; Bikfalvi, A.; Prats, H.; Pyronnet, S.; et al. Cxcl4l1-fibstatin cooperation inhibits tumor angiogenesis, lymphangiogenesis and metastasis. Microvasc. Res. 2013, 89, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wu, Y.; Qiu, Y.; Yao, Z.; Liu, S.; Liu, Y.; Shi, J.; Zheng, D. 2a peptide-based, lentivirus-mediated anti-death receptor 5 chimeric antibody expression prevents tumor growth in nude mice. Mol. Ther. 2012, 20, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Molgaard, K.; Compte, M.; Nunez-Prado, N.; Harwood, S.L.; Sanz, L.; Alvarez-Vallina, L. Balanced secretion of anti-cea x anti-cd3 diabody chains using the 2a self-cleaving peptide maximizes diabody assembly and tumor-specific cytotoxicity. Gene Ther. 2017, 24, 208–214. [Google Scholar] [CrossRef]
- Burkart, C.; Mukhopadhyay, A.; Shirley, S.A.; Connolly, R.J.; Wright, J.H.; Bahrami, A.; Campbell, J.S.; Pierce, R.H.; Canton, D.A. Improving therapeutic efficacy of il-12 intratumoral gene electrotransfer through novel plasmid design and modified parameters. Gene Ther. 2018, 25, 93–103. [Google Scholar] [CrossRef]
- Khodamoradi, S.; Shenagari, M.; Kheiri, M.T.; Sabahi, F.; Jamali, A.; Heidari, A.; Ashrafkhani, B. Ires-based co-expression of influenza virus conserved genes can promote synergistic antiviral effects both in vitro and in vivo. Arch. Virol. 2018, 163, 877–886. [Google Scholar] [CrossRef]
- Shkreta, L.; Talbot, B.G.; Diarra, M.S.; Lacasse, P. Immune responses to a DNA/protein vaccination strategy against staphylococcus aureus induced mastitis in dairy cows. Vaccine 2004, 23, 114–126. [Google Scholar] [CrossRef]
- He, X.; Zhang, L.; Liu, P.; Liu, L.; Deng, H.; Huang, J. Construction and characterization of vl-vh tail-parallel genetically engineered antibodies against staphylococcal enterotoxins. Immunol. Res. 2015, 61, 281–293. [Google Scholar] [CrossRef]
- Ma, Y.; An, H.J.; Wei, X.Q.; Xu, Q.; Yu, Y.Z.; Sun, Z.W. Enhanced potency of replicon vaccine using one vector to simultaneously co-express antigen and interleukin-4 molecular adjuvant. Hum. Vaccines Immunother. 2013, 9, 242–249. [Google Scholar] [CrossRef] [Green Version]
- Barbieri, A.; Panigada, M.; Soprana, E.; Di Mario, G.; Gubinelli, F.; Bernasconi, V.; Recagni, M.; Donatelli, I.; Castrucci, M.R.; Siccardi, A.G. Strategies to obtain multiple recombinant modified vaccinia ankara vectors. Applications to influenza vaccines. J. Virol. Methods 2018, 251, 7–14. [Google Scholar] [CrossRef] [PubMed]

| Characteristic | Properties of Protein Synthesis | Size | Gene Expression Level | Cleavage |
|---|---|---|---|---|
| IRES | Ribosome dissociates when the synthesis of the first gene is complete, the synthesis is interrupted, a new translation initiation complex is assembled in the IRES region | Large (over 500 bp) | The translation efficiency of the gene located downstream of the IRES is much lower than that of the gene located upstream of the IRES | The resulting proteins are always separated from each other |
| 2A peptides | Continuous synthesis of two proteins. Ribosomal skipping occurs after the synthesis of the first gene is complete, synthesis continues without the dissociation of ribosome | Small (54–66 bp) | Better correlation of the expression of genes placed upstream and downstream of the peptide sequence | Incomplete digestion of protein products is possible |
| Multiple promoters | Two separate transcription units, completely separated synthesis | Large | Poor correlation of the expression of two genes | Independent products |
| Splicing signals | Two separate transcription units, completely separated synthesis | Small | Uncertain correlation of the expression of two genes | Independent products |
| Fusion of genes | One chimeric polypeptide is translated, which can lead to impaired function | No intermediate sequences | Guaranteed co-expression | Not cleaved product |
| Cleavage factors | Two proteins are translated together | Small | Guaranteed co-expression | Cleaved by cellular proteases after protein synthesis |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shaimardanova, A.A.; Chulpanova, D.S.; Kitaeva, K.V.; Abdrakhmanova, I.I.; Chernov, V.M.; Rutland, C.S.; Rizvanov, A.A.; Solovyeva, V.V. Production and Application of Multicistronic Constructs for Various Human Disease Therapies. Pharmaceutics 2019, 11, 580. https://doi.org/10.3390/pharmaceutics11110580
Shaimardanova AA, Chulpanova DS, Kitaeva KV, Abdrakhmanova II, Chernov VM, Rutland CS, Rizvanov AA, Solovyeva VV. Production and Application of Multicistronic Constructs for Various Human Disease Therapies. Pharmaceutics. 2019; 11(11):580. https://doi.org/10.3390/pharmaceutics11110580
Chicago/Turabian StyleShaimardanova, Alisa A., Daria S. Chulpanova, Kristina V. Kitaeva, Ilmira I. Abdrakhmanova, Vladislav M. Chernov, Catrin S. Rutland, Albert A. Rizvanov, and Valeriya V. Solovyeva. 2019. "Production and Application of Multicistronic Constructs for Various Human Disease Therapies" Pharmaceutics 11, no. 11: 580. https://doi.org/10.3390/pharmaceutics11110580