Doxorubicin and Varlitinib Delivery by Functionalized Gold Nanoparticles Against Human Pancreatic Adenocarcinoma
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Gold Nanoparticles
2.3. Conjugation of Doxorubicin and Varlitining to Pegylated Gold Nanoparticles
2.4. Gold Nanoparticles Physicochemical Characterization
2.5. Varlitinib and Doxorubicin Conjugation Efficiencies and Release from PEGAuNPs
2.6. Evaluation of In Vitro Cytotoxicity
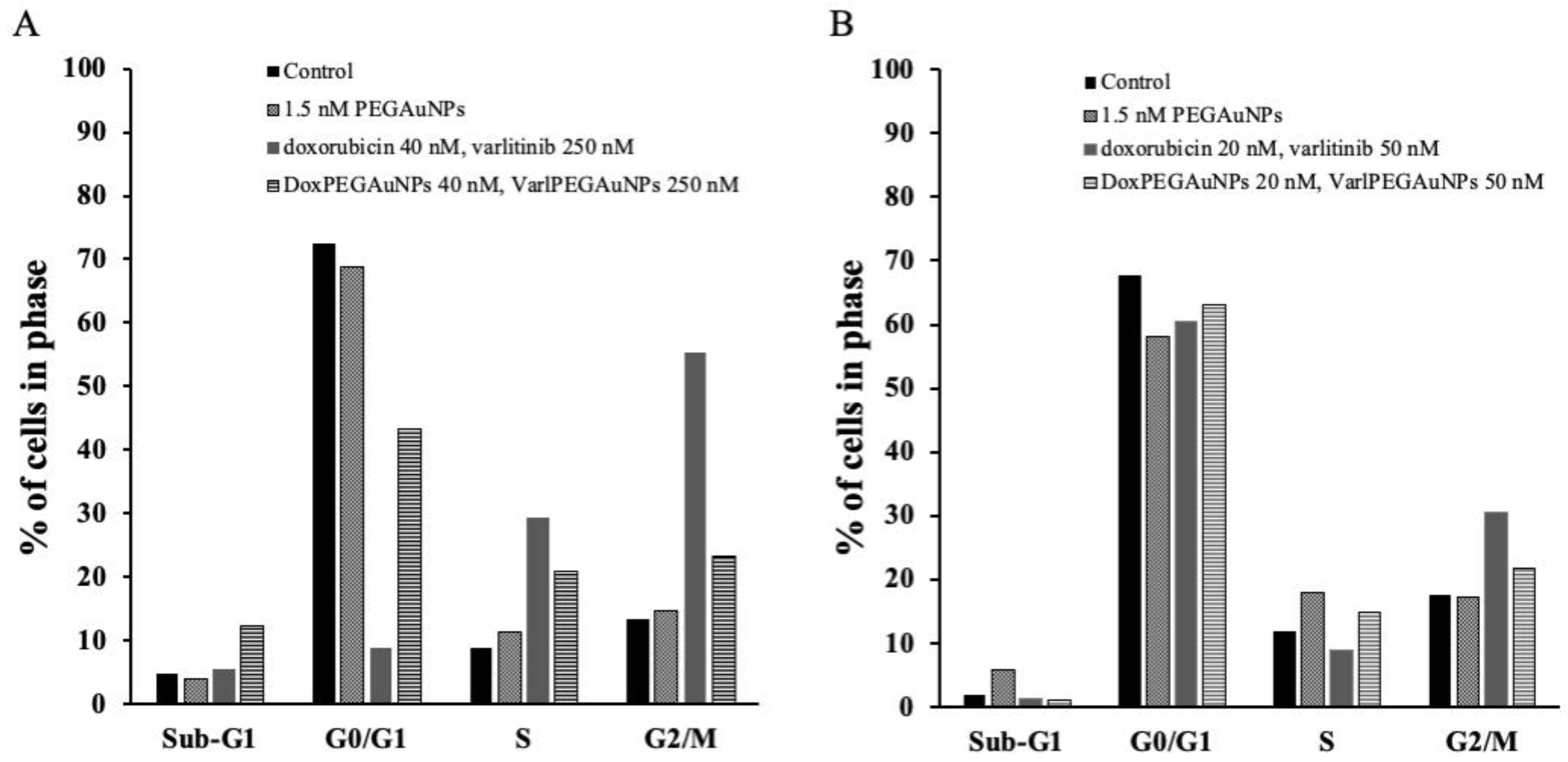
2.7. Flow Cytometry
2.8. Statistical Analysis
3. Results and Discussion
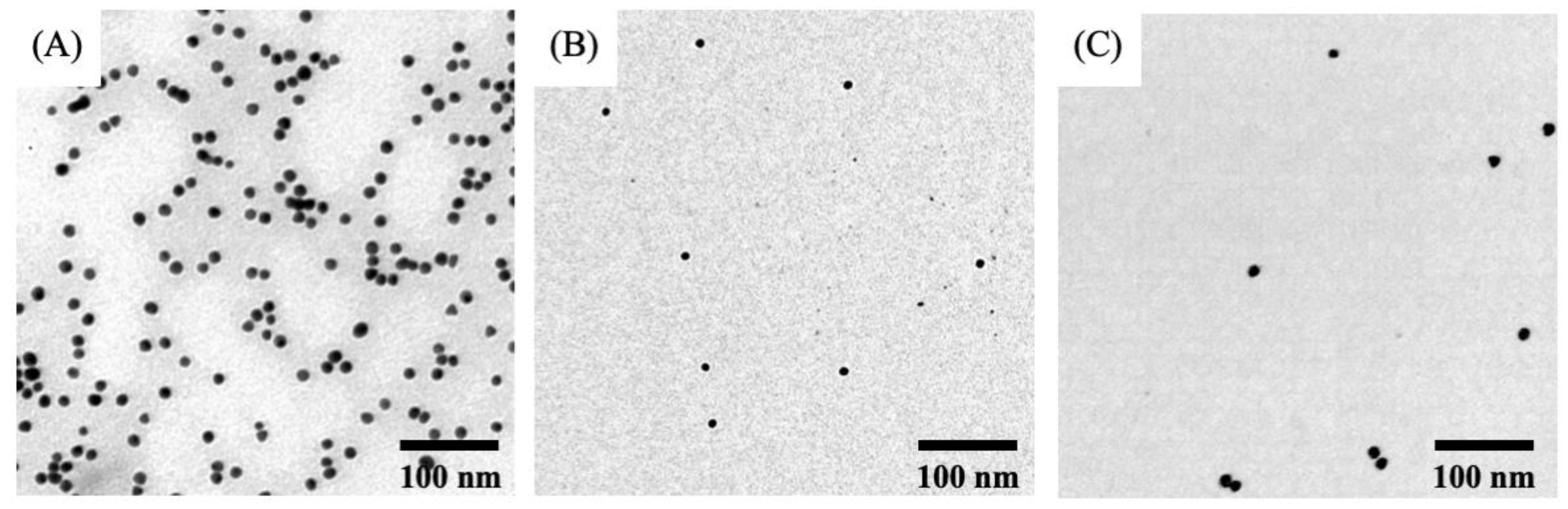
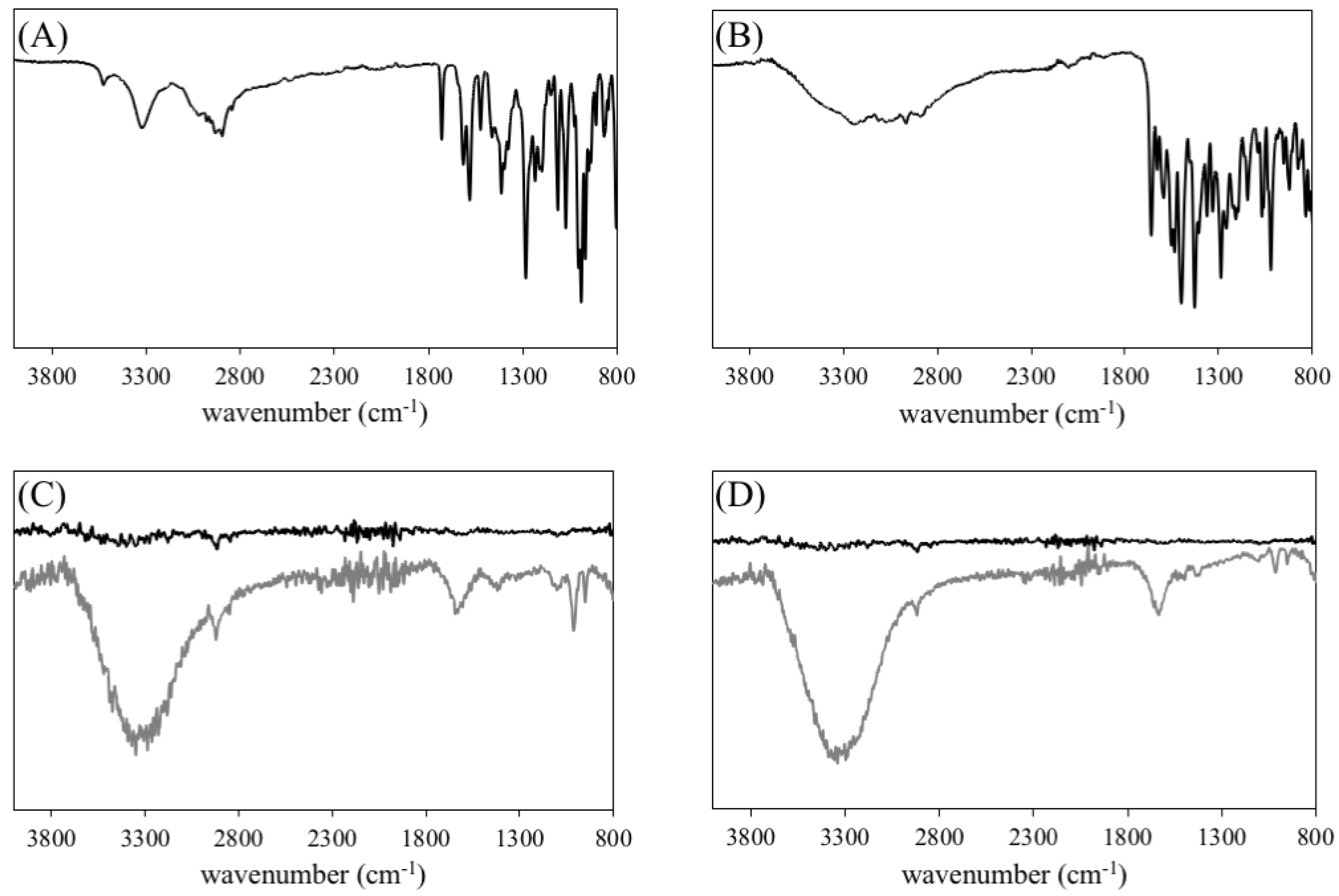
3.1. Synthesis and Characterization of Gold Nanoparticles and Nanoconjugates
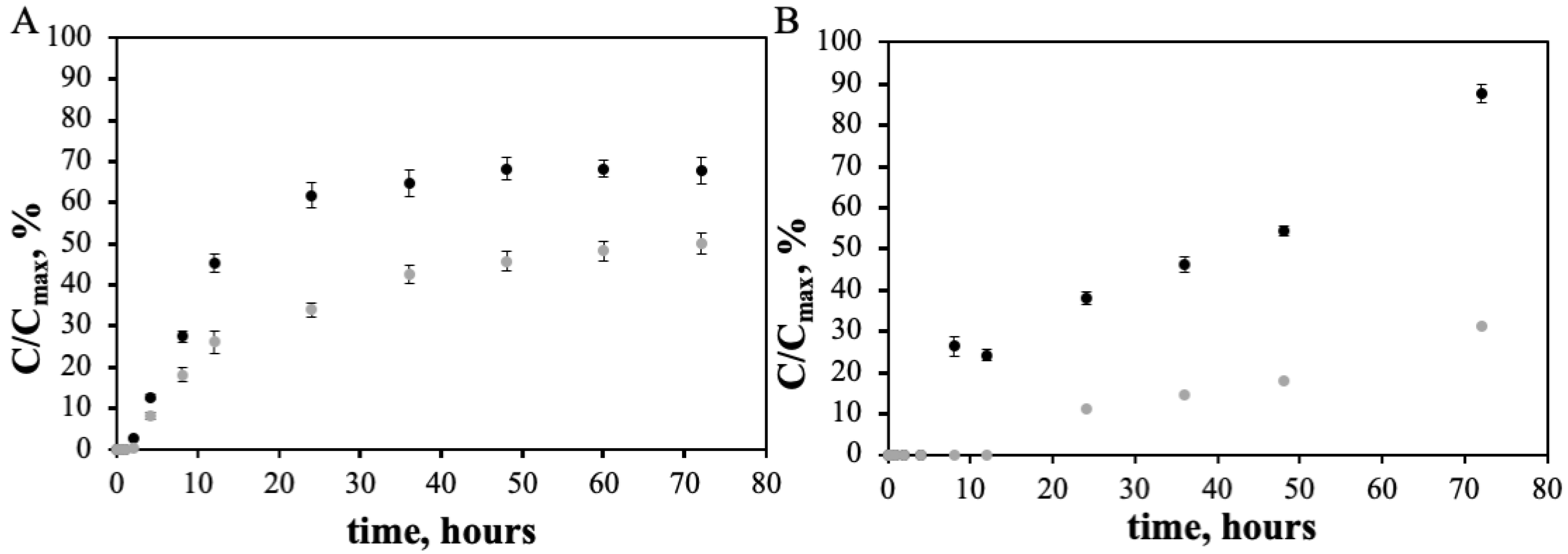
3.2. Doxorubicin and Varlitinib Conjugation Efficiency and In Vitro Release from the PEGAuNPs
3.3. Cytotoxicity Experiments
SRB Assay
4. Conclusions
Supplementary Materials
 ) free Dox plus 250 nM of free varlinitib on the cell survival (IC50 determination) of MIA PaCa-2 (A), S2-013 (B) and hTERT-HPNE (C) cells. Doxorubicin concentrations range is between 10 and 100 nM.
) free Dox plus 250 nM of free varlinitib on the cell survival (IC50 determination) of MIA PaCa-2 (A), S2-013 (B) and hTERT-HPNE (C) cells. Doxorubicin concentrations range is between 10 and 100 nM.Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Boisselier, E.; Astruc, D. Gold nanoparticles in nanomedicine: Preparations, imaging, diagnostics, therapies and toxicity. Chem. Soc. Rev. 2009, 38, 1759–1782. [Google Scholar] [CrossRef] [PubMed]
- Coelho, S.C.; Pereira, M.C.; Juzeniene, A.; Juzenas, P.; Coelho, M.A. Supramolecular nanoscale assemblies for cancer diagnosis and therapy. J. Controlled Release 2015, 213, 152–167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li Volsi, A.; Jimenez de Aberasturi, D.; Henriksen-Lacey, M.; Giammona, G.; Licciardi, M.; Liz-Marzan, L.M. Inulin coated plasmonic gold nanoparticles as a tumor-selective tool for cancer therapy. J. Mater. Chem. B 2016, 4, 1150–1155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maney, V.; Singh, M. The Synergism of Platinum-Gold Bimetallic Nanoconjugates Enhances 5-Fluorouracil Delivery In Vitro. Pharmaceutics 2019, 11, 439. [Google Scholar] [CrossRef] [PubMed]
- Cole, L.E.; Ross, R.D.; Tilley, J.M.; Vargo-Gogola, T.; Roeder, R.K. Gold nanoparticles as contrast agents in X-ray imaging and computed tomography. Nanomedicine 2015, 10, 321–341. [Google Scholar] [CrossRef] [PubMed]
- Rahman, W.N.; Bishara, N.; Ackerly, T.; He, C.F.; Jackson, P.; Wong, C.; Davidson, R.; Geso, M. Enhancement of radiation effects by gold nanoparticles for superficial radiation therapy. Nanomed. Nanotechnol. Biol. Med. 2009, 5, 136–142. [Google Scholar] [CrossRef]
- Kong, F.Y.; Zhang, J.W.; Li, R.F.; Wang, Z.X.; Wang, W.J.; Wang, W. Unique Roles of Gold Nanoparticles in Drug Delivery, Targeting and Imaging Applications. Molecules 2017, 22, 1445. [Google Scholar] [CrossRef]
- Gao, J.; Huang, X.; Liu, H.; Zan, F.; Ren, J. Colloidal stability of gold nanoparticles modified with thiol compounds: Bioconjugation and application in cancer cell imaging. Langmuir 2012, 28, 4464–4471. [Google Scholar] [CrossRef]
- Bhattacharya, R.; Patra, C.R.; Verma, R.; Kumar, S.; Greipp, P.R.; Mukherjee, P. Gold Nanoparticles Inhibit the Proliferation of Multiple Myeloma Cells. Adv. Mater. 2007, 19, 711–716. [Google Scholar] [CrossRef]
- Keizer, H.G. Doxorubicin (adriamycin): A critical review of free radical-dependent mechanisms of cytotoxicity. Pharmac. Ther. 1990, 47, 219–231. [Google Scholar] [CrossRef]
- Mandili, G.; Khadjavi, A.; Gallo, V.; Minero, V.G.; Bessone, L.; Carta, F.; Giribaldi, G.; Turrini, F. Characterization of the protein ubiquitination response induced by Doxorubicin. FEBS J. 2012, 279, 2182–2191. [Google Scholar] [CrossRef] [PubMed]
- Juzenas, P.; Chen, W.; Sun, Y.-P.; Coelho, M.A.N.; Generalov, R.; Generalova, N.; Christensen, I.L. Quantum dots and nanoparticles for photodynamic and radiation therapies of cancer. Adv. Drug Deliv. Rev. 2008, 60, 1600–1614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coelho, S.C.; Reis, D.P.; Pereira, M.C.; Coelho, M.A.N. Gold Nanoparticles for Targeting Varlitinib to Human Pancreatic Cancer Cells. Pharmaceutics 2018, 10, 91. [Google Scholar] [CrossRef] [PubMed]
- Pissuwan, D.; Niidome, T.; Cortie, M.B. The forthcoming applications of gold nanoparticles in drug and gene delivery systems. J. Controlled Release 2011, 149, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Pandit, S.; Mokkapati, V.R.S.S.; Garg, A.; Ravikumar, V.; Mijakovic, I. Gold Nanoparticles in Diagnostics and Therapeutics for Human Cancer. Int. J. Mol. Sci. 2018, 19, 1979. [Google Scholar] [CrossRef] [PubMed]
- Au, M.; Emeto, T.I.; Power, J.; Vangaveti, V.N.; Lai, H.C. Emerging Therapeutic Potential of Nanoparticles in Pancreatic Cancer: A Systematic Review of Clinical Trials. Biomedicines 2016, 4, 20. [Google Scholar] [CrossRef]
- Adamska, A.; Domenichini, A.; Falasca, M. Pancreatic Ductal Adenocarcinoma: Current and Evolving Therapies. Int. J. Mol. Sci. 2017, 18, 1338. [Google Scholar] [CrossRef]
- Manzur, A.; Oluwasanmi, A.; Moss, D.; Curtis, A.; Hoskins, C. Nanotechnologies in Pancreatic Cancer Therapy. Pharmaceutics 2017, 9, 39. [Google Scholar] [CrossRef]
- Desir, S.; O’Hare, P.; Vogel, R.I.; Sperduto, W.; Sarkari, A.; Dickson, E.L.; Wong, P.; Nelson, A.C.; Fong, Y.; Steer, C.J.; et al. Chemotherapy-Induced Tunneling Nanotubes Mediate Intercellular Drug Efflux in Pancreatic Cancer. Sci. Rep. 2018, 8, 9484. [Google Scholar] [CrossRef]
- Bardeesy, N.; DePinho, R.A. Pancreatic cancer biology and genetics. Nat. Rev. Cancer 2002, 2, 897–909. [Google Scholar] [CrossRef]
- Sun, J.; Wei, Q.; Zhou, Y.; Wang, J.; Liu, Q.; Xu, H. A systematic analysis of FDA-approved anticancer drugs. BMC Syst. Biol. 2017, 11, 87. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Yue, Z.; Eccleston, M.E.; Swartling, J.; Slater, N.K.; Kaminski, C.F. Fluorescence intensity and lifetime imaging of free and micellar-encapsulated doxorubicin in living cells. Nanomedicine 2008, 4, 49–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, W.; Kalen, A.L.; Smith, B.J.; Cullen, J.J.; Oberley, L.W. Enhancing the antitumor activity of adriamycin and ionizing radiation. Cancer Res. 2009, 69, 4294–4300. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.S.; Patel, A.; Sim, H.M.; Zhang, Y.K.; Wang, Y.J.; Kathawala, R.J.; Zhang, H.; Talele, T.T.; Ambudkar, S.V.; Xu, R.H.; et al. ARRY-334543 reverses multidrug resistance by antagonizing the activity of ATP-binding cassette subfamily G member 2. J. Cell. Biochem. 2014, 115, 1381–1391. [Google Scholar] [CrossRef] [PubMed]
- Coelho, S.C.; Rocha, S.; Juzenas, P.; Sampaio, P.; Almeida, G.M.; Silva, F.S.; Pereira, M.C.; Coelho, M.A. Gold nanoparticle delivery-enhanced proteasome inhibitor effect in adenocarcinoma cells. Expert Opin. Drug Deliv. 2013, 10, 1345–1352. [Google Scholar] [CrossRef]
- Skehan, P.; Storeng, R.; Scudiero, D.; Monks, A.; McMahon, J.; Vistica, D.; Warren, J.T.; Bokesch, H.; Kenney, S.; Boyd, M.R. New colorimetric cytotoxicity assay for anticancer-drug screening. J. Natl. Cancer Inst. 1990, 82, 1107–1112. [Google Scholar] [CrossRef]
- Elbialy, N.S.; Fathy, M.M.; Khalil, W.M. Doxorubicin loaded magnetic gold nanoparticles for in vivo targeted drug delivery. Int. J. Pharm. 2015, 490, 190–199. [Google Scholar] [CrossRef]
- Sahlberg, S.H.; Spiegelberg, D.; Glimelius, B.; Stenerlow, B.; Nestor, M. Evaluation of Cancer Stem Cell Markers CD133, CD44, CD24: Association with AKT Isoforms and Radiation Resistance in Colon Cancer Cells. PLoS ONE 2014, 9, e94621. [Google Scholar] [CrossRef]
- Mirza, A.Z.; Shamshad, H. Preparation and characterization of doxorubicin functionalized gold nanoparticles. Eur. J. Med. Chem. 2011, 46, 1857–1860. [Google Scholar] [CrossRef]
- Gallagher, W. Infrared Spectroscopy: Theory. J. Mol. Spectrosc. 2002, 214, 155–164. [Google Scholar]
- Thorn, C.F.; Oshiro, C.; Marsh, S.; Hernandez-Boussard, T.; McLeod, H.; Klein, T.E.; Altman, R.B. Doxorubicin pathways: Pharmacodynamics and adverse effects. Pharmacogenet Genom. 2011, 21, 440–446. [Google Scholar] [CrossRef] [PubMed]
- DeArmond, D.; Brattain, M.G.; Jessup, J.M.; Kreisberg, J.; Malik, S.; Zhao, S.; Freeman, J.W. Autocrine-mediated ErbB-2 kinase activation of STAT3 is required for growth factor independence of pancreatic cancer cell lines. Oncogene 2003, 22, 7781–7795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siu, W.Y.; Yam, C.H.; Poon, R.Y.C. G1 versus G2 cell cycle arrest after adriamycin-induced damage in mouse Swiss3T3 cells. FEBS Lett. 1999, 461, 299–305. [Google Scholar] [CrossRef]


 ) 40 nM of free Dox plus free varlinitib on the cell survival (IC50 determination) of MIA PaCa-2 (A), S2-013 (B) and hTERT-HPNE (C) cells. Varltinib concentrations range is between 50 and 750 nM.
) 40 nM of free Dox plus free varlinitib on the cell survival (IC50 determination) of MIA PaCa-2 (A), S2-013 (B) and hTERT-HPNE (C) cells. Varltinib concentrations range is between 50 and 750 nM.
 ) 40 nM of free Dox plus free varlinitib on the cell survival (IC50 determination) of MIA PaCa-2 (A), S2-013 (B) and hTERT-HPNE (C) cells. Varltinib concentrations range is between 50 and 750 nM.
) 40 nM of free Dox plus free varlinitib on the cell survival (IC50 determination) of MIA PaCa-2 (A), S2-013 (B) and hTERT-HPNE (C) cells. Varltinib concentrations range is between 50 and 750 nM.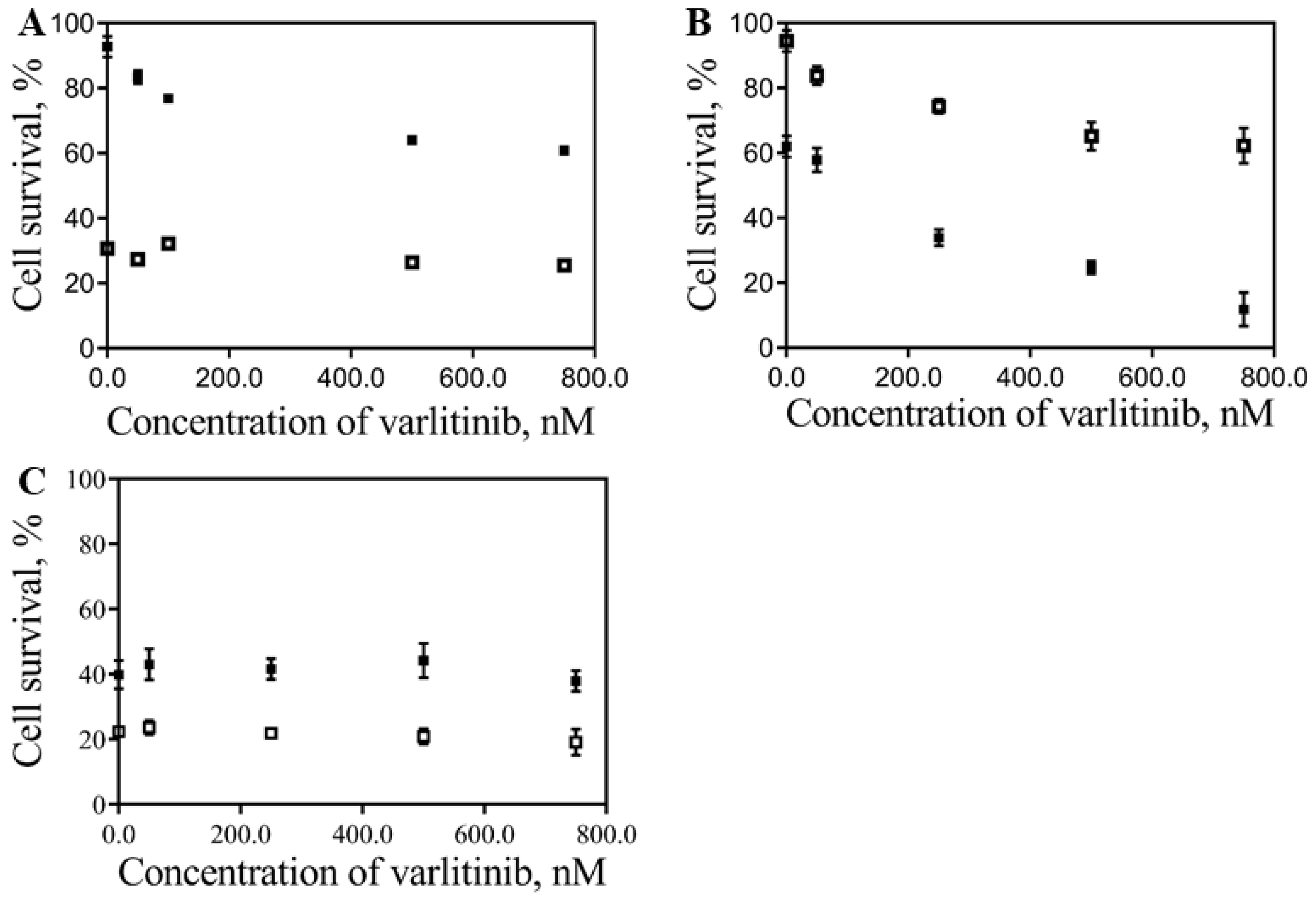
| Sample | Diameter (nm) | PdI | Zeta Potential (mV) | Concentration of AuNPs (nM) | Efficiency (%) | Drug Concentration (µM) |
|---|---|---|---|---|---|---|
| AuNPs | 18 ± 1 | 0.3 | −37 ± 2 | 15.2 | - | - |
| PEGAuNPs | 24 ± 1 | 0.4 | −41 ± 2 | 9.8 | - | - |
| DoxPEGAuNPs | 29 ± 2 | 0.5 | −40 ± 3 | 4.2 | 49.5 ± 5.0 | 3.6 |
| VarlPEGAuNPs | 29 ± 2 | 0.5 | −27 ± 2 | 3.5 | 95.0 ± 3.0 | 4.0 |
| Concentration of Varlitinib | MIA PaCa-2 | S2-013 | hTERT-HPNE | |||
|---|---|---|---|---|---|---|
| Doxorubicin | DoxPEGAuNPs | Doxorubicin | DoxPEGAuNPs | Doxorubicin | DoxPEGAuNPs | |
| 0 nM varlitinib | 24.2 ± 0.5 | 93.4 ± 2.0 | 17.7 ± 0.8 | |||
| 50 nM varlitinib | 27.0 ± 0.2 | 91.0 ± 0.5 | 17.5 ± 0.7 | |||
| 250 nM varlitinib | 27.5 ± 0.8 | 91.7 ± 0.2 | 17.3 ± 1.2 | |||
| 750 nM varlitinib | 26.4 ± 0.6 | 67.5 ± 0.2 | 12.7 ± 0.7 | |||
| 0 nM VarlPEGAuNPs | 112.0 ± 0.3 | 23.5 ± 0.3 | 21.4 ± 0.9 | |||
| 50 nM VarlPEGAuNPs | 105.0 ± 0.5 | 46.7 ± 0.3 | 27.5 ± 1.1 | |||
| 250 nM VarlPEGAuNPs | 99.0 ± 0.8 | 29.9 ± 0.1 | 30.2 ± 1.0 | |||
| 750 nM VarlPEGAuNPs | 51.2 ± 0.3 | 4.70 ± 0.2 | 27.5 ± 1.1 | |||
| Concentration of Varlitinib | MIA PaCa-2 | S2-013 | hTERT-HPNE | |||
|---|---|---|---|---|---|---|
| Doxorubicin | DoxPEGAuNPs | Doxorubicin | DoxPEGAuNPs | Doxorubicin | DoxPEGAuNPs | |
| 0 nM varlitinib | 23.6 ± 1.5 | 94.8 ± 0.3 | 17.1 ± 1.3 | |||
| 50 nM varlitinib | 25.3 ± 1.4 | 86.8 ± 0.5 | 18.0 ± 0.7 | |||
| 250 nM varlitinib | 32.6 ± 0.8 | 85.4 ± 1.6 | 17.9 ± 0.6 | |||
| 750 nM varlitinib | 30.1 ± 0.3 | 81.2 ± 0.4 | 12.2 ± 0.7 | |||
| 0nM VarlPEGAuNPs | 102.0 ± 1.0 | 50.8 ± 5.1 | 19.7 ± 0.9 | |||
| 50 nM VarlPEGAuNPs | 98.5 ± 0.5 | 35.7 ± 0.4 | 26.0 ± 1.1 | |||
| 250 nM VarlPEGAuNPs | 41.83 ± 0.8 | 27.6 ± 0.4 | 22.6 ± 1.0 | |||
| 750 nM VarlPEGAuNPs | 40.09 ± 0.7 | 24.8 ± 0.2 | 27.5 ± 1.1 | |||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coelho, S.C.; Reis, D.P.; Pereira, M.C.; Coelho, M.A.N. Doxorubicin and Varlitinib Delivery by Functionalized Gold Nanoparticles Against Human Pancreatic Adenocarcinoma. Pharmaceutics 2019, 11, 551. https://doi.org/10.3390/pharmaceutics11110551
Coelho SC, Reis DP, Pereira MC, Coelho MAN. Doxorubicin and Varlitinib Delivery by Functionalized Gold Nanoparticles Against Human Pancreatic Adenocarcinoma. Pharmaceutics. 2019; 11(11):551. https://doi.org/10.3390/pharmaceutics11110551
Chicago/Turabian StyleCoelho, Sílvia Castro, Daniel Pires Reis, Maria Carmo Pereira, and Manuel A. N. Coelho. 2019. "Doxorubicin and Varlitinib Delivery by Functionalized Gold Nanoparticles Against Human Pancreatic Adenocarcinoma" Pharmaceutics 11, no. 11: 551. https://doi.org/10.3390/pharmaceutics11110551
APA StyleCoelho, S. C., Reis, D. P., Pereira, M. C., & Coelho, M. A. N. (2019). Doxorubicin and Varlitinib Delivery by Functionalized Gold Nanoparticles Against Human Pancreatic Adenocarcinoma. Pharmaceutics, 11(11), 551. https://doi.org/10.3390/pharmaceutics11110551








