Dendrimers Show Promise for siRNA and microRNA Therapeutics
Abstract
1. Introduction
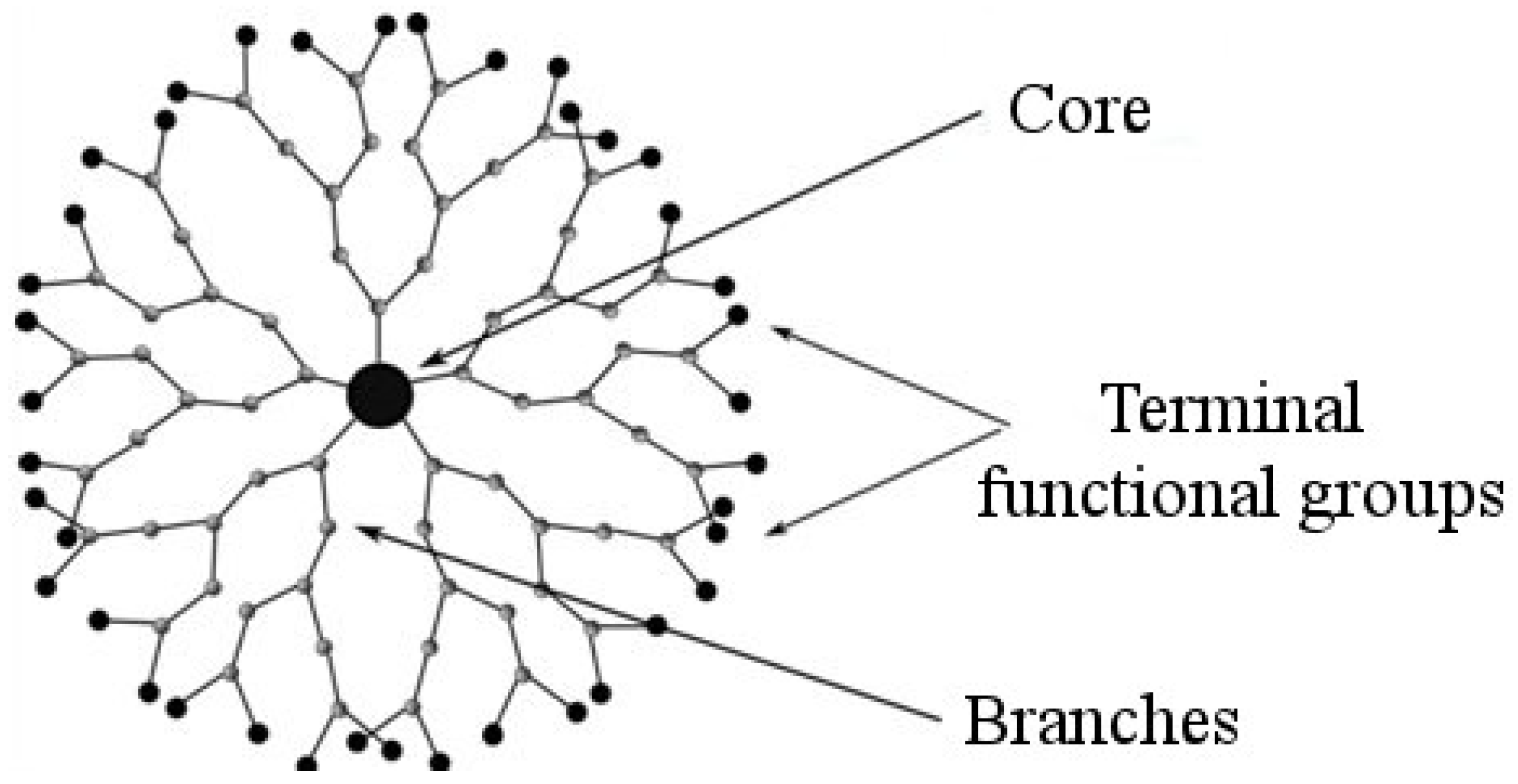
2. Dendrimers
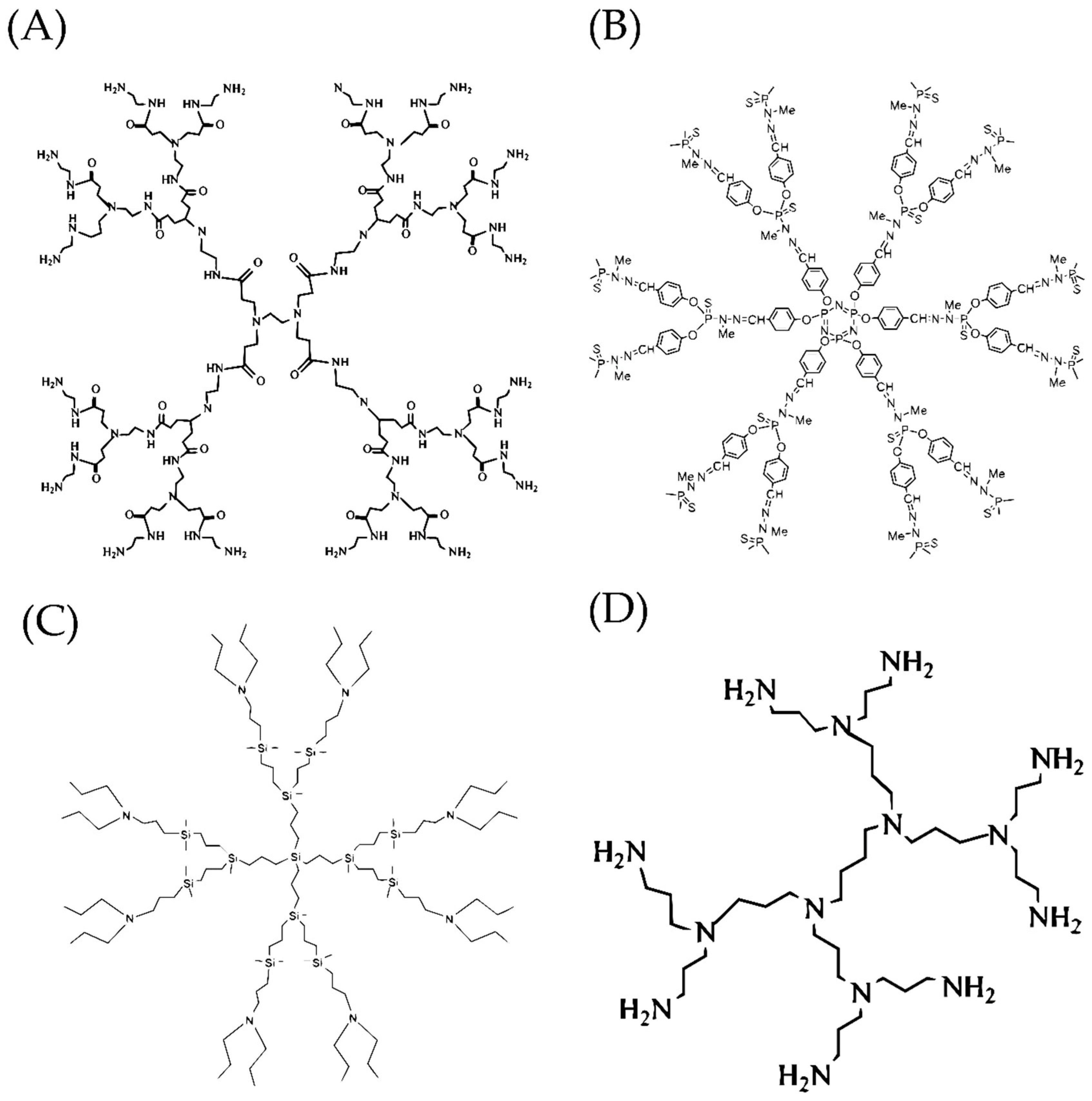
2.1. Polyamidoamine Dendrimers (PAMAMs)
2.2. Polypropylenimine Dendrimers (PPIs)
2.3. Carbosilane Dendrimers (CBS)
2.4. Polylysine Dendrimers (PLL Dendrimers)
2.5. Phosphorus-Containing Dendrimers
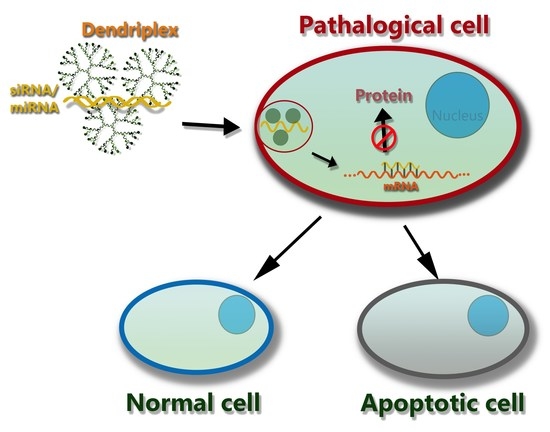
3. The Mechanism of Action of Dendriplexes in a Cell
4. PAMAM + siRNAs Dendriplexes
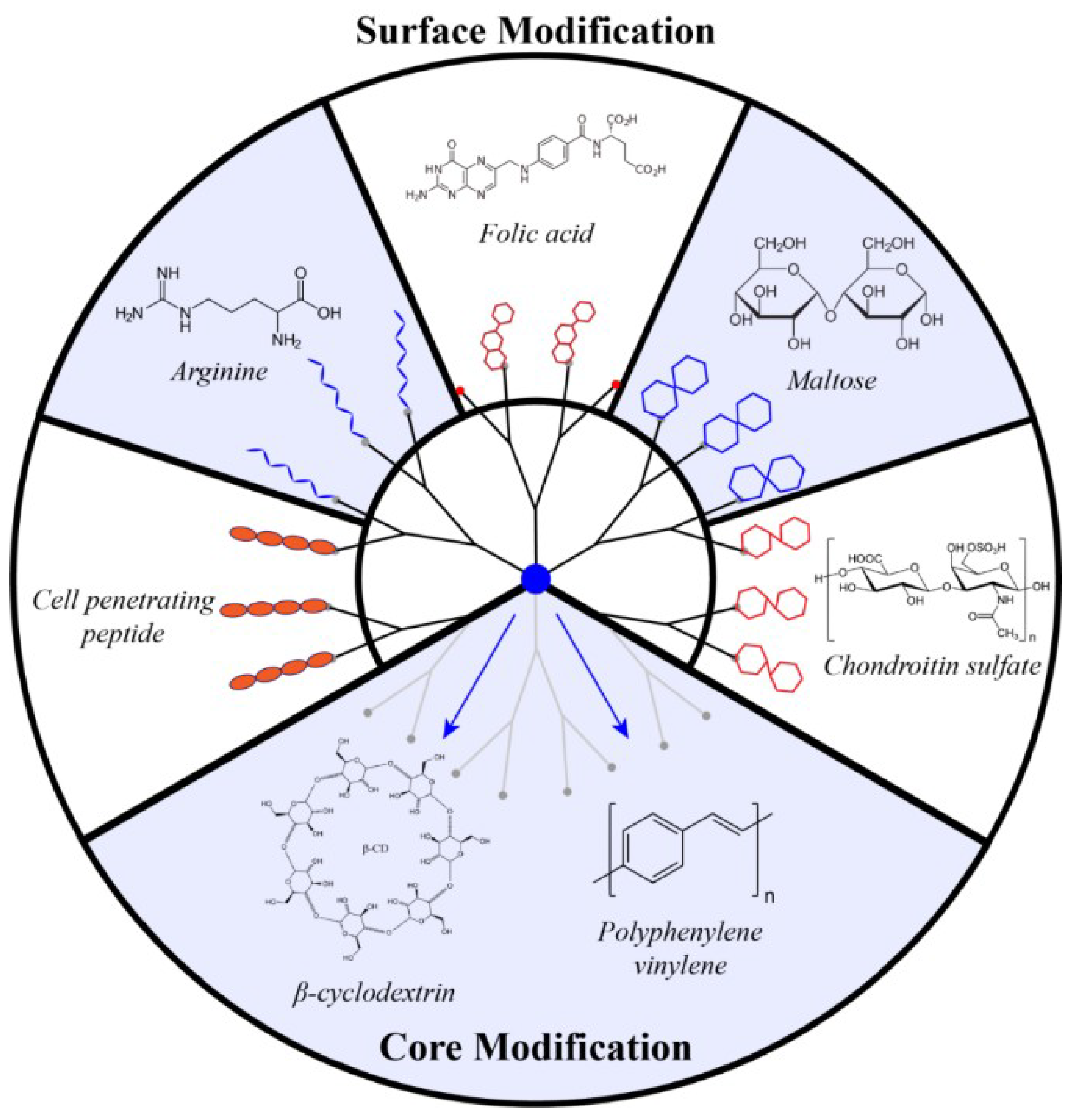
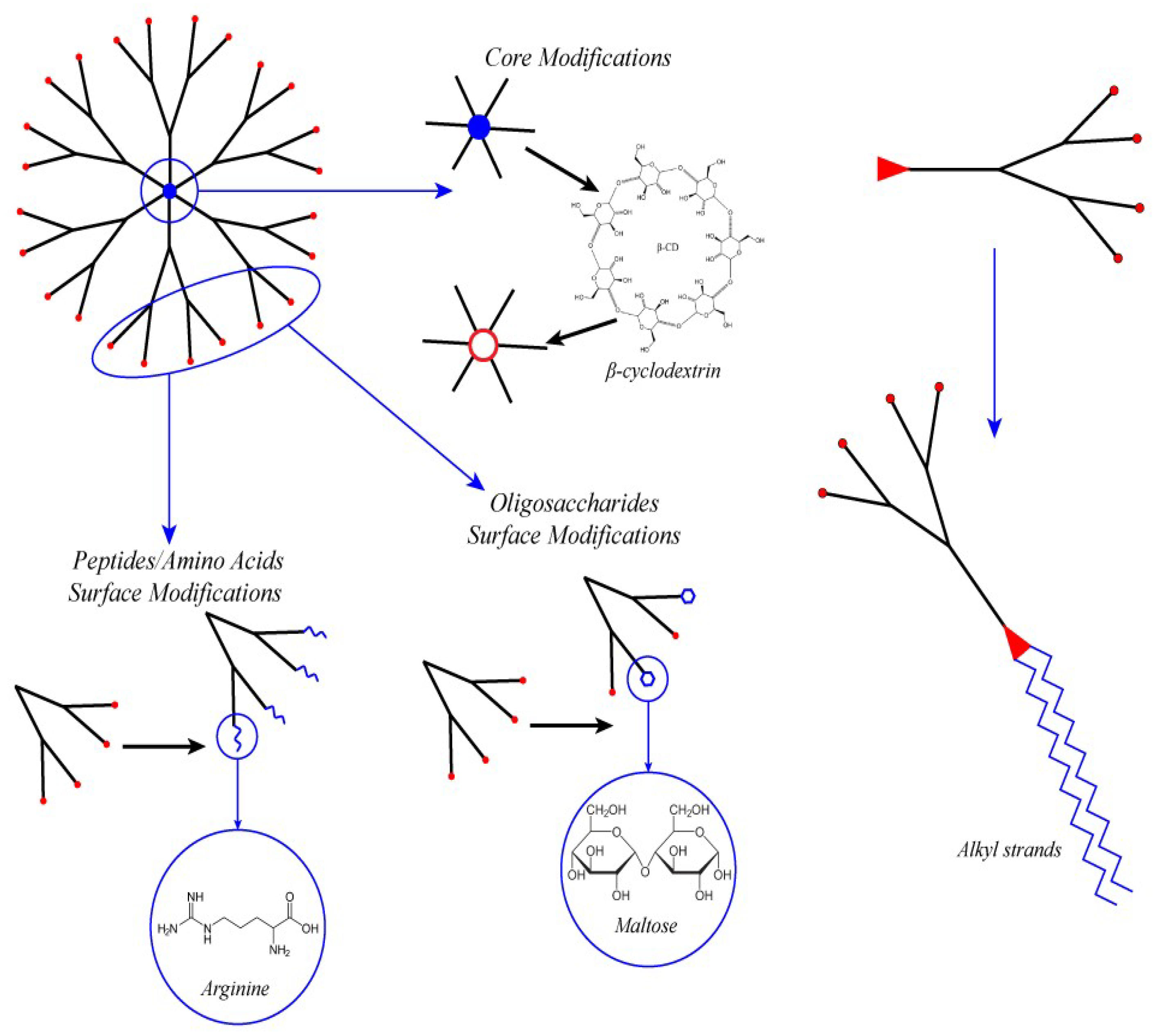
4.1. PAMAM Surface Decoration with Amino Acids and Peptides
4.2. Surface Decoration with Oligosaccharide
4.3. Core Modification
4.4. Amphiphilic Lipid-Like Dendrons
5. Non-PAMAM + siRNAs Dendriplexes
6. Dendriplexes with microRNA Mimics and Antagonists
- Similar to siRNA, the synthetic mimics of microRNAs (miRs) or their precursors can be introduced into the cell with subsequent target gene silencing triggered by RNA interference.
- Otherwise “malignant” endogenous microRNAs in the cells can be arrested by synthetic oligonucleotides called microRNA antagonists (antimiRs). These oligonucleotides form strong duplexes with microRNAs and block their activity [10].
7. Combined Effect of siRNA and microRNA with Therapeutic Drugs
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| TNA | therapeutic nucleic acid |
| RNAi | RNA interference |
| PAMAM | polyamidoamine |
| EDA | ethylenediamine |
| TEA | triethanolamine |
| PPI | polypropylenimine |
| PLL | poly-l-lysine |
| CBS | carbosilane dendrimer |
| Gn | dendrimer of nth generation |
| siRNA | small interfering RNA |
| dsiRNA | Dicer substrate siRNA (siRNA precursor) |
| miR | synthetic mimics of microRNA |
| antimiR | microRNA antagonist |
| PEG | polyethylene glycol |
| PBMC | peripheral blood mononuclear cell |
| CPP | cell-penetrating peptide |
| FA | folic acid |
References
- Kole, R.; Krainer, A.R.; Altman, S. RNA therapeutics: Beyond RNA interference and antisense oligonucleotides. Nat. Rev. Drug Discov. 2012, 11, 125–140. [Google Scholar] [CrossRef] [PubMed]
- Titze-de-Almeida, R.; David, C.; Titze-de-Almeida, S.S. The Race of 10 Synthetic RNAi-Based Drugs to the Pharmaceutical Market. Pharm. Res. 2017, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Borna, H.; Imani, S.; Iman, M.; Azimzadeh Jamalkandi, S. Therapeutic face of RNAi: In vivo challenges. Expert Opin. Biol. Ther. 2015, 15, 269–285. [Google Scholar] [CrossRef] [PubMed]
- Dufès, C.; Uchegbu, I.F.; Schätzlein, A.G. Dendrimers in gene delivery. Adv. Drug Deliv. Rev. 2005, 57, 2177–2202. [Google Scholar] [CrossRef] [PubMed]
- Niven, R.; Pearlman, R.; Wedeking, T.; Mackeigan, J.; Noker, P.; Simpson-Herren, L.; Smith, J.G. Biodistribution of radiolabeled lipid-DNA complexes and DNA in mice. J. Pharm. Sci. 1998, 87, 1292–1299. [Google Scholar] [CrossRef] [PubMed]
- Sen, G.L.; Blau, H.M. A brief history of RNAi: The silence of the genes. Faseb J. 2006, 20, 1293–1299. [Google Scholar] [CrossRef] [PubMed]
- Novina, C.D.; Sharp, P.A. The RNAi revolution. Nature 2004, 430, 161–164. [Google Scholar] [CrossRef] [PubMed]
- Sharp, P.A. RNA interference—2001. Genes Dev. 2001, 15, 485–490. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Rossi, J.J. Strategies for silencing human disease using RNA interference. Nat. Rev. Genet. 2007, 8, 173–184. [Google Scholar] [CrossRef] [PubMed]
- Lam, J.K.W.; Chow, M.Y.T.; Zhang, Y.; Leung, S.W.S. siRNA Versus miRNA as Therapeutics for Gene Silencing. Mol. Ther. Acids 2015, 4, e252. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yang, B.B. Friend or foe: The role of microRNA in chemotherapy resistance. Acta Pharmacol. Sin. 2013, 34, 870–879. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Dong, C.; Ji, C. MicroRNA and drug resistance. Cancer Gene Ther. 2010, 17, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Daka, A.; Peer, D. RNAi-based nanomedicines for targeted personalized therapy. Adv. Drug Deliv. Rev. 2012, 64, 1508–1521. [Google Scholar] [CrossRef] [PubMed]
- Ozcan, G.; Ozpolat, B.; Coleman, R.L.; Sood, A.K.; Lopez-Berestein, G. Preclinical and clinical development of siRNA-based therapeutics. Adv. Drug Deliv. Rev. 2015, 87, 108–119. [Google Scholar] [CrossRef] [PubMed]
- Kelnar, K.; Bader, A.G. A qRT-PCR method for determining the biodistribution profi le of a miR-34a mimic. Methods Mol. Biol. 2015, 1317, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Querfeld, C.; Pacheco, T.; Foss, F.M.; Halwani, A.S.; Porcu, P.; Seto, A.G.; Ruckman, J.; Landry, M.L.; Jackson, A.L.; Pestano, L.A.; et al. Preliminary Results of a Phase 1 Trial Evaluating MRG-106, a Synthetic microRNA Antagonist (LNA antimiR) of microRNA-155, in Patients with CTCL. Blood 2016, 128, 1829. [Google Scholar]
- Whitehead, K.A.; Langer, R.; Anderson, D.G. Knocking down barriers: Advances in siRNA delivery. Nat. Rev. Drug Discov. 2009, 8, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Vannucci, L.; Lai, M.; Chiuppesi, F.; Ceccherini-Nelli, L.; Pistello, M. Viral vectors: A look back and ahead on gene transfer technology. New Microbiol. 2013, 36, 1–22. [Google Scholar] [PubMed]
- Ylä-Herttuala, S. Endgame: Glybera Finally Recommended for Approval as the First Gene Therapy Drug in the European Union. Mol. Ther. 2012, 20, 1831–1832. [Google Scholar] [CrossRef] [PubMed]
- Touchot, N.; Flume, M. Early Insights from Commercialization of Gene Therapies in Europe. Genes 2017, 8, 78. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Jiang, Y.; Peng, H.; Chen, Y.; Zhu, P.; Huang, Y. Recent progress in microRNA delivery for cancer therapy by non-viral synthetic vectors. Adv. Drug Deliv. Rev. 2015, 81, 142–160. [Google Scholar] [CrossRef] [PubMed]
- Kanasty, R.; Dorkin, J.R.; Vegas, A.; Anderson, D. Delivery materials for siRNA therapeutics. Nat. Mater. 2013, 12, 967–977. [Google Scholar] [CrossRef] [PubMed]
- Dahlman, J.E.; Kauffman, K.J.; Langer, R.; Anderson, D.G. Nanotechnology for In vivo Targeted siRNA Delivery. Adv. Genet. 2014, 88, 37–69. [Google Scholar] [CrossRef] [PubMed]
- Tomalia, D.A.; Baker, H.; Dewald, J.; Hall, M.; Kallos, G.; Martin, S.; Roeck, J.; Ryder, J.; Smith, P. A New Class of Polymers: Starburst-Dendritic Macromolecules. Polym. J. 1985, 17, 117–132. [Google Scholar] [CrossRef]
- Caminade, A.-M.; Ouali, A.; Laurent, R.; Turrin, C.-O.; Majoral, J.-P. The dendritic effect illustrated with phosphorus dendrimers. Chem. Soc. Rev. 2015. [Google Scholar] [CrossRef] [PubMed]
- Haensler, J.; Szoka, F.C.J. Polyamidoamine cascade polymers mediate efficient transfection of cells in culture. Bioconjugate Chem. 1993, 4, 372–379. [Google Scholar] [CrossRef]
- Bauer, B.J.; Amis, E.J. Characterization of Dendritically Branched Polymers by Small Angle Neutron Scattering (SANS), Small Angle X-Ray Scattering (SAXS) and Transmission Electron Microscopy (TEM). In Dendrimers and Other Dendritic Polymers; John Wiley & Sons, Ltd.: Chichester, UK, 2001; pp. 255–284. ISBN 9780470845820. [Google Scholar]
- Mintzer, M.A.; Grinstaff, M.W. Biomedical applications of dendrimers: A tutorial. Chem. Soc. Rev. 2011, 40, 173–190. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, E.; Aval, S.; Akbarzadeh, A.; Milani, M.; Nasrabadi, H.; Joo, S.; Hanifehpour, Y.; Nejati-Koshki, K.; Pashaei-Asl, R. Dendrimers: Synthesis, applications, and properties. Nanoscale Res. Lett. 2014, 9, 247. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H. Tumor-selective delivery of macromolecular drugs via the EPR effect: Background and future prospects. Bioconjug. Chem. 2010, 21, 797–802. [Google Scholar] [CrossRef] [PubMed]
- Dendrimers: Towards Catalytic, Material and Biomedical Uses; Caminade, A.-M., Turrin, C.-O., Laurent, R., Ouali, A., Delavaux-Nicot, B., Eds.; John Wiley & Sons Ltd.: Chichester, UK, 2011; ISBN 9781119976530. [Google Scholar]
- Dendrimers in Biomedical Applications; Klajnert, B., Peng, L., Cena, V., Eds.; Royal Society of Chemistry: Cambridge, UK, 2013; ISBN 978-1-84973-611-4. [Google Scholar]
- Caminade, A.M.; Turrin, C.O.; Majoral, J.P. Biological properties of phosphorus dendrimers. New J. Chem. 2010. [Google Scholar]
- Khan, O.F.; Zaia, E.W.; Yin, H.; Bogorad, R.L.; Pelet, J.M.; Webber, M.J.; Zhuang, I.; Dahlman, J.E.; Langer, R.; Anderson, D.G. Ionizable amphiphilic dendrimer-based nanomaterials with alkyl-chain-substituted amines for tunable sirna delivery to the liver endothelium in vivo. Angew. Chem. Int. Ed. 2014, 53, 14397–14401. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Neff, C.P.; Liu, X.; Zhang, J.; Li, H.; Smith, D.D.; Swiderski, P.; Aboellail, T.; Huang, Y.; Du, Q.; et al. Systemic administration of combinatorial dsiRNAs via nanoparticles efficiently suppresses HIV-1 infection in humanized mice. Mol. Ther. 2011, 19, 2228–2238. [Google Scholar] [CrossRef] [PubMed]
- Rodrigo, A.C.; Rivilla, I.; Perez-Martinez, F.C.; Monteagudo, S.; Ocana, V.; Guerra, J.; Garcia-Martinez, J.C.; Merino, S.; Sanchez-Verdu, P.; Cena, V.; et al. Efficient, non-toxic hybrid PPV-PAMAM dendrimer as a gene carrier for neuronal cells. Biomacromolecules 2011, 12, 1205–1213. [Google Scholar] [CrossRef] [PubMed]
- Tomalia, D.A.; Christensen, J.B.; Boas, U. Dendrimers, Dendrons, and Dendritic Polymers, Discovery, Applications, and the Future; Cambridge University Press: Cambridge, UK, 2012; 412p. [Google Scholar]
- Liu, X.; Li, G.; Su, Z.; Jiang, Z.; Chen, L.; Wang, J.; Yu, S.; Liu, Z. Poly(amido amine) is an ideal carrier of miR-7 for enhancing gene silencing effects on the EGFR pathway in U251 glioma cells. Oncol. Rep. 2013, 29, 1387–1394. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liu, C.; Zhou, J.; Chen, C.; Qu, F.; Rossi, J.J.; Rocchi, P.; Peng, L. Promoting siRNA delivery via enhanced cellular uptake using an arginine-decorated amphiphilic dendrimer. Nanoscale 2015, 7, 3867–3875. [Google Scholar] [CrossRef] [PubMed]
- Dehshahri, A.; Sadeghpour, H. Surface decorations of poly(amidoamine) dendrimer by various pendant moieties for improved delivery of nucleic acid materials. Colloids Surf. B Biointerfaces 2015, 132, 85–102. [Google Scholar] [CrossRef] [PubMed]
- Van Duijvenbode, R.C.; Borkovec, M.; Koper, G.J.M. Acid-base properties of poly(propylene imine)dendrimers. Polymer 1998, 39, 2657–2664. [Google Scholar] [CrossRef]
- Kesharwani, P.; Jain, K.; Jain, N.K. Dendrimer as nanocarrier for drug delivery. Prog. Polym. Sci. 2014, 39, 268–307. [Google Scholar] [CrossRef]
- Santhakumaran, L.M. Enhanced cellular uptake of a triplex-forming oligonucleotide by nanoparticle formation in the presence of polypropylenimine dendrimers. Nucleic Acids Res. 2004, 32, 2102–2112. [Google Scholar] [CrossRef] [PubMed]
- Zinselmeyer, B.H.; Mackay, S.P.; Schatzlein, A.G.; Uchegbu, I.F. The lower-generation polypropylenimine dendrimers are effective gene-transfer agents. Pharm. Res. 2002, 19, 960–967. [Google Scholar] [CrossRef] [PubMed]
- Bermejo, J.F.; Ortega, P.; Chonco, L.; Eritja, R.; Samaniego, R.; Müllner, M.; De Jesus, E.; De La Mata, F.J.; Flores, J.C.; Gomez, R.; et al. Water-soluble carbosilane dendrimers: Synthesis biocompatibility and complexation with oligonucleotides; evaluation for medical applications. Chem. A Eur. J. 2007, 13, 483–495. [Google Scholar] [CrossRef] [PubMed]
- Ortega, P.; Bermejo, J.F.; Chonco, L.; De Jesus, E.; De La Mata, F.J.; Fernández, G.; Flores, J.C.; Gómez, R.; Serramía, M.J.; Angeles Muñoz-Fernandez, M. Novel water-soluble carbosilane dendrimers: Synthesis and biocompatibility. Eur. J. Inorg. Chem. 2006, 1388–1396. [Google Scholar] [CrossRef]
- Pedziwiatr-Werbicka, E.; Fuentes, E.; Dzmitruk, V.; Sánchez-Nieves, J.; Sudas, M.; Drozd, E.; Shakhbazau, A.; Shcharbin, D.; de la Mata, F.J.; Gomez-Ramirez, R.; et al. Novel “SiC” carbosilane dendrimers as carriers for anti-HIV nucleic acids: Studies on complexation and interaction with blood cells. Colloids Surf. B Biointerfaces 2013, 109, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Ionov, M.; Lazniewska, J.; Dzmitruk, V.; Halets, I.; Loznikova, S.; Novopashina, D.; Apartsin, E.; Krasheninina, O.; Venyaminova, A.; Milowska, K.; et al. Anticancer siRNA cocktails as a novel tool to treat cancer cells. Part (A). Mechanisms of interaction. Int. J. Pharm. 2015, 485, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Dzmitruk, V.; Szulc, A.; Shcharbin, D.; Janaszewska, A.; Shcharbina, N.; Lazniewska, J.; Novopashina, D.; Buyanova, M.; Ionov, M.; Klajnert-Maculewicz, B.; et al. Anticancer siRNA cocktails as a novel tool to treat cancer cells. Part (B). Efficiency of pharmacological action. Int. J. Pharm. 2015, 485, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, J.L.; Clemente, M.I.; Weber, N.D.; Sanchez, J.; Ortega, P.; de la Mata, F.J.; Gomez, R.; Garcia, D.; Lopez-Fernandez, L.A.; Munoz-Fernandez, M.A. Carbosilane dendrimers to transfect human astrocytes with small interfering RNA targeting human immunodeficiency virus. BioDrugs 2010, 24, 331–343. [Google Scholar] [CrossRef] [PubMed]
- Posadas, I.; López-Hernández, B.; Clemente, M.I.; Jiménez, J.L.; Ortega, P.; De La Mata, J.; Gómez, R.; Muñoz-Fernández, M.A.; Ceña, V. Highly efficient transfection of rat cortical neurons using carbosilane dendrimers unveils a neuroprotective Role for HIF-1α in early chemical hypoxia-mediated neurotoxicity. Pharm. Res. 2009, 26, 1181–1191. [Google Scholar] [CrossRef] [PubMed]
- Serramía, M.J.; Álvarez, S.; Fuentes-Paniagua, E.; Clemente, M.I.; Sánchez-Nieves, J.; Gómez, R.; De La Mata, J.; Muñoz-Fernández, M.Á. In vivo delivery of siRNA to the brain by carbosilane dendrimer. J. Control. Release 2015, 200, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Kadlecova, Z.; Rajendra, Y.; Matasci, M.; Baldi, L.; Hacker, D.L.; Wurm, F.M.; Klok, H.A. DNA delivery with hyperbranched polylysine: A comparative study with linear and dendritic polylysine. J. Control. Release 2013, 169, 276–288. [Google Scholar] [CrossRef] [PubMed]
- Ohsaki, M.; Okuda, T.; Wada, A.; Hirayama, T.; Niidome, T.; Aoyagi, H. In Vitro Gene Transfection Using Dendritic Poly(l-lysine). Bioconjug. Chem. 2002, 13, 510–517. [Google Scholar] [CrossRef] [PubMed]
- Okuda, T.; Kidoaki, S.; Ohsaki, M.; Koyama, Y.; Yoshikawa, K.; Niidome, T.; Aoyagi, H. Time-dependent complex formation of dendritic poly(l-lysine) with plasmid DNA and correlation with in vitro transfection efficiencies. Org. Biomol. Chem. 2003, 1, 1270–1273. [Google Scholar] [CrossRef] [PubMed]
- Okuda, T.; Sugiyama, A.; Niidome, T.; Aoyagi, H. Characters of dendritic poly(l-lysine) analogues with the terminal lysines replaced with arginines and histidines as gene carriers in vitro. Biomaterials 2004, 25, 537–544. [Google Scholar] [CrossRef]
- Yamagata, M.; Kawano, T.; Shiba, K.; Mori, T.; Katayama, Y.; Niidome, T. Structural advantage of dendritic poly(l-lysine) for gene delivery into cells. Bioorg. Med. Chem. 2007, 15, 526–532. [Google Scholar] [CrossRef] [PubMed]
- Caminade, A.-M.; Majoral, J.-P. Positively charged phosphorus dendrimers. An overview of their properties. New J. Chem. 2013, 37, 3358–3373. [Google Scholar] [CrossRef]
- Caminade, A.-M.; Majoral, J.-P. Water-soluble phosphorus-containing dendrimers. Prog. Polym. Sci. 2005, 30, 491–505. [Google Scholar] [CrossRef]
- Majoral, J.-P.; Caminade, A.-M.; Laurent, R.; Sutra, P. Phosphorus-containing dendrimers: From material science to biology. Heteroat. Chem. 2002, 13, 474–485. [Google Scholar] [CrossRef]
- Mu, R.; Laschober, C.; Szymanski, W.W. Determination of Molecular Weight, Particle Size, and Density of High Number Generation PAMAM Dendrimers Using MALDI-TOF-MS and nES-GEMMA. Macromolecules 2007, 40, 5599–5605. [Google Scholar] [CrossRef]
- Loup, C.; Zanta, M.; Caminade, A. Preparation of Water-Soluble Cationic Phosphorus-Containing Dendrimers. Chem. Eur. J. 1999, 5, 3644–3650. [Google Scholar] [CrossRef]
- Padié, C.; Maszewska, M.; Majchrzak, K.; Nawrot, B.; Caminade, A.-M.; Majoral, J.-P. Polycationic phosphorus dendrimers: Synthesis, characterization, study of cytotoxicity, complexation of DNA, and transfection experiments. New J. Chem. 2009, 33, 318–326. [Google Scholar] [CrossRef]
- D’Emanuele, A.; Attwood, D. Dendrimer-drug interactions. Adv. Drug Deliv. Rev. 2005, 57, 2147–2162. [Google Scholar] [CrossRef] [PubMed]
- Shcharbin, D.; Pedziwiatr, E.; Bryszewska, M. How to study dendriplexes I: Characterization. J. Control. Release 2009, 135, 186–197. [Google Scholar] [CrossRef] [PubMed]
- Shcharbin, D.; Pedziwiatr, E.; Blasiak, J.; Bryszewska, M. How to study dendriplexes II: Transfection and cytotoxicity. J. Control. Release 2010, 141, 110–127. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.; van Dongen, M.A.; Han, Y.; Yu, M.; Li, Y.; Liu, G.; Banaszak Holl, M.M.; Qi, R. The role of caveolin-1 and syndecan-4 in the internalization of PEGylated PAMAM dendrimer polyplexes into myoblast and hepatic cells. Eur. J. Pharm. Biopharm. 2014, 88, 658–663. [Google Scholar] [CrossRef] [PubMed]
- Sahay, G.; Alakhova, D.Y.; Kabanov, A. V Endocytosis of nanomedicines. J. Control. Release 2010, 145, 182–195. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, Y.; Hatakeyama, H.; Sato, Y.; Akita, H.; Takayama, K.; Kobayashi, S.; Futaki, S.; Harashima, H. Endosomal escape and the knockdown efficiency of liposomal-siRNA by the fusogenic peptide shGALA. Biomaterials 2011, 32, 5733–5742. [Google Scholar] [CrossRef] [PubMed]
- Rejman, J.; Bragonzi, A.; Conese, M. Role of clathrin- and caveolae-mediated endocytosis in gene transfer mediated by lipo- and polyplexes. Mol. Ther. 2005, 12, 468–474. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, J.; Szoka, F.C. Nucleic acid delivery: The missing pieces of the puzzle? Acc. Chem. Res. 2012, 45, 1153–1162. [Google Scholar] [CrossRef] [PubMed]
- Shakya, A.; Dougherty, C.A.; Xue, Y.; Al-Hashimi, H.M.; Banaszak Holl, M.M. Rapid Exchange Between Free and Bound States in RNA–Dendrimer Polyplexes: Implications on the Mechanism of Delivery and Release. Biomacromolecules 2016, 17, 154–164. [Google Scholar] [CrossRef] [PubMed]
- Szewczyk, M.; Drzewinska, J.; Dzmitruk, V.; Shcharbin, D.; Klajnert, B.; Appelhans, D.; Bryszewska, M. Stability of Dendriplexes Formed by Anti-HIV Genetic Material and Poly(propylene imine) Dendrimers in the Presence of Glucosaminoglycans. J. Phys. Chem. B 2012, 116, 14525–14532. [Google Scholar] [CrossRef] [PubMed]
- Jain, K.; Kesharwani, P.; Gupta, U.; Jain, N.K. Dendrimer toxicity: Let’s meet the challenge. Int. J. Pharm. 2010, 394, 122–142. [Google Scholar] [CrossRef] [PubMed]
- Shcharbin, D.; Ionov, M.; Abashkin, V.; Loznikova, S.; Dzmitruk, V.; Shcharbina, N.; Matusevich, L.; Milowska, K.; Gałecki, K.; Wysocki, S.; et al. Nanoparticle corona for proteins: Mechanisms of interaction between dendrimers and proteins. Colloids Surf. B Biointerfaces 2015, 134, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Akesson, A.; Cardenas, M.; Elia, G.; Monopoli, M.P.; Dawson, K.A. The protein corona of dendrimers: PAMAM binds and activates complement proteins in human plasma in a generation dependent manner. RSC Adv. 2012, 2, 11245–11248. [Google Scholar] [CrossRef]
- Shcharbin, D.; Janaszewska, A.; Klajnert-Maculewicz, B.; Ziemba, B.; Dzmitruk, V.; Halets, I.; Loznikova, S.; Shcharbina, N.; Milowska, K.; Ionov, M.; et al. How to study dendrimers and dendriplexes III. Biodistribution, pharmacokinetics and toxicity in vivo. J. Control. Release 2014, 181, 40–52. [Google Scholar] [CrossRef] [PubMed]
- Russ, V.; Gnther, M.; Halama, A.; Ogris, M.; Wagner, E. Oligoethylenimine-grafted polypropylenimine dendrimers as degradable and biocompatible synthetic vectors for gene delivery. J. Control. Release 2008, 132, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Dennig, J.; Duncan, E. Gene transfer into eukaryotic cells using activated polyamidoamine dendrimers. J. Biotechnol. 2002, 90, 339–347. [Google Scholar] [CrossRef]
- Al-Jamal, K.T.; Ramaswamy, C.; Florence, A.T. Supramolecular structures from dendrons and dendrimers. Adv. Drug Deliv. Rev. 2005, 57, 2238–2270. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, J.; Xiao, C.; Li, M.; Tian, H.; Chen, X. Cationic dendron-bearing lipids: Investigating structure-activity relationships for small interfering RNA delivery. Biomacromolecules 2013, 14, 4289–4300. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Liu, X.; Rocchi, P.; Qu, F.; Iovanna, J.L.; Peng, L. Arginine-terminated generation 4 PAMAM dendrimer as an effective nanovector for functional siRNA delivery in vitro and in vivo. Bioconjug. Chem. 2014, 25, 521–532. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liu, C.; Chen, C.; Bentobji, M.; Cheillan, F.A.; Piana, J.T.; Qu, F.; Rocchi, P.; Peng, L. Targeted delivery of Dicer-substrate siRNAs using a dual targeting peptide decorated dendrimer delivery system. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 1627–1636. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Liu, X.; Bolcato-Bellemin, A.-L.; Wang, Y.; Liu, C.; Erbacher, P.; Qu, F.; Rocchi, P.; Behr, J.-P.; Peng, L. An amphiphilic dendrimer for effective delivery of small interfering RNA and gene silencing in vitro and in vivo. Angew. Chem. Int. Ed. Engl. 2012, 51, 8478–8484. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhou, J.; Yu, T.; Chen, C.; Cheng, Q.; Sengupta, K.; Huang, Y.; Li, H.; Liu, C.; Wang, Y.; et al. Adaptive amphiphilic dendrimer-based nanoassemblies as robust and versatile siRNA delivery systems. Angew. Chem. Int. Ed. 2014, 53, 11822–11827. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.; Zhang, Y.; Li, L.; Cheng, Y. Efficient delivery of small interfering RNA into cancer cells using dodecylated dendrimers. J. Mater. Chem. B 2015, 3, 8197–8202. [Google Scholar] [CrossRef]
- Patil, M.L.; Zhang, M.; Betigeri, S.; Taratula, O.; He, H.; Minko, T. Surface-modified and internally cationic polyamidoamine dendrimers for efficient siRNA delivery. Bioconjug. Chem. 2008, 19, 1396–1403. [Google Scholar] [CrossRef] [PubMed]
- Patil, M.L.; Zhang, M.; Taratula, O.; Garbuzenko, O.B.; He, H.; Minko, T. Internally cationic polyamidoamine PAMAM-OH dendrimers for siRNA delivery: Effect of the degree of Quaternization and cancer targeting. Biomacromolecules 2009, 10, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Patil, M.L.; Zhang, M.; Minko, T. Multifunctional triblock nanocarrier (PAMAM-PEG-PLL) for the efficient intracellular siRNA delivery and gene silencing. ACS Nano 2011, 5, 1877–1887. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; DeLong, R.; Fisher, M.H.; Juliano, R.L. Tat-Conjugated PAMAM Dendrimers as Delivery Agents for Antisense and siRNA Oligonucleotides. Pharm. Res. 2005, 22, 2099–2106. [Google Scholar] [CrossRef] [PubMed]
- Anno, T.; Higashi, T.; Hayashi, Y.; Motoyama, K.; Jono, H.; Ando, Y.; Arima, H. Potential use of glucuronylglucosyl-β-cyclodextrin/dendrimer conjugate (G2) as a siRNA carrier for the treatment of familial amyloidotic polyneuropathy. J. Drug Target. 2014, 22, 883–890. [Google Scholar] [CrossRef] [PubMed]
- Motoyama, K.; Mitsuyasu, R.; Akao, C.; Abu Hashim, I.I.; Sato, N.; Tanaka, T.; Higashi, T.; Arima, H. Potential Use of Thioalkylated Mannose-Modified Dendrimer (G3)/α-Cyclodextrin Conjugate as an NF-κB siRNA Carrier for the Treatment of Fulminant Hepatitis. Mol. Pharm. 2015, 12, 3129–3136. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Luo, H.-C.; Zhang, L.-M.; Deng, J.-J.; Xie, X.-Y.; Lin, D.-Z.; Ren, M.; Yang, C.; Yan, L. Cationic star-shaped polymer as an siRNA carrier for reducing MMP-9 expression in skin fibroblast cells and promoting wound healing in diabetic rats. Int. J. Nanomed. 2014, 9, 3377. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Gu, C.; Cabigas, E.B.; Pendergrass, K.D.; Brown, M.E.; Luo, Y.; Davis, M.E. Functionalized dendrimer-based delivery of angiotensin type 1 receptor siRNA for preserving cardiac function following infarction. Biomaterials 2013, 34, 3729–3736. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Carriõn, M.D.; Pérez-Martínez, F.C.; Merino, S.; Sánchez-Verdã, P.; Martínez-Hernández, J.; Luján, R.; Ceña, V. Dendrimer-mediated siRNA delivery knocks down Beclin 1 and potentiates NMDA-mediated toxicity in rat cortical neurons. J. Neurochem. 2012, 120, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Finlay, J.; Roberts, C.M.; Lowe, G.; Loeza, J.; Rossi, J.J.; Glackin, C.A. RNA-based TWIST1 inhibition via dendrimer complex to reduce breast cancer cell metastasis. Biomed. Res. Int. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Harada-Shiba, M.; Suzuki, A.; Gokuden, R.; Kurihara, R.; Sugao, Y.; Mori, T.; Katayama, Y.; Niidome, T. In vivo siRNA delivery with dendritic poly(l-lysine) for the treatment of hypercholesterolemia. Mol. Biosyst. 2009, 5, 1306–1310. [Google Scholar] [CrossRef] [PubMed]
- Inoue, Y.; Kurihara, R.; Tsuchida, A.; Hasegawa, M.; Nagashima, T.; Mori, T.; Niidome, T.; Katayama, Y.; Okitsu, O. Efficient delivery of siRNA using dendritic poly(l-lysine) for loss-of-function analysis. J. Control. Release 2008, 126, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Perisé-Barrios, A.J.; Jiménez, J.L.; D’Omínguez-Soto, A.; De La Mata, F.J.; Corbí, A.L.; Gomez, R.; Muñoz-Fernandez, M.Á. Carbosilane dendrimers as gene delivery agents for the treatment of HIV infection. J. Control. Release 2014, 184, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Briz, V.; Serramía, M.J.; Madrid, R.; Hameau, A.; Caminade, A.-M.; Majoral, J.P.; Muñoz-Fernández, M.A. Validation of a generation 4 phosphorus-containing polycationic dendrimer for gene delivery against HIV-1. Curr. Med. Chem. 2012, 19, 5044–5051. [Google Scholar] [CrossRef] [PubMed]
- Gonzalo, T.; Clemente, M.I.; Chonco, L.; Weber, N.D.; Díaz, L.; Serramía, M.J.; Gras, R.; Ortega, P.; De La Mata, F.J.; Gómez, R.; et al. Gene therapy in HIV-infected cells to decrease viral impact by using an alternative delivery method. ChemMedChem 2010, 5, 921–929. [Google Scholar] [CrossRef] [PubMed]
- Weber, N.; Ortega, P.; Clemente, M.I.; Shcharbin, D.; Bryszewska, M.; de la Mata, F.J.; Gómez, R.; Muñoz-Fernández, M.A. Characterization of carbosilane dendrimers as effective carriers of siRNA to HIV-infected lymphocytes. J. Control. Release 2008, 132, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Taratula, O.; Garbuzenko, O.B.; Kirkpatrick, P.; Pandya, I.; Savla, R.; Pozharov, V.P.; He, H.; Minko, T. Surface-engineered targeted PPI dendrimer for efficient intracellular and intratumoral siRNA delivery. J. Control. Release 2009, 140, 284–293. [Google Scholar] [CrossRef] [PubMed]
- Ziemba, B.; Halets, I.; Shcharbin, D.; Appelhans, D.; Voit, B.; Pieszynski, I.; Bryszewska, M.; Klajnert, B. Influence of fourth generation poly(propyleneimine) dendrimers on blood cells. J. Biomed. Mater. Res. Part A 2012, 100A, 2870–2880. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Posocco, P.; Liu, X.; Cheng, Q.; Laurini, E.; Zhou, J.; Liu, C.; Wang, Y.; Tang, J.; Col, V.D.; et al. Mastering Dendrimer Self-Assembly for Efficient siRNA Delivery: From Conceptual Design to In Vivo Efficient Gene Silencing. Small 2016, 3667–3676. [Google Scholar] [CrossRef] [PubMed]
- Shcharbin, D.; Pedziwiatr, E.; Nowacka, O.; Kumar, M.; Zaborski, M.; Ortega, P.; Javier de la Mata, F.; Gómez, R.; Muñoz-Fernandez, M.A.; Bryszewska, M. Carbosilane dendrimers NN8 and NN16 form a stable complex with siGAG1. Colloids Surf. B Biointerfaces 2011, 83, 388–391. [Google Scholar] [CrossRef] [PubMed]
- Pedziwiatr-Werbicka, E.; Shcharbin, D.; Maly, J.; Maly, M.; Zaborski, M.; Gabara, B.; Ortega, P.; Javier De La Mata, F.; Gómez, R.; Angeles Muñoz-Fernandez, M.; et al. Carbosilane dendrimers are a non-viral delivery system for antisense oligonucleotides: Characterization of dendriplexes. J. Biomed. Nanotechnol. 2012, 8, 57–73. [Google Scholar] [CrossRef] [PubMed]
- Elbashir, S.M.; Harborth, J.; Lendeckel, W.; Yalcin, A.; Weber, K.; Tuschl, T. Duplexes of 21 ± nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature 2001, 411, 494–498. [Google Scholar] [CrossRef] [PubMed]
- Davidson, B.L.; McCray, P.B. Current prospects for RNA interference-based therapies. Nat. Rev. Genet. 2011, 12, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Ha, M.; Kim, V.N. Regulation of microRNA biogenesis. Nat. Rev. Mol. Cell Biol. 2014, 15, 509–524. [Google Scholar] [CrossRef] [PubMed]
- Lim, L.P.; Lau, N.C.; Garrett-Engele, P.; Grimson, A.; Schelter, J.M.; Castle, J.; Bartel, D.P.; Linsley, P.S.; Johnson, J.M. Microarray analysis shows that some microRNAs downregulate large numbers of target mRNAs. Nature 2005, 433, 769–773. [Google Scholar] [CrossRef] [PubMed]
- Vasudevan, S.; Yingchun Tong, J.A.S. Switching from Repression to Activation: MicroRNAs Can Up-Regulate Translation. Science 2007, 318, 1931–1934. [Google Scholar] [CrossRef] [PubMed]
- Bartel, D.P. MicroRNA Target Recognition and Regulatory Functions. Cell 2009, 136, 215–233. [Google Scholar] [CrossRef] [PubMed]
- Sethi, S.; Ali, S.; Sethi, S.; Sarkar, F.H. MicroRNAs in personalized cancer therapy. Clin. Genet. 2014, 86, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Budak, H.; Bulut, R.; Kantar, M.; Alptekin, B. MicroRNA nomenclature and the need for a revised naming prescription. Brief. Funct. Genom. 2016, 15, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Desvignes, T.; Batzel, P.; Berezikov, E.; Eilbeck, K.; Eppig, J.T.; McAndrews, M.S.; Singer, A.; Postlethwait, J.H. MiRNA Nomenclature: A View Incorporating Genetic Origins, Biosynthetic Pathways, and Sequence Variants. Trends Genet. 2015, 31, 613–626. [Google Scholar] [CrossRef] [PubMed]
- Rupaimoole, R.; Slack, F.J. MicroRNA therapeutics: Towards a new era for the management of cancer and other diseases. Nat. Rev. Drug Discov. 2017, 16, 203–222. [Google Scholar] [CrossRef] [PubMed]
- Gray, W.D.; Wu, R.J.; Yin, X.; Zhou, J.; Davis, M.E.; Luo, Y. Dendrimeric bowties featuring hemispheric-selective decoration of ligands for microRNA-based therapy. Biomacromolecules 2013, 14, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K.; Nguyen, L.H.; Miller, J.B.; Yan, Y.; Kos, P.; Xiong, H.; Li, L.; Hao, J.; Minnig, J.T.; Zhu, H.; et al. Modular degradable dendrimers enable small RNAs to extend survival in an aggressive liver cancer model. Proc. Natl. Acad. Sci. USA 2016, 113, 520–525. [Google Scholar] [CrossRef] [PubMed]
- Tiram, G.; Segal, E.; Krivitsky, A.; Shreberk-Hassidim, R.; Ferber, S.; Ofek, P.; Udagawa, T.; Edry, L.; Shomron, N.; Roniger, M.; et al. Identification of Dormancy-Associated MicroRNAs for the Design of Osteosarcoma-Targeted Dendritic Polyglycerol Nanopolyplexes. ACS Nano 2016, 10, 2028–2045. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Xing, Z.; Yang, J.; Wang, Y.; Chen, J.; Zhang, Y.; Shi, W.; Li, Q. Chondroitin sulfate-functionalized polyamidoamine-mediated miR-34a delivery for inhibiting the proliferation and migration of pancreatic cancer. RSC Adv. 2016, 6, 70870–70876. [Google Scholar] [CrossRef]
- Campbell, M.A.; Wengel, J. Locked vs. unlocked nucleic acids (LNAvs.UNA): Contrasting structures work towards common therapeutic goals. Chem. Soc. Rev. 2011, 40, 5680–5689. [Google Scholar] [CrossRef] [PubMed]
- Kala, S.; Mak, A.S.C.; Liu, X.; Posocco, P.; Pricl, S.; Peng, L.; Wong, A.S.T. Combination of dendrimer-nanovector-mediated small interfering RNA delivery to target akt with the clinical anticancer drug paclitaxel for effective and potent anticancer activity in treating ovarian cancer. J. Med. Chem. 2014, 57, 2634–2642. [Google Scholar] [CrossRef] [PubMed]
- Monteagudo, S.; Pérez-Martínez, F.C.; Pérez-Carrión, M.D.; Guerra, J.; Merino, S.; Sánchez-Verdú, M.P.; Ceña, V. Inhibition of p42 MAPK using a nonviral vector-delivered siRNA potentiates the anti-tumor effect of metformin in prostate cancer cells. Nanomedicine 2012, 7, 493–506. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Lv, Q.; Tang, X.J.; Hu, Y.L.; Xu, D.H.; Li, F.Z.; Liang, W.Q.; Gao, J.Q. Overcoming drug resistance of MCF-7/ADR cells by altering intracellular distribution of doxorubicin via MVP knockdown with a novel siRNA polyamidoamine-hyaluronic acid complex. J. Control. Release 2012, 163, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Ihnatsyeu-Kachan, A.; Dzmitruk, V.; Apartsin, E.; Krasheninina, O.; Ionov, M.; Loznikova, S.; Venyaminova, A.; Miłowska, K.; Shcharbin, D.; Mignani, S.; et al. Multi-Target Inhibition of Cancer Cell Growth by siRNA Cocktails and 5-Fluorouracil Using Effective Piperidine-Terminated Phosphorus Dendrimers. Colloids and Interfaces 2017, 1, 6. [Google Scholar] [CrossRef]
- Ren, Y.; Kang, C.-S.; Yuan, X.-B.; Zhou, X.; Xu, P.; Han, L.; Wang, G.X.; Jia, Z.; Zhong, Y.; Yu, S.; et al. Co-delivery of as-miR-21 and 5-FU by poly(amidoamine) dendrimer attenuates human glioma cell growth in vitro. J. Biomater. Sci. Polym. Ed. 2010, 21, 303–314. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Zhou, X.; Mei, M.; Yuan, X.-B.; Han, L.; Wang, G.-X.; Jia, Z.-F.; Xu, P.; Pu, P.-Y.; Kang, C.-S. MicroRNA-21 inhibitor sensitizes human glioblastoma cells U251 (PTEN-mutant) and LN229 (PTEN-wild type) to taxol. BMC Cancer 2010, 10, 27. [Google Scholar] [CrossRef] [PubMed]
- Mei, M.; Ren, Y.; Zhou, X.; Yuan, X.-B.; Li, F.; Jiang, L.-H.; Kang, C.-S.; Yao, Z. Suppression of breast cancer cells in vitro by polyamidoamine-dendrimer-mediated 5-fluorouracil chemotherapy combined with antisense micro-RNA 21 gene therapy. J. Appl. Polym. Sci. 2009, 114, 3760–3766. [Google Scholar] [CrossRef]
- Mei, M.; Ren, Y.; Zhou, X.; Yuan, X.; Han, L.; Wang, G.; Jia, Z.; Pu, P.; Kang, C.; Yao, Z. Downregulation of miR-21 enhances chemotherapeutic effect of taxol in breast carcinoma cells. Technol. cancer Res. Treat. 2010, 9, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.-M.; Shi, Z.-D.; Ren, Y.; Liu, C.-Y.; Ji, Y.-R.; Long, L.-X.; Pu, P.; Sheng, J.; Yuan, X.-B.; Kang, C.-S. Synergistic inhibition of human glioma cell line by temozolomide and PAMAM-mediated miR-21i. J. Appl. Polym. Sci. 2013, 127, 570–576. [Google Scholar] [CrossRef]
- Qian, X.; Ren, Y.; Shi, Z.; Long, L.; Pu, P.; Sheng, J.; Yuan, X.; Kang, C. Sequence-dependent synergistic inhibition of human glioma cell lines by combined Temozolomide and miR-21 inhibitor gene therapy. Mol. Pharm. 2012, 9, 2636–2645. [Google Scholar] [CrossRef] [PubMed]
- Shcharbin, D.; Shakhbazau, A.; Bryszewska, M. Poly(amidoamine) dendrimer complexes as a platform for gene delivery. Expert Opin. Drug Deliv. 2013, 10, 1687–1698. [Google Scholar] [CrossRef] [PubMed]
- Mello, C.C.; Conte, D. Revealing the world of RNA interference. Nature 2004, 431, 338–342. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, C.; Sharma, A.R.; Sharma, G.; Doss, C.G.P.; Lee, S.S. Therapeutic miRNA and siRNA: Moving from Bench to Clinic as Next Generation Medicine. Mol. Ther. Nucleic Acids 2017, 8, 132–143. [Google Scholar] [CrossRef] [PubMed]
- Rittenhouse, K.D.; Johnson, T.R.; Vicini, P.; Hirakawa, B.; Kalabat, D.; Yang, A.H.; Huang, W.; Basile, A.S. RTP801 Gene Expression Is Differentially Upregulated in Retinopathy and Is Silenced by PF-04523655, a 19-Mer siRNA Directed Against RTP801Pharmacodynamics of siRNA PF-655 in Retinopathy. Investig. Ophthalmol. Vis. Sci. 2014, 55, 1232–1240. [Google Scholar] [CrossRef] [PubMed]
| Target Protein/Type of Short RNA | Object | Dendrimer/Dendrimer Based Construction | Effect | Ref. |
|---|---|---|---|---|
| PAMAM dendrimers | ||||
| Hsp27/siRNA | Human prostate cancer cells (PC-3) | Arginine-terminated TEA-PAMAM G4 (G4Arg) dendrimer | 80% reduction of Hsp27-mRNA, Hsp27 protein expression dropped by 85% | [82] |
| PC-3 prostate cancer xenografts in nude mice | Hsp27 protein expression decreased by 55% | |||
| Human prostate cancer cells (PC-3) | Complex of TEA-PAMAM G5/siRNA/oligopeptide E16G6RGDK | Hsp27 mRNA reduction by 60%, decrease of Hsp27 protein expression by 85%, reduction of cell viability by 55% | [83] | |
| PC-3 prostate cancer xenografts in nude mice | Hsp27 expression decrease by 70%, 5-fold inhibition of tumor growth | |||
| Human prostate cancer cells (PC-3) | Amphiphilic TEA-PAMAM dendrons G1,2,3, bearing C18 alkyl chain in focal point | Target mRNA decrease of 75%, protein expression decrease of 80%, 2.7-fold increase of apoptotic cells | [84] | |
| PC-3 prostate cancer xenografts in nude mice | Decrease of target mRNA by 50%, decrease of Hsp27 protein expression by 50% | |||
| Human prostate cancer cells (PC-3) | Arginine-decorated TEA-PAMAM Dendron G3, bearing an alkyl chain in the focal point | Decrease of target mRNA by 80% | [39] | |
| Amphiphilic Janus-type PAMAM G2 dendron bearing two alkyl chains in focal point | Decrease of Hsp27 mRNA by 80%, decrease of Hsp27 protein expression by 95% | [85] | ||
| PC-3 prostate cancer xenografts in nude mice | Decrease of hsp27 mRNA by 60%, decrease of Hsp27 protein expression by 75%, 2.5-fold inhibition of tumor growth in vivo | |||
| Cocktail of viral (HIV) Tat and Rev, lymphocytic CD4/TNPO3/dsiRNAs | T-cells and primary human PBMC | TEA-PAMAM G5 dendrimer | Decrease of viral p24 expression by >50% and CD4 expression by 60–75% | [35] |
| HIV-infected humanized Rag2−/−γc−/− mouse model | Decrement of viral load up to 0%, prevent CD4+ T-cell level fall | |||
| Bcl-2 (inhibitor of apoptosis)/siRNA | Human cervical adenocarcinoma cells (HeLa) | Dodecylated PAMAM G4 bearing 23 chains of C12 | Decrease of target mRNA by 90%, protein Bcl-2 expression inhibition by 40% | [86] |
| Human ovarian carcinoma cells (A2780) | QPAMAM-NHAc, internally quaternized and surface-acetylated PAMAM G4 modified with LHRH at the periphery | Inhibition of target mRNA by 85% | [87,88] | |
| Triblock PAMAM-PEG-PLL nanocarrier | Inhibition of target mRNA by up to 80% | [89] | ||
| Alpha-fetoprotein (AFP)/siRNA | C57BL/6 mice, hepatocarcinoma model | EDA-PAMAM G1 substituted by alkyl chains on the periphery | Selective accumulation in hepatocytes, decrease of target protein expression by 50% (C12) and by 90% (C15) | [34] |
| Multiple drug resistance protein 1 (MDR1)/siRNA | MDR1-positive mouse embryonic fibroblast (NIH 3T3) cells | Tat-Conjugated EDA-PAMAM G5 dendrimers | Target protein (MDR1) expression decreased by 35% | [90] |
| Transthyretin—transport protein (TTR)/siRNA | Hepato-carcinoma cells (HepG2) | EDA-PAMAM G2 decorated with glucuronylglucosyl-β-cyclodextrin | Decrease of target mRNA level by 60% | [91] |
| Mice model BALB/c | Decrease of target protein (TTR) expression by 10% | |||
| NF-κB p65- (regulator of inflammatory response)/siRNA | Rat alveolar macrophages (NR8383) | EDA-PAMAM G3- decorated with cyclodextrin and thioalkylated mannose fragments | Decrease of target NF-κB p65 mRNA level by 85% | [92] |
| Mice model C57BL/6 | Reduction of proinflammatory cytokines p65, TNF-α, IL-1β secretion by 75–85% | |||
| MMP-9 (diabetic wound healing regulator)/siRNA | Rat fibroblasts (CRL1213) | PAMAM G3 with β- cyclodextrin core | Decrease of target MMP-9 mRNA level by 68%, decrease of target protein expression by 94% | [93] |
| Sprague Dawley rats with induced diabetes | Enhancement wound healing (52% against 38% in control) | |||
| Cocktail of Bcl-2, Bcl-xL, Mcl-1 (apoptosis inhibitors)/siRNAs | Human cervical adenocarcinoma cells (HeLa), human promyelocytic leukemia cells (HL-60) | PAMAM G3 and G4 | Increased apoptotic cell fraction up to 30–40% | [48,49] |
| Angiotensin II receptor type 1 (AT1R)/siRNA | Cardiomyoblastic cells (H9C2) | EDA-PAMAM G4 dendrons conjugated with PEG-R9peptide | Reduction of protein AT1R expression by 60% | [94] |
| Rats with induced ischemia | 2.5-fold decrease of heart attack risk | |||
| Cofilin-1 (regulator of neuronal death)/siRNA | Rat cerebellar granular neurons (CGNs) | TRANSGEDEN: Polyphenylenevinylene (PPV) core with flexible PAMAM branches | Knockdown of target mRNA by 85%, reduction of protein Ccofilin-1 expression by 80% | [36] |
| Beclin 1 (autophagy regulator)/siRNA | Rat brain rat neurons | Decrease of Beclin 1 mRNA by 90%, knockdown of Beclin 1 protein expression by 80% | [95] | |
| TWIST1 (marker of breast cancer)/siRNA | Breast cancer cells (SUM1315) | YTZ3-15, TEA-PAMAM dendron G3 with two lipid tails in focal point | Decrease of TWIST1 mRNA and protein by 75–95%, reduction of epithelial-mesenchymal transition (EMT)-related (N-cadherin and vimentin) gene mRNA | [96] |
| CD4 (primary HIV receptor)/dsiRNA | Human hematopoietic CD34+ stem cells | Amphiphilic TEA-PAMAM dendron G3 bearing alkyl chain C18 in focal point, decorated with arginine | Decrease of CD-4 mRNA by 60% | [39] |
| Acute lymphoblastic leukemia T-cells (CCRF-CEM) | Amphiphilic Janus-type- TEA-PAMAM dendrons bearing two alkyl chains | Decrease of CD4-mRNA by 55%, knockdown of CD4 protein expression by 80% | [85] | |
| Cocktail of HIV-1 Tat/Rev (viral integrase)/dsiRNAs | PBMC CD4+, hematopoietic stem cells CD34+ | Decrease of Tat/Rev mRNA level by 50–55%, inhibition of HIV replication in infected cells by 30–40% | ||
| Delivery of siRNA by non-PAMAM constructions | ||||
| Cocktail Bcl-2, Bcl-xL, Mcl-1 (apoptosis inhibitors)/siRNA | Human cervical cancer cells (HeLa), human acute promyelocytic cells (HL-60) | PAMAM G3, G4; carbosilane G2; phosphorous G3, G4 (comparison study) | Apoptosis induction by cocktail of 3 siRNA: carbosilane (15–20%) < PAMAM (30–40%) < phosphorous G3 (45%) << phosphorous G4 (95%) | [48,49] |
| Apolipo-protein B (ApoB)/siRNA | Mice model C57BL/6 | Poly-l-lysine G6 (KG6) | Decrease of mRNA in hepatocytes by 22% (aiApoBI) and by 50% (aiApoBII), low and very low density lipoprotein level in blood by 20–25% | [97] |
| PEPCK (glucose production regulator)/siRNA | Rat hepatocytes H4IIEC3 | Combination of KG6 (dendritic poly(L-lysine) G6) and Endo-Porter peptide | Decrease of PEPCK-mRNA by 80%, knockdown of PEPCK protein expression by 95%, blood glucose level decrease by 70% | [98] |
| OCT1 (gluconeogenesis regulator via influence on metformin)/siRNA | Decrease of OCT1-mRNA by 80%, metformin (inhibitor of gluconeogenesis) action arrest | |||
| Nef (necessary protein for HIV reproduction)/siRNA | CD4+-lymphocytes | Carbosilane (CBS) G2, G3 dendrimers | HIV-1 reproduction inhibition in vitro by 35% (G2) and by 50% (G3) | [99] |
| PBMCs | Phosphorous G4 dendrimer | HIV-1 reproduction inhibition by 60% | [100] | |
| COX2 (cyclooxygenase-2, stimulator of HIV propagation in brain)/pool of four siRNA sequences | Astroglioma cells (U87MG) | NN-16 G2 (carbosilane dendrimer) | Decrease of COX2 expression in HIV-infected cells to the level of uninfected cells | [101] |
| P24, NEF (HIV structural proteins)/siRNA | 50% inhibition of HIV-1 propagation | [50] | ||
| P24, GAG1, NEF (HIV structural proteins)/cocktail of three siRNAs | T-cell lymphoma lymphoblasts (SupT1), primary PBMCs | 35% inhibition of HIV-1 propagation | [102] | |
| Bcl-2 (apoptosis inhibitor)/siRNA | Cell Line human ovarian carcinoma (A2780) | PPI G5-PEG-LHRH conjugate | Decrease of Bcl-2 mRNA level by 75% | [103] |
| Human lung carcinoma (A549) | Decrease of Bcl-2 mRNA level by >95% | |||
| A549-derived lung carcinoma xenografts in a nude mouse model | LHRH conjugates increase accumulation of dendriplexes in tumor xenografts | |||
| microRNA (Target) | Object | Dendrimer/Dendrimer Based Construction | Effect | Ref. |
|---|---|---|---|---|
| microRNA Mimic Delivery | ||||
| miR-7 (epidermal growth factor receptor) | Human glioblastoma cells U251 | Conjugate of PAMAM folic acid (FA/PAMAM) | Decreased expression of proteins EGFR by 90%, PI3K by 50%, AKT-2 by 30% | [38] |
| Immunodeficient mouse with induced glioma | Decreased expression of proteins EGFR by 50%, AKT-2 by 60%, reduction of tumor size | |||
| duplex miR-126 (signal protein SPRED1) | Human umbilical vein endothelial cells (HUVECs) | Amphiphilic Janus-type-PAMAM dendrimer, consisting of dendron G3, bound to the penetrating peptide CR9 or targeting peptide CGGRGDS | Decrease of the level SPRED1-mRNA by 50% | [120] |
| let-7g (target is unknown) | Mice with induced aggressive hepatocarcinoma | Hybrid carbosilane dendrimer G2 with a polyamine core and thiol-containing surface groups | Inhibition of liver tumor growth in mice in vivo, let-7g expression was increased 13-fold in liver tissues after 48 h post intravenous (i.v.) injection | [121] |
| miR-34a (cMET, angiogenesis and tumori-genesis regulator), miR-93 (angiogenesis regulator factor HIF1α), miR-200c (prevents metastatic spread, pathway unknown) | Human osteosarcoma cells (Saos-2 and MG-63), SCID mice with Saos-2 derived tumors | Aminated polyglycerol dendrimer (dPG-NH2) | 2–3 fold times increase in latent phase of osteosarcoma duration in vivo | [118] |
| miR-34a (procaspase-3 and Bcl-2) | Pancreatic cancer cells (MiaPaCa-2) | PAMAM dendrimer functionalized by chondroitin sulfate on the surface (CS-PAMAM) | Decreased viability of MiaPaCa-2 cells by 35%, 6.5-fold increase of the cell fraction in the apoptosis phase in vitro | [119] |
| microRNA/siRNA (Its Target) | Object | Dendrimer/Dendrimer Based Construction | Effect | Ref. |
|---|---|---|---|---|
| siRNA | ||||
| p42 MAPK-siRNA (a protein of MAPK/ERK signaling cascade regulating transcription) + metformin | Prostate cancer cells (PCa) | EDA-PAMAM G1 | Decrease of p42-mRNA by 85%, decrease of p42 protein expression by 70%, increased cells sensitivity to metformin | [124] |
| Akt-siRNA (ovarian cancer stimulator protein) + paclitaxel | Human ovarian carcinoma cells (SKOV-3) | TEA-PAMAM G6 | Decrease of Akt-mRNA by 60%, decrease of the Akt protein expression by 40%, in cell viability decreased by 40% (dendriplex) and by 60% (dendriplex + paclitaxel) | [123] |
| SKOV-3 xenograft nude mice model | Reduction of xenograft tumor size by 2 times (dendriplex) and by 4 times (dendriplex + paclitaxel) | |||
| MVP-siRNA (major vault protein involved in breast cancer drug resistance) + doxorubicin (DOX) | Breast cancer cells (MCF-7/ADR) | EDA-PAMAM/hyaluronic acid conjugate | Significant knockdown of MVP protein expression, increased cytotoxicity of the dendriplex + DOX (IC50 = 11.3 μM) compared to DOX alone (IC50 = 48.5 μM) | [125] |
| Xenograft of MCF-7/ADR in Nude BALB/c mice | Enhanced tumor target, higher intracellular accumulation, increased blood circulating time and reduced vitrotoxicity of DOX/denpriplex co-delivery compared to DOX alone | |||
| Cocktail Bcl-2, Bcl-xL, Mcl-1 (apoptosis inhibitors) / siRNA + 5- fluorouracil | Human cervical cancer cells (HeLa) | Aminopiperidine-terminated phosphorus dendrimers G3 and G4 | Synergistic effect of two anti-cancer agents (siRNA and chemodrug), enhancement of the apopotosis induction | [126] |
| microRNA Antagonists | ||||
| antimiR-21 + 5- fluorouracil | Glioblastoma cells (U251 and LN229) | TEA-PAMAM G5 | Addition of dendriplex increase cell chemosensitivity to 5-fluorouracil | [127] |
| antimiR-21 + taxol | Decrease of miR-21 level by 90–95%, increase in cells chemosensitivity to taxol (IC50 = 60–160 nM) | [128] | ||
| antimiR-21 + 5- fluorouracil | Breast cancer cells (MCF7) | Increased chemosensitivity of cells to 5-fluorouracil, a prolonged cytotoxic effect | [129] | |
| antimiR-21 + taxol | Decreased expression of p-AKT, Bcl-2, EGFR, STAT-3 proteins, increased sensitivity of cells to taxol | [130] | ||
| antimiR-21 + temozolomide | Glioma cells (U87) | Decrease of miR-21 level by 80–90%, an increase in the sensitivity of cells to temozolomide (IC50 = 7.5 μM) | [131] | |
| antimiR-21 + temozolomide | Glioma cells (U251, LN229, U87) | Decreased expression of STAT-3 and p-STAT proteins, increased chemosensitivity of cells to temozolomide | [132] | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dzmitruk, V.; Apartsin, E.; Ihnatsyeu-Kachan, A.; Abashkin, V.; Shcharbin, D.; Bryszewska, M. Dendrimers Show Promise for siRNA and microRNA Therapeutics. Pharmaceutics 2018, 10, 126. https://doi.org/10.3390/pharmaceutics10030126
Dzmitruk V, Apartsin E, Ihnatsyeu-Kachan A, Abashkin V, Shcharbin D, Bryszewska M. Dendrimers Show Promise for siRNA and microRNA Therapeutics. Pharmaceutics. 2018; 10(3):126. https://doi.org/10.3390/pharmaceutics10030126
Chicago/Turabian StyleDzmitruk, Volha, Evgeny Apartsin, Aliaksei Ihnatsyeu-Kachan, Viktar Abashkin, Dzmitry Shcharbin, and Maria Bryszewska. 2018. "Dendrimers Show Promise for siRNA and microRNA Therapeutics" Pharmaceutics 10, no. 3: 126. https://doi.org/10.3390/pharmaceutics10030126
APA StyleDzmitruk, V., Apartsin, E., Ihnatsyeu-Kachan, A., Abashkin, V., Shcharbin, D., & Bryszewska, M. (2018). Dendrimers Show Promise for siRNA and microRNA Therapeutics. Pharmaceutics, 10(3), 126. https://doi.org/10.3390/pharmaceutics10030126