Lysine Dendrigraft Nanocontainers. Influence of Topology on Their Size and Internal Structure
Abstract
:1. Introduction
2. Materials and Methods
3. Results
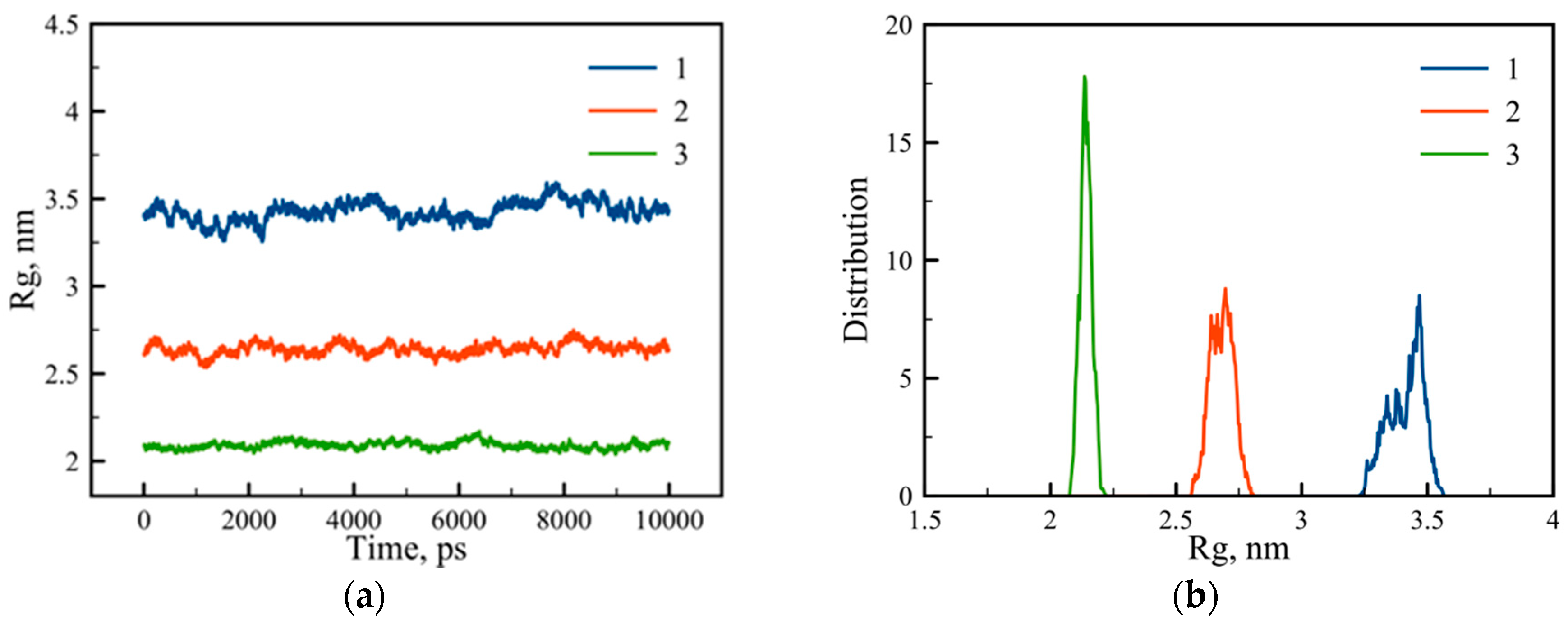
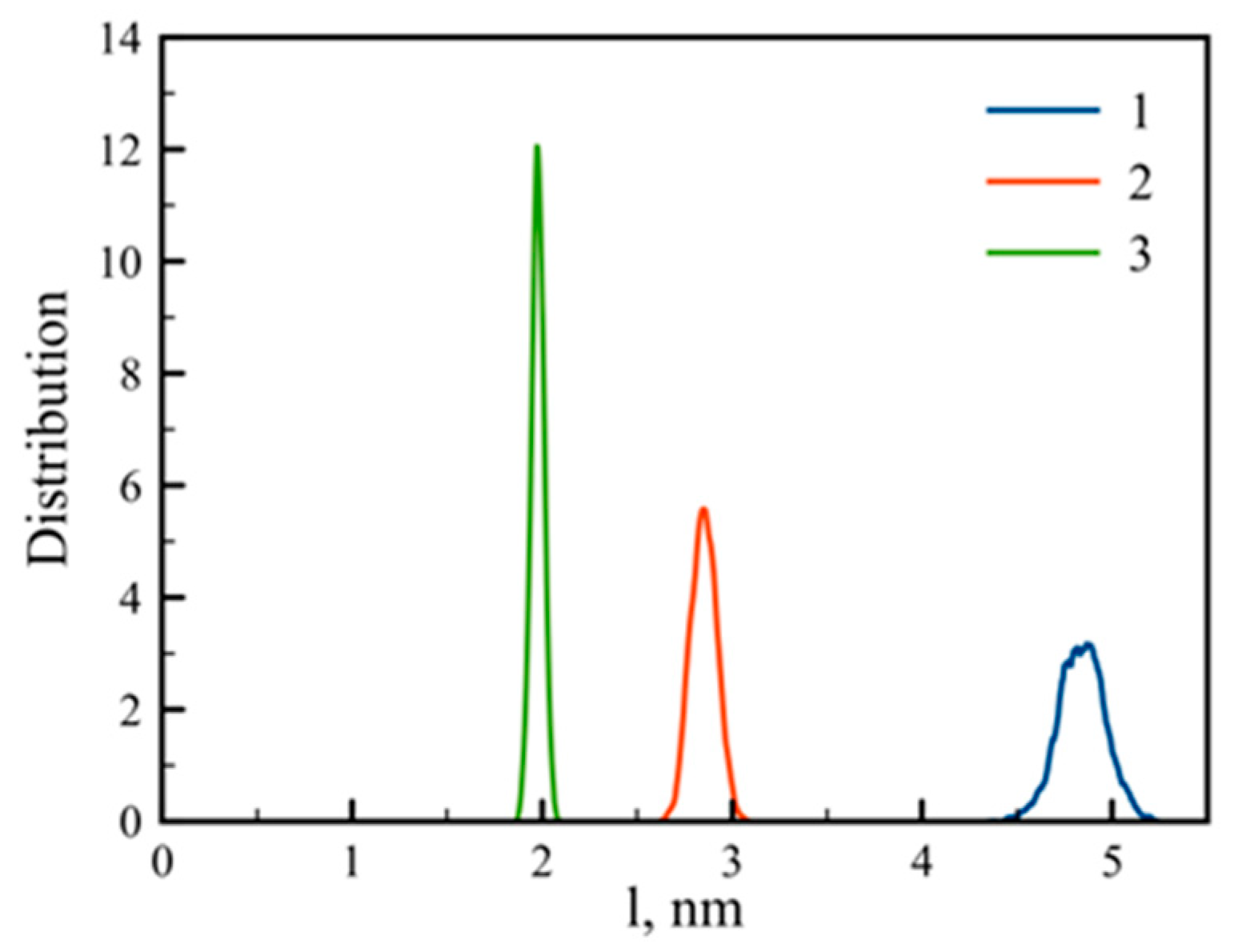
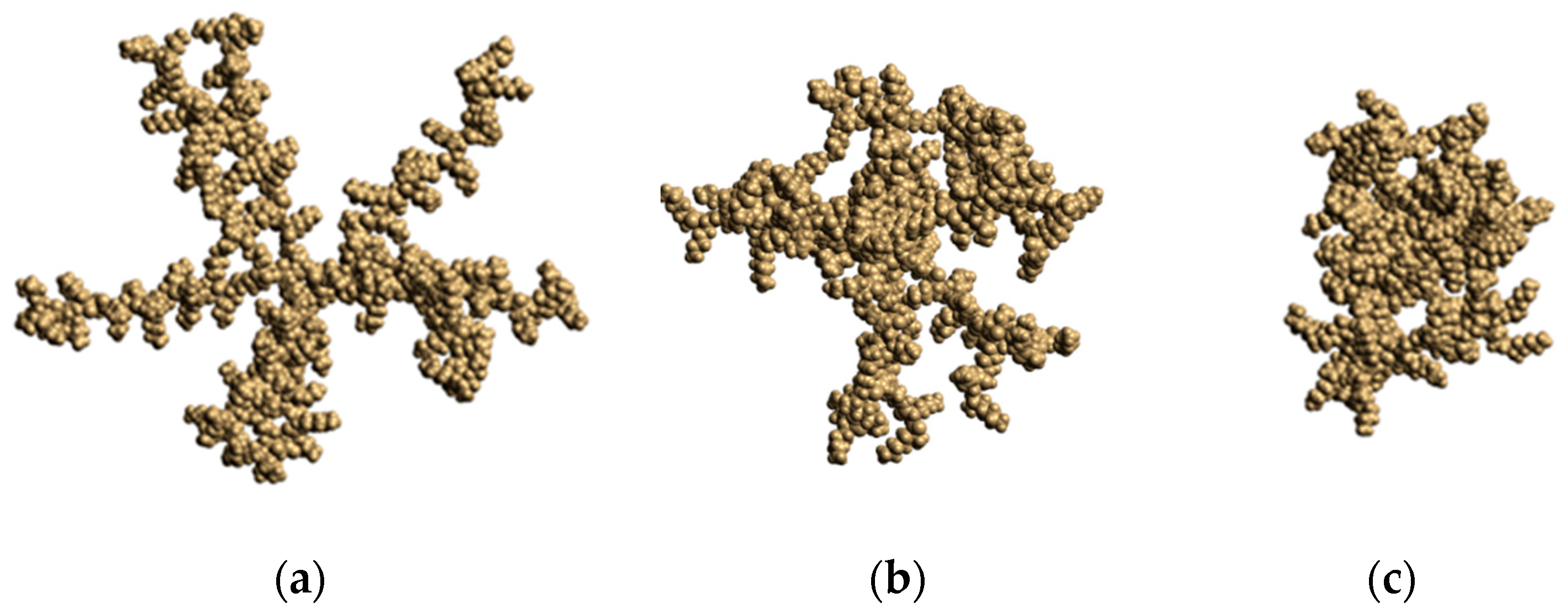
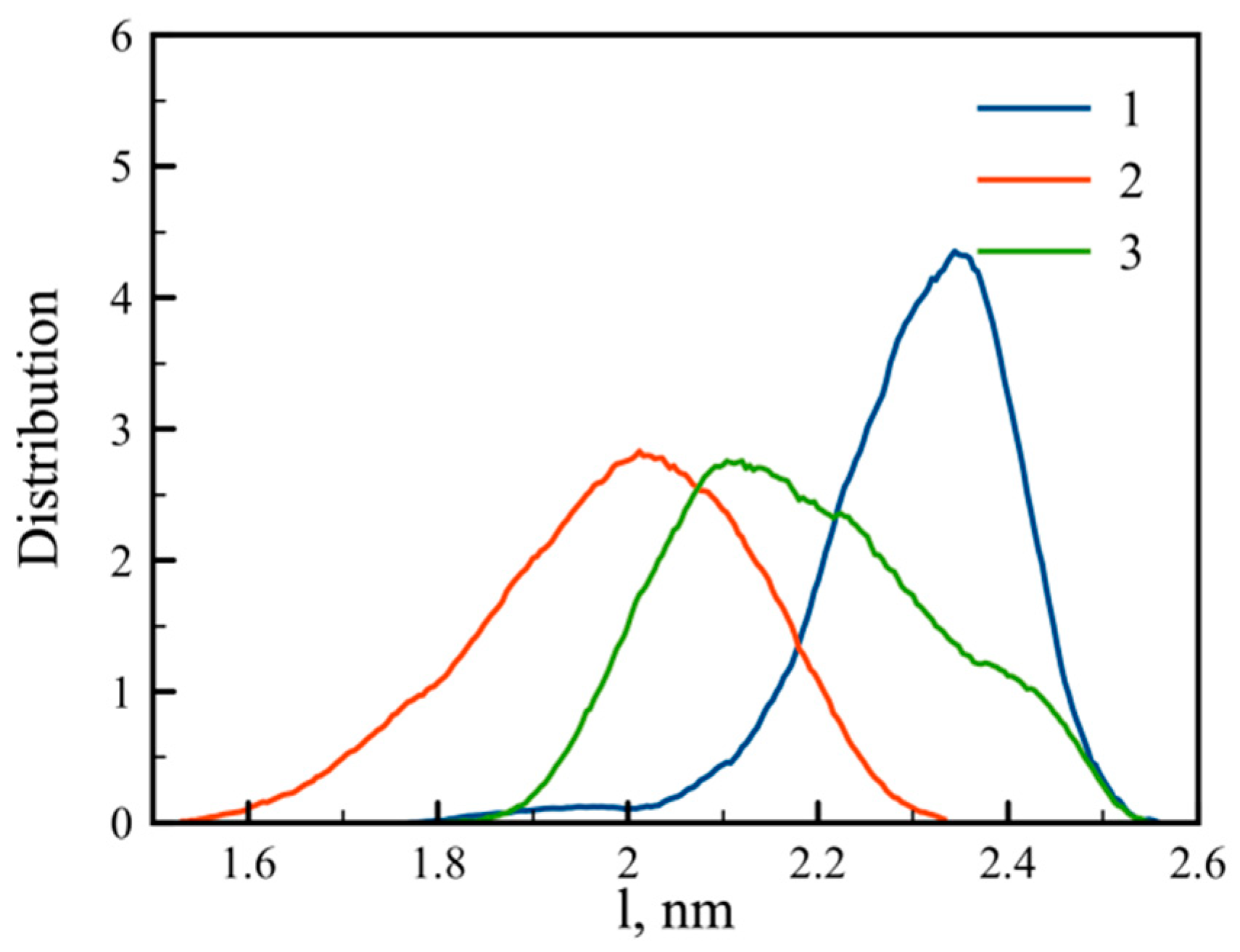
3.1. Large-Scale Properties
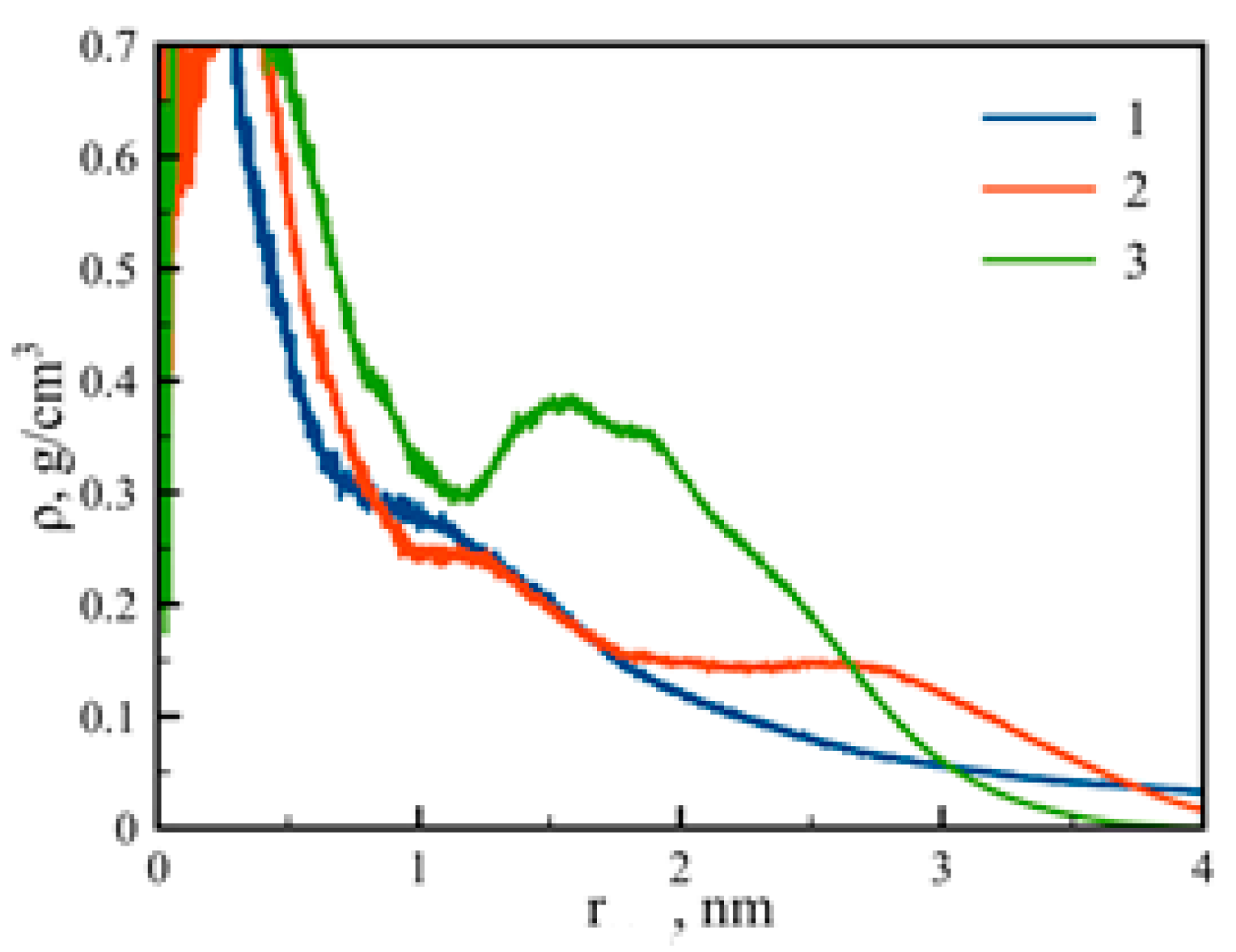
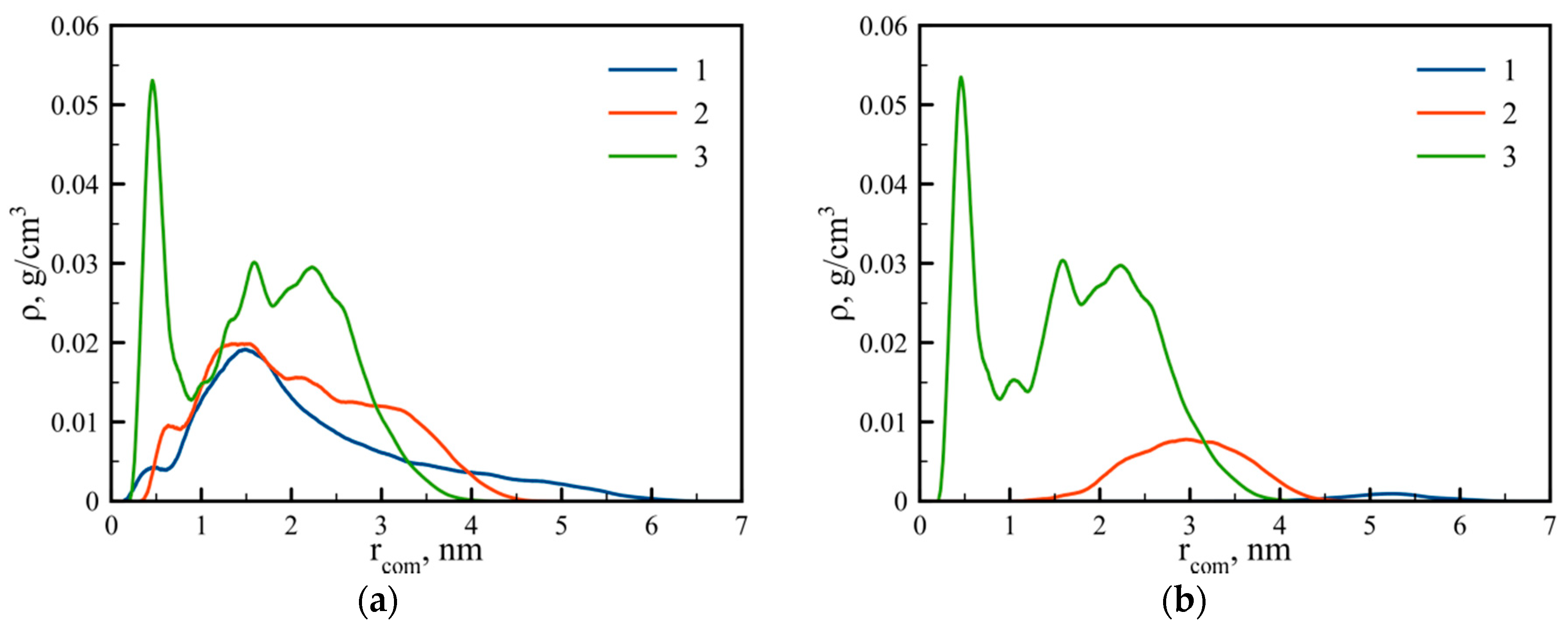
3.2. Internal Structure
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bosman, A.W.; Janssen, H.M.; Meijer, E.W. About Dendrimers: Structure, Physical Properties, and Applications. Chem. Rev. 1999, 99, 1665–1688. [Google Scholar] [CrossRef] [PubMed]
- Dykes, G.M. Dendrimers: A review of their appeal and applications. J. Chem. Technol. Biotechnol. 2001, 76, 903–918. [Google Scholar] [CrossRef]
- Gittins, P.J.; Twyman, L.J. Dendrimers and supramolecular chemistry. Supramol. Chem. 2002, 99, 4782–4787. [Google Scholar] [CrossRef]
- Gajbhiye, V.; Palanirajan, V.K.; Tekade, R.K.; Jain, N.K. Dendrimers as therapeutic agents: a systematic review. J. Pharm. Pharmacol. 2009, 61, 989–1003. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, S.C.; Zeng, F.; Reichert, D.E.C.; Kolotuchin, S.V. Self-assembling dendrimers. Science 1996, 271, 1095–1098. [Google Scholar] [CrossRef] [PubMed]
- Grayson, S.M.; Fréchet, J.M.J. Convergent dendrons and dendrimers: From synthesis to applications. Chem. Rev. 2001, 101, 3819–3868. [Google Scholar] [CrossRef] [PubMed]
- Crespo, L.; Sanclimens, G.; Pons, M.; Giralt, E.; Royo, M.; Albericio, F. Peptide and amide bond-containing dendrimers. Chem. Rev. 2005, 105, 1663–1681. [Google Scholar] [CrossRef] [PubMed]
- Rao, C.; Tam, J.P. Synthesis of Peptide Dendrimer. J. Am. Chem. Soc. 1994, 116, 6975–6976. [Google Scholar] [CrossRef]
- Darbre, T.; Reymond, J.L. Peptide dendrimers as artificial enzymes, receptors, and drug-delivery agents. Acc. Chem. Res. 2006, 39, 925–934. [Google Scholar] [CrossRef] [PubMed]
- Klajnert, B.; Janiszewska, J.; Urbanczyk-Lipkowska, Z.; Bryszewska, M.; Shcharbin, D.; Labieniec, M. Biological properties of low molecular mass peptide dendrimers. Int. J. Pharm. 2006, 309, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Veine, D.M.; Fay, K.S.; Staszewski, E.D.; Zeng, Z.Z.; Livant, D.L. The PHSCN dendrimer as a more potent inhibitor of human breast cancer cell invasion, extravasation, and lung colony formation. Breast Cancer Res. Treat. 2011, 125, 363–375. [Google Scholar] [CrossRef] [PubMed]
- Tyssen, D.; Henderson, S.A.; Johnson, A.; Sterjovski, J.; Moore, K.; La, J.; Zanin, M.; Sonza, S.; Karellas, P.; Giannis, M.P.; et al. Structure activity relationship of dendrimer microbicides with dual action antiviral activity. PLoS ONE 2010, 5, e12309. [Google Scholar] [CrossRef] [PubMed]
- Boyd, B.J.; Kaminskas, L.M.; Karellas, P.; Krippner, G.; Lessene, R.; Porter, C.J.H. Cationic poly-l-lysine dendrimers: Pharmacokinetics, biodistribution and evidence for metabolism and bioresorption after intravenous administration to rats. Mol. Pharm. 2006, 3, 614–627. [Google Scholar] [CrossRef] [PubMed]
- Neelov, I.M.; Janaszewska, A.; Klajnert, B.; Bryszewska, M.; Makova, N.Z.; Hicks, D.; Pearson, H.; Vlasov, G.P.; Ilyash, M.Y.; Vasilev, D.S.; et al. Molecular properties of lysine dendrimers and their interactions with A β-peptides and neuronal cells. Curr. Med. Chem. 2013, 20, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Kodama, Y.; Nakamura, T.; Kurosaki, T.; Egashira, K.; Mine, T.; Nakagawa, H.; Muro, T.; Kitahara, T.; Higuchi, N.; Sasaki, H. Biodegradable nanoparticles composed of dendrigraft poly-l-lysine for gene delivery. Eur. J. Pharm. Biopharm. 2014, 87, 472–479. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Dong, H.; Li, Y.; Ren, T. Harnessing the PEG-cleavable strategy to balance cytotoxicity, intracellular release and the therapeutic effect of dendrigraft poly-l-lysine for cancer gene therapy. J. Mater. Chem. B 2016, 4, 1284–1295. [Google Scholar] [CrossRef]
- Romestand, B.; Rolland, J.; Commeyras, A.; Desvignes, I.; Pascal, R.; Vandenabeele-trambouze, O. Dendrigraft Poly-l-lysine: A Non-Immunogenic Synthetic Carrier for Antibody Production. Biomacromolecules 2010, 11, 1169–1173. [Google Scholar] [CrossRef] [PubMed]
- Francoia, J.-P.; Rossi, J.-C.; Monard, G.; Vial, L. Digitizing poly-l-lysine dendrigrafts: from experimental data to molecular dynamics simulations. J. Chem. Inf. Model. 2017, 57, 2173–2180. [Google Scholar] [CrossRef] [PubMed]
- Roberts, B.P.; Scanlon, M.J.; Krippner, G.Y.; Chalmers, D.K. Molecular dynamics of poly(l-lysine) dendrimers with naphthalene disulfonate caps. Macromolecules 2009, 42, 2775–2783. [Google Scholar] [CrossRef]
- Markelov, D.A.; Falkovich, S.G.; Neelov, I.M.; Ilyash, M.Y.; Matveev, V.; Lahderanta, E.; Ingman, P.; Darinskii, A. Molecular dynamics simulation of spin-lattice NMR relaxation in poly-l-Lysine dendrimers: Manifestation of the semiflexibility effect. Phys. Chem. Chem. Phys. 2015, 17, 3214–3226. [Google Scholar] [CrossRef] [PubMed]
- Falkovich, S.; Markelov, D.; Neelov, I.; Darinskii, A. Are structural properties of dendrimers sensitive to the symmetry of branching? Computer simulation of lysine dendrimers. J. Chem. Phys. 2013, 139, 064903. [Google Scholar] [CrossRef] [PubMed]
- Neelov, I.; Markelov, D.; Falkovich, S.; Ilyash, M.; Okrugin, B.; Darinskii, A. Mathematical modeling of lysine dendrimers temperature dependencies. Polym. Sci. Ser. C 2013, 55, 154–161. [Google Scholar] [CrossRef]
- Neelov, I.; Ilyash, M.; Falkovich, S.; Darinskii, A. Computer simulation of lysine dendrimers by molecular dynamics method. Smart Nanocompos. 2014, 5, 127. [Google Scholar]
- Neelov, I.M.; Okrugin, B.M.; Falkovich, S.G.; Darinskii, A.A. Computer simulation of new type of branched peptides-lysine dendrigrafts. In Proceedings of the 8th International Symposium: Molecular Order and Mobility in Polymers, St. Petersburg, Russia, 2–6 June 2014; Abstract Number O-13. p. 31. [Google Scholar]
- Neelov, I.; Popova, E. Interaction of Lysine Dendrigraft of 2nd Generation and Semax Peptide. Molecular Dynamics Simulation. In Proceedings of the International Conference on Control, Artificial Intelligence, Robotics & Optimization (ICCAIRO), Prague, Czech Republic, 20–22 May 2017; pp. 182–186. [Google Scholar]
- Hess, B.; Kutzner, C.; Van Der Spoel, D.; Lindahl, E. GROMACS 4: Algorithms for highly efficient, load-balanced, and scalable molecular simulation. J. Chem. Theor. Comput. 2008, 4, 435–447. [Google Scholar] [CrossRef] [PubMed]
- Berendsen, H.J.C.; Postma, J.P.M.; Gunsteren, W.F.; Dinola, A.; Haak, J.R. Molecular dynamics with coupling to an external bath. J. Chem. Phys. 1984, 81, 3684–3690. [Google Scholar] [CrossRef]
- Cottet, H.; Martin, M.; Papillaud, A.; Souaıd, E.; Collet, H.; Commeyras, A. Determination of dendrigraft poly-l-lysine diffusion coefficients by taylor dispersion analysis. Biomacromolecules 2007, 8, 3235–3243. [Google Scholar] [CrossRef] [PubMed]
- Yevlampieva, N.; Dobrodumov, A.; Nazarova, O.; Okatova, O.; Cottet, H. Hydrodynamic behavior of dendrigraft polylysines in water and dimethylformamide. Polymers 2012, 4, 20–31. [Google Scholar] [CrossRef]
- Baigude, H.; Katsuraya, K.; Okuyama, K.; Tokunaga, S.; Uryu, T. Synthesis of sphere-type monodispersed oligosaccharide-polypeptide dendrimers. Macromolecules 2003, 36, 7100–7106. [Google Scholar] [CrossRef]
- Lederer, A.; Hartmann, T.; Komber, H. Sphere-like fourth generation pseudo-dendrimers with a hyperbranched core. Macromol. Rapid Commun. 2012, 33, 1440–1444. [Google Scholar] [CrossRef] [PubMed]
- Maiti, P.K.; Cagin, T.; Wang, G.; Goddard, W.A. Structure of PAMAM dendrimers: Generations 1 through 11. Macromolecules 2004, 37, 6236–6254. [Google Scholar] [CrossRef]
- Götze, I.O.; Likos, C.N. Conformations of flexible dendrimers: A simulation study. Macromolecules 2003, 36, 8189–8197. [Google Scholar] [CrossRef]
- Kłos, J.S.; Sommer, J.-U. Properties of dendrimers with flexible spacer-chains: A Monte Carlo study. Macromolecules 2009, 42, 4878–4886. [Google Scholar] [CrossRef]
- González, M.A.; Abascal, J.L.F. The shear viscosity of rigid water models. J. Chem. Phys. 2010, 132, 096101. [Google Scholar] [CrossRef] [PubMed]
- Venable, R.M.; Hatcher, E.; Guvench, O.; MacKerell, A.D.; Pastor, R.W. Comparing simulated and experimental translation and rotation constants: Range of validity for viscosity scaling. J. Phys. Chem. B 2010, 114, 12501–12507. [Google Scholar] [CrossRef] [PubMed]
- Timoshenko, E.G.; Kuznetsov, Y.A.; Connolly, R. Conformations of dendrimers in dilute solution. J. Chem. Phys. 2002, 117, 9050. [Google Scholar] [CrossRef]
- Markelov, D.A.; Matveev, V.V.; Ingman, P.; Nikolaeva, M.N.; Lähderanta, E.; Shevelev, V.A.; Boiko, N.I. NMR studies of carbosilanedendrimer with terminal mesogenic groups. J. Phys. Chem. B 2010, 114, 4159–4165. [Google Scholar] [CrossRef] [PubMed]
- Markelov, D.A.; Mazo, M.A.; Balabaev, N.K.; Gotlib, Y.Y. Temperature dependence of the structure of a carbosilanedendrimer with terminal cyanobiphenyl groups: Molecular-dynamics simulation. Polym. Sci. Ser. A 2013, 55, 53–60. [Google Scholar] [CrossRef]
- Markelov, D.A.; Polotsky, A.A.; Birshtein, T.M. Formation of a “Hollow” interior in the fourth-generation dendrimer with attached oligomeric terminal segments. J. Phys. Chem. B 2014, 118, 14961–14971. [Google Scholar] [CrossRef] [PubMed]
- Neelov, I.; Falkovich, S.; Markelov, D.; Paci, E.; Darinskii, A.; Tenhu, H. Molecular dinamics of lysine dendrimers: Computer simulation and NMR. In Dendrimers in Biomedical Applications; Klajnert, B., Ed.; Royal Society of Chemistry: London, UK, 2013; pp. 99–114. [Google Scholar] [CrossRef]
- Neelov, I.; Adolf, D. Brownian dynamics simulations of dendrimers under elongational flow: Bead-rod model with hydro-dynamic interactions. Macromolecules 2003, 36, 6914–6924. [Google Scholar] [CrossRef]
- Ennari, J.; Elomaa, M.; Neelov, I. Modeling of water-free and water containing solid polyelectrolytes. Polymer 2000, 41, 985–990. [Google Scholar] [CrossRef]
- Neelov, I.M.; Binder, K. Brownian dynamics of grafted polymer chains-time-dependent properties. Macromol. Theor. Simul. 1995, 4, 1063–1084. [Google Scholar] [CrossRef]
- Darinskii, A.; Gotlib, Y.; Lyulin, A.; Neelov, I. Computer simulation of local dynamics of a polymer chain in the orienting field of the liquid crystal type. Polym. Sci. USSR 1991, 33, 1116–1125. [Google Scholar] [CrossRef]
- Darinsky, A.; Lyulin, A.; Neelov, I. Computer simulations of molecular motion in liquid crystals by the method of brownian dynamics. Macromol. Chem. Theory Simul. 1993, 2, 523–530. [Google Scholar] [CrossRef]
- Ennari, J.; Neelov, I.; Sundholm, F. Simulation of a PEO based solid polyelectrolyte, comparison of the CMM and the Ewald summation method. Polymer 2000, 41, 2149–2155. [Google Scholar] [CrossRef]
- Mazo, M.A.; Shamaev, M.Y.; Balabaev, N.K.; Darinskii, A.A. Conformational mobility of carbosilanedendrimer: Molecular dynamics simulation. Phys. Chem. Chem. Phys. 2004, 6, 1285–1289. [Google Scholar] [CrossRef]
- Neelov, I.M.; Adolf, D.B.; McLeish, T.C.; Paci, E. Molecular dynamics simulation of dextran extension by constant force in single molecule AFM. Biophys. J. 2006, 91, 3579–3588. [Google Scholar] [CrossRef] [PubMed]
- Shavykin, O.V.; Neelov, I.M.; Darinskii, A.A. Is the manifestation of the local dynamics in the spin-lattice NMR relaxation in dendrimers sensitive to excluded volume interactions? Phys. Chem. Chem. Phys. 2016, 18, 24307–24317. [Google Scholar] [CrossRef] [PubMed]
- Shavykin, O.V.; Mikhailov, I.V.; Neelov, I.M.; Darinskii, A.A.; Leermakers, F.A.M. Effect of an asymmetry of branching on structural characteristics of dendrimers revealed by Brownian dynamics simulations. Polymer 2018, 146, 256–266. [Google Scholar] [CrossRef]
- Ennari, J.; Neelov, I.; Sundholm, F. Molecular dynamics simulation of the structure of PEO based solid polymer electrolytes. Polymer 2000, 41, 4057–4063. [Google Scholar] [CrossRef]
- Neelov, I.M.; Adolf, D.B. Brownian dynamics simulation of hyperbranched polymers under elongational flow. J. Phys. Chem. B 2004, 108, 7627–7636. [Google Scholar] [CrossRef]
- Ennari, J.; Neelov, I.; Sundholm, F. Estimation of the ion conductivity of a PEO-based polyelectrolyte system by molecular modeling. Polymer 2001, 42, 8043–8050. [Google Scholar] [CrossRef]
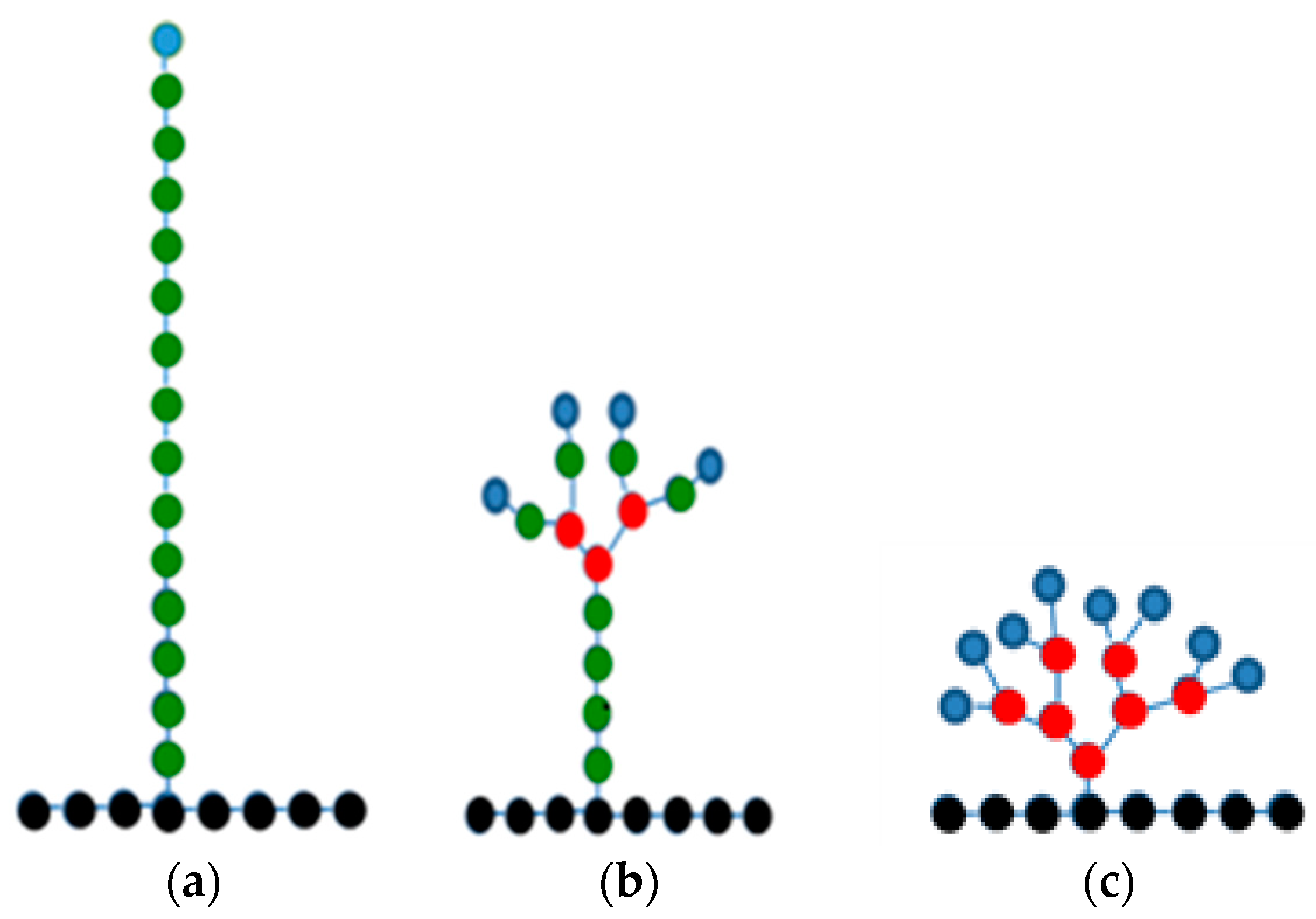
| Type | M (g/mol) | Nt |
|---|---|---|
| Dendrigraft 1 | 2947 | 8 |
| Dendrigraft 2 | 2947 | 32 |
| Dendrigraft 3 | 2947 | 64 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okrugin, B.; Ilyash, M.; Markelov, D.; Neelov, I. Lysine Dendrigraft Nanocontainers. Influence of Topology on Their Size and Internal Structure. Pharmaceutics 2018, 10, 129. https://doi.org/10.3390/pharmaceutics10030129
Okrugin B, Ilyash M, Markelov D, Neelov I. Lysine Dendrigraft Nanocontainers. Influence of Topology on Their Size and Internal Structure. Pharmaceutics. 2018; 10(3):129. https://doi.org/10.3390/pharmaceutics10030129
Chicago/Turabian StyleOkrugin, Boris, Maxim Ilyash, Denis Markelov, and Igor Neelov. 2018. "Lysine Dendrigraft Nanocontainers. Influence of Topology on Their Size and Internal Structure" Pharmaceutics 10, no. 3: 129. https://doi.org/10.3390/pharmaceutics10030129
APA StyleOkrugin, B., Ilyash, M., Markelov, D., & Neelov, I. (2018). Lysine Dendrigraft Nanocontainers. Influence of Topology on Their Size and Internal Structure. Pharmaceutics, 10(3), 129. https://doi.org/10.3390/pharmaceutics10030129





