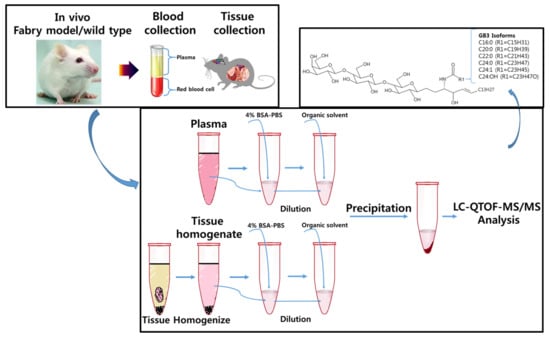
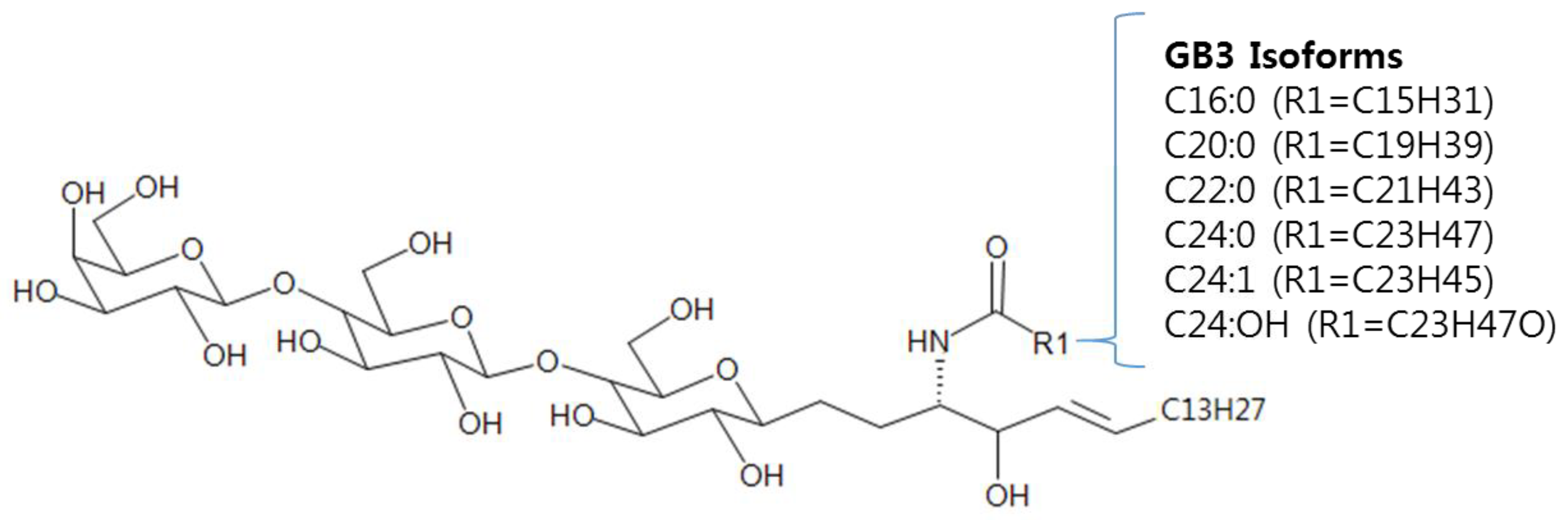
A Liquid Chromatography-Quadrupole-Time-of-Flight Mass Spectrometric Assay for the Quantification of Fabry Disease Biomarker Globotriaosylceramide (GB3) in Fabry Model Mouse
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of Stock Solution
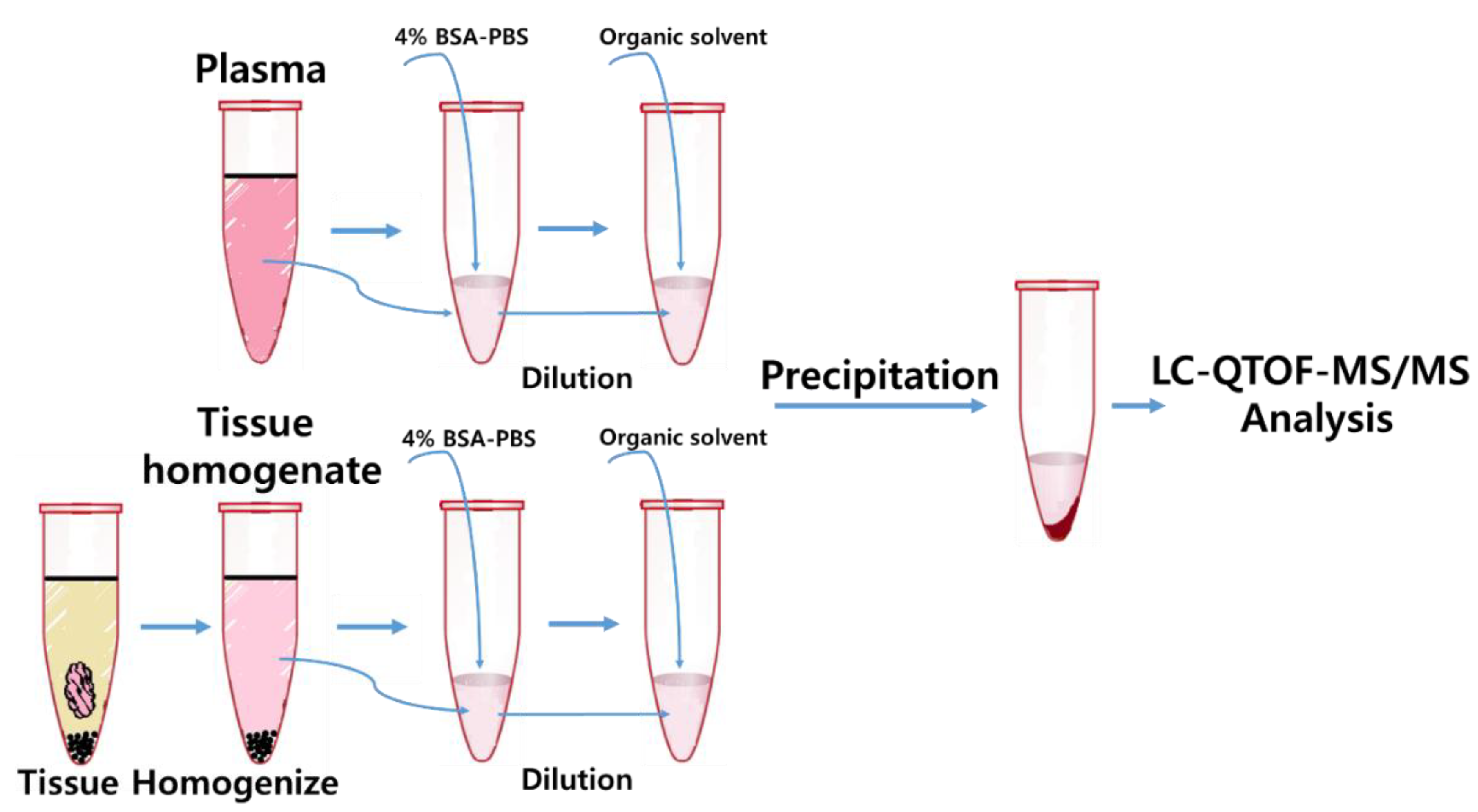
2.3. Sample Preparation-Plasma
2.4. Sample Preparation–Tissues (Heart, Liver, Spleen, Kidney, Brain)
2.5. Liquid Chromatographic Mass Spectrometry (LC-MS/MS) Condition
2.6. Method Qualification
2.7. Software
2.8. Application for Animal Study
3. Results
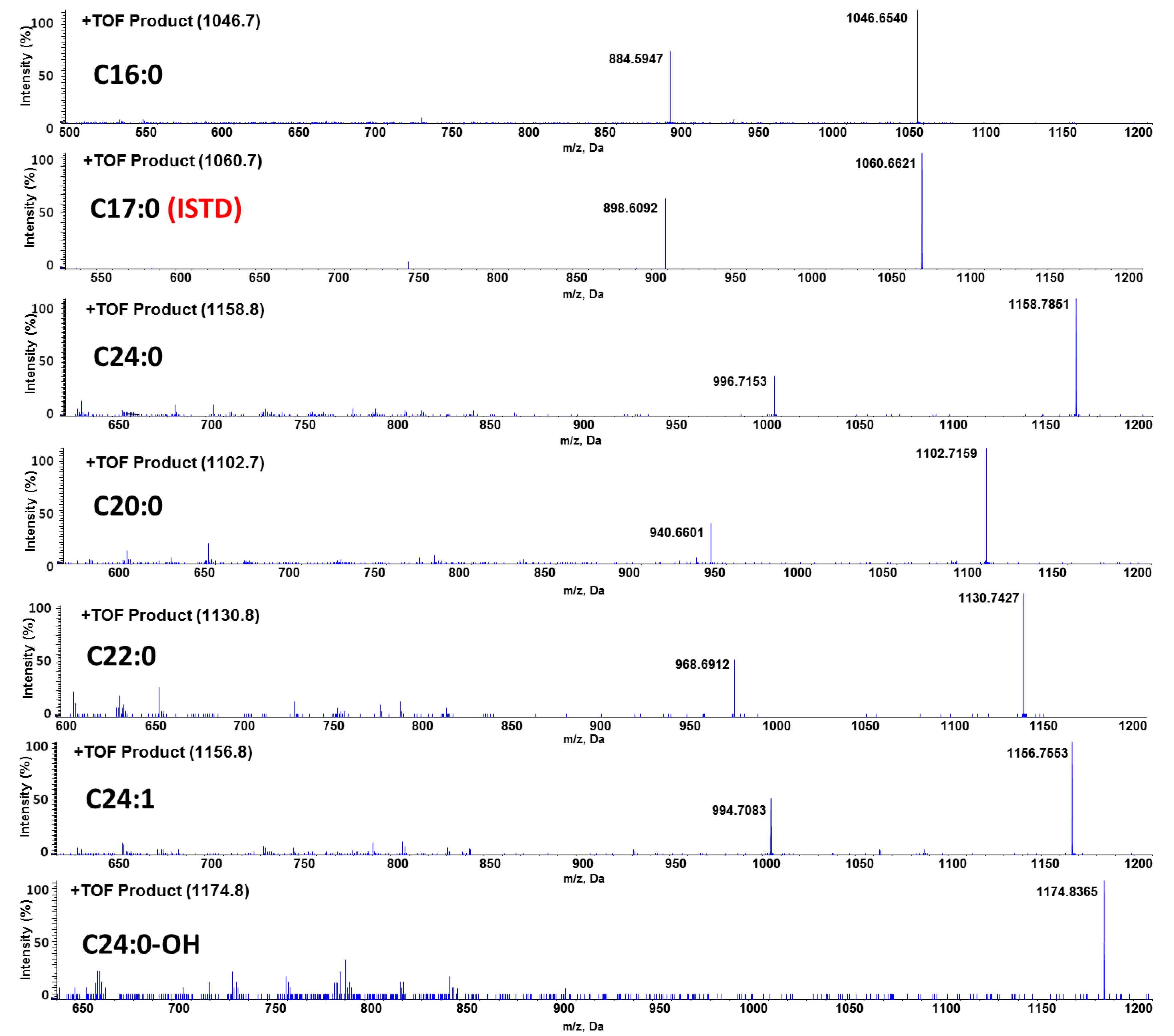
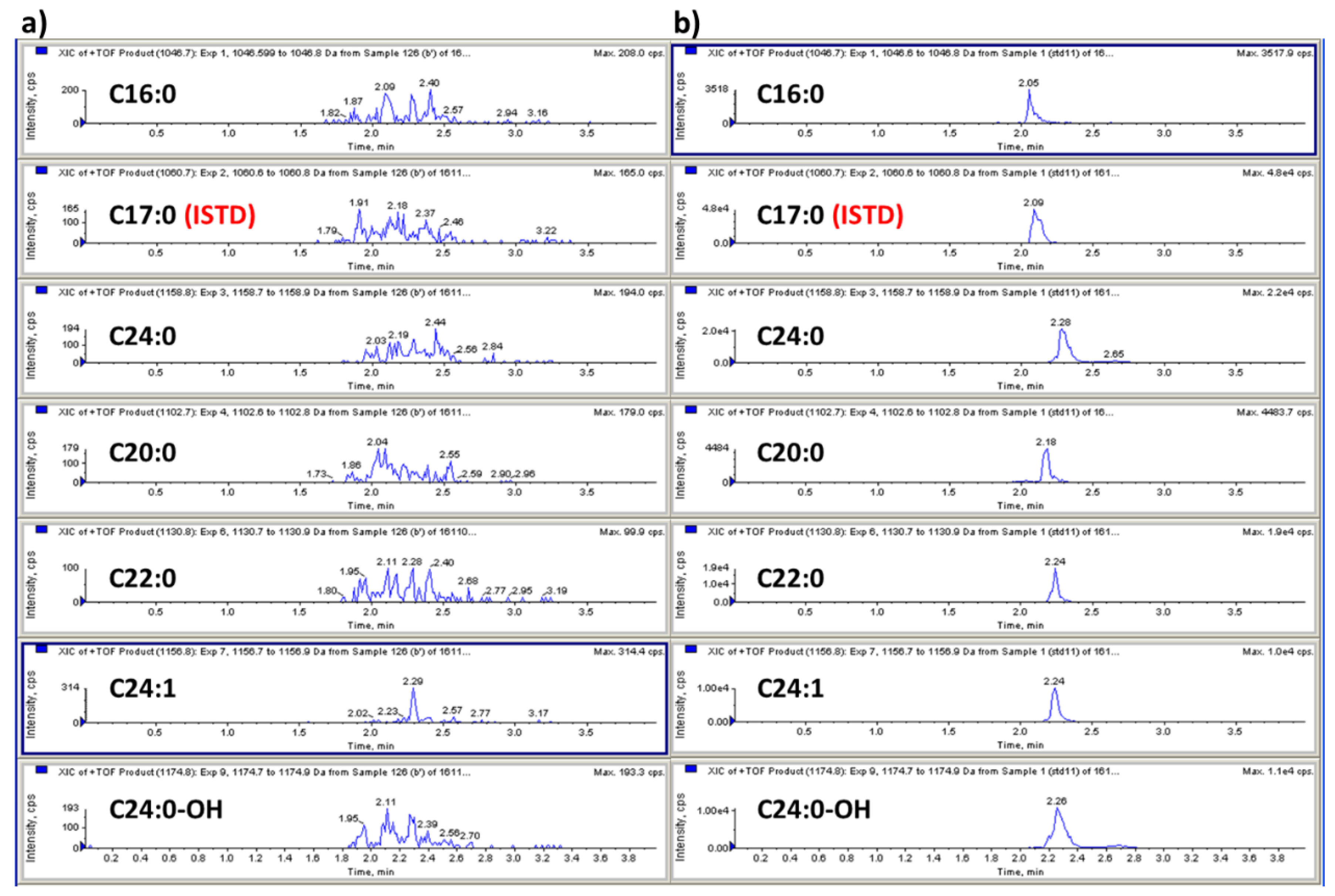
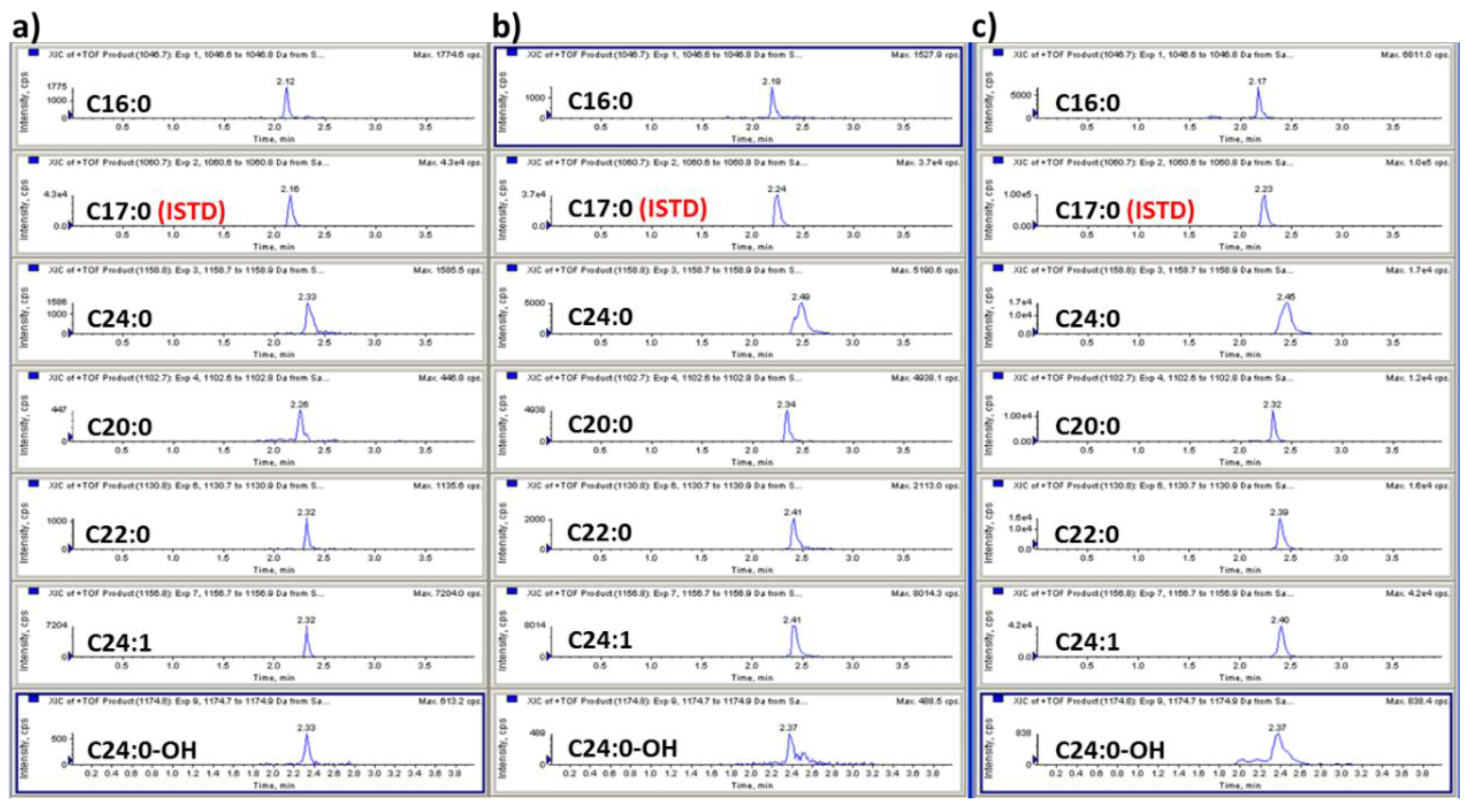
3.1. Method Development: Sample Preparation and LC-MS/MS Analysis
3.2. Method Qualification
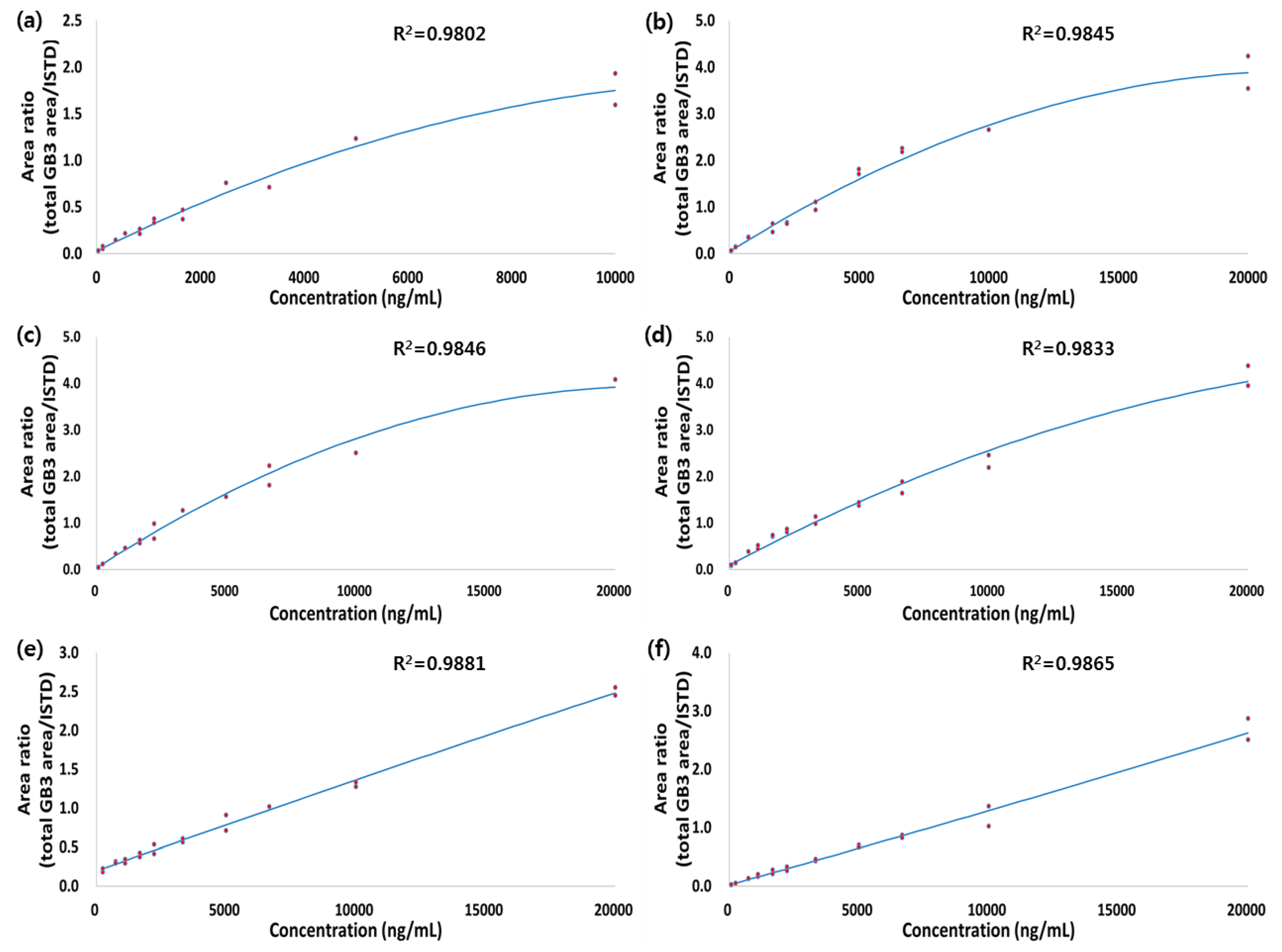
3.2.1. Calibration Curve, Accuracy, and Precision
3.2.2. Preliminary Stability
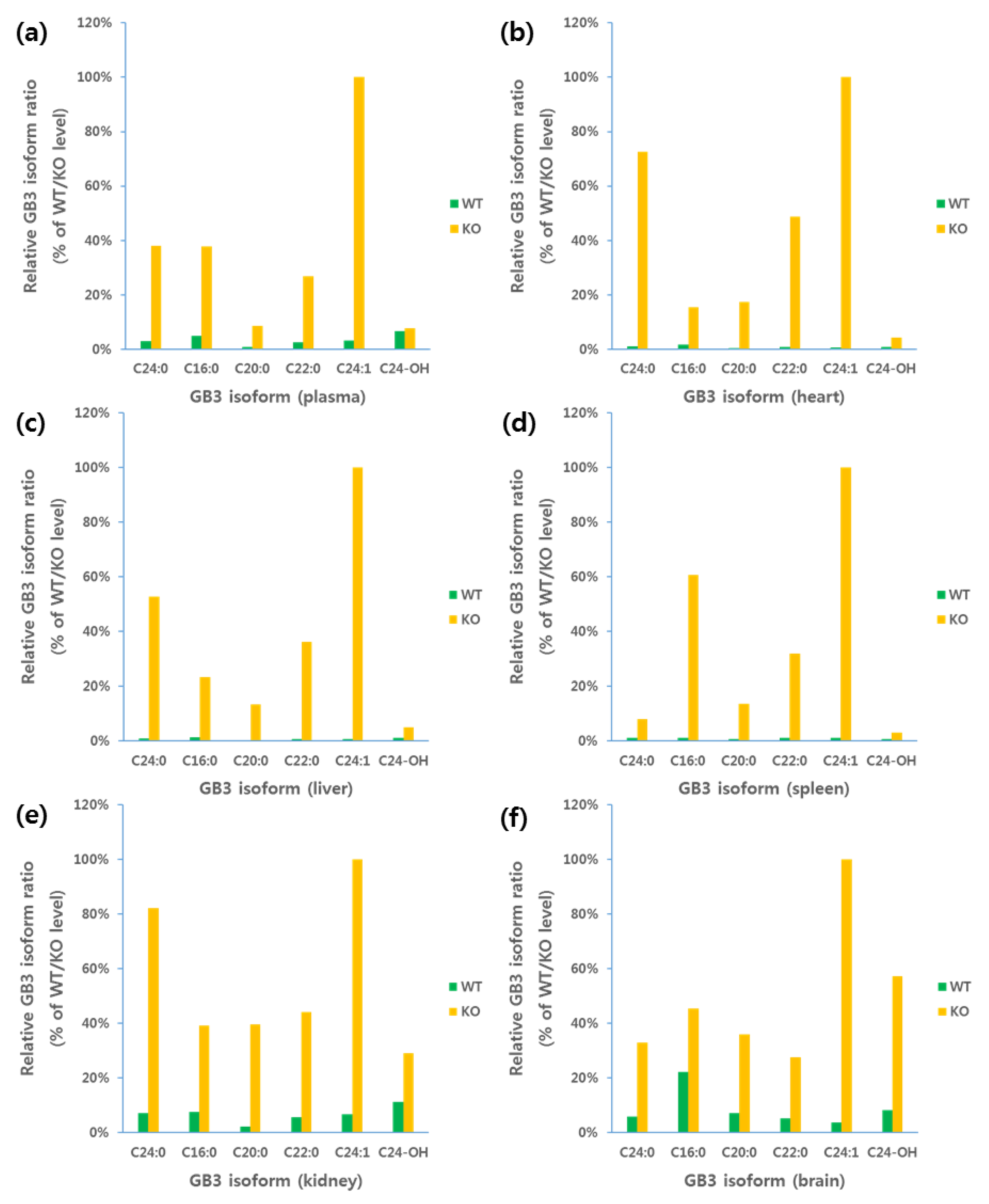
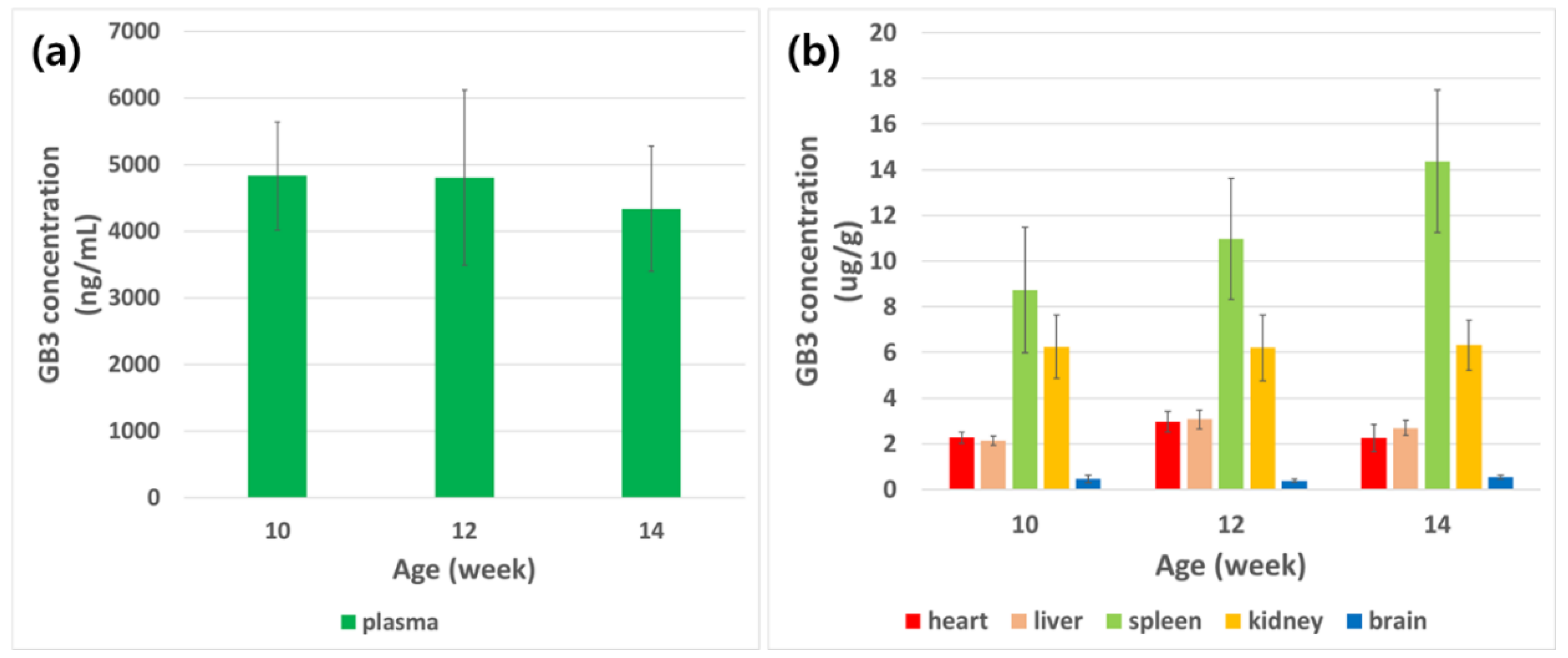
3.3. Application for Animal Study
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Brady, R.O. Enzymatic abnormalities in diseases of sphingolipid metabolism. Clin. Chem. 1967, 13, 565–577. [Google Scholar] [PubMed]
- Fabry, H. An historical overview of Fabry disease. J. Inherit. Metab. Dis. 2001, 24 (Suppl. 2), 3–7. [Google Scholar] [CrossRef] [PubMed]
- Desnick, R.J.; Allen, K.Y.; Simmons, R.L.; Woods, J.E.; Anderson, C.F.; Najarian, J.S.; Krivit, W. Fabry disease: Correction of the enzymatic deficiency by renal transplantation. Birth Defects Orig. Artic. Ser. 1973, 9, 88–96. [Google Scholar] [PubMed]
- Desnick, R.J.; Brady, R.; Barranger, J.; Collins, A.J.; Germain, D.P.; Goldman, M.; Grabowski, G.; Packman, S.; Wilcox, W.R. Fabry disease, an under-recognized multisystemic disorder: Expert recommendations for diagnosis, management, and enzyme replacement therapy. Ann. Intern. Med. 2003, 138, 338–346. [Google Scholar] [CrossRef] [PubMed]
- Thurberg, B.L.; Rennke, H.; Colvin, R.B.; Dikman, S.; Gordon, R.E.; Collins, A.B.; Desnick, R.J.; O’Callaghan, M. Globotriaosylceramide accumulation in the Fabry kidney is cleared from multiple cell types after enzyme replacement therapy. Kidney Int. 2002, 62, 1933–1946. [Google Scholar] [CrossRef] [PubMed]
- MacDermot, K.D.; Holmes, A.; Miners, A.H. Anderson-Fabry disease: Clinical manifestations and impact of disease in a cohort of 98 hemizygous males. J. Med. Genet. 2001, 38, 750–760. [Google Scholar] [CrossRef] [PubMed]
- MacDermot, K.D.; Holmes, A.; Miners, A.H. Anderson-Fabry disease: Clinical manifestations and impact of disease in a cohort of 60 obligate carrier females. J. Med. Genet. 2001, 38, 769–775. [Google Scholar] [CrossRef] [PubMed]
- Brady, R.O.; Tallman, J.F.; Johnson, W.G.; Gal, A.E.; Leahy, W.R.; Quirk, J.M.; Dekaban, A.S. Replacement therapy for inherited enzyme deficiency. Use of purified ceramidetrihexosidase in Fabry’s disease. N. Engl. J. Med. 1973, 289, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Eng, C.M.; Guffon, N.; Wilcox, W.R.; Germain, D.P.; Lee, P.; Waldek, S.; Caplan, L.; Linthorst, G.E.; Desnick, R.J.; International Collaborative Fabry Disease Study Group. Safety and efficacy of recombinant human α-galactosidase A replacement therapy in Fabry’s disease. N. Engl. J. Med. 2001, 345, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Schiffmann, R.; Ries, M.; Timmons, M.; Flaherty, J.T.; Brady, R.O. Long-term therapy with agalsidase alfa for Fabry disease: Safety and effects on renal function in a home infusion setting. Nephrol. Dial. Transplant. 2006, 21, 345–354. [Google Scholar] [CrossRef] [PubMed]
- Valenzano, K.J.; Khanna, R.; Powe, A.C.; Boyd, R.; Lee, G.; Flanagan, J.J.; Benjamin, E.R. Identification and characterization of pharmacological chaperones to correct enzyme deficiencies in lysosomal storage disorders. Assay Drug Dev. Technol. 2011, 9, 213–235. [Google Scholar] [CrossRef] [PubMed]
- Yam, G.H.; Bosshard, N.; Zuber, C.; Steinmann, B.; Roth, J. Pharmacological chaperone corrects lysosomal storage in Fabry disease caused by trafficking-incompetent variants. Am. J. Physiol. Cell Physiol. 2006, 290, C1076–C1082. [Google Scholar] [CrossRef] [PubMed]
- Young-Gqamana, B.; Brignol, N.; Chang, H.H.; Khanna, R.; Soska, R.; Fuller, M.; Sitaraman, S.A.; Germain, D.P.; Giugliani, R.; Hughes, D.A.; et al. Migalastat HCl reduces globotriaosylsphingosine (lyso-Gb3) in Fabry transgenic mice and in the plasma of Fabry patients. PLoS ONE 2013, 8, e57631. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, T.; Ishige, N.; Suzuki, K.; Owada, M.; Ohashi, T.; Kobayashi, M.; Eto, Y.; Tanaka, A.; Mills, K.; Winchester, B.; et al. Non-invasive screening method for Fabry disease by measuring globotriaosylceramide in whole urine samples using tandem mass spectrometry. Mol. Genet. Metab. 2005, 85, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Whitfield, P.D.; Calvin, J.; Hogg, S.; O’Driscoll, E.; Halsall, D.; Burling, K.; Maguire, G.; Wright, N.; Cox, T.M.; Meikle, P.J.; et al. Monitoring enzyme replacement therapy in Fabry disease—Role of urine globotriaosylceramide. J. Inherit. Metab. Dis. 2005, 28, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Auray-Blais, C.; Cyr, D.; Ntwari, A.; West, M.L.; Cox-Brinkman, J.; Bichet, D.G.; Germain, D.P.; Laframboise, R.; Melancon, S.B.; Stockley, T.; et al. Urinary globotriaosylceramide excretion correlates with the genotype in children and adults with Fabry disease. Mol. Genet. Metab. 2008, 93, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Nelson, B.C.; Roddy, T.; Araghi, S.; Wilkens, D.; Thomas, J.J.; Zhang, K.; Sung, C.C.; Richards, S.M. Globotriaosylceramide isoform profiles in human plasma by liquid chromatography-tandem mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2004, 805, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Hozumi, I.; Nishizawa, M.; Ariga, T.; Miyatake, T. Biochemical and clinical analysis of accumulated glycolipids in symptomatic heterozygotes of angiokeratoma corporis diffusum (Fabry’s disease) in comparison with hemizygotes. J. Lipid Res. 1990, 31, 335–340. [Google Scholar] [PubMed]
- Kniep, B.; Muhlradt, P.F. Immunochemical detection of glycosphingolipids on thin-layer chromatograms. Anal. Biochem. 1990, 188, 5–8. [Google Scholar] [CrossRef]
- Groener, J.E.; Poorthuis, B.J.; Kuiper, S.; Helmond, M.T.; Hollak, C.E.; Aerts, J.M. HPLC for simultaneous quantification of total ceramide, glucosylceramide, and ceramide trihexoside concentrations in plasma. Clin. Chem. 2007, 53, 742–747. [Google Scholar] [CrossRef] [PubMed]
- Fauler, G.; Rechberger, G.N.; Devrnja, D.; Erwa, W.; Plecko, B.; Kotanko, P.; Breunig, F.; Paschke, E. Rapid determination of urinary globotriaosylceramide isoform profiles by electrospray ionization mass spectrometry using stearoyl-d35-globotriaosylceramide as internal standard. Rapid Commun. Mass Spectrom. 2005, 19, 1499–1506. [Google Scholar] [CrossRef] [PubMed]
- Auray-Blais, C.; Boutin, M. Novel Gb(3) isoforms detected in urine of fabry disease patients: A metabolomic study. Curr. Med. Chem. 2012, 19, 3241–3252. [Google Scholar] [CrossRef] [PubMed]
- Sueoka, H.; Aoki, M.; Tsukimura, T.; Togawa, T.; Sakuraba, H. Distributions of Globotriaosylceramide Isoforms, and Globotriaosylsphingosine and Its Analogues in an α-Galactosidase A Knockout Mouse, a Model of Fabry Disease. PLoS ONE 2015, 10, e0144958. [Google Scholar] [CrossRef] [PubMed]
- Alharbi, F.J.; Geberhiwot, T.; Hughes, D.A.; Ward, D.G. A Novel Rapid MALDI-TOF-MS-Based Method for Measuring Urinary Globotriaosylceramide in Fabry Patients. J. Am. Soc. Mass Spectrom. 2016, 27, 719–725. [Google Scholar] [CrossRef] [PubMed]
- Kruger, R.; Bruns, K.; Grunhage, S.; Rossmann, H.; Reinke, J.; Beck, M.; Lackner, K.J. Determination of globotriaosylceramide in plasma and urine by mass spectrometry. Clin. Chem. Lab. Med. 2010, 48, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Boutin, M.; Menkovic, I.; Martineau, T.; Vaillancourt-Lavigueur, V.; Toupin, A.; Auray-Blais, C. Separation and Analysis of Lactosylceramide, Galabiosylceramide, and Globotriaosylceramide by LC-MS/MS in Urine of Fabry Disease Patients. Anal. Chem. 2017, 89, 13382–13390. [Google Scholar] [CrossRef] [PubMed]
- Polo, G.; Burlina, A.P.; Kolamunnage, T.B.; Zampieri, M.; Dionisi-Vici, C.; Strisciuglio, P.; Zaninotto, M.; Plebani, M.; Burlina, A.B. Diagnosis of sphingolipidoses: A new simultaneous measurement of lysosphingolipids by LC-MS/MS. Clin. Chem. Lab. Med. 2017, 55, 403–414. [Google Scholar] [CrossRef] [PubMed]
- Boutin, M.; Gagnon, R.; Lavoie, P.; Auray-Blais, C. LC-MS/MS analysis of plasma lyso-Gb3 in Fabry disease. Clin. Chim. Acta 2012, 414, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Ioannou, Y.A.; Zeidner, K.M.; Gordon, R.E.; Desnick, R.J. Fabry disease: Preclinical studies demonstrate the effectiveness of α-galactosidase A replacement in enzyme-deficient mice. Am. J. Hum. Genet. 2001, 68, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Ohshima, T.; Murray, G.J.; Swaim, W.D.; Longenecker, G.; Quirk, J.M.; Cardarelli, C.O.; Sugimoto, Y.; Pastan, I.; Gottesman, M.M.; Brady, R.O.; et al. α-Galactosidase A deficient mice: A model of Fabry disease. Proc. Natl. Acad. Sci. USA 1997, 94, 2540–2544. [Google Scholar] [CrossRef] [PubMed]
- Ramagiri, S.; Garofolo, F. Large molecule bioanalysis using Q-TOF without predigestion and its data processing challenges. Bioanalysis 2012, 4, 529–540. [Google Scholar] [CrossRef] [PubMed]
| TOF-MS Condition | |||
| GS1 | 50 | CUR (Curtain Gas) | 30 |
| GS2 | 50 | ISVP (Ion Spray Voltage) | 5500 |
| SRM High Sensitive Scan Mode, Positive | |||
| GB3 Isoform | Parent-To-Parent Transition | Declustering Voltage (DP) | Collision Energy (CE) |
| C16:0-GB3 | 1046.7→1046.7 | 100 | 66 |
| C17:0-GB3 | 1060.7→1060.7 (ISTD) | 100 | 66 |
| C20:0-GB3 | 1102.7→1102.7 | 100 | 66 |
| C22:0-GB3 | 1130.8→1130.8 | 100 | 66 |
| C24:1-GB3 | 1156.8→1156.8 | 100 | 66 |
| C24:0-GB3 | 1158.8→1158.8 | 100 | 66 |
| C24:0-OH-GB3 | 1174.8→1174.8 | 100 | 66 |
| Matrix | QC Samples | Mean Concentration (ng/mL) | RSD (%) | Mean Accuracy (%) | n |
|---|---|---|---|---|---|
| Plasma | QC medium (400 ng/mL) | 314.28 | 15.06 | 78.57 | 3 |
| QC high (2000 ng/mL) | 2131.34 | 5.92 | 106.57 | 3 | |
| Heart | QC medium (800 ng/mL) | 779.48 | 11.96 | 99.02 | 3 |
| QC high (4000 ng/mL) | 3286.93 | 8.41 | 83.23 | 3 | |
| Liver | QC medium (800 ng/mL) | 823.21 | 14.53 | 102.90 | 3 |
| QC high (4000 ng/mL) | 3395.26 | 8.66 | 84.88 | 3 | |
| Spleen | QC medium (800 ng/mL) | 848.22 | 10.64 | 106.03 | 3 |
| QC high (4000 ng/mL) | 3861.45 | 25.49 | 96.54 | 3 | |
| Kidney | QC medium (800 ng/mL) | 839.15 | 26.16 | 104.89 | 3 |
| QC high (4000 ng/mL) | 4275.53 | 9.38 | 106.89 | 3 | |
| Brain | QC medium (800 ng/mL) | 740.97 | 11.60 | 92.62 | 3 |
| QC high (4000 ng/mL) | 3826.03 | 15.16 | 95.65 | 3 |
| Matrix | Time Point (min) | Mean Area Ratio | RSD (%) | Mean Accuracy (%) | n |
|---|---|---|---|---|---|
| Plasma | 0 | 3.78 | 6.54 | 100.00 | 3 |
| 60 | 3.27 | 6.02 | 86.66 | ||
| 120 | 3.64 | 9.04 | 96.49 | ||
| 180 | 4.12 | 13.53 | 109.20 | ||
| Heart | 0 | 3.06 | 12.10 | 100.00 | 3 |
| 60 | 3.09 | 1.70 | 101.09 | ||
| 120 | 2.88 | 9.98 | 94.34 | ||
| 180 | 2.53 | 12.23 | 82.83 | ||
| Liver | 0 | 3.14 | 9.73 | 100.00 | 3 |
| 60 | 3.07 | 12.30 | 97.80 | ||
| 120 | 3.17 | 12.58 | 100.97 | ||
| 180 | 3.01 | 3.92 | 95.85 | ||
| Spleen | 0 | 3.51 | 8.45 | 100.00 | 3 |
| 60 | 2.89 | 8.54 | 82.31 | ||
| 120 | 3.02 | 11.61 | 86.14 | ||
| 180 | 2.50 | 3.18 | 71.32 | ||
| Kidney | 0 | 2.42 | 10.26 | 100.00 | 3 |
| 60 | 3.03 | 2.88 | 125.16 | ||
| 120 | 2.95 | 14.21 | 121.72 | ||
| 180 | 2.69 | 5.63 | 111.00 | ||
| Brain | 0 | 1.48 | 3.70 | 100.00 | 3 |
| 60 | 1.37 | 16.01 | 92.77 | ||
| 120 | 1.37 | 16.97 | 92.42 | ||
| 180 | 1.55 | 16.97 | 105.07 |
| Matrix | Control/FT-3 Cycle | Mean Area Ratio | RSD (%) | Mean Accuracy (%) | n |
|---|---|---|---|---|---|
| Plasma | Control | 2.31 | 1.91 | 100.00 | 3 |
| FT-3 cycle | 2.24 | 7.97 | 97.14 | ||
| Heart | Control | 2.27 | 11.81 | 100.00 | 3 |
| FT-3 cycle | 2.19 | 7.70 | 96.63 | ||
| Liver | Control | 1.93 | 1.43 | 100.00 | 3 |
| FT-3 cycle | 1.97 | 21.40 | 102.16 | ||
| Spleen | Control | 2.23 | 6.15 | 100.00 | 3 |
| FT-3 cycle | 2.12 | 10.07 | 95.20 | ||
| Kidney | Control | 2.14 | 5.96 | 100.00 | 3 |
| FT-3 cycle | 2.11 | 14.91 | 98.69 | ||
| Brain | Control | 1.34 | 7.09 | 100.00 | 3 |
| FT-3 cycle | 1.32 | 5.68 | 98.21 |
| Organ | Week | Mean Area Ratio | RSD (%) | Mean Accuracy (%) | n |
|---|---|---|---|---|---|
| Plasma | 0 week | 2.26 | 3.36 | 100 | 3 |
| 1 week | 2.29 | 14.55 | 101.46 | 3 | |
| 2 week | 2.27 | 11.23 | 100.23 | 3 | |
| Heart | 0 week | 3.62 | 17.56 | 100 | 3 |
| 1 week | 3.81 | 8.19 | 105.32 | 3 | |
| 2 week | 3.94 | 12.43 | 108.84 | 3 | |
| Liver | 0 week | 2.76 | 12.83 | 100 | 3 |
| 1 week | 2.52 | 0.73 | 91.18 | 3 | |
| 2 week | 3.08 | 6.72 | 111.51 | 3 | |
| Spleen | 0 week | 4.05 | 4.44 | 100 | 3 |
| 1 week | 3.73 | 15.32 | 91.91 | 3 | |
| 2 week | 3.72 | 5.13 | 91.8 | 3 | |
| Kidney | 0 week | 2.63 | 14.47 | 100 | 3 |
| 1 week | 2.69 | 10.44 | 102.31 | 3 | |
| 2 week | 2.77 | 11.44 | 105.4 | 3 | |
| Brain | 0 week | 0.82 | 10.86 | 100 | 3 |
| 1 week | 0.8 | 7.71 | 97.46 | 3 | |
| 2 week | 0.77 | 21.72 | 93.86 | 3 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, S.-H.; Park, M.-H.; Byeon, J.-J.; Lee, B.I.; Park, Y.; Ko, A.-r.; Seong, M.-r.; Lee, S.; Kim, M.R.; Seo, J.; et al. A Liquid Chromatography-Quadrupole-Time-of-Flight Mass Spectrometric Assay for the Quantification of Fabry Disease Biomarker Globotriaosylceramide (GB3) in Fabry Model Mouse. Pharmaceutics 2018, 10, 69. https://doi.org/10.3390/pharmaceutics10020069
Shin S-H, Park M-H, Byeon J-J, Lee BI, Park Y, Ko A-r, Seong M-r, Lee S, Kim MR, Seo J, et al. A Liquid Chromatography-Quadrupole-Time-of-Flight Mass Spectrometric Assay for the Quantification of Fabry Disease Biomarker Globotriaosylceramide (GB3) in Fabry Model Mouse. Pharmaceutics. 2018; 10(2):69. https://doi.org/10.3390/pharmaceutics10020069
Chicago/Turabian StyleShin, Seok-Ho, Min-Ho Park, Jin-Ju Byeon, Byeong Ill Lee, Yuri Park, Ah-ra Ko, Mi-ran Seong, Soyeon Lee, Mi Ra Kim, Jinwook Seo, and et al. 2018. "A Liquid Chromatography-Quadrupole-Time-of-Flight Mass Spectrometric Assay for the Quantification of Fabry Disease Biomarker Globotriaosylceramide (GB3) in Fabry Model Mouse" Pharmaceutics 10, no. 2: 69. https://doi.org/10.3390/pharmaceutics10020069
APA StyleShin, S.-H., Park, M.-H., Byeon, J.-J., Lee, B. I., Park, Y., Ko, A.-r., Seong, M.-r., Lee, S., Kim, M. R., Seo, J., Jung, M. E., Jin, D.-K., & Shin, Y. G. (2018). A Liquid Chromatography-Quadrupole-Time-of-Flight Mass Spectrometric Assay for the Quantification of Fabry Disease Biomarker Globotriaosylceramide (GB3) in Fabry Model Mouse. Pharmaceutics, 10(2), 69. https://doi.org/10.3390/pharmaceutics10020069