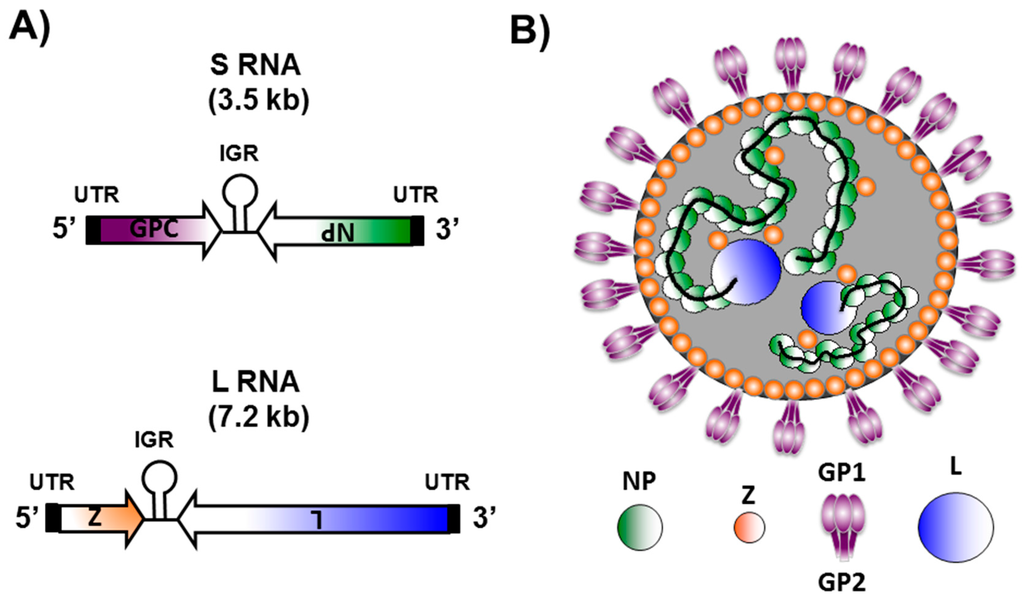
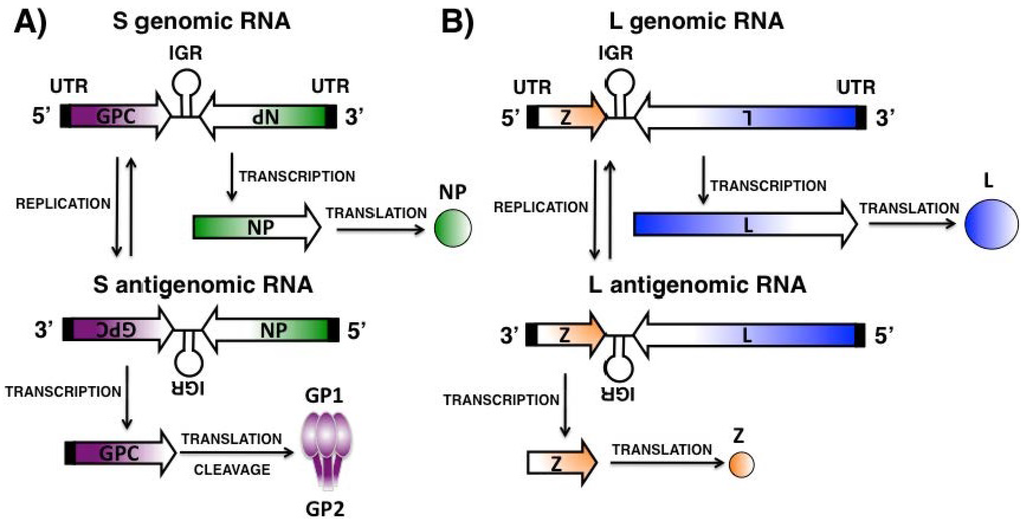
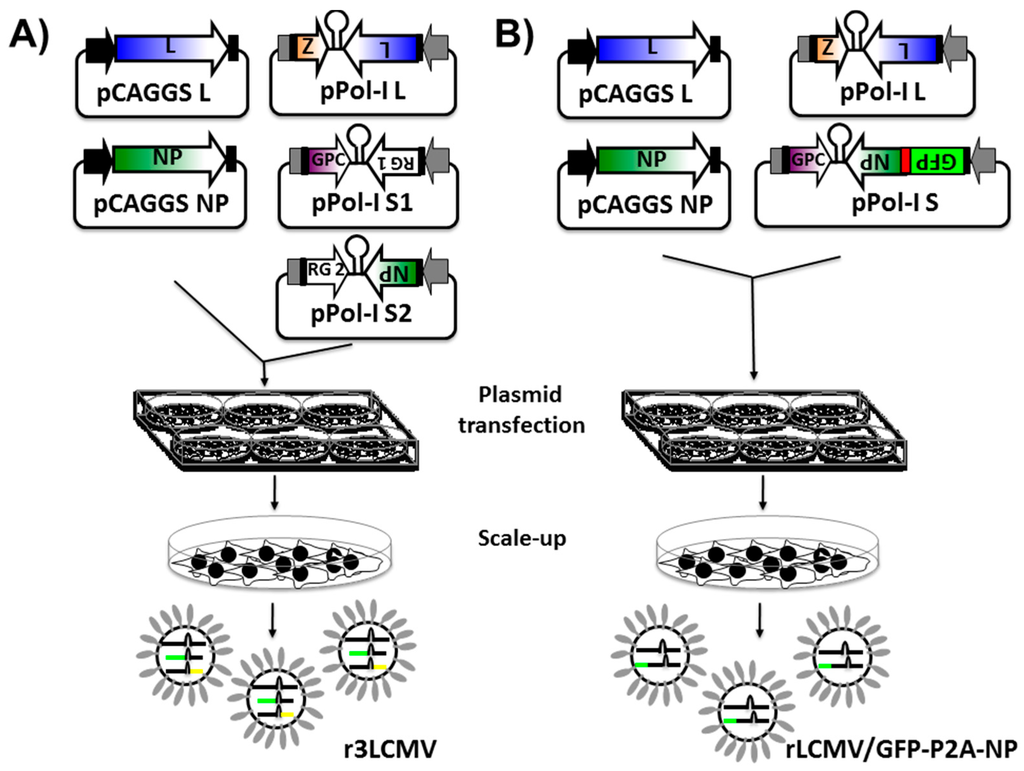
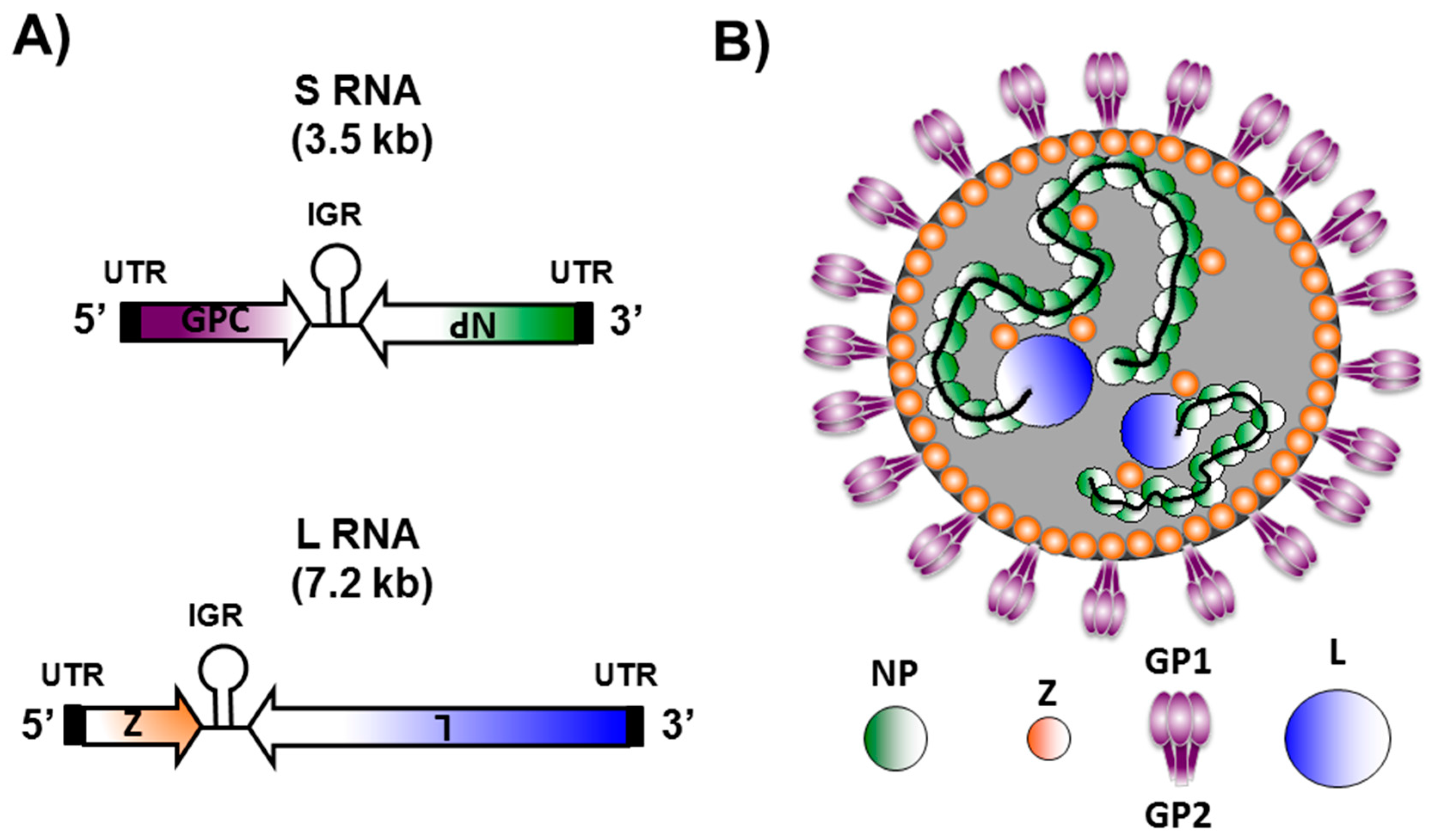
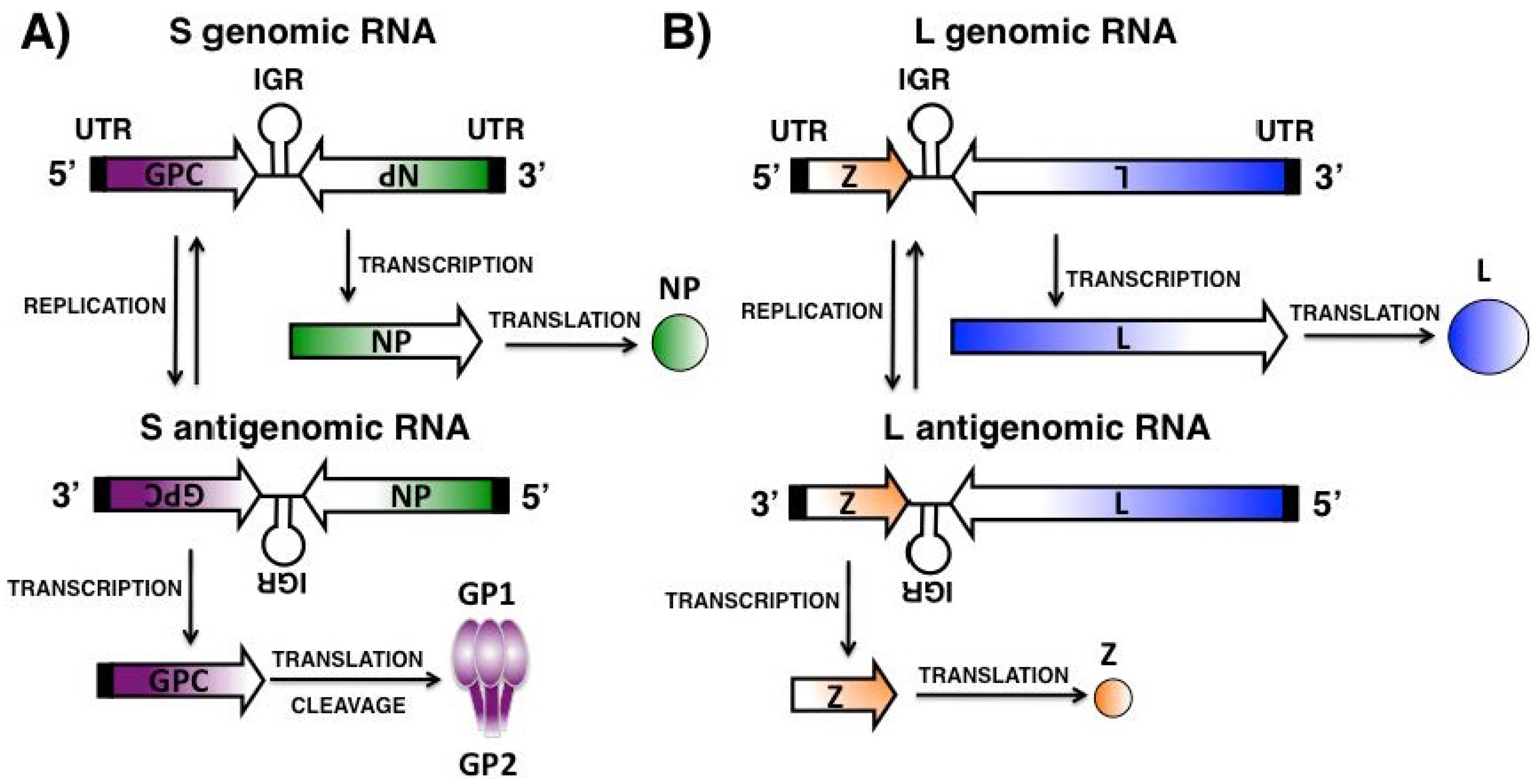
Abstract
Several arenaviruses cause hemorrhagic fever (HF) disease in humans and pose an important public health problem in their endemic regions. To date, no Food and Drug Administration (FDA)-licensed vaccines are available to combat human arenavirus infections, and current anti-arenaviral drug therapy is limited to an off-label use of ribavirin that is only partially effective. The development of arenavirus reverse genetic approaches has provided investigators with a novel and powerful approach for the study of arenavirus biology including virus–host interactions underlying arenavirus induced disease. The use of cell-based minigenome systems has allowed examining the cis- and trans-acting factors involved in arenavirus replication and transcription, as well as particle assembly and budding. Likewise, it is now feasible to rescue infectious arenaviruses containing predetermined mutations in their genomes to investigate virus-host interactions and mechanisms of pathogenesis. The use of reverse genetics approaches has also allowed the generation of recombinant arenaviruses expressing additional genes of interest. These advances in arenavirus molecular genetics have also facilitated the implementation of novel screens to identify anti-arenaviral drugs, and the development of novel strategies for the generation of arenavirus live-attenuated vaccines. In this review, we will summarize the current knowledge on reporter-expressing, replicating-competent arenaviruses harboring reporter genes in different locations of the viral genome and their use for studying and understanding arenavirus biology and the identification of anti-arenaviral drugs to combat these important human pathogens.
7. Conclusions
The development of arenavirus reverse genetics systems has provided investigators with a novel and powerful experimental approach to study basic aspects of arenavirus biology, including the identification of viral determinants, and their mechanisms of action, which contribute to arenaviral human diseases. The ability to manipulate the genome of arenaviruses has proven to be a superb model system to study virus–host interactions and associated disease. Moreover, generating recombinant arenaviruses with predetermined mutations allows investigators to gain a detailed understanding of arenavirus–host interactions and phenotypic outcomes of virus infection. Furthermore, the generation of r3 or bicistronic arenaviruses expressing appropriate RG, together with the development of specific cell-based assays for each of the different steps of the arenavirus life cycle, is facilitating novel approaches to discover and characterize antiviral drugs against these important human pathogens.
Acknowledgments
We want to thank past and current members in our laboratories and arenavirus virologists whose work has contributed to the generation of recombinant replicating-competent, reporter-expressing arenaviruses. Current arenavirus research in the Luis Martínez-Sobrido laboratory is funded by the National Institutes of Health (NIH) grants R21 AI119775-01 and R43 AI119775-01 and 1R21AI121550-01A1. Research in the Juan Carlos de la Torre laboratory is supported by grants RO1 AI047140, RO1 AI077719, and RO1 AI079665.
Author Contributions
Luis Martínez-Sobrido and Juan Carlos de la Torre wrote the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Buchmeier, M.J.; Peter, C.J.; de la Torre, J.C. Arenaviridae: The Viruses and Their Replication; Lippincott William and Wilkins: Philadelphia, PA, USA, 2007; Volume 2. [Google Scholar]
- Radoshitzky, S.R.; Bao, Y.; Buchmeier, M.J.; Charrel, R.N.; Clawson, A.N.; Clegg, C.S.; DeRisi, J.L.; Emonet, S.; Gonzalez, J.P.; Kuhn, J.H.; et al. Past, present, and future of arenavirus taxonomy. Arch. Virol. 2015, 160, 1851–1874. [Google Scholar] [CrossRef] [PubMed]
- Stenglein, M.D.; Jacobson, E.R.; Chang, L.W.; Sanders, C.; Hawkins, M.G.; Guzman, D.S.; Drazenovich, T.; Dunker, F.; Kamaka, E.K.; Fisher, D.; et al. Widespread recombination, reassortment, and transmission of unbalanced compound viral genotypes in natural arenavirus infections. PLoS Pathog. 2015, 11, e1004900. [Google Scholar] [CrossRef] [PubMed]
- Stenglein, M.D.; Leavitt, E.B.; Abramovitch, M.A.; McGuire, J.A.; DeRisi, J.L. Genome sequence of a bornavirus recovered from an African garter snake (Elapsoidea loveridgei). Genome Announc. 2014, 2. [Google Scholar] [CrossRef] [PubMed]
- Stenglein, M.D.; Sanders, C.; Kistler, A.L.; Ruby, J.G.; Franco, J.Y.; Reavill, D.R.; Dunker, F.; Derisi, J.L. Identification, characterization, and in vitro culture of highly divergent arenaviruses from boa constrictors and annulated tree boas: Candidate etiological agents for snake inclusion body disease. mBio 2012, 3. [Google Scholar] [CrossRef] [PubMed]
- Enria, D.A.; Briggiler, A.M.; Sanchez, Z. Treatment of argentine hemorrhagic fever. Antivir. Res. 2008, 78, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Geisbert, T.W.; Jahrling, P.B. Exotic emerging viral diseases: Progress and challenges. Nat. Med. 2004, 10, S110–S121. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.H.; Goba, A.; Chu, M.; Roth, C.; Healing, T.; Marx, A.; Fair, J.; Guttieri, M.C.; Ferro, P.; Imes, T.; et al. New opportunities for field research on the pathogenesis and treatment of Lassa fever. Antivir. Res. 2008, 78, 103–115. [Google Scholar] [CrossRef] [PubMed]
- McCormick, J.B.; Fisher-Hoch, S.P. Lassa fever. In Arenaviruses i; Oldstone, M.B., Ed.; Springer-Verlag: Berlin/Heidelberg, Germany, 2002; Volume 262, pp. 75–110. [Google Scholar]
- Peters, C.J. Human infection with arenaviruses in the Americas. In Arenaviruses I; Oldstone, M.B., Ed.; Springer-Verlag: Berlin/Heidelberg, Germany, 2002; Volume 262, pp. 65–74. [Google Scholar]
- Freedman, D.O.; Woodall, J. Emerging infectious diseases and risk to the traveler. Med. Clin. N. Am. 1999, 83, 865–883. [Google Scholar] [PubMed]
- Holmes, G.P.; McCormick, J.B.; Trock, S.C.; Chase, R.A.; Lewis, S.M.; Mason, C.A.; Hall, P.A.; Brammer, L.S.; Perez-Oronoz, G.I.; McDonnell, M.K.; et al. Lassa fever in the United States. Investigation of a case and new guidelines for management. N. Engl. J. Med. 1990, 323, 1120–1123. [Google Scholar] [CrossRef] [PubMed]
- Isaacson, M. Viral hemorrhagic fever hazards for travelers in Africa. Clin. Infect. Dis. 2001, 33, 1707–1712. [Google Scholar] [CrossRef] [PubMed]
- Richmond, J.K.; Baglole, D.J. Lassa fever: Epidemiology, clinical features, and social consequences. BMJ 2003, 327, 1271–1275. [Google Scholar] [CrossRef] [PubMed]
- Briese, T.; Paweska, J.T.; McMullan, L.K.; Hutchison, S.K.; Street, C.; Palacios, G.; Khristova, M.L.; Weyer, J.; Swanepoel, R.; Egholm, M.; et al. Genetic detection and characterization of Lujo virus, a new hemorrhagic fever-associated arenavirus from Southern Africa. PLoS Pathog. 2009, 5, e1000455. [Google Scholar] [CrossRef] [PubMed]
- Kuns, M.L. Epidemiology of Machupo virus infection. II. Ecological and control studies of hemorrhagic fever. Am. J. Trop. Med. Hyg. 1965, 14, 813–816. [Google Scholar] [PubMed]
- Webb, P.A.; Johnson, K.M.; Mackenzie, R.B.; Kuns, M.L. Some characteristics of Machupo virus, causative agent of Bolivian hemorrhagic fever. Am. J. Trop. Med. Hyg. 1967, 16, 531–538. [Google Scholar] [PubMed]
- Delgado, S.; Erickson, B.R.; Agudo, R.; Blair, P.J.; Vallejo, E.; Albarino, C.G.; Vargas, J.; Comer, J.A.; Rollin, P.E.; Ksiazek, T.G.; et al. Chapare virus, a newly discovered arenavirus isolated from a fatal hemorrhagic fever case in Bolivia. PLoS Pathog. 2008, 4, e1000047. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, J.P.; Bowen, M.D.; Nichol, S.T.; Rico-Hesse, R. Genetic characterization and phylogeny of Sabia virus, an emergent pathogen in Brazil. Virology 1996, 221, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, L.R.; Dembry, L.M.; Rainey, P.M.; Russi, M.B.; Khan, A.S.; Fischer, S.H.; Edberg, S.C.; Ksiazek, T.G.; Rollin, P.E.; Peters, C.J. Management of a Sabia virus-infected patients in a US hospital. Infect. Control Hosp. Epidemiol. 1999, 20, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Tesh, R.B.; Jahrling, P.B.; Salas, R.; Shope, R.E. Description of Guanarito virus (Arenaviridae: Arenavirus), the etiologic agent of Venezuelan hemorrhagic fever. Am. J. Trop. Med. Hyg. 1994, 50, 452–459. [Google Scholar] [PubMed]
- Weaver, S.C.; Salas, R.A.; de Manzione, N.; Fulhorst, C.F.; Duno, G.; Utrera, A.; Mills, J.N.; Ksiazek, T.G.; Tovar, D.; Tesh, R.B. Guanarito virus (Arenaviridae) isolates from endemic and outlying localities in Venezuela: Sequence comparisons among and within strains isolated from Venezuelan hemorrhagic fever patients and rodents. Virology 2000, 266, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, J.P.; Sanchez, A.; Rico-Hesse, R. Molecular phylogeny of Guanarito virus, an emerging arenavirus affecting humans. Am. J. Trop. Med. Hyg. 1995, 53, 1–6. [Google Scholar] [PubMed]
- Fulhorst, C.F.; Bowen, M.D.; Ksiazek, T.G.; Rollin, P.E.; Nichol, S.T.; Kosoy, M.Y.; Peters, C.J. Isolation and characterization of whitewater arroyo virus, a novel North American arenavirus. Virology 1996, 224, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Charrel, R.N.; de Lamballerie, X.; Fulhorst, C.F. The Whitewater Arroyo virus: Natural evidence for genetic recombination among Tacaribe serocomplex viruses (family Arenaviridae). Virology 2001, 283, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Cajimat, M.N.; Milazzo, M.L.; Bradley, R.D.; Fulhorst, C.F. Ocozocoautla de espinosa virus and hemorrhagic fever, Mexico. Emerg. Infect. Dis. 2012, 18, 401–405. [Google Scholar] [CrossRef] [PubMed]
- Barton, L.L.; Mets, M.B. Lymphocytic choriomeningitis virus: Pediatric pathogen and fetal teratogen. Pediatr. Infect. Dis. J. 1999, 18, 540–541. [Google Scholar] [CrossRef] [PubMed]
- Barton, L.L.; Mets, M.B. Congenital lymphocytic choriomeningitis virus infection: Decade of rediscovery. Clin. Infect. Dis. 2001, 33, 370–374. [Google Scholar] [CrossRef] [PubMed]
- Barton, L.L.; Mets, M.B.; Beauchamp, C.L. Lymphocytic choriomeningitis virus: Emerging fetal teratogen. Am. J. Obstet. Gynecol. 2002, 187, 1715–1716. [Google Scholar] [CrossRef] [PubMed]
- Jahrling, P.B.; Peters, C.J. Lymphocytic choriomeningitis virus. A neglected pathogen of man. Arch. Pathol. Lab. Med. 1992, 116, 486–488. [Google Scholar] [PubMed]
- Mets, M.B.; Barton, L.L.; Khan, A.S.; Ksiazek, T.G. Lymphocytic choriomeningitis virus: An underdiagnosed cause of congenital chorioretinitis. Am. J. Ophthalmol. 2000, 130, 209–215. [Google Scholar] [CrossRef]
- Fischer, S.A.; Graham, M.B.; Kuehnert, M.J.; Kotton, C.N.; Srinivasan, A.; Marty, F.M.; Comer, J.A.; Guarner, J.; Paddock, C.D.; DeMeo, D.L.; et al. Transmission of lymphocytic choriomeningitis virus by organ transplantation. N. Engl. J. Med. 2006, 354, 2235–2249. [Google Scholar] [CrossRef] [PubMed]
- Palacios, G.; Druce, J.; Du, L.; Tran, T.; Birch, C.; Briese, T.; Conlan, S.; Quan, P.L.; Hui, J.; Marshall, J.; et al. A new arenavirus in a cluster of fatal transplant-associated diseases. N. Engl. J. Med. 2008, 358, 991–998. [Google Scholar] [CrossRef] [PubMed]
- Peters, C.J. Lymphocytic choriomeningitis virus—An old enemy up to new tricks. N. Engl. J. Med. 2006, 354, 2208–2211. [Google Scholar] [CrossRef] [PubMed]
- Borio, L.; Inglesby, T.; Peters, C.J.; Schmaljohn, A.L.; Hughes, J.M.; Jahrling, P.B.; Ksiazek, T.; Johnson, K.M.; Meyerhoff, A.; O’Toole, T.; et al. Hemorrhagic fever viruses as biological weapons: Medical and public health management. JAMA 2002, 287, 2391–2405. [Google Scholar] [CrossRef] [PubMed]
- Damonte, E.B.; Coto, C.E. Treatment of arenavirus infections: From basic studies to the challenge of antiviral therapy. Adv. Virus Res. 2002, 58, 125–155. [Google Scholar] [PubMed]
- Harvie, P.; Omar, R.F.; Dusserre, N.; Desormeaux, A.; Gourde, P.; Tremblay, M.; Beauchamp, D.; Bergeron, M.G. Antiviral efficacy and toxicity of ribavirin in murine acquired immunodeficiency syndrome model. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 1996, 12, 451–461. [Google Scholar] [CrossRef] [PubMed]
- Omar, R.F.; Harvie, P.; Gourde, P.; Desormeaux, A.; Tremblay, M.; Beauchamp, D.; Bergeron, M.G. Antiviral efficacy and toxicity of ribavirin and foscarnet each given alone or in combination in the murine aids model. Toxicol. Appl. Pharmacol. 1997, 143, 140–151. [Google Scholar] [CrossRef] [PubMed]
- Snell, N.J. Ribavirin—Current status of a broad spectrum antiviral agent. Expert Opin. Pharmacother. 2001, 2, 1317–1324. [Google Scholar] [CrossRef] [PubMed]
- Oldstone, M.B. Biology and pathogenesis of lymphocytic choriomeningitis virus infection. In Arenaviruses; Berlin/Heidelberg, Germany; New York, NY, USA, 2002; Volume 263, pp. 83–118. [Google Scholar]
- Zinkernagel, R.M. Lymphocytic choriomeningitis virus and immunology. Curr. Top. Microbiol. Immunol. 2002, 263, 1–5. [Google Scholar] [PubMed]
- Lee, K.J.; Novella, I.S.; Teng, M.N.; Oldstone, M.B.; de La Torre, J.C. NP and L proteins of lymphocytic choriomeningitis virus (LCMV) are sufficient for efficient transcription and replication of LCMV genomic RNA analogs. J. Virol. 2000, 74, 3470–3477. [Google Scholar] [CrossRef] [PubMed]
- Strecker, T.; Eichler, R.; Meulen, J.; Weissenhorn, W.; Dieter Klenk, H.; Garten, W.; Lenz, O. Lassa virus Z protein is a matrix protein and sufficient for the release of virus-like particles [corrected]. J. Virol. 2003, 77, 10700–10705. [Google Scholar] [CrossRef] [PubMed]
- Perez, M.; Craven, R.C.; de la Torre, J.C. The small RING finger protein Z drives arenavirus budding: Implications for antiviral strategies. Proc. Natl. Acad. Sci. USA 2003, 100, 12978–12983. [Google Scholar] [CrossRef] [PubMed]
- Urata, S.; Noda, T.; Kawaoka, Y.; Yokosawa, H.; Yasuda, J. Cellular factors required for Lassa virus budding. J. Virol. 2006, 80, 4191–4195. [Google Scholar] [CrossRef] [PubMed]
- Pinschewer, D.D.; Perez, M.; Sanchez, A.B.; de la Torre, J.C. Recombinant lymphocytic choriomeningitis virus expressing vesicular stomatitis virus glycoprotein. Proc. Natl. Acad. Sci. USA 2003, 100, 7895–7900. [Google Scholar] [CrossRef] [PubMed]
- Beyer, W.R.; Popplau, D.; Garten, W.; von Laer, D.; Lenz, O. Endoproteolytic processing of the lymphocytic choriomeningitis virus glycoprotein by the subtilase SKI-1/S1P. J. Virol. 2003, 77, 2866–2872. [Google Scholar] [CrossRef] [PubMed]
- Rojek, J.M.; Sanchez, A.B.; Nguyen, N.T.; de la Torre, J.C.; Kunz, S. Different mechanisms of cell entry by human-pathogenic Old World and New World arenaviruses. J. Virol. 2008, 82, 7677–7687. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.J.; Perez, M.; Pinschewer, D.D.; de la Torre, J.C. Identification of the lymphocytic choriomeningitis virus (LCMV) proteins required to rescue LCMV RNA analogs into LCMV-like particles. J. Virol. 2002, 76, 6393–6397. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Riano, E.; Cheng, B.Y.; de la Torre, J.C.; Martinez-Sobrido, L. Self-association of lymphocytic choriomeningitis virus nucleoprotein is mediated by its N-terminal region and is not required for its anti-interferon function. J. Virol. 2012, 86, 3307–3317. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Riano, E.; Cheng, B.Y.; de la Torre, J.C.; Martinez-Sobrido, L. The C-terminal region of lymphocytic choriomeningitis virus nucleoprotein contains distinct and segregable functional domains involved in NP-Z interaction and counteraction of the type I interferon response. J. Virol. 2011, 85, 13038–13048. [Google Scholar] [CrossRef] [PubMed]
- Pythoud, C.; Rodrigo, W.W.; Pasqual, G.; Rothenberger, S.; Martinez-Sobrido, L.; de la Torre, J.C.; Kunz, S. Arenavirus nucleoprotein targets interferon regulatory factor-activating kinase IKKepsilon. J. Virol. 2012, 86, 7728–7738. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Sobrido, L.; Emonet, S.; Giannakas, P.; Cubitt, B.; Garcia-Sastre, A.; de la Torre, J.C. Identification of amino acid residues critical for the anti-interferon activity of the nucleoprotein of the prototypic arenavirus lymphocytic choriomeningitis virus. J. Virol. 2009, 83, 11330–11340. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Sobrido, L.; Giannakas, P.; Cubitt, B.; Garcia-Sastre, A.; de la Torre, J.C. Differential inhibition of type I interferon induction by arenavirus nucleoproteins. J. Virol. 2007, 81, 12696–12703. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Sobrido, L.; Zuniga, E.I.; Rosario, D.; Garcia-Sastre, A.; de la Torre, J.C. Inhibition of the type I interferon response by the nucleoprotein of the prototypic arenavirus lymphocytic choriomeningitis virus. J. Virol. 2006, 80, 9192–9199. [Google Scholar] [CrossRef] [PubMed]
- Borrow, P.; Martinez-Sobrido, L.; de la Torre, J.C. Inhibition of the type I interferon antiviral response during arenavirus infection. Viruses 2010, 2, 2443–2480. [Google Scholar] [CrossRef] [PubMed]
- Pythoud, C.; Rothenberger, S.; Martinez-Sobrido, L.; de la Torre, J.C.; Kunz, S. Lymphocytic choriomeningitis virus differentially affects the virus-induced type I interferon response and mitochondrial apoptosis mediated by RIG-I/MAVS. J. Virol. 2015, 89, 6240–6250. [Google Scholar] [CrossRef] [PubMed]
- Rodrigo, W.W.; Ortiz-Riano, E.; Pythoud, C.; Kunz, S.; de la Torre, J.C.; Martinez-Sobrido, L. Arenavirus nucleoproteins prevent activation of nuclear factor Kappa B. J. Virol. 2012, 86, 8185–8197. [Google Scholar] [CrossRef] [PubMed]
- Igonet, S.; Vaney, M.C.; Vonrhein, C.; Bricogne, G.; Stura, E.A.; Hengartner, H.; Eschli, B.; Rey, F.A. X-ray structure of the arenavirus glycoprotein GP2 in its postfusion hairpin conformation. Proc. Natl. Acad. Sci. USA 2011, 108, 19967–19972. [Google Scholar] [CrossRef] [PubMed]
- Burri, D.J.; da Palma, J.R.; Kunz, S.; Pasquato, A. Envelope glycoprotein of arenaviruses. Viruses 2012, 4, 2162–2181. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Henry, M.D.; Borrow, P.; Yamada, H.; Elder, J.H.; Ravkov, E.V.; Nichol, S.T.; Compans, R.W.; Campbell, K.P.; Oldstone, M.B. Identification of alpha-dystroglycan as a receptor for lymphocytic choriomeningitis virus and Lassa fever virus. Science 1998, 282, 2079–2081. [Google Scholar] [CrossRef] [PubMed]
- Kunz, S.; Borrow, P.; Oldstone, M.B. Receptor structure, binding, and cell entry of arenaviruses. Curr. Top. Microbiol. Immunol. 2002, 262, 111–137. [Google Scholar] [PubMed]
- Kunz, S.; Sevilla, N.; McGavern, D.B.; Campbell, K.P.; Oldstone, M.B. Molecular analysis of the interaction of LCMV with its cellular receptor [alpha]-dystroglycan. J. Cell Biol. 2001, 155, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Radoshitzky, S.R.; Abraham, J.; Spiropoulou, C.F.; Kuhn, J.H.; Nguyen, D.; Li, W.; Nagel, J.; Schmidt, P.J.; Nunberg, J.H.; Andrews, N.C.; et al. Transferrin receptor 1 is a cellular receptor for new world haemorrhagic fever arenaviruses. Nature 2007, 446, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Pasqual, G.; Rojek, J.M.; Masin, M.; Chatton, J.Y.; Kunz, S. Old World arenaviruses enter the host cell via the multivesicular body and depend on the endosomal sorting complex required for transport. PLoS Pathog. 2011, 7, e1002232. [Google Scholar] [CrossRef]
- Pinschewer, D.D.; Perez, M.; de la Torre, J.C. Dual role of the lymphocytic choriomeningitis virus intergenic region in transcription termination and virus propagation. J. Virol. 2005, 79, 4519–4526. [Google Scholar] [CrossRef] [PubMed]
- Capul, A.A.; Perez, M.; Burke, E.; Kunz, S.; Buchmeier, M.J.; de la Torre, J.C. Arenavirus Z-glycoprotein association requires Z myristoylation but not functional RING or late domains. J. Virol. 2007, 81, 9451–9460. [Google Scholar] [CrossRef] [PubMed]
- Perez, M.; Greenwald, D.L.; de la Torre, J.C. Myristoylation of the RING finger Z protein is essential for arenavirus budding. J. Virol. 2004, 78, 11443–11448. [Google Scholar] [CrossRef] [PubMed]
- Strecker, T.; Maisa, A.; Daffis, S.; Eichler, R.; Lenz, O.; Garten, W. The role of myristoylation in the membrane association of the Lassa virus matrix protein Z. Virol. J. 2006, 3, 93. [Google Scholar] [CrossRef] [PubMed]
- Enria, D.A.; Barrera Oro, J.G. Junin virus vaccines. Curr. Top. Microbiol. Immunol. 2002, 263, 239–261. [Google Scholar] [PubMed]
- Maiztegui, J.I.; McKee, K.T., Jr.; Barrera Oro, J.G.; Harrison, L.H.; Gibbs, P.H.; Feuillade, M.R.; Enria, D.A.; Briggiler, A.M.; Levis, S.C.; Ambrosio, A.M.; et al. Protective efficacy of a live attenuated vaccine against Argentine hemorrhagic fever. AHF Study Group. J. Infect. Dis. 1998, 177, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Falzarano, D.; Feldmann, H. Vaccines for viral hemorrhagic fevers—Progress and shortcomings. Curr. Opin. Virol. 2013, 3, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Albarino, C.G.; Bergeron, E.; Erickson, B.R.; Khristova, M.L.; Rollin, P.E.; Nichol, S.T. Efficient reverse genetics generation of infectious Junin viruses differing in glycoprotein processing. J. Virol. 2009, 83, 5606–5614. [Google Scholar] [CrossRef] [PubMed]
- Emonet, S.F.; Seregin, A.V.; Yun, N.E.; Poussard, A.L.; Walker, A.G.; de la Torre, J.C.; Paessler, S. Rescue from cloned cDNAs and in vivo characterization of recombinant pathogenic Romero and live-attenuated Candid#1 strains of Junin virus, the causative agent of Argentine hemorrhagic fever disease. J. Virol. 2011, 85, 1473–1483. [Google Scholar] [PubMed]
- Albarino, C.G.; Bird, B.H.; Chakrabarti, A.K.; Dodd, K.A.; Erickson, B.R.; Nichol, S.T. Efficient rescue of recombinant Lassa virus reveals the influence of S segment noncoding regions on virus replication and virulence. J. Virol. 2011, 85, 4020–4024. [Google Scholar] [CrossRef] [PubMed]
- Yun, N.E.; Seregin, A.V.; Walker, D.H.; Popov, V.L.; Walker, A.G.; Smith, J.N.; Miller, M.; de la Torre, J.C.; Smith, J.K.; Borisevich, V.; et al. Mice lacking functional STAT1 are highly susceptible to lethal infection with Lassa virus. J. Virol. 2013, 87, 10908–10911. [Google Scholar] [CrossRef] [PubMed]
- McCormick, J.B.; King, I.J.; Webb, P.A.; Scribner, C.L.; Craven, R.B.; Johnson, K.M.; Elliott, L.H.; Belmont-Williams, R. Lassa fever. Effective therapy with ribavirin. N. Engl. J. Med. 1986, 314, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Kilgore, P.E.; Ksiazek, T.G.; Rollin, P.E.; Mills, J.N.; Villagra, M.R.; Montenegro, M.J.; Costales, M.A.; Paredes, L.C.; Peters, C.J. Treatment of Bolivian hemorrhagic fever with intravenous ribavirin. Clin. Infect. Dis. 1997, 24, 718–722. [Google Scholar] [CrossRef] [PubMed]
- McKee, K.T., Jr.; Huggins, J.W.; Trahan, C.J.; Mahlandt, B.G. Ribavirin prophylaxis and therapy for experimental Argentine hemorrhagic fever. Antimicrob. Agents Chemother. 1988, 32, 1304–1309. [Google Scholar] [CrossRef] [PubMed]
- Leyssen, P.; De Clercq, E.; Neyts, J. Molecular strategies to inhibit the replication of RNA viruses. Antivir. Res. 2008, 78, 9–25. [Google Scholar] [CrossRef] [PubMed]
- Parker, W.B. Metabolism and antiviral activity of ribavirin. Virus Res. 2005, 107, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Cameron, C.E.; Castro, C. The mechanism of action of ribavirin: Lethal mutagenesis of RNA virus genomes mediated by the viral RNA-dependent RNA polymerase. Curr. Opin. Infect. Dis. 2001, 14, 757–764. [Google Scholar] [CrossRef] [PubMed]
- Crotty, S.; Maag, D.; Arnold, J.J.; Zhong, W.; Lau, J.Y.N.; Hong, Z.; Andino, R.; Cameron, C.E. The broad-spectrum antiviral ribonucleotide, ribavirin, is an RNA virus mutagen. Nat. Med. 2000, 6, 1375–1379. [Google Scholar] [PubMed]
- Ruiz-Jarabo, C.M.; Ly, C.; Domingo, E.; de la Torre, J.C. Lethal mutagenesis of the prototypic arenavirus lymphocytic choriomeningitis virus (LCMV). Virology 2003, 308, 37–47. [Google Scholar] [CrossRef]
- Hoffmann, H.H.; Kunz, A.; Simon, V.A.; Palese, P.; Shaw, M.L. Broad-spectrum antiviral that interferes with de novo pyrimidine biosynthesis. Proc. Natl. Acad. Sci. USA 2011, 108, 5777–5782. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Riano, E.; Ngo, N.; Devito, S.; Eggink, D.; Munger, J.; Shaw, M.L.; de la Torre, J.C.; Martinez-Sobrido, L. Inhibition of arenavirus by A3, a pyrimidine biosynthesis inhibitor. J. Virol. 2014, 88, 878–889. [Google Scholar] [CrossRef] [PubMed]
- Gowen, B.B.; Juelich, T.L.; Sefing, E.J.; Brasel, T.; Smith, J.K.; Zhang, L.; Tigabu, B.; Hill, T.E.; Yun, T.; Pietzsch, C.; et al. Favipiravir (T-705) inhibits Junin virus infection and reduces mortality in a guinea pig model of Argentine hemorrhagic fever. PLoS Negl. Trop. Dis. 2013, 7, e2614. [Google Scholar] [CrossRef] [PubMed]
- Mendenhall, M.; Russell, A.; Juelich, T.; Messina, E.L.; Smee, D.F.; Freiberg, A.N.; Holbrook, M.R.; Furuta, Y.; de la Torre, J.C.; Nunberg, J.H.; et al. T-705 (favipiravir) inhibition of arenavirus replication in cell culture. Antimicrob. Agents Chemother. 2011, 55, 782–787. [Google Scholar] [CrossRef] [PubMed]
- Bolken, T.C.; Laquerre, S.; Zhang, Y.; Bailey, T.R.; Pevear, D.C.; Kickner, S.S.; Sperzel, L.E.; Jones, K.F.; Warren, T.K.; Amanda Lund, S.; et al. Identification and characterization of potent small molecule inhibitor of hemorrhagic fever New World arenaviruses. Antivir. Res. 2006, 69, 86–97. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.M.; Rojek, J.M.; Spiropoulou, C.F.; Gundersen, A.T.; Jin, W.; Shaginian, A.; York, J.; Nunberg, J.H.; Boger, D.L.; Oldstone, M.B.; et al. Unique small molecule entry inhibitors of hemorrhagic fever arenaviruses. J. Biol. Chem. 2008, 283, 18734–18742. [Google Scholar] [CrossRef] [PubMed]
- Ngo, N.; Cubitt, B.; Iwasaki, M.; de la Torre, J.C. Identification and mechanism of action of a novel small-molecule inhibitor of arenavirus multiplication. J. Virol. 2015, 89, 10924–10933. [Google Scholar] [CrossRef] [PubMed]
- Loureiro, M.E.; D’Antuono, A.; Levingston Macleod, J.M.; Lopez, N. Uncovering viral protein-protein interactions and their role in arenavirus life cycle. Viruses 2012, 4, 1651–1667. [Google Scholar] [CrossRef] [PubMed]
- De la Torre, J.C. Reverse genetics approaches to combat pathogenic arenaviruses. Antivir. Res. 2008, 80, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Emonet, S.E.; Urata, S.; de la Torre, J.C. Arenavirus reverse genetics: New approaches for the investigation of arenavirus biology and development of antiviral strategies. Virology 2011, 411, 416–425. [Google Scholar] [CrossRef] [PubMed]
- Cheng, B.Y.; Ortiz-Riano, E.; de la Torre, J.C.; Martinez-Sobrido, L. Arenavirus genome rearrangement for the development of live-attenuated vaccines. J. Virol. 2015. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Riano, E.; Cheng, B.Y.; de la Torre, J.C.; Martinez-Sobrido, L. D471G mutation in LCMV-NP affects its ability to self-associate and results in a dominant negative effect in viral RNA synthesis. Viruses 2012, 4, 2137–2161. [Google Scholar] [CrossRef] [PubMed]
- Russier, M.; Reynard, S.; Carnec, X.; Baize, S. The exonuclease domain of Lassa virus nucleoprotein is involved in antigen-presenting-cell-mediated NK cell responses. J. Virol. 2014, 88, 13811–13820. [Google Scholar] [CrossRef] [PubMed]
- Reynard, S.; Russier, M.; Fizet, A.; Carnec, X.; Baize, S. Exonuclease domain of the Lassa virus nucleoprotein is critical to avoid RIG-I signaling and to inhibit the innate immune response. J. Virol. 2014, 88, 13923–13927. [Google Scholar] [CrossRef] [PubMed]
- Seregin, A.V.; Yun, N.E.; Miller, M.; Aronson, J.; Smith, J.K.; Walker, A.G.; Smith, J.N.; Huang, C.; Manning, J.T.; de la Torre, J.C.; et al. The glycoprotein precursor gene of Junin virus determines the virulence of Romero strain and attenuation of Candid#1 strain in a representative animal model of Argentine hemorrhagic fever. J. Virol. 2015, 89, 5949–5956. [Google Scholar] [PubMed]
- Ortiz-Riano, E.; Cheng, B.Y.; Carlos de la Torre, J.; Martinez-Sobrido, L. Arenavirus reverse genetics for vaccine development. J. Gen. Virol. 2013, 94, 1175–1188. [Google Scholar] [CrossRef] [PubMed]
- Cheng, B.Y.; Ortiz-Riano, E.; de la Torre, J.C.; Martinez-Sobrido, L. Generation of recombinant arenavirus for vaccine development in FDA-approved Vero cells. J. Vis. Exp. 2013. [Google Scholar] [CrossRef] [PubMed]
- Cheng, B.Y.; Ortiz-Riano, E.; Nogales, A.; de la Torre, J.C.; Martinez-Sobrido, L. Development of live-attenuated arenavirus vaccines based on codon deoptimization. J. Virol. 2015. [Google Scholar] [CrossRef] [PubMed]
- Emonet, S.F.; Garidou, L.; McGavern, D.B.; de la Torre, J.C. Generation of recombinant lymphocytic choriomeningitis viruses with trisegmented genomes stably expressing two additional genes of interest. Proc. Natl. Acad. Sci. USA 2009, 106, 3473–3478. [Google Scholar] [CrossRef] [PubMed]
- Dhanwani, R.; Zhou, Y.; Huang, Q.; Verma, V.; Dileepan, M.; Ly, H.; Liang, Y. A novel live pichinde virus-based vaccine vector induces enhanced humoral and cellular immunity after a booster dose. J. Virol. 2015, 90, 2551–2560. [Google Scholar] [CrossRef] [PubMed]
- Popkin, D.L.; Teijaro, J.R.; Lee, A.M.; Lewicki, H.; Emonet, S.; de la Torre, J.C.; Oldstone, M. Expanded potential for recombinant trisegmented lymphocytic choriomeningitis viruses: Protein production, antibody production, and in vivo assessment of biological function of genes of interest. J. Virol. 2011, 85, 7928–7932. [Google Scholar] [CrossRef] [PubMed]
- Rodrigo, W.W.; de la Torre, J.C.; Martinez-Sobrido, L. Use of single-cycle infectious lymphocytic choriomeningitis virus to study hemorrhagic fever arenaviruses. J. Virol. 2011, 85, 1684–1695. [Google Scholar] [CrossRef] [PubMed]
- Flatz, L.; Hegazy, A.N.; Bergthaler, A.; Verschoor, A.; Claus, C.; Fernandez, M.; Gattinoni, L.; Johnson, S.; Kreppel, F.; Kochanek, S.; et al. Development of replication-defective lymphocytic choriomeningitis virus vectors for the induction of potent CD8+ T cell immunity. Nat. Med. 2010, 16, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Nisii, C.; Castilletti, C.; Raoul, H.; Hewson, R.; Brown, D.; Gopal, R.; Eickmann, M.; Gunther, S.; Mirazimi, A.; Koivula, T.; et al. Biosafety level-4 laboratories in europe: Opportunities for public health, diagnostics, and research. PLoS Pathog. 2013, 9, e1003105. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, A.B.; de la Torre, J.C. Rescue of the prototypic arenavirus LCMV entirely from plasmid. Virology 2006, 350, 370–380. [Google Scholar] [CrossRef] [PubMed]
- Flatz, L.; Bergthaler, A.; de la Torre, J.C.; Pinschewer, D.D. Recovery of an arenavirus entirely from RNA polymerase I/II-driven cDNA. Proc. Natl. Acad. Sci. USA 2006, 103, 4663–4668. [Google Scholar] [CrossRef] [PubMed]
- Hass, M.; Golnitz, U.; Muller, S.; Becker-Ziaja, B.; Gunther, S. Replicon system for Lassa virus. J. Virol. 2004, 78, 13793–13803. [Google Scholar] [CrossRef] [PubMed]
- Bergeron, E.; Chakrabarti, A.K.; Bird, B.H.; Dodd, K.A.; McMullan, L.K.; Spiropoulou, C.F.; Nichol, S.T.; Albarino, C.G. Reverse genetics recovery of Lujo virus and role of virus RNA secondary structures in efficient virus growth. J. Virol. 2012, 86, 10759–10765. [Google Scholar] [CrossRef] [PubMed]
- Lan, S.; McLay Schelde, L.; Wang, J.; Kumar, N.; Ly, H.; Liang, Y. Development of infectious clones for virulent and avirulent pichinde viruses: A model virus to study arenavirus-induced hemorrhagic fevers. J. Virol. 2009, 83, 6357–6362. [Google Scholar] [CrossRef] [PubMed]
- Patterson, M.; Seregin, A.; Huang, C.; Kolokoltsova, O.; Smith, J.; Miller, M.; Smith, J.; Yun, N.; Poussard, A.; Grant, A.; et al. Rescue of a recombinant Machupo virus from cloned cDNAs and in vivo characterization in interferon (alphabeta/gamma) receptor double knockout mice. J. Virol. 2014, 88, 1914–1923. [Google Scholar] [CrossRef] [PubMed]
- Flick, R.; Hobom, G. Transient bicistronic vRNA segments for indirect selection of recombinant influenza viruses. Virology 1999, 262, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Vieira Machado, A.; Naffakh, N.; Gerbaud, S.; van der Werf, S.; Escriou, N. Recombinant influenza a viruses harboring optimized dicistronic NA segment with an extended native 5′ terminal sequence: Induction of heterospecific B and T cell responses in mice. Virology 2006, 345, 73–87. [Google Scholar] [CrossRef] [PubMed]
- Marschalek, A.; Finke, S.; Schwemmle, M.; Mayer, D.; Heimrich, B.; Stitz, L.; Conzelmann, K.K. Attenuation of rabies virus replication and virulence by picornavirus internal ribosome entry site elements. J. Virol. 2009, 83, 1911–1919. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Sastre, A.; Muster, T.; Barclay, W.S.; Percy, N.; Palese, P. Use of a mammalian internal ribosomal entry site element for expression of a foreign protein by a transfectant influenza virus. J. Virol. 1994, 68, 6254–6261. [Google Scholar] [PubMed]
- Goto, H.; Muramoto, Y.; Noda, T.; Kawaoka, Y. The genome-packaging signal of the influenza a virus genome comprises a genome incorporation signal and a genome-bundling signal. J. Virol. 2013, 87, 11316–11322. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Hong, Y.; Parslow, T.G. Cis-acting packaging signals in the influenza virus PB1, PB2, and PA genomic RNA segments. J. Virol. 2005, 79, 10348–10355. [Google Scholar] [CrossRef] [PubMed]
- Meyer, B.J.; de la Torre, J.C.; Southern, P.J. Arenaviruses: Genomic RNAs, transcription, and replication. Curr. Top. Microbiol. Immunol. 2002, 262, 139–157. [Google Scholar] [PubMed]
- Buchmeier, M.J. Arenaviruses: Protein structure and function. Curr. Top. Microbiol. Immunol. 2002, 262, 159–173. [Google Scholar] [PubMed]
- Young, P.R.; Howard, C.R. Fine structure analysis of pichinde virus nucleocapsids. J. Gen. Virol. 1983, 64, 833–842. [Google Scholar] [CrossRef] [PubMed]
- Lavanya, M.; Cuevas, C.D.; Thomas, M.; Cherry, S.; Ross, S.R. SiRNA screen for genes that affect Junin virus entry uncovers voltage-gated calcium channels as a therapeutic target. Sci. Transl. Med. 2013, 5, 204ra131. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, M.; Urata, S.; Cho, Y.; Ngo, N.; de la Torre, J.C. Cell entry of lymphocytic choriomeningitis virus is restricted in myotubes. Virology 2014, 458–459, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Nogales, A.; Baker, S.F.; Martinez-Sobrido, L. Replication-competent influenza a viruses expressing a red fluorescent protein. Virology 2015, 476, 206–216. [Google Scholar] [CrossRef] [PubMed]
- Breen, M.; Nogales, A.; Baker, S.F.; Perez, D.R.; Martinez-Sobrido, L. Replication-competent influenza A and B viruses expressing a fluorescent dynamic timer protein for in vitro and in vivo studies. PLoS ONE 2016, 11, e0147723. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).