Distinct Morphology of Human T-Cell Leukemia Virus Type 1-Like Particles
Abstract
:1. Introduction
2. Materials and Methods
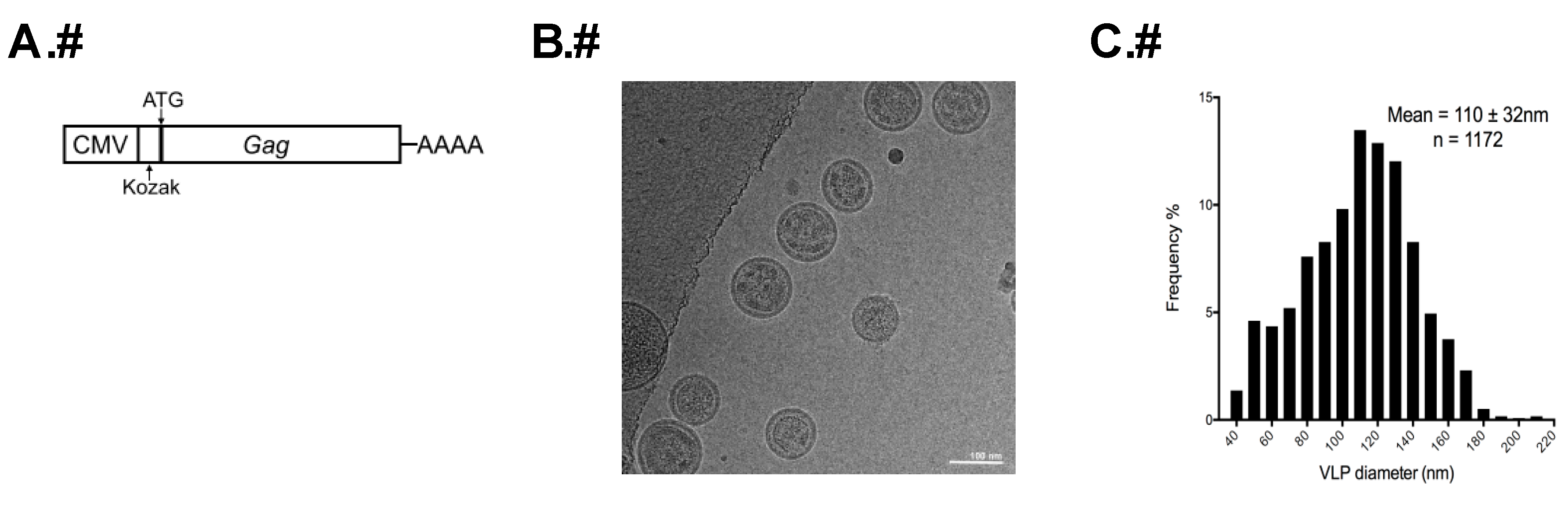
2.1. Transfection and HTLV-1-Like Particle Production
2.2. Gradient Purification of Authentic Virus Particles and VLPs
2.3. Cryo-TEM of HTLV-1-Like Particles and Authentic Virus Particles
2.4. Determination of Particle Size
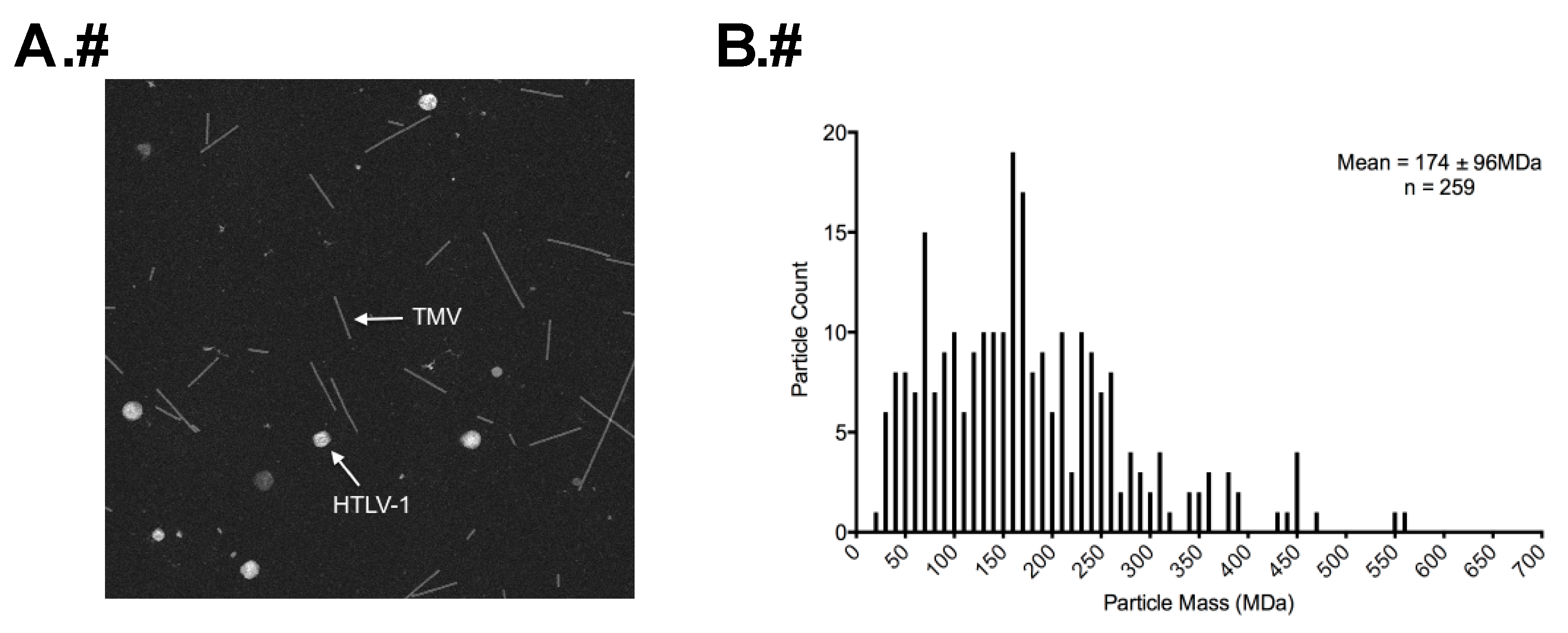
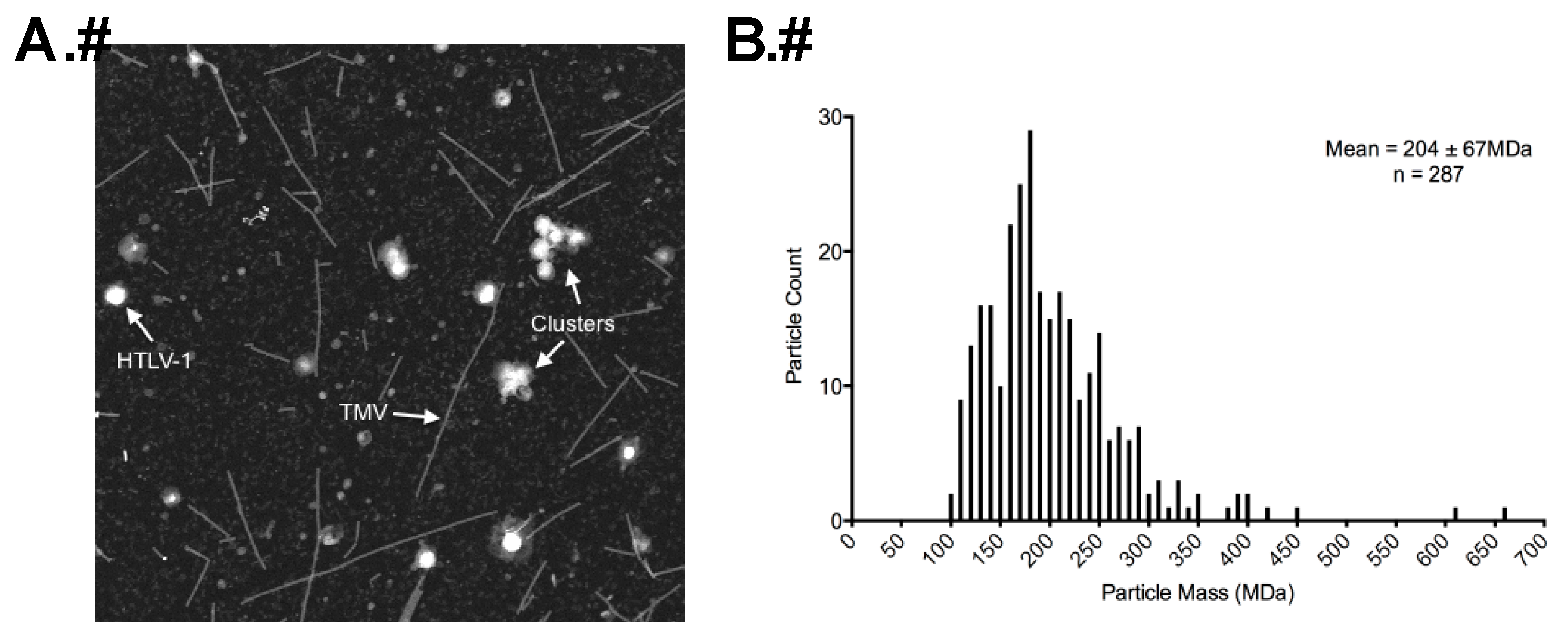
2.5. Determination of Particle Mass by STEM
3. Results
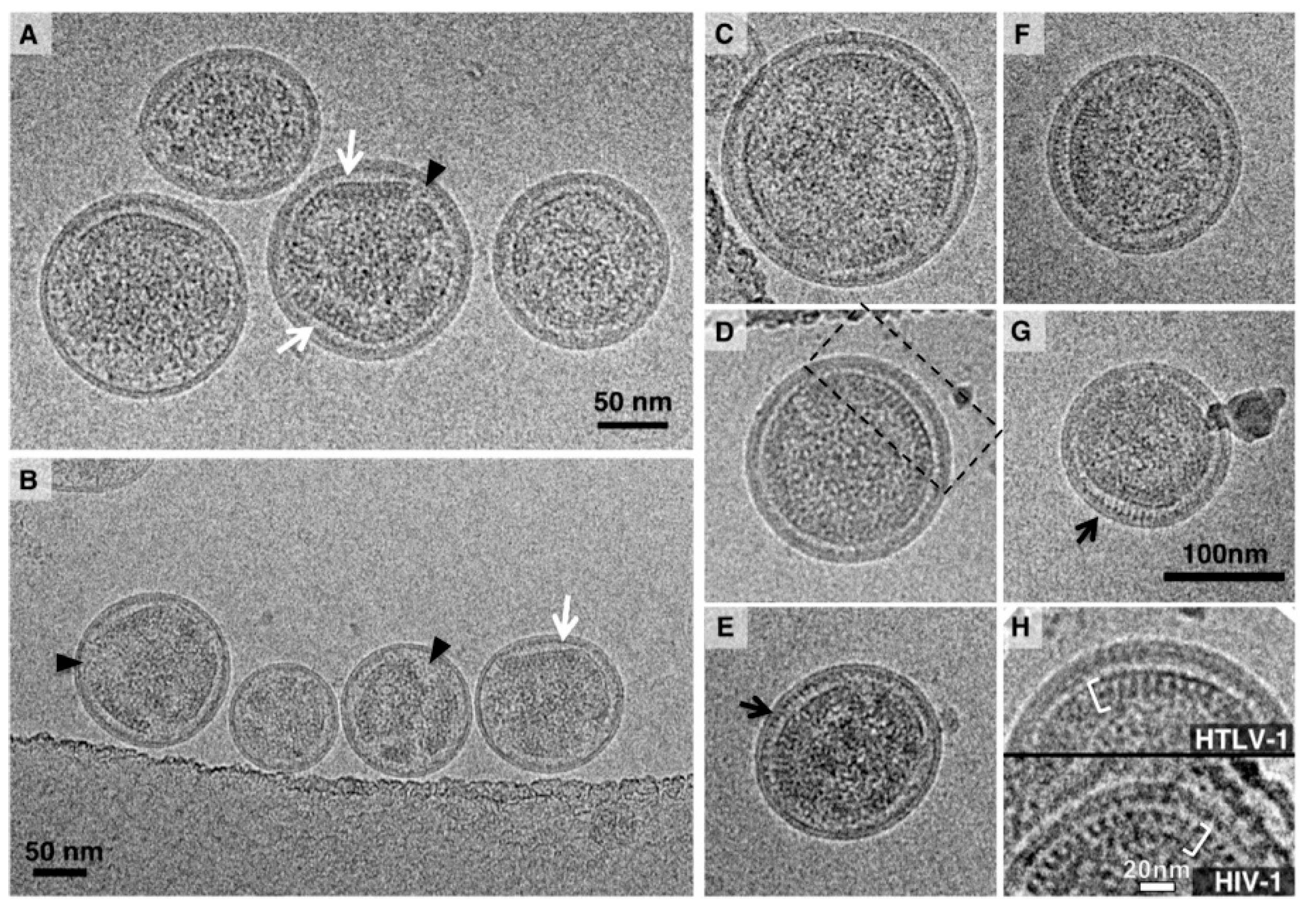
3.1. Analysis of the Morphology of HTLV-1-Like Particles
3.2. Morphology of Authentic HTLV-1 Mature Particles Produced from MT-2 Cells
3.3. STEM Analyses of HTLV-1-Like Particles and Authentic Mature HTLV-1 Particles
3.4. Calculation of Gag Stoichiometry in HTLV-1-Like Particles
3.5. Estimating Gag Stoichiometry in Authentic Immature HTLV-1 Particles by Calculating Gag Copy Number in Authentic Mature HTLV-1 Particles
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| AT-2 | 2,2′-dithiodipyridine |
| BNL | Brookhaven National Laboratory |
| Cryo-TEM | cryogenic transmission electron microscopy |
| HIV-1 | human immunodeficiency virus type 1 |
| HTLV-1 | human T-cell leukemia virus type 1 |
| MPMV | Mason-Pfizer monkey virus |
| RSV | sarcoma virus |
| STEM | scanning transmission electron microscopy |
| TMV | tobacco mosaic virus |
| VLP | virus-like particle |
References
- De The, G.; Bomford, R. An HTLV-1 vaccine: Why, how, for whom? AIDS Res. Hum. Retrovir. 1993, 9, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Gessain, A.; Cassar, O. Epidemiological aspects and world distribution of HTLV-1 infection. Front. Microbiol. 2012, 3, 388. [Google Scholar] [CrossRef] [PubMed]
- Gessain, A.; Barin, F.; Vernant, J.C.; Gout, O.; Maurs, L.; Calender, A.; de The, G. Antibodies to human T-lymphotropic virus type-1 in patients with tropical spastic paraparesis. Lancet 1985, 2, 407–410. [Google Scholar] [CrossRef]
- Osame, M.; Usuku, K.; Izumo, S.; Ijichi, N.; Amitani, H.; Igata, A.; Matsumoto, M.; Tara, M. HTLV-1 associated myelopathy, a new clinical entity. Lancet 1986, 1, 1031–1032. [Google Scholar] [CrossRef]
- Maldonado, J.; Martin, J.; Mueller, J.; Zhang, W.; Mansky, L. New insights into retroviral Gag-Gag and Gag-membrane interactions. Front. Microbiol. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Fogarty, K.H.; Chen, Y.; Grigsby, I.F.; Macdonald, P.J.; Smith, E.M.; Johnson, J.L.; Rawson, J.M.; Mansky, L.M.; Mueller, J.D. Characterization of cytoplasmic Gag-Gag interactions by dual-color z-scan fluorescence fluctuation spectroscopy. Biophys. J. 2011, 100, 1587–1595. [Google Scholar] [CrossRef] [PubMed]
- Jouvenet, N.; Simon, S.M.; Bieniasz, P.D. Imaging the interaction of HIV-1 genomes and Gag during assembly of individual viral particles. Proc. Natl. Acad. Sci. USA 2009, 106, 19114–19119. [Google Scholar] [CrossRef] [PubMed]
- Kingston, R.L.; Olson, N.H.; Vogt, V.M. The organization of mature Rous sarcoma virus as studied by cryoelectron microscopy. J. Struct. Biol. 2001, 136, 67–80. [Google Scholar] [CrossRef] [PubMed]
- Briggs, J.A.; Johnson, M.C.; Simon, M.N.; Fuller, S.D.; Vogt, V.M. Cryo-electron microscopy reveals conserved and divergent features of Gag packing in immature particles of Rous sarcoma virus and human immunodeficiency virus. J. Mol. Biol. 2006, 355, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Briggs, J.A.; Simon, M.N.; Gross, I.; Krausslich, H.G.; Fuller, S.D.; Vogt, V.M.; Johnson, M.C. The stoichiometry of Gag protein in HIV-1. Nat. Struct. Mol. Biol. 2004, 11, 672–675. [Google Scholar] [CrossRef] [PubMed]
- Briggs, J.A.; Watson, B.E.; Gowen, B.E.; Fuller, S.D. Cryoelectron microscopy of mouse mammary tumor virus. J. Virol. 2004, 78, 2606–2608. [Google Scholar] [CrossRef] [PubMed]
- Yeager, M.; Wilson-Kubalek, E.M.; Weiner, S.G.; Brown, P.O.; Rein, A. Supramolecular organization of immature and mature murine leukemia virus revealed by electron cryo-microscopy: Implications for retroviral assembly mechanisms. Proc. Natl. Acad. Sci. USA 1998, 95, 7299–7304. [Google Scholar] [CrossRef] [PubMed]
- Fuller, S.D.; Wilk, T.; Gowen, B.E.; Krausslich, H.G.; Vogt, V.M. Cryo-electron microscopy reveals ordered domains in the immature HIV-1 particle. Curr. Biol. 1997, 7, 729–738. [Google Scholar] [CrossRef]
- Butan, C.; Winkler, D.C.; Heymann, J.B.; Craven, R.C.; Steven, A.C. RSV capsid polymorphism correlates with polymerization efficiency and envelope glycoprotein content: Implications that nucleation controls morphogenesis. J. Mol. Biol. 2008, 376, 1168–1181. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Joshi, S.M.; Ma, Y.M.; Kingston, R.L.; Simon, M.N.; Vogt, V.M. Characterization of Rous sarcoma virus Gag particles assembled in vitro. J. Virol. 2001, 75, 2753–2764. [Google Scholar] [CrossRef] [PubMed]
- Carlson, L.A.; Briggs, J.A.; Glass, B.; Riches, J.D.; Simon, M.N.; Johnson, M.C.; Muller, B.; Grunewald, K.; Krausslich, H.G. Three-dimensional analysis of budding sites and released virus suggests a revised model for HIV-1 morphogenesis. Cell Host Microbe 2008, 4, 592–599. [Google Scholar] [CrossRef] [PubMed]
- Vogt, V.M.; Simon, M.N. Mass determination of Rous sarcoma virus virions by scanning transmission electron microscopy. J. Virol. 1999, 73, 7050–7055. [Google Scholar] [PubMed]
- Parker, S.D.; Wall, J.S.; Hunter, E. Analysis of Mason-Pfizer monkey virus Gag particles by scanning transmission electron microscopy. J. Virol. 2001, 75, 9543–9548. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wu, B.; Musier-Forsyth, K.; Mansky, L.M.; Mueller, J.D. Fluorescence fluctuation spectroscopy on viral-like particles reveals variable gag stoichiometry. Biophys. J. 2009, 96, 1961–1969. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Cao, S.; Martin, J.L.; Mueller, J.D.; Mansky, L.M. Morphology and ultrastructure of retrovirus particles. AIMS Biophys. 2015, 2, 343–369. [Google Scholar] [CrossRef] [PubMed]
- Grigsby, I.F.; Zhang, W.; Johnson, J.L.; Fogarty, K.H.; Chen, Y.; Rawson, J.M.; Crosby, A.J.; Mueller, J.D.; Mansky, L.M. Biophysical analysis of HTLV-1 particles reveals novel insights into particle morphology and Gag stochiometry. Retrovirology 2010, 7. [Google Scholar] [CrossRef] [PubMed]
- Haertle, T.; Carrera, C.J.; Wasson, D.B.; Sowers, L.C.; Richman, D.D.; Carson, D.A. Metabolism and anti-human immunodeficiency virus-1 activity of 2-halo-2′, 3′-dideoxyadenosine derivatives. J. Biol. Chem. 1988, 263, 5870–5875. [Google Scholar] [PubMed]
- Harada, S.; Koyanagi, Y.; Yamamoto, N. Infection of HTLV-III/LAV in HTLV-I-carrying cells MT-2 and MT-4 and application in a plaque assay. Science 1985, 229, 563–566. [Google Scholar] [CrossRef] [PubMed]
- Rossio, J.L.; Esser, M.T.; Suryanarayana, K.; Schneider, D.K.; Bess, J.W., Jr.; Vasquez, G.M.; Wiltrout, T.A.; Chertova, E.; Grimes, M.K.; Sattentau, Q.; et al. Inactivation of human immunodeficiency virus type 1 infectivity with preservation of conformational and functional integrity of virion surface proteins. J. Virol. 1998, 72, 7992–8001. [Google Scholar] [PubMed]
- Baker, T.S.; Olson, N.H.; Fuller, S.D. Adding the third dimension to virus life cycles: Three-dimensional reconstruction of icosahedral viruses from cryo-electron micrographs. Microbiol. Mol. Biol. Rev. 1999, 63, 862–922. [Google Scholar] [CrossRef] [PubMed]
- Wall, J.S.; Hainfeld, J.F.; Simon, M.N. Scanning transmission electron microscopy of nuclear structures. Methods Cell Biol. 1998, 53, 139–164. [Google Scholar] [PubMed]
- Namba, K.; Stubbs, G. Structure of tobacco mosaic virus at 3.6 Å resolution: Implications for assembly. Science 1986, 231, 1401–1406. [Google Scholar] [CrossRef] [PubMed]
- Bharat, T.A.; Davey, N.E.; Ulbrich, P.; Riches, J.D.; de Marco, A.; Rumlova, M.; Sachse, C.; Ruml, T.; Briggs, J.A. Structure of the immature retroviral capsid at 8 Å resolution by cryo-electron microscopy. Nature 2012, 487, 385–389. [Google Scholar] [CrossRef] [PubMed]
- de Marco, A.; Davey, N.E.; Ulbrich, P.; Phillips, J.M.; Lux, V.; Riches, J.D.; Fuzik, T.; Ruml, T.; Krausslich, H.G.; Vogt, V.M.; et al. Conserved and variable features of Gag structure and arrangement in immature retrovirus particles. J. Virol. 2010, 84, 11729–11736. [Google Scholar] [CrossRef] [PubMed]
- Frank, G.A.; Narayan, K.; Bess, J.W., Jr.; Del Prete, G.Q.; Wu, X.; Moran, A.; Hartnell, L.M.; Earl, L.A.; Lifson, J.D.; Subramaniam, S. Maturation of the HIV-1 core by a non-diffusional phase transition. Nat. Commun. 2015, 6, 5854. [Google Scholar] [CrossRef] [PubMed]
- Briggs, J.A.; Riches, J.D.; Glass, B.; Bartonova, V.; Zanetti, G.; Krausslich, H.G. Structure and assembly of immature HIV. Proc. Natl. Acad. Sci. USA 2009, 106, 11090–11095. [Google Scholar] [CrossRef] [PubMed]
- Wright, E.R.; Schooler, J.B.; Ding, H.J.; Kieffer, C.; Fillmore, C.; Sundquist, W.I.; Jensen, G.J. Electron cryotomography of immature HIV-1 virions reveals the structure of the CA and SP1 Gag shells. EMBO J. 2007, 26, 2218–2226. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Maldonado, J.O.; Grigsby, I.F.; Mansky, L.M.; Zhang, W. Analysis of human T-cell leukemia virus type 1 particles by using cryo-electron tomography. J. Virol. 2015, 89, 2430–2435. [Google Scholar] [CrossRef] [PubMed]
- Cockburn, J.J.; Abrescia, N.G.; Grimes, J.M.; Sutton, G.C.; Diprose, J.M.; Benevides, J.M.; Thomas, G.J., Jr.; Bamford, J.K.; Bamford, D.H.; Stuart, D.I. Membrane structure and interactions with protein and DNA in bacteriophage PRD1. Nature 2004, 432, 122–125. [Google Scholar] [CrossRef] [PubMed]
- Fleissner, E. Chromatographic separation and antigenic analysis of proteins of the oncornaviruses. I. Avian leukemia-sarcoma viruses. J. Virol. 1971, 8, 778–785. [Google Scholar] [PubMed]
- Nam, S.H.; Kidokoro, M.; Shida, H.; Hatanaka, M. Processing of Gag precursor polyprotein of human T-cell leukemia virus type I by virus-encoded protease. J. Virol. 1988, 62, 3718–3728. [Google Scholar] [PubMed]
- Nam, S.H.; Copeland, T.D.; Hatanaka, M.; Oroszlan, S. Characterization of ribosomal frameshifting for expression of pol gene products of human T-cell leukemia virus type I. J. Virol. 1993, 67, 196–203. [Google Scholar] [PubMed]
- Mador, N.; Panet, A.; Honigman, A. Translation of gag, pro, and pol gene products of human T-cell leukemia virus type 2. J. Virol. 1989, 63, 2400–2404. [Google Scholar] [PubMed]
- Poiesz, B.J.; Ruscetti, F.W.; Gazdar, A.F.; Bunn, P.A.; Minna, J.D.; Gallo, R.C. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc. Natl. Acad. Sci. USA 1980, 77, 7415–7419. [Google Scholar] [CrossRef] [PubMed]
- Poiesz, B.J.; Ruscetti, F.W.; Reitz, M.S.; Kalyanaraman, V.S.; Gallo, R.C. Isolation of a new type C retrovirus (HTLV) in primary uncultured cells of a patient with sezary T-cell leukaemia. Nature 1981, 294, 268–271. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.S.; Rich, D.H.; Ikeda, R.A. Substrates and inhibitors of human T-cell leukemia virus type I protease. Biochemistry 1998, 37, 17514–17518. [Google Scholar] [CrossRef] [PubMed]
- Pettit, S.C.; Sanchez, R.; Smith, T.; Wehbie, R.; Derse, D.; Swanstrom, R. HIV type 1 protease inhibitors fail to inhibit HTLV-I Gag processing in infected cells. AIDS Res. Hum. Retrovir. 1998, 14, 1007–1014. [Google Scholar] [CrossRef] [PubMed]
- Louis, J.M.; Oroszlan, S.; Tozser, J. Stabilization from autoproteolysis and kinetic characterization of the human T-cell leukemia virus type 1 proteinase. J. Biol. Chem. 1999, 274, 6660–6666. [Google Scholar] [CrossRef] [PubMed]
- Tozser, J.; Weber, I.T. The protease of human T-cell leukemia virus type-1 is a potential therapeutic target. Curr. Pharm. Des. 2007, 13, 1285–1294. [Google Scholar] [CrossRef] [PubMed]
- Schur, F.K.; Hagen, W.J.; Rumlova, M.; Ruml, T.; Muller, B.; Krausslich, H.G.; Briggs, J.A. Structure of the immature HIV-1 capsid in intact virus particles at 8.8 Å resolution. Nature 2015, 517, 505–508. [Google Scholar] [CrossRef] [PubMed]

| HTLV-1 Particle Sample | |||
|---|---|---|---|
| Measurement | Virus-Like Particle | Authentic Particle | |
| Average Diameter (nm) a | 110 | 113 | |
| Average Particle Mass (MDa) b | 174 | 204 | |
| Mass of RNA, Lipid and Protein (MDa) | RNA c | 7 | 7 |
| Lipid d | 70 | 80 | |
| Total protein e | 97 | 118 | |
| Mass of Gag Molecules (MDa) | Total Gag polyprotein f | 70–87 | 82–106 |
| Gag | 70–87 | 70–90 | |
| Gag-Pro | N/A | 10–13 | |
| Gag-Pro-Pol | N/A | 2.5–3 | |
| Gag polyprotein copy number g | 1300–1600 | 1500–1900 | |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maldonado, J.O.; Cao, S.; Zhang, W.; Mansky, L.M. Distinct Morphology of Human T-Cell Leukemia Virus Type 1-Like Particles. Viruses 2016, 8, 132. https://doi.org/10.3390/v8050132
Maldonado JO, Cao S, Zhang W, Mansky LM. Distinct Morphology of Human T-Cell Leukemia Virus Type 1-Like Particles. Viruses. 2016; 8(5):132. https://doi.org/10.3390/v8050132
Chicago/Turabian StyleMaldonado, José O., Sheng Cao, Wei Zhang, and Louis M. Mansky. 2016. "Distinct Morphology of Human T-Cell Leukemia Virus Type 1-Like Particles" Viruses 8, no. 5: 132. https://doi.org/10.3390/v8050132
APA StyleMaldonado, J. O., Cao, S., Zhang, W., & Mansky, L. M. (2016). Distinct Morphology of Human T-Cell Leukemia Virus Type 1-Like Particles. Viruses, 8(5), 132. https://doi.org/10.3390/v8050132








