Zoonotic Hepatitis E Virus: Classification, Animal Reservoirs and Transmission Routes
Abstract
:1. Introduction
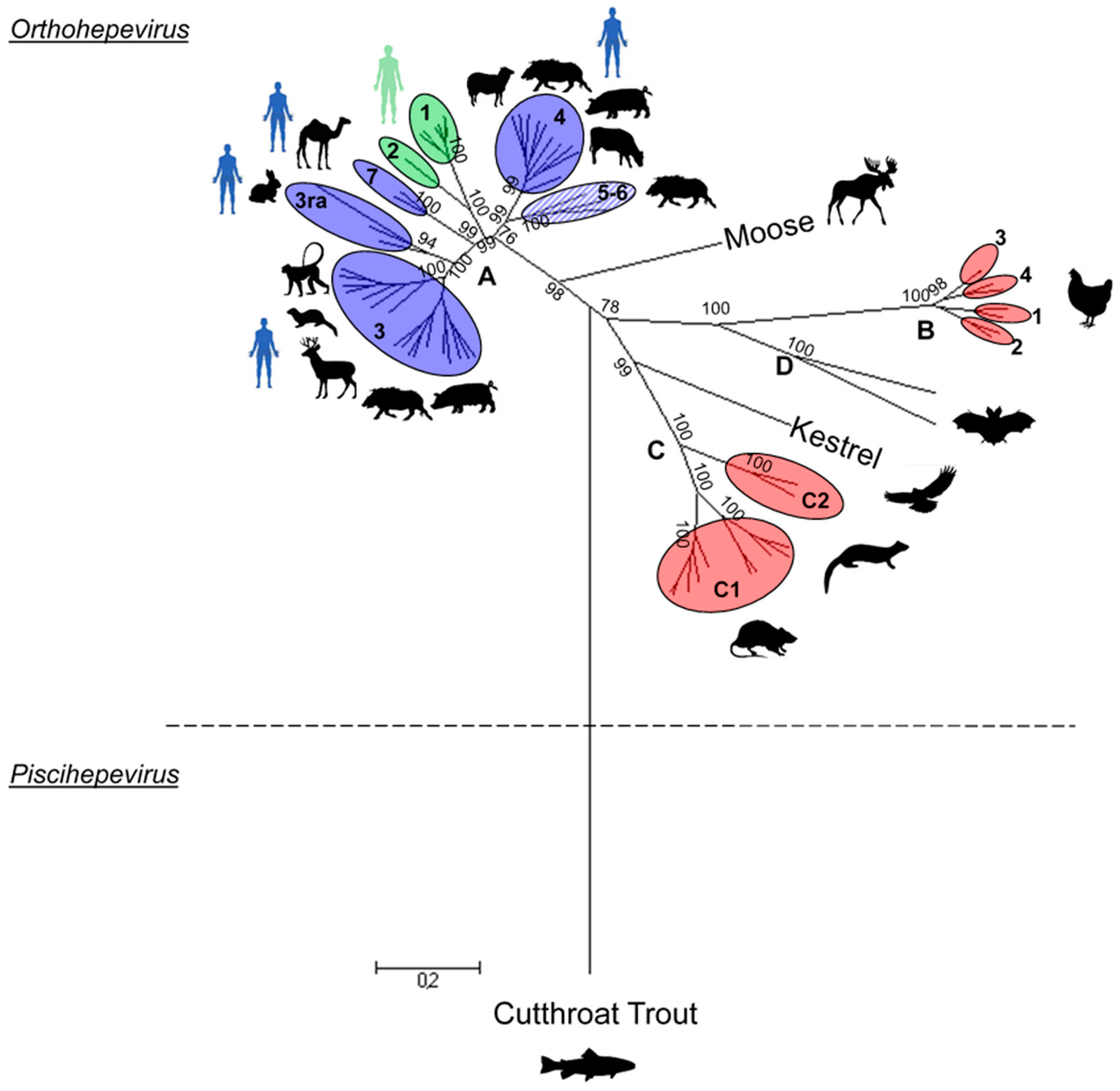
2. HEV Phylogeny
2.1. Piscihepevirus Genus
2.2. Orthohepevirus Genus
2.2.1. Orthohepevirus A
Genotypes 1 and 2
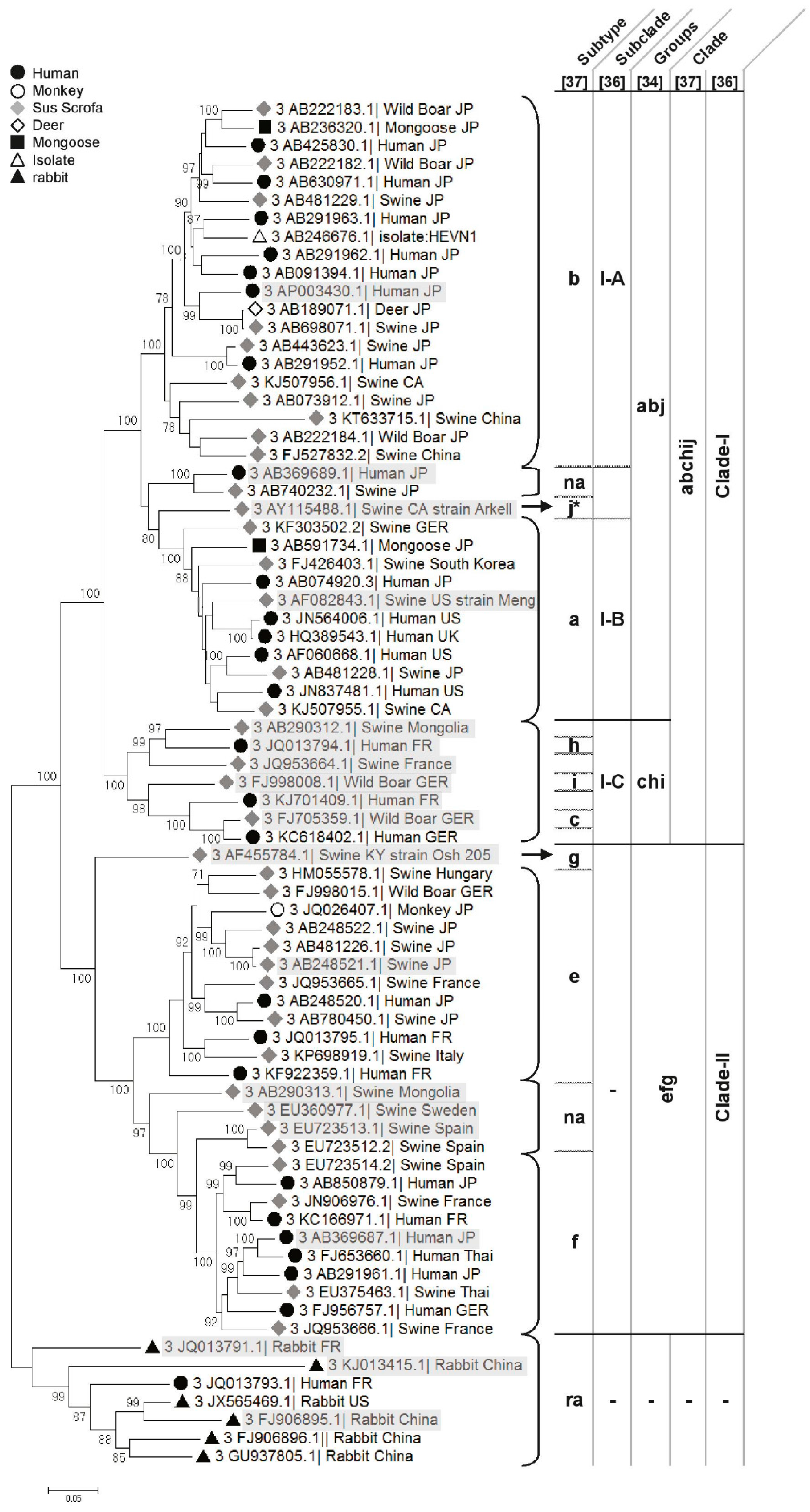
Genotype 3
- Clade 3abchij: Within this first clade, HEV strains can be separated into two subclades, 3abj and 3chi, sharing 81.16% to 85.33% nt identity (Figure 2) [35]. HEV strains that cluster in the 3chi subclade share 84.7% to 96.46% nt identity and originate from Europe (France and Germany) and Mongolia. HEV strains within the 3abj subclade share more than 83.75% nt identity. They were isolated in Asia, Europe and North America and are predominantly circulating in Asia and North America. Complete genomes of subtype 3a are from North America and evolutionary studies suggest that it came from Asia and diverged from subtype 3b to subtype 3a in the early 1920s [37,40]. In the subtype 3b, nearly 90% of the full genomes are from Japan, sharing more than 95% nt identity [35,37] (Figure 2). Studies on the origin of HEV in Japan suggest that HEV-3 was imported from Europe in the early 20th century and then diverged into the 3b subtype [37,40]. The subtype 3j was isolated from a pool of pig faecal samples in North America [48], thus, full genome sequences from single animals must be added to validate this subtype [35].
- Clade 3efg: This clade includes the three subtypes e, f and g, sharing 82.75%–90.57% nt identity, and 3 non-assigned subtypes. Subtypes 3e and 3f are mainly found in Asia and Europe [49,50]. Evolutionary studies have hypothesised that these HEV strains have emerged in Europe around 1871 [37]. There is only one complete sequence for the subtype 3g from Kirgizstan, which is the most divergent virus of this clade (Figure 2). The classification of the subtype 3d is based on one partial ORF2 sequence from Taiwan, it shares 86.18% and 84.87% nt identity with the subtypes 3g and 3h, respectively. Subtype 3d does not belong to any clade so far.
- Subtype 3ra (rabbit): These strains share 73% to 80% nt identity with other HEV-3 subtypes and form a distinct clade within genotype 3 (Figure 2). This divergence is mainly due to numerous substitutions and insertions in the rabbit HEV genome compared to the other Orthohepevirus A HEV strains [8,51]. As rabbit strains better cluster with other genotype 3 strains, they are provisionally assigned as subtype 3ra [8,38] and divided into 2 subclades [38]. This subtype includes a strain isolated from a human case of hepatitis E in France that shares 80.12% to 86.14% nt identity with the other rabbit strains [51].
Genotype 4
Genotypes 5 and 6
Genotype 7
2.2.2. Orthohepevirus B
2.2.3. Orthohepevirus C
2.2.4. Orthohepevirus D
2.2.5. Unassigned Orthohepeviruses
Swedish Moose
Kestrel (Falconidae)
3. Animal models of HEV
3.1. Non-Human Primates (Historical Model)
3.2. Swine
3.3. Chickens
3.4. Rabbits
3.5. Rats
3.6. Ferrets
3.7. Mongolian Gerbils
3.8. Human Liver Chimeric Mice
4. Inter-Species Transmission of HEV
5. Transmission Pathways of Zoonotic HEV
6. Conclusions
Acknowledgments
Conflicts of Interest
References
- Kamar, N.; Marion, O.; Abravanel, F.; Izopet, J.; Dalton, H.R. Extrahepatic manifestations of hepatitis E virus. Liver Int. 2016, 36, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Sayed, I.M.; Vercouter, A.-S.; Abdelwahab, S.F.; Vercauteren, K.; Meuleman, P. Is hepatitis E virus an emerging problem in industrialized countries? Hepatology 2015, 62, 1883–1892. [Google Scholar] [CrossRef] [PubMed]
- Murali, A.R.; Kotwal, V.; Chawla, S. Chronic hepatitis E: A brief review. World J. Hepatol. 2015, 7, 2194–2201. [Google Scholar] [CrossRef] [PubMed]
- Khuroo, M.S. Study of an epidemic of non-A, non-B hepatitis. Possibility of another human hepatitis virus distinct from post-transfusion non-A, non-B type. Am. J. Med. 1980, 68, 818–824. [Google Scholar] [CrossRef]
- Balayan, M.S.; Andjaparidze, A.G.; Savinskaya, S.S.; Ketiladze, E.S.; Braginsky, D.M.; Savinov, A.P.; Poleschuk, V.F. Evidence for a virus in non-A, non-B hepatitis transmitted via the fecal-oral route. Intervirology 1983, 20, 23–31. [Google Scholar] [PubMed]
- Reyes, G.R.; Purdy, M.A.; Kim, J.P.; Luk, K.C.; Young, L.M.; Fry, K.E.; Bradley, D.W. Isolation of a cDNA from the virus responsible for enterically transmitted non-A, non-B hepatitis. Science 1990, 247, 1335–1339. [Google Scholar] [CrossRef] [PubMed]
- Tam, A.W.; Smith, M.M.; Guerra, M.E.; Huang, C.C.; Bradley, D.W.; Fry, K.E.; Reyes, G.R. Hepatitis E virus (HEV): Molecular cloning and sequencing of the full-length viral genome. Virology 1991, 185, 120–131. [Google Scholar] [CrossRef]
- Smith, D.B.; Simmonds, P.; Jameel, S.; Emerson, S.U.; Harrison, T.J.; Meng, X.-J.; Okamoto, H.; Van der Poel, W.H.M.; Purdy, M.A.; et al. Consensus proposals for classification of the family Hepeviridae. J. Gen. Virol. 2014, 95, 2223–2232. [Google Scholar] [CrossRef] [PubMed]
- Clayson, E.T.; Innis, B.L.; Myint, K.S.; Narupiti, S.; Vaughn, D.W.; Giri, S.; Ranabhat, P.; Shrestha, M.P. Detection of hepatitis E virus infections among domestic swine in the Kathmandu Valley of Nepal. Am. J. Trop. Med. Hyg. 1995, 53, 228–232. [Google Scholar] [PubMed]
- Meng, X.J.; Purcell, R.H.; Halbur, P.G.; Lehman, J.R.; Webb, D.M.; Tsareva, T.S.; Haynes, J.S.; Thacker, B.J.; Emerson, S.U. A novel virus in swine is closely related to the human hepatitis E virus. Proc. Natl. Acad. Sci. USA 1997, 94, 9860–9865. [Google Scholar] [CrossRef] [PubMed]
- Halbur, P.G.; Kasorndorkbua, C.; Gilbert, C.; Guenette, D.; Potters, M.B.; Purcell, R.H.; Emerson, S.U.; Toth, T.E.; Meng, X.J. Comparative pathogenesis of infection of pigs with hepatitis E viruses recovered from a pig and a human. J. Clin. Microbiol. 2001, 39, 918–923. [Google Scholar] [CrossRef] [PubMed]
- Pavio, N.; Meng, X.-J.; Renou, C. Zoonotic hepatitis E: Animal reservoirs and emerging risks. Vet. Res. 2010, 41, 46. [Google Scholar] [CrossRef] [PubMed]
- Pavio, N.; Meng, X.-J.; Doceul, V. Zoonotic origin of hepatitis E. Curr. Opin. Virol. 2015, 10, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Yugo, D.M.; Meng, X.-J. Hepatitis E virus: Foodborne, waterborne and zoonotic transmission. Int. J. Environ. Res. Public. Health 2013, 10, 4507–4533. [Google Scholar] [CrossRef] [PubMed]
- Li, T.-C.; Saito, M.; Ogura, G.; Ishibashi, O.; Miyamura, T.; Takeda, N. Serologic evidence for hepatitis E virus infection in mongoose. Am. J. Trop. Med. Hyg. 2006, 74, 932–936. [Google Scholar] [PubMed]
- Nakamura, M.; Takahashi, K.; Taira, K.; Taira, M.; Ohno, A.; Sakugawa, H.; Arai, M.; Mishiro, S. Hepatitis E virus infection in wild mongooses of Okinawa, Japan: Demonstration of anti-HEV antibodies and a full-genome nucleotide sequence. Hepatol. Res. 2006, 34, 137–140. [Google Scholar] [CrossRef] [PubMed]
- Nidaira, M.; Takahashi, K.; Ogura, G.; Taira, K.; Okano, S.; Kudaka, J.; Itokazu, K.; Mishiro, S.; Nakamura, M. Detection and phylogenetic analysis of hepatitis E viruses from mongooses in Okinawa, Japan. J. Vet. Med. Sci. 2012, 74, 1665–1668. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Ma, Z.; Harrison, T.J.; Feng, R.; Zhang, C.; Qiao, Z.; Fan, J.; Ma, H.; Li, M.; Song, A.; Wang, Y. A novel genotype of hepatitis E virus prevalent among farmed rabbits in China. J. Med. Virol. 2009, 81, 1371–1379. [Google Scholar] [CrossRef] [PubMed]
- Cossaboom, C.M.; Córdoba, L.; Dryman, B.A.; Meng, X.-J. Hepatitis E virus in rabbits, Virginia, USA. Emerg. Infect. Dis. 2011, 17, 2047–2049. [Google Scholar] [CrossRef] [PubMed]
- Lhomme, S.; Dubois, M.; Abravanel, F.; Top, S.; Bertagnoli, S.; Guerin, J.-L.; Izopet, J. Risk of zoonotic transmission of HEV from rabbits. J. Clin. Virol. 2013, 58, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Caruso, C.; Modesto, P.; Prato, R.; Scaglione, F.E.; De Marco, L.; Bollo, E.; Acutis, P.L.; Masoero, L.; Peletto, S. Hepatitis E Virus: First description in a pet house rabbit. A new transmission route for human? Transbound. Emerg. Dis. 2015, 62, 229–232. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M.; Nishizawa, T.; Sato, H.; Sato, Y.; Jirintai; Nagashima, S.; Okamoto, H. Analysis of the full-length genome of a hepatitis E virus isolate obtained from a wild boar in Japan that is classifiable into a novel genotype. J. Gen. Virol. 2011, 92, 902–908. [Google Scholar] [CrossRef] [PubMed]
- Woo, P.C.Y.; Lau, S.K.P.; Teng, J.L.L.; Tsang, A.K.L.; Joseph, M.; Wong, E.Y.M.; Tang, Y.; Sivakumar, S.; Xie, J.; Bai, R.; Wernery, R.; et al. New hepatitis E virus genotype in camels, the Middle East. Emerg. Infect. Dis. 2014, 20, 1044–1048. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Karlsson, M.; Olofson, A.-S.; Belák, S.; Malmsten, J.; Dalin, A.-M.; Widén, F.; Norder, H. High prevalence of hepatitis e virus in Swedish moose—A phylogenetic characterization and comparison of the virus from different regions. PLoS ONE 2015, 10, e0122102. [Google Scholar] [CrossRef] [PubMed]
- Haqshenas, G.; Shivaprasad, H.L.; Woolcock, P.R.; Read, D.H.; Meng, X.J. Genetic identification and characterization of a novel virus related to human hepatitis E virus from chickens with hepatitis-splenomegaly syndrome in the United States. J. Gen. Virol. 2001, 82, 2449–2462. [Google Scholar] [CrossRef] [PubMed]
- Payne, C.J.; Ellis, T.M.; Plant, S.L.; Gregory, A.R.; Wilcox, G.E. Sequence data suggests big liver and spleen disease virus (BLSV) is genetically related to hepatitis E virus. Vet. Microbiol. 1999, 68, 119–125. [Google Scholar] [CrossRef]
- Huang, F.F.; Haqshenas, G.; Shivaprasad, H.L.; Guenette, D.K.; Woolcock, P.R.; Larsen, C.T.; Pierson, F.W.; Elvinger, F.; Toth, T.E.; Meng, X.J. Heterogeneity and seroprevalence of a newly identified avian hepatitis e virus from chickens in the United States. J. Clin. Microbiol. 2002, 40, 4197–4202. [Google Scholar] [CrossRef] [PubMed]
- Johne, R.; Heckel, G.; Plenge-Bönig, A.; Kindler, E.; Maresch, C.; Reetz, J.; Schielke, A.; Ulrich, R.G. Novel hepatitis E virus genotype in Norway rats, Germany. Emerg. Infect. Dis. 2010, 16, 1452–1455. [Google Scholar] [CrossRef] [PubMed]
- Raj, V.S.; Smits, S.L.; Pas, S.D.; Provacia, L.B. V.; Moorman-Roest, H.; Osterhaus, A.D. M.E.; Haagmans, B.L. Novel Hepatitis E Virus in Ferrets, The Netherlands. Emerg. Infect. Dis. 2012, 18, 1369–1370. [Google Scholar] [CrossRef] [PubMed]
- Krog, J.S.; Breum, S.Ø.; Jensen, T.H.; Larsen, L.E. Hepatitis E Virus variant in farmed mink, Denmark. Emerg. Infect. Dis. 2013, 19, 2028–2030. [Google Scholar] [CrossRef] [PubMed]
- Bodewes, R.; van der Giessen, J.; Haagmans, B.L.; Osterhaus, A.D.M.E.; Smits, S.L. Identification of multiple novel viruses, including a parvovirus and a hepevirus, in feces of red foxes. J. Virol. 2013, 87, 7758–7764. [Google Scholar] [CrossRef] [PubMed]
- Drexler, J.F.; Seelen, A.; Corman, V.M.; Tateno, A.F.; Cottontail, V.; Zerbinati, R.M.; Gloza-Rausch, F.; Klose, S.M.; Adu-Sarkodie, Y.; Oppong, S.K.; et al. Bats worldwide carry hepatitis E virus-related viruses that form a putative novel genus within the family Hepeviridae. J. Virol. 2012, 86, 9134–9147. [Google Scholar] [CrossRef] [PubMed]
- Reuter, G.; Boros, Á.; Mátics, R.; Kapusinszky, B.; Delwart, E.; Pankovics, P. Divergent hepatitis E virus in birds of prey, common kestrel (Falco tinnunculus) and red-footed falcon (F. vespertinus), Hungary. Infect. Genet. Evol. 2016, 43, 343–346. [Google Scholar] [CrossRef] [PubMed]
- Batts, W.; Yun, S.; Hedrick, R.; Winton, J. A novel member of the family Hepeviridae from cutthroat trout (Oncorhynchus clarkii). Virus Res. 2011, 158, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Vina-Rodriguez, A.; Schlosser, J.; Becher, D.; Kaden, V.; Groschup, M.H.; Eiden, M. Hepatitis E virus genotype 3 diversity: Phylogenetic analysis and presence of subtype 3b in wild boar in Europe. Viruses 2015, 7, 2704–2726. [Google Scholar] [CrossRef] [PubMed]
- Oliveira-Filho, E.F.; König, M.; Thiel, H.-J. Genetic variability of HEV isolates: Inconsistencies of current classification. Vet. Microbiol. 2013, 165, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Mirazo, S.; Mir, D.; Bello, G.; Ramos, N.; Musto, H.; Arbiza, J. New insights into the hepatitis E virus genotype 3 phylodynamics and evolutionary history. Infect. Genet. Evol. 2016, 43, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.B.; Simmonds, P.; Izopet, J.; Oliveira-Filho, E.F.; Ulrich, R.G.; Johne, R.; Koenig, M.; Jameel, S.; Harrison, T.J.; Meng, X.-J.; et al. Proposed reference sequences for hepatitis E virus subtypes. J. Gen. Virol. 2016, 97, 537–542. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Li, C.; Hagedorn, C.H. Phylogenetic analysis of global hepatitis E virus sequences: Genetic diversity, subtypes and zoonosis. Rev. Med. Virol. 2006, 16, 5–36. [Google Scholar] [CrossRef] [PubMed]
- Zehender, G.; Ebranati, E.; Lai, A.; Luzzago, C.; Paladini, S.; Tagliacarne, C.; Galli, C.; Galli, M.; Ciccozzi, M.; Zanetti, A.R.; et al. Phylogeography and phylodynamics of European genotype 3 hepatitis E virus. Infect. Genet. Evol. 2014, 25, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.C.; Nguyen, D.; Fernandez, J.; Yun, K.Y.; Fry, K.E.; Bradley, D.W.; Tam, A.W.; Reyes, G.R. Molecular cloning and sequencing of the Mexico isolate of hepatitis E virus (HEV). Virology 1992, 191, 550–558. [Google Scholar] [CrossRef]
- Buisson, Y.; Grandadam, M.; Nicand, E.; Cheval, P.; van Cuyck-Gandre, H.; Innis, B.; Rehel, P.; Coursaget, P.; Teyssou, R.; Tsarev, S. Identification of a novel hepatitis E virus in Nigeria. J. Gen. Virol. 2000, 81, 903–909. [Google Scholar] [CrossRef] [PubMed]
- Nicand, E.; Armstrong, G.L.; Enouf, V.; Guthmann, J.P.; Guerin, J.-P.; Caron, M.; Nizou, J.Y.; Andraghetti, R. Genetic heterogeneity of hepatitis E virus in Darfur, Sudan, and neighboring Chad. J. Med. Virol. 2005, 77, 519–521. [Google Scholar] [CrossRef] [PubMed]
- Ijaz, S.; Said, B.; Boxall, E.; Smit, E.; Morgan, D.; Tedder, R.S. Indigenous hepatitis E in England and wales from 2003 to 2012: Evidence of an emerging novel phylotype of viruses. J. Infect. Dis. 2014, 209, 1212–1218. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.B.; Ijaz, S.; Tedder, R.S.; Hogema, B.; Zaaijer, H.L.; Izopet, J.; Bradley-Stewart, A.; Gunson, R.; Harvala, H.; Kokki, I.; Simmonds, P. Variability and pathogenicity of hepatitis E virus genotype 3 variants. J. Gen. Virol. 2015, 96, 3255–3264. [Google Scholar] [CrossRef] [PubMed]
- Purdy, M.A.; Khudyakov, Y.E. Evolutionary history and population dynamics of hepatitis E virus. PLoS ONE 2010, 5, e14376. [Google Scholar] [CrossRef] [PubMed]
- Teo, C.-G. Fatal outbreaks of jaundice in pregnancy and the epidemic history of hepatitis E. Epidemiol. Infect. 2012, 140, 767–787. [Google Scholar] [CrossRef] [PubMed]
- Pei, Y.; Yoo, D. Genetic characterization and sequence heterogeneity of a Canadian isolate of Swine hepatitis E virus. J. Clin. Microbiol. 2002, 40, 4021–4029. [Google Scholar] [CrossRef] [PubMed]
- Bouquet, J.; Tesse, S.; Lunazzi, A.; Eloit, M.; Rose, N.; Nicand, E.; Pavio, N. Close similarity between sequences of hepatitis E virus recovered from humans and swine, France, 2008–2009. Emerg. Infect. Dis. 2011, 17, 2018–2025. [Google Scholar] [CrossRef] [PubMed]
- Lhomme, S.; Abravanel, F.; Dubois, M.; Chapuy-Regaud, S.; Sandres-Saune, K.; Mansuy, J.-M.; Rostaing, L.; Kamar, N.; Izopet, J. Temporal evolution of the distribution of hepatitis E virus genotypes in Southwestern France. Infect. Genet. Evol. 2015, 35, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Izopet, J.; Dubois, M.; Bertagnoli, S.; Lhomme, S.; Marchandeau, S.; Boucher, S.; Kamar, N.; Abravanel, F.; Guérin, J.-L. Hepatitis E virus strains in rabbits and evidence of a closely related strain in humans, France. Emerg. Infect. Dis. 2012, 18, 1274–1281. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Si, F.; Jiang, C.; Li, T.; Jin, M. Molecular detection of hepatitis E virus in sheep from southern Xinjiang, China. Virus Genes 2015, 50, 410–417. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Li, Y.; Yu, W.; Jing, S.; Wang, J.; Long, F.; He, Z.; Yang, C.; Bi, Y.; Cao, W.; et al. Excretion of infectious hepatitis E virus into milk in cows imposes high risks of zoonosis. Hepatology 2016, 64, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.-H.; Tan, B.-H.; Teo, E.C.-Y.; Lim, S.-G.; Dan, Y.-Y.; Wee, A.; Aw, P.P.K.; Zhu, Y.; Hibberd, M.L.; Tan, C.-K.; et al. Chronic infection with camelid hepatitis E virus in a liver transplant recipient who regularly consumes camel meat and milk. Gastroenterology 2016, 150, 355–357. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.F.; Sun, Z.F.; Emerson, S.U.; Purcell, R.H.; Shivaprasad, H.L.; Pierson, F.W.; Toth, T.E.; Meng, X.J. Determination and analysis of the complete genomic sequence of avian hepatitis E virus (avian HEV) and attempts to infect rhesus monkeys with avian HEV. J. Gen. Virol. 2004, 85, 1609–1618. [Google Scholar] [CrossRef] [PubMed]
- Bilic, I.; Jaskulska, B.; Basic, A.; Morrow, C.J.; Hess, M. Sequence analysis and comparison of avian hepatitis E viruses from Australia and Europe indicate the existence of different genotypes. J. Gen. Virol. 2009, 90, 863–873. [Google Scholar] [CrossRef] [PubMed]
- Hsu, I.W.-Y.; Tsai, H.-J. Avian hepatitis E virus in chickens, Taiwan, 2013. Emerg. Infect. Dis. 2014, 20, 149–151. [Google Scholar] [CrossRef] [PubMed]
- Guan, D.; Li, W.; Su, J.; Fang, L.; Takeda, N.; Wakita, T.; Li, T.-C.; Ke, C. Asian musk shrew as a reservoir of rat hepatitis E virus, China. Emerg. Infect. Dis. 2013, 19, 1341–1343. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Guan, D.; Su, J.; Takeda, N.; Wakita, T.; Li, T.-C.; Ke, C.W. High prevalence of rat hepatitis E virus in wild rats in China. Vet. Microbiol. 2013, 165, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Norder, H.; Uhlhorn, H.; Belák, S.; Widén, F. Novel hepatitis E like virus found in Swedish moose. J. Gen. Virol. 2014, 95, 557–570. [Google Scholar] [CrossRef] [PubMed]
- Johne, R.; Dremsek, P.; Reetz, J.; Heckel, G.; Hess, M.; Ulrich, R.G. Hepeviridae: An expanding family of vertebrate viruses. Infect. Genet. Evol. 2014, 27, 212–229. [Google Scholar] [CrossRef] [PubMed]
- Vitral, C.L.; Yoshida, C.F.; Gaspar, A.M. The use of non-human primates as animal models for the study of hepatitis viruses. Braz. J. Med. Biol. Res. Rev. 1998, 31, 1035–1048. [Google Scholar] [CrossRef]
- Yamamoto, H.; Suzuki, J.; Matsuda, A.; Ishida, T.; Ami, Y.; Suzaki, Y.; Adachi, I.; Wakita, T.; Takeda, N.; Li, T.-C. Hepatitis E virus outbreak in monkey facility, Japan. Emerg. Infect. Dis. 2012, 18, 2032–2034. [Google Scholar] [CrossRef] [PubMed]
- Tsarev, S.A.; Tsareva, T.S.; Emerson, S.U.; Rippy, M.K.; Zack, P.; Shapiro, M.; Purcell, R.H. Experimental hepatitis E in pregnant rhesus monkeys: Failure to transmit hepatitis E virus (HEV) to offspring and evidence of naturally acquired antibodies to HEV. J. Infect. Dis. 1995, 172, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Kamili, S.; Krawczynski, K. Quantitative detection of hepatitis E virus RNA and dynamics of viral replication in experimental infection. J. Viral Hepat. 2006, 13, 835–839. [Google Scholar] [CrossRef] [PubMed]
- Erker, J.C.; Desai, S.M.; Schlauder, G.G.; Dawson, G.J.; Mushahwar, I.K. A hepatitis E virus variant from the United States: Molecular characterization and transmission in cynomolgus macaques. J. Gen. Virol. 1999, 80, 681–690. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Song, X.; Harrison, T.J.; Li, R.; Huang, G.; Zhang, H.; Kong, W.; Wang, Y. Immunogenicity and efficacy of a bacterially expressed HEV ORF3 peptide, assessed by experimental infection of primates. Arch. Virol. 2009, 154, 1641–1648. [Google Scholar] [CrossRef] [PubMed]
- Yugo, D.M.; Cossaboom, C.M.; Meng, X.-J. Naturally occurring animal models of human hepatitis E virus infection. ILAR J. 2014, 55, 187–199. [Google Scholar] [CrossRef] [PubMed]
- Bradley, D.W.; Krawczynski, K.; Cook, E.H.; McCaustland, K.A.; Humphrey, C.D.; Spelbring, J.E.; Myint, H.; Maynard, J.E. Enterically transmitted non-A, non-B hepatitis: Serial passage of disease in cynomolgus macaques and tamarins and recovery of disease-associated 27- to 34-nm viruslike particles. Proc. Natl. Acad. Sci. USA 1987, 84, 6277–6281. [Google Scholar] [CrossRef] [PubMed]
- Krawczynski, K.; Bradley, D.W. Enterically transmitted non-A, non-B hepatitis: Identification of virus-associated antigen in experimentally infected cynomolgus macaques. J. Infect. Dis. 1989, 159, 1042–1049. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, R.; Kamili, S.; Spelbring, J.; Krawczynski, K. Experimental studies on subclinical hepatitis E virus infection in cynomolgus macaques. J. Infect. Dis. 2001, 184, 1380–1385. [Google Scholar] [CrossRef] [PubMed]
- Longer, C.F.; Denny, S.L.; Caudill, J.D.; Miele, T.A.; Asher, L.V.; Myint, K.S.; Huang, C.C.; Engler, W.F.; LeDuc, J.W.; Binn, L.N. Experimental hepatitis E: Pathogenesis in cynomolgus macaques (Macaca fascicularis). J. Infect. Dis. 1993, 168, 602–609. [Google Scholar] [CrossRef] [PubMed]
- Tsarev, S.A.; Emerson, S.U.; Tsareva, T.S.; Yarbough, P.O.; Lewis, M.; Govindarajan, S.; Reyes, G.R.; Shapiro, M.; Purcell, R.H. Variation in course of hepatitis E in experimentally infected cynomolgus monkeys. J. Infect. Dis. 1993, 167, 1302–1306. [Google Scholar] [CrossRef] [PubMed]
- Purdy, M.A.; McCaustland, K.A.; Krawczynski, K.; Spelbring, J.; Reyes, G.R.; Bradley, D.W. Preliminary evidence that a trpE-HEV fusion protein protects cynomolgus macaques against challenge with wild-type hepatitis E virus (HEV). J. Med. Virol. 1993, 41, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Tsarev, S.A.; Tsareva, T.S.; Emerson, S.U.; Govindarajan, S.; Shapiro, M.; Gerin, J.L.; Purcell, R.H. Successful passive and active immunization of cynomolgus monkeys against hepatitis E. Proc. Natl. Acad. Sci. USA 1994, 91, 10198–10202. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.J.; Halbur, P.G.; Shapiro, M.S.; Govindarajan, S.; Bruna, J.D.; Mushahwar, I.K.; Purcell, R.H.; Emerson, S.U. Genetic and experimental evidence for cross-species infection by swine hepatitis E virus. J. Virol. 1998, 72, 9714–9721. [Google Scholar] [PubMed]
- Arankalle, V.A.; Chobe, L.P.; Chadha, M.S. Type-IV Indian swine HEV infects rhesus monkeys. J. Viral Hepat. 2006, 13, 742–745. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Bu, Q.-N.; Wang, L.; Han, J.; Du, R.-J.; Lei, Y.-X.; Ouyang, Y.-Q.; Li, J.; Zhu, Y.-H.; Lu, F.-M.; et al. Transmission of hepatitis E virus from rabbits to cynomolgus macaques. Emerg. Infect. Dis. 2013, 19, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Li, T.-C.; Yoshizaki, S.; Ami, Y.; Suzaki, Y.; Yang, T.; Takeda, N.; Takaji, W. Monkeys and rats are not susceptible to ferret hepatitis E virus infection. Intervirology 2015, 58, 139–142. [Google Scholar] [CrossRef] [PubMed]
- Feagins, A.R.; Opriessnig, T.; Huang, Y.W.; Halbur, P.G.; Meng, X.J. Cross-species infection of specific-pathogen-free pigs by a genotype 4 strain of human hepatitis E virus. J. Med. Virol. 2008, 80, 1379–1386. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.J.; Halbur, P.G.; Haynes, J.S.; Tsareva, T.S.; Bruna, J.D.; Royer, R.L.; Purcell, R.H.; Emerson, S.U. Experimental infection of pigs with the newly identified swine hepatitis E virus (swine HEV), but not with human strains of HEV. Arch. Virol. 1998, 143, 1405–1415. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Ha, Y.; Ahn, K.K.; Chae, C. Localisation of swine hepatitis E virus in experimentally infected pigs. Vet. J. Lond. Engl. 1997 2009, 179, 417–421. [Google Scholar] [CrossRef] [PubMed]
- Williams, T.P.; Kasorndorkbua, C.; Halbur, P.G.; Haqshenas, G.; Guenette, D.K.; Toth, T.E.; Meng, X.J. Evidence of extrahepatic sites of replication of the hepatitis E virus in a swine model. J. Clin. Microbiol. 2001, 39, 3040–3046. [Google Scholar] [CrossRef] [PubMed]
- Cossaboom, C.M.; Córdoba, L.; Sanford, B.J.; Piñeyro, P.; Kenney, S.P.; Dryman, B.A.; Wang, Y.; Meng, X.-J. Cross-species infection of pigs with a novel rabbit, but not rat, strain of hepatitis E virus isolated in the United States. J. Gen. Virol. 2012, 93, 1687–1695. [Google Scholar] [CrossRef] [PubMed]
- Schlosser, J.; Eiden, M.; Vina-Rodriguez, A.; Fast, C.; Dremsek, P.; Lange, E.; Ulrich, R.G.; Groschup, M.H. Natural and experimental hepatitis E virus genotype 3-infection in European wild boar is transmissible to domestic pigs. Vet. Res. 2014, 45, 121. [Google Scholar] [CrossRef] [PubMed]
- Schlosser, J.; Vina-Rodriguez, A.; Fast, C.; Groschup, M.H.; Eiden, M. Chronically infected wild boar can transmit genotype 3 hepatitis E virus to domestic pigs. Vet. Microbiol. 2015, 180, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Marek, A.; Bilic, I.; Prokofieva, I.; Hess, M. Phylogenetic analysis of avian hepatitis E virus samples from European and Australian chicken flocks supports the existence of a different genus within the Hepeviridae comprising at least three different genotypes. Vet. Microbiol. 2010, 145, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Billam, P.; Huang, F.F.; Sun, Z.F.; Pierson, F.W.; Duncan, R.B.; Elvinger, F.; Guenette, D.K.; Toth, T.E.; Meng, X.J. Systematic pathogenesis and replication of avian hepatitis E virus in specific-pathogen-free adult chickens. J. Virol. 2005, 79, 3429–3437. [Google Scholar] [CrossRef] [PubMed]
- Billam, P.; Pierson, F.W.; Li, W.; LeRoith, T.; Duncan, R.B.; Meng, X.J. Development and validation of a negative-strand-specific reverse transcription-PCR assay for detection of a chicken strain of hepatitis E virus: Identification of nonliver replication sites. J. Clin. Microbiol. 2008, 46, 2630–2634. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.F.; Larsen, C.T.; Huang, F.F.; Billam, P.; Pierson, F.W.; Toth, T.E.; Meng, X.J. Generation and infectivity titration of an infectious stock of avian hepatitis E virus (HEV) in chickens and cross-species infection of turkeys with avian HEV. J. Clin. Microbiol. 2004, 42, 2658–2662. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.F.; Pierson, F.W.; Toth, T.E.; Meng, X.J. Construction and characterization of infectious cDNA clones of a chicken strain of hepatitis E virus (HEV), avian HEV. J. Gen. Virol. 2005, 86, 2585–2593. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.M.; LeRoith, T.; Pudupakam, R.S.; Pierson, F.W.; Huang, Y.-W.; Dryman, B.A.; Meng, X.-J. Construction of an infectious cDNA clone of avian hepatitis E virus (avian HEV) recovered from a clinically healthy chicken in the United States and characterization of its pathogenicity in specific-pathogen-free chickens. Vet. Microbiol. 2011, 147, 310–319. [Google Scholar] [CrossRef] [PubMed]
- Pudupakam, R.S.; Huang, Y.W.; Opriessnig, T.; Halbur, P.G.; Pierson, F.W.; Meng, X.J. Deletions of the hypervariable region (HVR) in open reading frame 1 of hepatitis E virus do not abolish virus infectivity: Evidence for attenuation of HVR deletion mutants in vivo. J. Virol. 2009, 83, 384–395. [Google Scholar] [CrossRef] [PubMed]
- Pudupakam, R.S.; Kenney, S.P.; Córdoba, L.; Huang, Y.-W.; Dryman, B.A.; Leroith, T.; Pierson, F.W.; Meng, X.-J. Mutational analysis of the hypervariable region of hepatitis e virus reveals its involvement in the efficiency of viral RNA replication. J. Virol. 2011, 85, 10031–10040. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Wang, S.; Dai, X.; Shi, C.; Wen, Y.; Zhu, M.; Zhan, S.; Meng, J. Rabbit as a novel animal model for hepatitis E virus infection and vaccine evaluation. PLoS ONE 2012, 7, e51616. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Lei, Y.; Liu, L.; Liu, P.; Xia, J.; Zhang, Y.; Zeng, H.; Wang, L.; Wang, L.; Zhuang, H. SPF rabbits infected with rabbit hepatitis E virus isolate experimentally showing the chronicity of hepatitis. PLoS ONE 2014, 9, e99861. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Zheng, L.; Liu, Y.; Zhao, C.; Harrison, T.J.; Ma, Y.; Sun, S.; Zhang, J.; Wang, Y. Experimental infection of rabbits with rabbit and genotypes 1 and 4 hepatitis E viruses. PLoS ONE 2010, 5, e9160. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.; Zhao, Y.; She, R.; Cao, B.; Xiao, P.; Wu, Q.; Guo, Z.; Ma, L.; Soomro, M.H. Detection and localization of rabbit hepatitis e virus and antigen in systemic tissues from experimentally intraperitoneally infected rabbits. PLoS ONE 2014, 9, e88607. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Liu, L.; Wang, L.; Zhang, Y.; Zeng, H.; Liu, P.; Zou, Q.; Wang, L.; Zhuang, H. Experimental infection of pregnant rabbits with hepatitis E virus demonstrating high mortality and vertical transmission. J. Viral Hepat. 2015, 22, 850–857. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wang, L.; Xia, J.; Zhang, Y.; Zeng, H.; Liu, P.; Zou, Q.; Wang, L.; Zhuang, H. Mix-breeding with HEV-infected swine induced inapparent HEV infection in SPF rabbits. J. Med. Virol. 2016, 88, 681–685. [Google Scholar] [CrossRef] [PubMed]
- Li, T.-C.; Yoshizaki, S.; Ami, Y.; Suzaki, Y.; Yasuda, S.P.; Yoshimatsu, K.; Arikawa, J.; Takeda, N.; Wakita, T. Susceptibility of laboratory rats against genotypes 1, 3, 4, and rat hepatitis E viruses. Vet. Microbiol. 2013, 163, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Maneerat, Y.; Clayson, E.T.; Myint, K.S.; Young, G.D.; Innis, B.L. Experimental infection of the laboratory rat with the hepatitis E virus. J. Med. Virol. 1996, 48, 121–128. [Google Scholar] [CrossRef]
- Purcell, R.H.; Engle, R.E.; Rood, M.P.; Kabrane-Lazizi, Y.; Nguyen, H.T.; Govindarajan, S.; St Claire, M.; Emerson, S.U. Hepatitis E virus in rats, Los Angeles, California, USA. Emerg. Infect. Dis. 2011, 17, 2216–2222. [Google Scholar] [CrossRef] [PubMed]
- Li, T.-C.; Yang, T.; Yoshizaki, S.; Ami, Y.; Suzaki, Y.; Ishii, K.; Haga, K.; Nakamura, T.; Ochiai, S.; Takaji, W.; et al. Construction and characterization of an infectious cDNA clone of rat hepatitis E virus. J. Gen. Virol. 2015, 96, 1320–1327. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Yu, X.; Zhang, Y.; Ni, Y.; Si, F.; Yu, R.; Dong, S.; Huang, Y.; Li, Z. Infectivity of a genotype 4 hepatitis E virus cDNA clone by intrahepatic inoculation of laboratory rats. Vet. Microbiol. 2013, 166, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Si, F.; Shi, B.; Wang, X.; Zhu, Y.; Liu, X.; Yang, Q.; Li, Z. Construction of an infectious cDNA clone of a swine genotype 3 HEV strain isolated in Shanghai, China. Intervirology 2014, 57, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Li, T.-C.; Yang, T.; Yoshizaki, S.; Ami, Y.; Suzaki, Y.; Ishii, K.; Kishida, N.; Shirakura, M.; Asanuma, H.; Takeda, N.; et al. Ferret hepatitis E virus infection induces acute hepatitis and persistent infection in ferrets. Vet. Microbiol. 2016, 183, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Sun, Q.; She, R.; Wang, D.; Duan, X.; Yin, J.; Ding, Y. Experimental infection of Mongolian gerbils by a genotype 4 strain of swine hepatitis E virus. J. Med. Virol. 2009, 81, 1591–1596. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Shi, R.; She, R.; Soomro, M.H.; Mao, J.; Du, F.; Zhao, Y.; Liu, C. Effect of swine hepatitis E virus on the livers of experimentally infected Mongolian gerbils by swine hepatitis E virus. Virus Res. 2015, 208, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Bouwknegt, M.; Rutjes, S.A.; Reusken, C.B.; Stockhofe-Zurwieden, N.; Frankena, K.; de Jong, M.C.M.; de Roda Husman, A.M.; van der Poel, W.H.M. The course of hepatitis E virus infection in pigs after contact-infection and intravenous inoculation. BMC Vet. Res. 2009, 5. [Google Scholar] [CrossRef] [PubMed]
- Shi, R.; Soomro, M.H.; She, R.; Yang, Y.; Wang, T.; Wu, Q.; Li, H.; Hao, W. Evidence of hepatitis E virus breaking through the blood-brain barrier and replicating in the central nervous system. J. Viral Hepat. 2016. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; He, Z.-J.; Tao, W.; Fu, T.; Wang, Y.-K.; Chen, Y. Experimental infection of Z:ZCLA Mongolian gerbils with human hepatitis E virus. World J. Gastroenterol. 2015, 21, 862–867. [Google Scholar] [PubMed]
- Li, T.-C.; Suzaki, Y.; Ami, Y.; Tsunemitsu, H.; Miyamura, T.; Takeda, N. Mice are not susceptible to hepatitis E virus infection. J. Vet. Med. Sci. 2008, 70, 1359–1362. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Zhang, W.; Gong, G.; Yuan, C.; Yan, Y.; Yang, S.; Cui, L.; Zhu, J.; Yang, Z.; Hua, X. Experimental infection of Balb/c nude mice with Hepatitis E virus. BMC Infect. Dis. 2009, 9, 93. [Google Scholar] [CrossRef] [PubMed]
- Allweiss, L.; Gass, S.; Giersch, K.; Groth, A.; Kah, J.; Volz, T.; Rapp, G.; Schöbel, A.; Lohse, A.W.; Polywka, S.; et al. Human liver chimeric mice as a new model of chronic hepatitis E virus infection and preclinical drug evaluation. J. Hepatol. 2016, 64, 1033–1040. [Google Scholar] [CrossRef] [PubMed]
- Sayed, I.M.; Verhoye, L.; Cocquerel, L.; Abravanel, F.; Foquet, L.; Montpellier, C.; Debing, Y.; Farhoudi, A.; Wychowski, C.; Dubuisson, J.; et al. Study of hepatitis E virus infection of genotype 1 and 3 in mice with humanised liver. Gut 2016. [Google Scholar] [CrossRef] [PubMed]
- Van de Garde, M.D.B.; Pas, S.D.; van der Net, G.; de Man, R.A.; Osterhaus, A.D.M.E.; Haagmans, B.L.; Boonstra, A.; Vanwolleghem, T. Hepatitis E virus (HEV) genotype 3 infection of human liver chimeric mice as a model for chronic HEV infection. J. Virol. 2016, 90, 4394–4401. [Google Scholar] [CrossRef] [PubMed]
- Córdoba, L.; Feagins, A.R.; Opriessnig, T.; Cossaboom, C.M.; Dryman, B.A.; Huang, Y.-W.; Meng, X.-J. Rescue of a genotype 4 human hepatitis E virus from cloned cDNA and characterization of intergenotypic chimeric viruses in cultured human liver cells and in pigs. J. Gen. Virol. 2012, 93, 2183–2194. [Google Scholar] [CrossRef] [PubMed]
- Feagins, A.R.; Córdoba, L.; Sanford, B.J.; Dryman, B.A.; Huang, Y.-W.; LeRoith, T.; Emerson, S.U.; Meng, X.-J. Intergenotypic chimeric hepatitis E viruses (HEVs) with the genotype 4 human HEV capsid gene in the backbone of genotype 3 swine HEV are infectious in pigs. Virus Res. 2011, 156, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.N.; Devhare, P.B.; Pingle, S.Y.; Paingankar, M.S.; Arankalle, V.A.; Lole, K.S. HEV-1 harbouring HEV-4 nonstructural protein (ORF1) replicates in transfected porcine kidney cells. J. Gen. Virol. 2016, 97, 1829–1840. [Google Scholar] [CrossRef] [PubMed]
- Lara, J.; Purdy, M.A.; Khudyakov, Y.E. Genetic host specificity of hepatitis E virus. Infect. Genet. Evol. 2014, 24, 127–139. [Google Scholar] [CrossRef] [PubMed]
- Bouquet, J.; Cheval, J.; Rogée, S.; Pavio, N.; Eloit, M. Identical consensus sequence and conserved genomic polymorphism of hepatitis E virus during controlled interspecies transmission. J. Virol. 2012, 86, 6238–6245. [Google Scholar] [CrossRef] [PubMed]
- Tei, S.; Kitajima, N.; Takahashi, K.; Mishiro, S. Zoonotic transmission of hepatitis E virus from deer to human beings. Lancet 2003, 362, 371–373. [Google Scholar] [CrossRef]
- Li, T.-C.; Chijiwa, K.; Sera, N.; Ishibashi, T.; Etoh, Y.; Shinohara, Y.; Kurata, Y.; Ishida, M.; Sakamoto, S.; Takeda, N.; Miyamura, T. Hepatitis E virus transmission from wild boar meat. Emerg. Infect. Dis. 2005, 11, 1958–1960. [Google Scholar] [CrossRef] [PubMed]
- Riveiro-Barciela, M.; Mínguez, B.; Gironés, R.; Rodriguez-Frías, F.; Quer, J.; Buti, M. Phylogenetic demonstration of hepatitis E infection transmitted by pork meat ingestion. J. Clin. Gastroenterol. 2015, 49, 165–168. [Google Scholar] [CrossRef] [PubMed]
- Renou, C.; Roque-Alfonso, A.; Pavio, N. Foodborne transmission of HEV from raw pork liver sausage in France. Emerg. Infect. Dis. 2014, in press. [Google Scholar] [CrossRef] [PubMed]
- Yazaki, Y.; Mizuo, H.; Takahashi, M.; Nishizawa, T.; Sasaki, N.; Gotanda, Y.; Okamoto, H. Sporadic acute or fulminant hepatitis E in Hokkaido, Japan, may be food-borne, as suggested by the presence of hepatitis E virus in pig liver as food. J. Gen. Virol. 2003, 84, 2351–2357. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, H.; Okada, K.; Takahashi, K.; Mishiro, S. Severe hepatitis E virus infection after ingestion of uncooked liver from a wild boar. J. Infect. Dis. 2003, 188, 944. [Google Scholar] [CrossRef] [PubMed]
- Colson, P.; Borentain, P.; Queyriaux, B.; Kaba, M.; Moal, V.; Gallian, P.; Heyries, L.; Raoult, D.; Gerolami, R. Pig liver sausage as a source of hepatitis E virus transmission to humans. J. Infect. Dis. 2010, 202, 825–834. [Google Scholar] [CrossRef] [PubMed]
- Yapa, C.M.; Furlong, C.; Rosewell, A.; Ward, K.A.; Adamson, S.; Shadbolt, C.; Kok, J.; Tracy, S.L.; Bowden, S.; Smedley, E.J.; et al. First reported outbreak of locally acquired hepatitis E virus infection in Australia. Med. J. Aust. 2016, 204, 274. [Google Scholar] [CrossRef] [PubMed]
- Masuda, J.-I.; Yano, K.; Tamada, Y.; Takii, Y.; Ito, M.; Omagari, K.; Kohno, S. Acute hepatitis E of a man who consumed wild boar meat prior to the onset of illness in Nagasaki, Japan. Hepatol. Res. 2005, 31, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Tamada, Y.; Yano, K.; Yatsuhashi, H.; Inoue, O.; Mawatari, F.; Ishibashi, H. Consumption of wild boar linked to cases of hepatitis E. J. Hepatol. 2004, 40, 869–870. [Google Scholar] [CrossRef] [PubMed]
- Guillois, Y.; Abravanel, F.; Miura, T.; Pavio, N.; Vaillant, V.; Lhomme, S.; Le Guyader, F.S.; Rose, N.; Le Saux, J.-C.; King, L.A.; et al. High proportion of asymptomatic infections in an outbreak of hepatitis E associated with a spit-roasted piglet, France, 2013. Clin. Infect. Dis. 2016, 62, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Wichmann, O.; Schimanski, S.; Koch, J.; Kohler, M.; Rothe, C.; Plentz, A.; Jilg, W.; Stark, K. Phylogenetic and case-control study on hepatitis E virus infection in Germany. J. Infect. Dis. 2008, 198, 1732–1741. [Google Scholar] [CrossRef] [PubMed]
- Mansuy, J.M.; Gallian, P.; Dimeglio, C.; Saune, K.; Arnaud, C.; Pelletier, B.; Morel, P.; Legrand, D.; Tiberghien, P.; Izopet, J. A nationwide survey of hepatitis E viral infection in French blood donors. Hepatology 2016, 63, 1145–1154. [Google Scholar] [CrossRef] [PubMed]
- Cossaboom, C.M.; Heffron, C.L.; Cao, D.; Yugo, D.M.; Houk-Miles, A.E.; Lindsay, D.S.; Zajac, A.M.; Bertke, A.S.; Elvinger, F.; Meng, X.-J. Risk factors and sources of foodborne hepatitis E virus infection in the United States. J. Med. Virol. 2016, 88, 1641–1645. [Google Scholar] [CrossRef] [PubMed]
- Chaussade, H.; Rigaud, E.; Allix, A.; Carpentier, A.; Touzé, A.; Delzescaux, D.; Choutet, P.; Garcia-Bonnet, N.; Coursaget, P. Hepatitis E virus seroprevalence and risk factors for individuals in working contact with animals. J. Clin. Virol. 2013, 58, 504–508. [Google Scholar] [CrossRef] [PubMed]
- Said, B.; Ijaz, S.; Chand, M.A.; Kafatos, G.; Tedder, R.; Morgan, D. Hepatitis E virus in England and Wales: Indigenous infection is associated with the consumption of processed pork products. Epidemiol. Infect. 2014, 142, 1467–1475. [Google Scholar] [CrossRef] [PubMed]
- Pavio, N.; Merbah, T.; Thébault, A. Frequent hepatitis E virus contamination in food containing raw pork liver, France. Emerg. Infect. Dis. 2014, 20, 1925–1927. [Google Scholar] [CrossRef] [PubMed]
- Di Bartolo, I.; Diez-Valcarce, M.; Vasickova, P.; Kralik, P.; Hernandez, M.; Angeloni, G.; Ostanello, F.; Bouwknegt, M.; Rodríguez-Lázaro, D.; Pavlik, I.; et al. Hepatitis E virus in pork production chain in Czech Republic, Italy, and Spain, 2010. Emerg. Infect. Dis. 2012, 18, 1282–1289. [Google Scholar] [CrossRef] [PubMed]
- Di Bartolo, I.; Angeloni, G.; Ponterio, E.; Ostanello, F.; Ruggeri, F.M. Detection of hepatitis E virus in pork liver sausages. Int. J. Food Microbiol. 2015, 193, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Szabo, K.; Trojnar, E.; Anheyer-Behmenburg, H.; Binder, A.; Schotte, U.; Ellerbroek, L.; Klein, G.; Johne, R. Detection of hepatitis E virus RNA in raw sausages and liver sausages from retail in Germany using an optimized method. Int. J. Food Microbiol. 2015, 215, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Grierson, S.; Heaney, J.; Cheney, T.; Morgan, D.; Wyllie, S.; Powell, L.; Smith, D.; Ijaz, S.; Steinbach, F.; Choudhury, B.; et al. Prevalence of Hepatitis E Virus Infection in Pigs at the Time of Slaughter, United Kingdom, 2013. Emerg. Infect. Dis. 2015, 21, 1396–1401. [Google Scholar] [CrossRef] [PubMed]
- Rose, N.; Lunazzi, A.; Dorenlor, V.; Merbah, T.; Eono, F.; Eloit, M.; Madec, F.; Pavio, N. High prevalence of Hepatitis E virus in French domestic pigs. Comp. Immunol. Microbiol. Infect. Dis. 2011, 34, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Bouwknegt, M.; Engel, B.; Herremans, M.M.P.T.; Widdowson, M.A.; Worm, H.C.; Koopmans, M.P.G.; Frankena, K.; de Roda Husman, A.M.; De Jong, M.C.M.; Van Der Poel, W.H.M. Bayesian estimation of hepatitis E virus seroprevalence for populations with different exposure levels to swine in The Netherlands. Epidemiol. Infect. 2008, 136, 567–576. [Google Scholar] [CrossRef] [PubMed]
- Berto, A.; Martelli, F.; Grierson, S.; Banks, M. Hepatitis E virus in pork food chain, United Kingdom, 2009–2010. Emerg. Infect. Dis. 2012, 18, 1358–1360. [Google Scholar] [CrossRef] [PubMed]
- Okano, H.; Takahashi, M.; Isono, Y.; Tanaka, H.; Nakano, T.; Oya, Y.; Sugimoto, K.; Ito, K.; Ohmori, S.; Maegawa, T.; et al. Characterization of sporadic acute hepatitis E and comparison of hepatitis E virus genomes in acute hepatitis patients and pig liver sold as food in Mie, Japan. Hepatol. Res. 2014, 44, E63–E76. [Google Scholar] [CrossRef] [PubMed]
- Feagins, A.R.; Opriessnig, T.; Guenette, D.K.; Halbur, P.G.; Meng, X.-J. Detection and characterization of infectious Hepatitis E virus from commercial pig livers sold in local grocery stores in the USA. J. Gen. Virol. 2007, 88, 912–917. [Google Scholar] [CrossRef] [PubMed]
- Lhomme, S.; Top, S.; Bertagnoli, S.; Dubois, M.; Guerin, J.-L.; Izopet, J. Wildlife reservoir for hepatitis E virus, southwestern France. Emerg. Infect. Dis. 2015, 21, 1224–1226. [Google Scholar] [CrossRef] [PubMed]
- Serracca, L.; Battistini, R.; Rossini, I.; Mignone, W.; Peletto, S.; Boin, C.; Pistone, G.; Ercolini, R.; Ercolini, C. Molecular investigation on the presence of hepatitis E virus (HEV) in wild game in north-western Italy. Food Environ. Virol. 2015, 7, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Heldt, F.H.; Staggmeier, R.; Gularte, J.S.; Demoliner, M.; Henzel, A.; Spilki, F.R. Hepatitis E virus in surface water, sediments, and pork products marketed in southern Brazil. Food Environ. Virol. 2016, 8, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Berto, A.; Grierson, S.; Hakze-van der Honing, R.; Martelli, F.; Johne, R.; Reetz, J.; Ulrich, R.G.; Pavio, N.; Van der Poel, W.H.M.; Banks, M. Hepatitis E virus in pork liver sausage, France. Emerg. Infect. Dis. 2013, 19, 264–266. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Tanaka, T.; Jirintai, S.; Nagashima, S.; Takahashi, M.; Nishizawa, T.; Mizuo, H.; Yazaki, Y.; Okamoto, H. A549 and PLC/PRF/5 cells can support the efficient propagation of swine and wild boar hepatitis E virus (HEV) strains: Demonstration of HEV infectivity of porcine liver sold as food. Arch. Virol. 2012, 157, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Mesquita, J.R.; Oliveira, D.; Rivadulla, E.; Abreu-Silva, J.; Varela, M.F.; Romalde, J.L.; Nascimento, M.S.J. Hepatitis E virus genotype 3 in mussels (Mytilus galloprovinciallis), Spain. Food Microbiol. 2016, 58, 13–15. [Google Scholar] [CrossRef] [PubMed]
- Crossan, C.; Baker, P.J.; Craft, J.; Takeuchi, Y.; Dalton, H.R.; Scobie, L. Hepatitis E virus genotype 3 in shellfish, United Kingdom. Emerg. Infect. Dis. 2012, 18, 2085–2087. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.-J.; Jeong, H.-J.; Kim, Y.-J.; Lee, S.-W.; Lee, J.-B.; Park, S.-Y.; Song, C.-S.; Park, H.-M.; Choi, I.-S. Analysis of complete genome sequences of swine hepatitis E virus and possible risk factors for transmission of HEV to humans in Korea. J. Med. Virol. 2010, 82, 583–591. [Google Scholar] [CrossRef] [PubMed]
- Li, T.-C.; Miyamura, T.; Takeda, N. Detection of hepatitis E virus RNA from the bivalve Yamato-Shijimi (Corbicula japonica) in Japan. Am. J. Trop. Med. Hyg. 2007, 76, 170–172. [Google Scholar] [PubMed]
- Gao, S.; Li, D.; Zha, E.; Zhou, T.; Wang, S.; Yue, X. Surveillance of hepatitis E virus contamination in shellfish in China. Int. J. Environ. Res. Public. Health 2015, 12, 2026–2036. [Google Scholar] [CrossRef] [PubMed]
- Grodzki, M.; Schaeffer, J.; Piquet, J.-C.; Le Saux, J.-C.; Chevé, J.; Ollivier, J.; Le Pendu, J.; Le Guyader, F.S. Bioaccumulation efficiency, tissue distribution, and environmental occurrence of hepatitis E virus in bivalve shellfish from France. Appl. Environ. Microbiol. 2014, 80, 4269–4276. [Google Scholar] [CrossRef] [PubMed]
- Koizumi, Y.; Isoda, N.; Sato, Y.; Iwaki, T.; Ono, K.; Ido, K.; Sugano, K.; Takahashi, M.; Nishizawa, T.; Okamoto, H. Infection of a Japanese patient by genotype 4 hepatitis e virus while traveling in Vietnam. J. Clin. Microbiol. 2004, 42, 3883–3885. [Google Scholar] [CrossRef] [PubMed]
- Said, B.; Ijaz, S.; Kafatos, G.; Booth, L.; Thomas, H.L.; Walsh, A.; Ramsay, M.; Morgan, D.; Hepatitis E Incident Investigation Team. Hepatitis E outbreak on cruise ship. Emerg. Infect. Dis. 2009, 15, 1738–1744. [Google Scholar] [CrossRef] [PubMed]
- Brassard, J.; Gagné, M.-J.; Généreux, M.; Côté, C. Detection of human food-borne and zoonotic viruses on irrigated, field-grown strawberries. Appl. Environ. Microbiol. 2012, 78, 3763–3766. [Google Scholar] [CrossRef] [PubMed]
- Maunula, L.; Kaupke, A.; Vasickova, P.; Söderberg, K.; Kozyra, I.; Lazic, S.; van der Poel, W.H.M.; Bouwknegt, M.; Rutjes, S.; Willems, K.A.; et al. Tracing enteric viruses in the European berry fruit supply chain. Int. J. Food Microbiol. 2013, 167, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Kokkinos, P.; Kozyra, I.; Lazic, S.; Bouwknegt, M.; Rutjes, S.; Willems, K.; Moloney, R.; de Roda Husman, A.M.; Kaupke, A.; Legaki, E.; et al. Harmonised investigation of the occurrence of human enteric viruses in the leafy green vegetable supply chain in three European countries. Food Environ. Virol. 2012, 4, 179–191. [Google Scholar] [CrossRef] [PubMed]
- Loisy-Hamon, F.; Leturnier, G. Autochthonous cases of hepatitis E: Where does the virus come from? Impact of pig slurry treatment on reduction of the viral load and prevalence of the virus in food substrates. EuroReference 2015, 13, 13–18. [Google Scholar]
- McCreary, C.; Martelli, F.; Grierson, S.; Ostanello, F.; Nevel, A.; Banks, M. Excretion of hepatitis E virus by pigs of different ages and its presence in slurry stores in the United Kingdom. Vet. Rec. 2008, 163, 261–265. [Google Scholar] [CrossRef] [PubMed]
- Pina, S.; Buti, M.; Cotrina, M.; Piella, J.; Girones, R. HEV identified in serum from humans with acute hepatitis and in sewage of animal origin in Spain. J. Hepatol. 2000, 33, 826–833. [Google Scholar] [CrossRef]
- Fernández-Barredo, S.; Galiana, C.; García, A.; Vega, S.; Gómez, M.T.; Pérez-Gracia, M.T. Detection of hepatitis E virus shedding in feces of pigs at different stages of production using reverse transcription-polymerase chain reaction. J. Vet. Diagn. Investig. 2006, 18, 462–465. [Google Scholar] [CrossRef]
- Kasorndorkbua, C.; Opriessnig, T.; Huang, F.F.; Guenette, D.K.; Thomas, P.J.; Meng, X.-J.; Halbur, P.G. Infectious swine hepatitis E virus is present in pig manure storage facilities on United States farms, but evidence of water contamination is lacking. Appl. Environ. Microbiol. 2005, 71, 7831–7837. [Google Scholar] [CrossRef] [PubMed]
- Steyer, A.; Naglič, T.; Močilnik, T.; Poljšak-Prijatelj, M.; Poljak, M. Hepatitis E virus in domestic pigs and surface waters in Slovenia: Prevalence and molecular characterization of a novel genotype 3 lineage. Infect. Genet. Evol. 2011, 11, 1732–1737. [Google Scholar] [CrossRef] [PubMed]
- Gentry-Shields, J.; Myers, K.; Pisanic, N.; Heaney, C.; Stewart, J. Hepatitis E virus and coliphages in waters proximal to swine concentrated animal feeding operations. Sci. Total Environ. 2015, 505, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Rutjes, S.A.; Lodder, W.J.; Lodder-Verschoor, F.; van den Berg, H.H.J.L.; Vennema, H.; Duizer, E.; Koopmans, M.; de Roda Husman, A.M. Sources of hepatitis E virus genotype 3 in The Netherlands. Emerg. Infect. Dis. 2009, 15, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Ishida, S.; Yoshizumi, S.; Ikeda, T.; Miyoshi, M.; Goto, A.; Matsubayashi, K.; Ikeda, H. Detection and molecular characterization of hepatitis E virus in clinical, environmental and putative animal sources. Arch. Virol. 2012, 157, 2363–2368. [Google Scholar] [CrossRef] [PubMed]
- Iaconelli, M.; Purpari, G.; Della Libera, S.D.; Petricca, S.; Guercio, A.; Ciccaglione, A.R.; Bruni, R.; Taffon, S.; Equestre, M.; Fratini, M.; et al. Hepatitis A and E Viruses in Wastewaters, in River Waters, and in Bivalve Molluscs in Italy. Food Environ. Virol. 2015, 7, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.-C.; Zhang, J.; Zhang, X.-F.; Zhou, C.; Wang, Z.-Z.; Huang, S.-J.; Wang, H.; Yang, C.-L.; Jiang, H.-M.; Cai, J.-P.; et al. Efficacy and safety of a recombinant hepatitis E vaccine in healthy adults: A large-scale, randomised, double-blind placebo-controlled, phase 3 trial. Lancet 2010, 376, 895–902. [Google Scholar] [CrossRef]
- Johne, R.; Trojnar, E.; Filter, M.; Hofmann, J. Thermal stability of hepatitis E virus estimated by a cell culture method. Appl. Environ. Microbiol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Emerson, S.U.; Arankalle, V.A.; Purcell, R.H. Thermal stability of hepatitis E virus. J. Infect. Dis. 2005, 192, 930–933. [Google Scholar] [CrossRef] [PubMed]
- Feagins, A.R.; Opriessnig, T.; Guenette, D.K.; Halbur, P.G.; Meng, X.J. Inactivation of infectious hepatitis E virus present in commercial pig livers sold in local grocery stores in the United States. Int. J. Food Microbiol. 2008, 123, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Barnaud, E.; Rogée, S.; Garry, P.; Rose, N.; Pavio, N. Thermal inactivation of infectious hepatitis E virus in experimentally contaminated food. Appl. Environ. Microbiol. 2012, 78, 5153–5159. [Google Scholar] [CrossRef] [PubMed]
- Withers, M.R.; Correa, M.T.; Morrow, M.; Stebbins, M.E.; Seriwatana, J.; Webster, W.D.; Boak, M.B.; Vaughn, D.W. Antibody levels to hepatitis E virus in North Carolina swine workers, non-swine workers, swine, and murids. Am. J. Trop. Med. Hyg. 2002, 66, 384–388. [Google Scholar] [PubMed]
- Meng, X.J.; Wiseman, B.; Elvinger, F.; Guenette, D.K.; Toth, T.E.; Engle, R.E.; Emerson, S.U.; Purcell, R.H. Prevalence of antibodies to hepatitis E virus in veterinarians working with swine and in normal blood donors in the United States and other countries. J. Clin. Microbiol. 2002, 40, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Olsen, B.; Axelsson-Olsson, D.; Thelin, A.; Weiland, O. Unexpected high prevalence of IgG-antibodies to hepatitis E virus in Swedish pig farmers and controls. Scand. J. Infect. Dis. 2006, 38, 55–58. [Google Scholar] [CrossRef] [PubMed]
- Drobeniuc, J.; Favorov, M.O.; Shapiro, C.N.; Bell, B.P.; Mast, E.E.; Dadu, A.; Culver, D.; Iarovoi, P.; Robertson, B.H.; Margolis, H.S. Hepatitis E virus antibody prevalence among persons who work with swine. J. Infect. Dis. 2001, 184, 1594–1597. [Google Scholar] [CrossRef] [PubMed]
- Traoré, K.A.; Ouoba, J.B.; Huot, N.; Rogée, S.; Dumarest, M.; Traoré, A.S.; Pavio, N.; Barro, N.; Roques, P. Hepatitis E virus exposure is increased in pork butchers from Burkina Faso. Am. J. Trop. Med. Hyg. 2015, 93, 1356–1359. [Google Scholar] [CrossRef] [PubMed]
- Carpentier, A.; Chaussade, H.; Rigaud, E.; Rodriguez, J.; Berthault, C.; Boué, F.; Tognon, M.; Touzé, A.; Garcia-Bonnet, N.; Choutet, P.; et al. High hepatitis E virus seroprevalence in forestry workers and in wild boars in France. J. Clin. Microbiol. 2012, 50, 2888–2893. [Google Scholar] [CrossRef] [PubMed]
- Dremsek, P.; Wenzel, J.J.; Johne, R.; Ziller, M.; Hofmann, J.; Groschup, M.H.; Werdermann, S.; Mohn, U.; Dorn, S.; Motz, M.; et al. Seroprevalence study in forestry workers from eastern Germany using novel genotype 3- and rat hepatitis E virus-specific immunoglobulin G ELISAs. Med. Microbiol. Immunol. 2012, 201, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Schielke, A.; Ibrahim, V.; Czogiel, I.; Faber, M.; Schrader, C.; Dremsek, P.; Ulrich, R.G.; Johne, R. Hepatitis E virus antibody prevalence in hunters from a district in Central Germany, 2013: A cross-sectional study providing evidence for the benefit of protective gloves during disembowelling of wild boars. BMC Infect. Dis. 2015, 15, 440. [Google Scholar] [CrossRef] [PubMed]
- Cui, W.; Sun, Y.; Xu, A.; Gao, R.; Gong, L.; Zhang, L.; Jiang, M. Hepatitis E seroprevalence and related risk factors among seafood processing workers: A cross-sectional survey in Shandong Province, China. Int. J. Infect. Dis. 2016, 49, 62–66. [Google Scholar] [CrossRef] [PubMed]
- Renou, C.; Cadranel, J.-F.; Bourlière, M.; Halfon, P.; Ouzan, D.; Rifflet, H.; Carenco, P.; Harafa, A.; Bertrand, J.J.; Boutrouille, A.; et al. Possible Zoonotic Transmission of Hepatitis E from Pet Pig to Its Owner. Emerg. Infect. Dis. 2007, 13, 1094–1096. [Google Scholar] [CrossRef] [PubMed]

© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Doceul, V.; Bagdassarian, E.; Demange, A.; Pavio, N. Zoonotic Hepatitis E Virus: Classification, Animal Reservoirs and Transmission Routes. Viruses 2016, 8, 270. https://doi.org/10.3390/v8100270
Doceul V, Bagdassarian E, Demange A, Pavio N. Zoonotic Hepatitis E Virus: Classification, Animal Reservoirs and Transmission Routes. Viruses. 2016; 8(10):270. https://doi.org/10.3390/v8100270
Chicago/Turabian StyleDoceul, Virginie, Eugénie Bagdassarian, Antonin Demange, and Nicole Pavio. 2016. "Zoonotic Hepatitis E Virus: Classification, Animal Reservoirs and Transmission Routes" Viruses 8, no. 10: 270. https://doi.org/10.3390/v8100270
APA StyleDoceul, V., Bagdassarian, E., Demange, A., & Pavio, N. (2016). Zoonotic Hepatitis E Virus: Classification, Animal Reservoirs and Transmission Routes. Viruses, 8(10), 270. https://doi.org/10.3390/v8100270



