Co-Detection of ADV, Influenza B, and HPIV: Independent Risk Factors for SMPP with Changes in NPIs
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Specimen Collection
2.3. Detection of Nine Respiratory Pathogens by RT-PCR Capillary Electrophoresis Fragment Analysis
2.4. Definition of MPP and Severe MPP
2.5. Statistical Analysis
3. Results
3.1. Demographic Characteristics in Children with ARTIs
3.2. Epidemiological Trend of MP in Children with ARTIs
3.3. Co-Detection of MP with Other Respiratory Pathogens
3.4. Risk Factors for MP Epidemic and Severe MP Pneumonia
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MP | mycoplasma pneumoniae |
| ARTIs | acute respiratory tract infections |
| SMPP | severe mycoplasma pneumoniae pneumonia |
| NPIs | non-pharmaceutical interventions |
| OR | odds ratio |
| CI | confidence interval |
| CAP | community-acquired pneumonia |
| SCH | Children’s Hospital of Soochow University |
| RT-PCR | real-time polymerase chain reaction |
| HPIV | human parainfluenza virus |
| HBoV | human bocaparvovirus |
| InfA | influenza A |
| InfB | influenza virus B |
| HRV | human rhinovirus |
| HRSV | human syncytial |
| HMPV | human metapneumovirus |
| ADV | adenovirus |
References
- Jain, S.; Self, W.H.; Wunderink, R.G.; Fakhran, S.; Balk, R.; Bramley, A.M.; Chappell, J.D. Community-Acquired Pneumonia Requiring Hospitalization among, U.S. Adults. N. Engl. J. Med. 2015, 373, 415–427. [Google Scholar] [CrossRef] [PubMed]
- Sauteur, P.M.M.; Jacobs, B.C.; Spuesens, E.B.M.; Jacobs, E.; Nadal, D.; Vink, C.; van Rossum, A.M.C.; Haldar, K. Antibody responses to Mycoplasma pneumoniae: Role in pathogenesis and diagnosis of encephalitis. PLoS Pathog. 2014, 10, e1003983. [Google Scholar] [CrossRef]
- Wang, X.; Li, M.; Luo, M.; Luo, Q.; Kang, L.; Xie, H.; Wang, Y.; Yu, X.; Li, A.; Dong, M.; et al. Mycoplasma pneumoniae triggers pneumonia epidemic in autumn and winter in Beijing: A multicentre, population-based epidemiological study between 2015 and 2020. Emerg. Microbes Infect. 2022, 11, 1508–1517. [Google Scholar] [CrossRef]
- Wang, M.; Wang, Y.; Yan, Y.; Zhu, C.; Huang, L.; Shao, X.; Xu, J.; Zhu, H.; Sun, X.; Ji, W.; et al. Clinical and laboratory profiles of refractory Mycoplasma pneumoniae pneumonia in children. Int. J. Infect. Dis. 2014, 29, 18–23. [Google Scholar] [CrossRef]
- Waites, K.B.; Talkington, D.F. Mycoplasma pneumoniae and its role as a human pathogen. Clin. Microbiol. Rev. 2004, 17, 697–728. [Google Scholar] [CrossRef]
- Gao, J.; Xu, L.; Xu, B.; Xie, Z.; Shen, K. Human adenovirus Coinfection aggravates the severity of Mycoplasma pneumoniae pneumonia in children. BMC Infect. Dis. 2020, 20, 420. [Google Scholar] [CrossRef]
- Lenglet, A.; Herrador, Z.; Magiorakos, A.P.; Leitmeyer, K.; Coulombier, D. European Working Group on Mycoplasma pneumoniae surveillance. Surveillance status and recent data for Mycoplasma pneumoniae infections in the European Union and European Economic Area, January 2012. Euro Surveill. 2012, 17, 20075. [Google Scholar] [CrossRef] [PubMed]
- Kutty, P.K.; Jain, S.; Taylor, T.H.; Bramley, A.M.; Diaz, M.H.; Ampofo, K.; Arnold, S.R.; Williams, D.J.; Edwards, K.M.; A McCullers, J.; et al. Mycoplasma pneumoniae Among Children Hospitalized with Community-acquired Pneumonia. Clin. Infect. Dis. 2019, 68, 5–12. [Google Scholar] [CrossRef]
- Meyer Sauteur, P.M.; Beeton, M.L.; Uldum, S.A.; Bossuyt, N.; Vermeulen, M.; Loens, K.; Pereyre, S.; Bébéar, C.; Keše, D.; Day, J.; et al. Mycoplasma pneumoniae detections before and during the COVID-19 pandemic: Results of a global survey, 2017 to 2021. Euro Surveill. 2022, 27, 2100746. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Xu, H.; Zhang, Z.; Liu, Q.; Zhao, R.; Zheng, G.; Wu, X. The Epidemiology of Pathogens in Community-Acquired Pneumonia Among Children in Southwest China Before, During and After COVID-19 Non-pharmaceutical Interventions: A Cross-Sectional Study. Influenza Other Respir. Viruses 2024, 18, e13361. [Google Scholar] [CrossRef]
- Yang, M.C.; Su, Y.T.; Chen, P.H.; Tsai, C.C.; Lin, T.I.; Wu, J.R. Changing patterns of infectious diseases in children during the COVID-19 pandemic. Front. Cell Infect. Microbiol. 2023, 13, 1200617. [Google Scholar] [CrossRef] [PubMed]
- Association RGotPBotCM; Clinics EBotCJoPP. Expert consensus on the diagnosis and management of Mycoplasma pneumoniae pneumonia in children. Chin. J. Pract. Paediatr. 2015, 30, 1304–1308. [Google Scholar]
- China NHCotPsRo. Medicine SAoC. diagnosis and treatment standard of community acquired pneumonia in Children (2019 Edition). Chin. J. Clin. Infect. Dis. 2019, 12, 6–13. [Google Scholar]
- Li, H.; Yang, Y.; Tao, R.; Shang, S. Analyzing infections caused by 11 respiratory pathogens in children: Pre- and post-COVID-19 pandemic trends in China. J. Med. Virol. 2024, 96, e29929. [Google Scholar] [CrossRef]
- Zhu, X.; Liu, P.; Yu, H.; Wang, L.; Zhong, H.; Xu, M.; Lu, L.; Jia, R.; Su, L.; Cao, L.; et al. An outbreak of Mycoplasma pneumoniae in children after the COVID-19 pandemic, Shanghai, China, 2023. Front Microbiol 2024, 15, 1427702. [Google Scholar] [CrossRef]
- Zhang, X.B.; He, W.; Gui, Y.H.; Lu, Q.; Yin, Y.; Zhang, J.H.; Dong, X.Y.; Wang, Y.W.; Ye, Y.Z.; Xu, H.; et al. Current Mycoplasma pneumoniae epidemic among children in Shanghai: Unusual pneumonia caused by usual pathogen. World J. Pediatr. 2024, 20, 5–10. [Google Scholar] [CrossRef]
- Liu, S.; Lei, Y.; Chen, X.; Wen, Z.; Mei, B. Epidemiological characteristics of respiratory pathogens infections among children after the removal of non-pharmaceutical interventions in central China. Virol. J. 2024, 21, 303. [Google Scholar] [CrossRef]
- Cheng, Y.; Cheng, Y.; Dai, S.; Hou, D.; Ge, M.; Zhang, Y.; Fan, L.; Pei, Y.; Yu, L.; Xue, G.; et al. The Prevalence of Mycoplasma Pneumoniae Among Children in Beijing Before and During the COVID-19 Pandemic. Front. Cell Infect. Microbiol. 2022, 12, 854505. [Google Scholar] [CrossRef]
- Miao, Y.; Li, J.; Huang, L.; Shi, T.; Jiang, T. Mycoplasma pneumoniae detections in children with acute respiratory infection, 2010–2023: A large sample study in China. Ital. J. Pediatr. 2025, 51, 11. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Chen, R.; Mo, D.; Wang, Y.; Xia, Y.; He, M. Analysis of Mycoplasma pneumoniae pneumonia and viral coinfection in southern Guangzhou after the COVID-19 pandemic. J. Thorac. Dis. 2024, 16, 6789–6798. [Google Scholar] [CrossRef]
- Wang, W.; Luo, X.; Ren, Z.; Fu, X.; Chen, Y.; Wang, W.; Bao, Y.; Zheng, Y.; Cao, K.; Chen, J. Impact of COVID-19 pandemic measures on hospitalizations and epidemiological patterns of twelve respiratory pathogens in children with acute respiratory infections in southern China. BMC Infect. Dis. 2025, 25, 103. [Google Scholar] [CrossRef]
- Zhao, P.; Zhang, Y.; Wang, J.; Li, Y.; Wang, Y.; Gao, Y.; Zhao, M.; Zhao, M.; Tan, H.; Tie, Y.; et al. Epidemiology of respiratory pathogens in patients with acute respiratory infections during the COVID-19 pandemic and after easing of COVID-19 restrictions. Microbiol. Spectr. 2024, 12, e0116124. [Google Scholar] [CrossRef]
- Pinky, L.; Dobrovolny, H.M. Coinfections of the Respiratory Tract: Viral Competition for Resources. PLoS ONE 2016, 11, e0155589. [Google Scholar] [CrossRef]
- Chiu, C.Y.; Chen, C.J.; Wong, K.S.; Tsai, M.H.; Chiu, C.H.; Huang, Y.C. Impact of bacterial and viral coinfection on mycoplasmal pneumonia in childhood community-acquired pneumonia. J. Microbiol. Immunol. Infect. 2015, 48, 51–56. [Google Scholar] [CrossRef]
- Scotta, M.C.; Chakr, V.C.; de Moura, A.; Becker, R.G.; de Souza, A.P.; Jones, M.H.; Pinto, L.A.; Sarria, E.E.; Pitrez, P.M.; Stein, R.T.; et al. Respiratory viral coinfection and disease severity in children: A systematic review and meta-analysis. J. Clin. Virol. 2016, 80, 45–56. [Google Scholar] [CrossRef]
- Lim, F.J.; de Klerk, N.; Blyth, C.C.; Fathima, P.; Moore, H.C. Systematic review and meta-analysis of respiratory viral coinfections in children. Respirology 2016, 21, 648–655. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.; Xu, B.P.; Shen, K.L. Effects of bacterial and viral co-infections of mycoplasma pneumoniae pneumonia in children: Analysis report from Beijing Children’s Hospital between 2010 and 2014. Int. J. Clin. Exp. Med. 2015, 8, 15666–15674. [Google Scholar]
- Cilla, G.; Oñate, E.; Perez-Yarza, E.G.; Montes, M.; Vicente, D.; Perez-Trallero, E. Viruses in community-acquired pneumonia in children aged less than 3 years old: High rate of viral coinfection. J. Med. Virol. 2008, 80, 1843–1849. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Lin, L.; Zhang, N.; Yang, Y. Adenovirus and Mycoplasma pneumoniae co-infection as a risk factor for severe community-acquired pneumonia in children. Front. Pediatr. 2024, 12, 1337786. [Google Scholar] [CrossRef]
- Li, F.; Zhang, Y.; Shi, P.; Cao, L.; Su, L.; Fu, P.; Abuduxikuer, K.; Wang, L.; Wang, Y.; Lu, R.; et al. Mycoplasma pneumoniae and Adenovirus Coinfection Cause Pediatric Severe Community-Acquired Pneumonia. Microbiol. Spectr. 2022, 10, e0002622. [Google Scholar] [CrossRef] [PubMed]
- Canter, J.A.; Tulman, E.R.; Beaudet, J.; Lee, D.H.; May, M.; Szczepanek, S.M.; Geary, S.J. Transcriptional and Pathological Host Responses to Coinfection with Virulent or Attenuated Mycoplasma gallisepticum and Low-Pathogenic Avian Influenza A Virus in Chickens. Infect. Immun. 2019, 88, e00607-19. [Google Scholar] [CrossRef] [PubMed]
- Mehinagic, K.; Pilo, P.; Vidondo, B.; Stokar-Regenscheit, N. Coinfection of Swiss cattle with bovine parainfluenza virus 3 and Mycoplasma bovis at acute and chronic stages of bovine respiratory disease complex. J. Vet. Diagn. Investig. 2019, 31, 674–680. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Yang, C.; Sun, P.; Zeng, F.; Wang, Q.; Wu, J.; Fang, C.; Zhang, C.; Wang, J.; Gu, Y.; et al. Epidemic features and megagenomic analysis of childhood Mycoplasma pneumoniae post COVID-19 pandemic: A 6-year study in southern China. Emerg. Microbes Infect. 2024, 13, 2353298. [Google Scholar] [CrossRef] [PubMed]
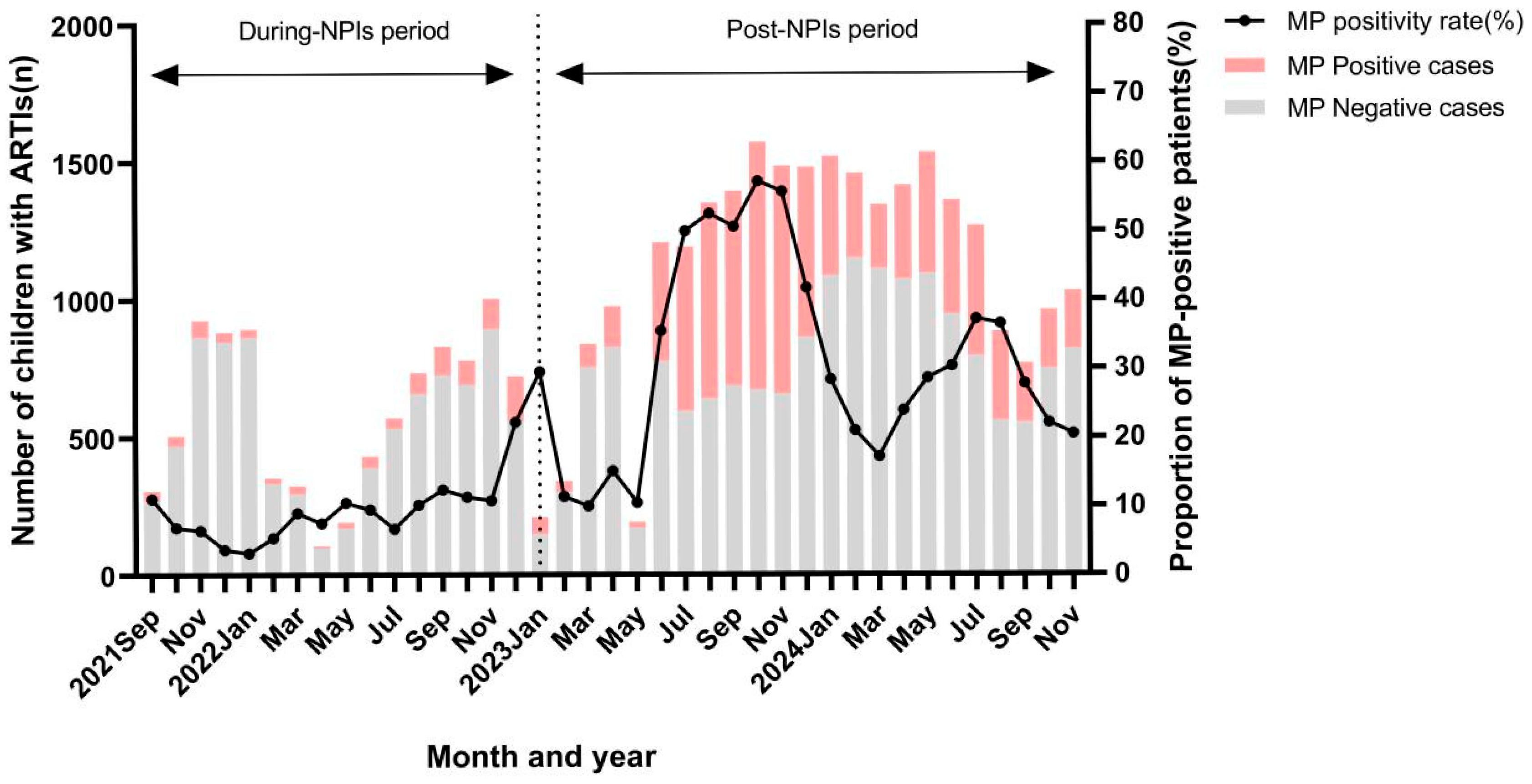
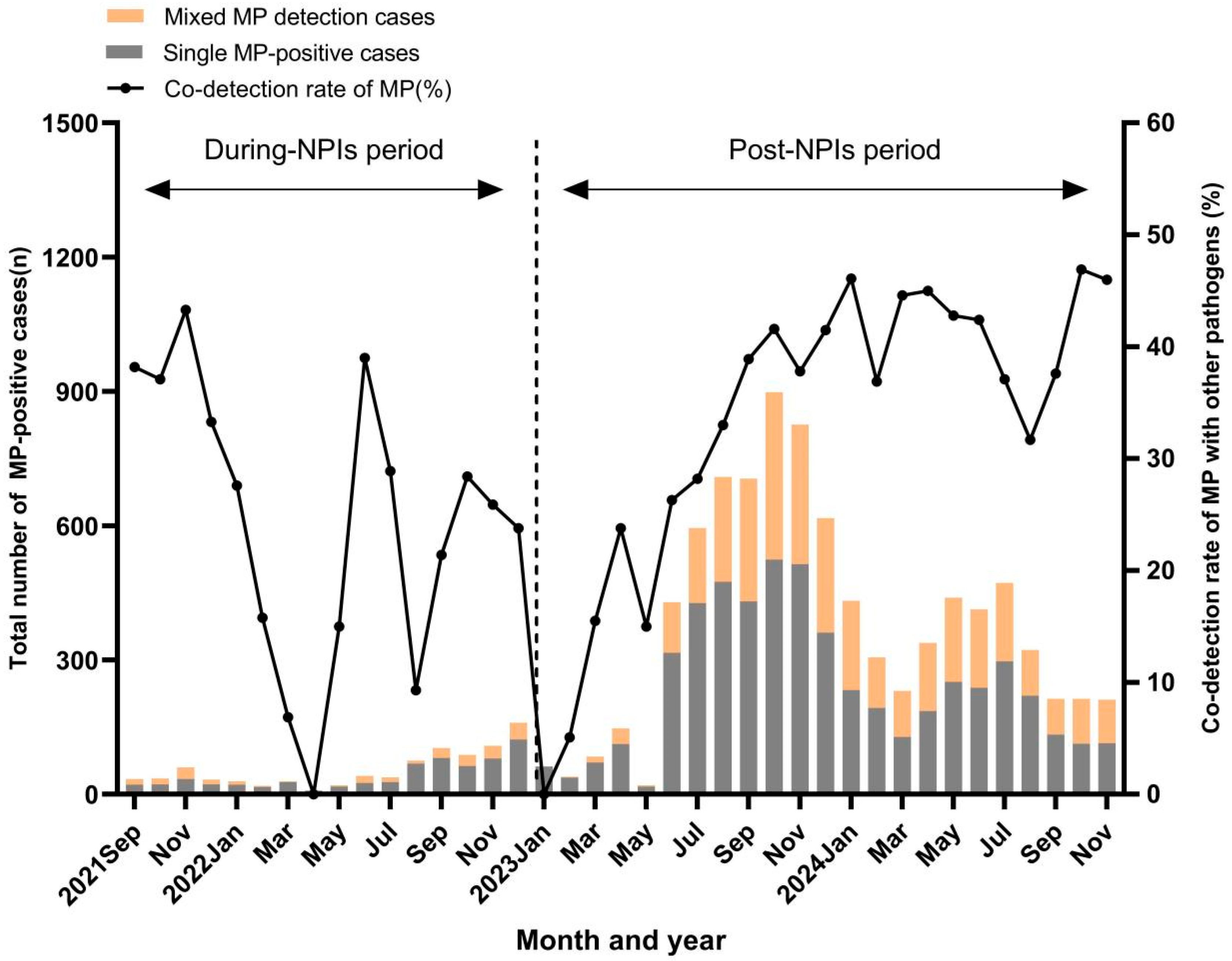
| Category | During-NPIs Period (n = 9554) | Post-NPIs Period (n = 26,826) | p |
|---|---|---|---|
| Number of patients with ARTIs (per month) | 597 ± 286 | 1120 ± 420 | <0.01 |
| Sex (male, n%) | 5394 (56.5) | 14,487 (54.0) | <0.01 |
| Age (years, n%) | |||
| ≤1 | 3290 (34.4) | 4714 (17.5) | <0.01 |
| 1–≤3 | 2835 (29.7) | 4925 (18.4) | <0.01 |
| 3–≤6 | 2246 (23.5) | 7382 (27.5) | <0.01 |
| >6 | 1183 (12.4) | 9805 (36.6) | <0.01 |
| Positive rate of MP | 880 (9.2) | 8942 (33.3) | <0.01 |
| Category | During-NPIs Period (n = 880) | Post-NPIs Period (n = 8942) | p |
|---|---|---|---|
| Sex (male, n%) | 469 (53.3) | 4588 (51.3) | 0.260 |
| Age (years, n%) | |||
| ≤1 | 70 (8.0) | 375 (4.2) | <0.01 |
| 1–≤3 | 156 (17.7) | 928 (10.4) | <0.01 |
| 3–≤6 | 236 (26.8) | 2371 (26.5) | 0.846 |
| >6 | 391 (44.5) | 5268 (58.9) | <0.01 |
| Detection rate of other pathogens | 226 (25.7) | 3268 (36.5) | <0.01 |
| Pathogens | During-NPIs Period | Post-NPIs Period | ||||
|---|---|---|---|---|---|---|
| MP Positive Group (n = 880) | MP Negative Group (n = 8674) | p | MP Positive Group (n = 8942) | MP Negative Group (n = 17,884) | p | |
| InfA (n,%) | 13 (1.5) | 329 (3.8) | <0.01 | 221 (2.5) | 1195 (6.7) | <0.01 |
| InfB (n,%) | 2 (0.2) | 288 (3.3) | <0.01 | 98 (1.1) | 528 (3.0) | <0.01 |
| HPIV (n,%) | 21 (2.4) | 1005 (11.6) | <0.01 | 457 (5.1) | 1703 (9.5) | <0.01 |
| HBoV (n,%) | 19 (2.2) | 564 (6.5) | <0.01 | 89 (1.0) | 419 (2.3) | <0.01 |
| HRV (n,%) | 160 (18.2) | 2123 (24.5) | <0.01 | 1969 (22.0) | 4976 (27.8) | <0.01 |
| ADV (n,%) | 3 (0.3) | 194 (2.2) | <0.01 | 554 (6.2) | 1935 (10.8) | <0.01 |
| HRSV (n,%) | 18 (2.0) | 1591 (18.3) | <0.01 | 311 (3.5) | 3242 (18.1) | <0.01 |
| HMPV (n,%) | 20 (2.3) | 1052 (12.1) | <0.01 | 143 (1.6) | 803 (4.5) | <0.01 |
| p | <0.01 | <0.01 | <0.01 | <0.01 | ||
| Variable | Model 1 | Model 2 | ||
|---|---|---|---|---|
| OR (95%CI) | p | OR (95%CI) | p | |
| Sex | ||||
| Female | 1 (reference) | 1 (reference) | ||
| Male | 0.840 (0.802–0.880) | <0.01 | 0.986 (0.934–1.040) | 0.986 |
| Age (year) | ||||
| ≤1 | 1 (reference) | 1 (reference) | ||
| 1–≤3 | 2.758 (2.458–3.095) | <0.01 | 2.752 (2.443–3.101) | <0.01 |
| 3–≤6 | 6.397 (5.756–7.110) | <0.01 | 5.140 (4.603–5.739) | <0.01 |
| >6 | 18.038 (16.278–19.989) | <0.01 | 10.795 (9.690–12.026) | <0.01 |
| Period | ||||
| During-NPIs | 1 (reference) | 1 (reference) | ||
| Post-NPIs | 4.928 (4.578–5.306) | <0.01 | 3.697 (3.408–4.011) | <0.01 |
| Season | ||||
| Spring | 1 (reference) | 1 (reference) | ||
| Summer | 2.197 (2.047–2.357) | <0.01 | 2.135 (1.971–2.313) | <0.01 |
| Autumn | 1.815 (1.695–1.943) | <0.01 | 2.228 (2.060–2.410) | <0.01 |
| Winter | 1.154 (1.068–1.247) | <0.01 | 1.567 (1.432–1.714) | <0.01 |
| Pathogen | ||||
| InfA | ||||
| Negative | 1 (reference) | 1 (reference) | ||
| Positive | 0.401 (0.349–0.461) | <0.01 | 0.232 (0.200–0.270) | <0.01 |
| InfB | ||||
| Negative | 1 (reference) | 1 (reference) | ||
| Positive | 0.324 (0.263–0.400) | <0.01 | 0.220 (0.176–0.276) | <0.01 |
| HPIV | ||||
| Negative | 1 (reference) | 1 (reference) | ||
| Positive | 0.451 (0.408–0.498) | <0.01 | 0.437 (0.392–0.489) | <0.01 |
| HBoV | ||||
| Negative | 1 (reference) | 1 (reference) | ||
| Positive | 0.289 (0.237–0.353) | <0.01 | 0.466 (0.376–0.578) | <0.01 |
| HRV | ||||
| Negative | 1 (reference) | 1 (reference) | ||
| Positive | 0.759 (0.718–0.802) | <0.01 | 0.590 (0.554–0.629) | <0.01 |
| ADV | ||||
| Negative | 1 (reference) | 1 (reference) | ||
| Positive | 0.690 (0.626–0.760) | <0.01 | 0.358 (0.322–0.397) | <0.01 |
| HRSV | ||||
| Negative | 1 (reference) | 1 (reference) | ||
| Positive | 0.156 (0.139–0.175) | <0.01 | 0.250 (0.221–0.282) | <0.01 |
| HMPV | ||||
| Negative | 1 (reference) | 1 (reference) | ||
| Positive | 0.225 (0.191–0.264) | <0.01 | 0.262 (0.220–0.311) | <0.01 |
| Variable | Model 1 | Model 2 | ||
|---|---|---|---|---|
| OR (95%CI) | p | OR (95%CI) | p | |
| Sex | ||||
| Female | 1 (reference) | |||
| Male | 1.166 (0.804–1.690) | 0.417 | ||
| Age (year) | ||||
| ≤1 | 1 (reference) | 1 (reference) | ||
| 1–≤3 | 0.446 (0.188–1.058) | 0.067 | 0474 (0.199–1.132) | 0.093 |
| 3–≤6 | 0.501 (0.243–1.033) | 0.061 | 0.542 (0.258–1.138) | 0.106 |
| >6 | 0.490 (0.250–0.961) | 0.038 | 0.514 (0.255–1.037) | 0.063 |
| Period | ||||
| During-NPIs | 1 (reference) | |||
| Post-NPIs | 0.921 (0.492–1.721) | 0.795 | ||
| Season | ||||
| Spring | 1 (reference) | |||
| Summer | 0.703 (0.390–1.269) | 0.242 | ||
| Autumn | 0.972 (0.564–1.677) | 0.919 | ||
| Winter | 1.243 (0.685–2.255) | 0.474 | ||
| Pathogen | ||||
| InfA | ||||
| Negative | 1 (reference) | |||
| Positive | 1.498 (0.548–4.099) | 0.431 | ||
| InfB | ||||
| Negative | 1 (reference) | 1 (reference) | ||
| Positive | 3.641 (1.316–10.074) | 0.013 | 3.009 (1.041–8.697) | 0.042 |
| HPIV | ||||
| Negative | 1 (reference) | 1 (reference) | ||
| Positive | 2.113 (1.127–3.962) | 0.020 | 2.226 (1.170–4.235) | 0.015 |
| HBoV | ||||
| Negative | 1 (reference) | |||
| Positive | 0.794 (0.110–5.739) | 0.819 | ||
| HRV | ||||
| Negative | 1 (reference) | |||
| Positive | 0.767 (0.472–1.245) | 0.283 | ||
| ADV | ||||
| Negative | 1 (reference) | 1 (reference) | ||
| Positive | 1.978 (1.081–3.620) | 0.027 | 2.035 (1.105–3.750) | 0.023 |
| HRSV | ||||
| Negative | 1 (reference) | |||
| Positive | 1.614 (0.704–3.700) | 0.258 | ||
| HMPV | ||||
| Negative | 1 (reference) | |||
| Positive | 1.059 (0.259–4.324) | 0.936 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, L.; Shi, T. Co-Detection of ADV, Influenza B, and HPIV: Independent Risk Factors for SMPP with Changes in NPIs. Viruses 2025, 17, 1266. https://doi.org/10.3390/v17091266
Huang L, Shi T. Co-Detection of ADV, Influenza B, and HPIV: Independent Risk Factors for SMPP with Changes in NPIs. Viruses. 2025; 17(9):1266. https://doi.org/10.3390/v17091266
Chicago/Turabian StyleHuang, Linlin, and Ting Shi. 2025. "Co-Detection of ADV, Influenza B, and HPIV: Independent Risk Factors for SMPP with Changes in NPIs" Viruses 17, no. 9: 1266. https://doi.org/10.3390/v17091266
APA StyleHuang, L., & Shi, T. (2025). Co-Detection of ADV, Influenza B, and HPIV: Independent Risk Factors for SMPP with Changes in NPIs. Viruses, 17(9), 1266. https://doi.org/10.3390/v17091266







