Abstract
Global efforts to eradicate smallpox—an Orthopoxvirus infection—began in the mid-20th century, with the last naturally occurring case reported in 1977. This was achieved through global solidarity efforts that expanded the smallpox eradication vaccination program. Approximately 50 years following the cessation of mass smallpox vaccination and in the absence of access to a sustainable boosting program, the population immunologically naïve to Orthopoxviruses has increased significantly. With increasing global movements and travels, we argue that the emergence of two back-to-back yet distinct mpox epidemics in the 21st century is a sign of humanity’s lost herd immunity to Orthopoxviruses. This needs concerted efforts to restore.
1. Introduction
Orthopoxviruses are a group of zoonotic, phylogenetically related, double-stranded DNA viruses. The genus Orthopoxvirus belongs to the family Poxviridae, under which there are several genera, including Parapoxvirus, Avipoxvirus, Capripoxvirus, Leporipoxvirus, Suipoxvirus, Molluscipoxvirus, and Yatapoxvirus. Historically, the most widely reported infection caused by an Orthopoxvirus is smallpox—a disease of humans from time immemorial. The global eradication program for smallpox started in the mid-20th century, with the World Health Organisation (WHO) proposing a global smallpox eradication program in 1959 [1]. The timeline of events towards eradication is summarized in Figure 1.
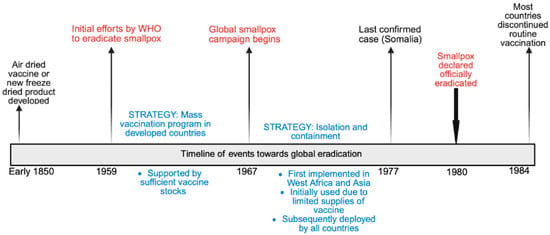
Figure 1.
Timeline of events towards global smallpox eradication.
Since 2022, humanity has faced two distinct but consecutive mpox (formerly monkeypox) outbreaks caused by the mpox virus (MPXV) [2,3]. The first outbreak (2022–2023) driven by Clade IIb spread, rapidly across Europe and North America. The current epidemic driven by Clade Ib began in August 2023 in South Kivu Province, Democratic Republic of Congo (DRC), and has since spread rapidly across East, Central, and Southern Africa [4,5,6].
These two discrete but closely related epidemics of mpox have not only increased interest in the role of Orthopoxviruses in causing pandemics but also serve as an indication of humanity’s prevailing risk to and/or immunity against Orthopoxviruses [7]. A growing school of thought attributes recent mpox outbreaks to waning Orthopoxvirus immunity, particularly among populations born after routine smallpox vaccination ceased [8,9]. It is estimated that up to 70% of the world’s population is no longer protected against smallpox and closely related Orthopoxviruses through cross-immunity [10].
Vaccination has emerged as a medical countermeasure for responding to mpox; however, this is affected by the high cost and unavailability of enough doses of the common vaccines like Bavarian Nordic’s MVA-BN vaccine that was initially licensed for smallpox. Evidence shows smallpox vaccine-induced immunity has the potential to protect against other Orthopoxviridae viruses, including mpox [11,12,13,14] (Table 1).

Table 1.
Summary of studies demonstrating evidence of mpox and cross protection with smallpox and smallpox vaccines.
2. A Case for a Global Vaccination Program to Restore Humanity’s Herd Immunity Against Orthopoxviruses: Strategies for Restoring Orthopoxviruses Herd Immunity
In the 1960s, the initial goal of the smallpox vaccination program was to vaccinate 80% of the population to achieve herd immunity [19]. Given the dwindled herd immunity of most of the world’s population, we argue that the world needs to come together to see to it that this shield is restored rather than leaving the mandate to individual countries, some of which are too poor to afford the vaccines. Below we highlight the strategies for restoring Orthopoxvirus herd immunity.
3. Targeted Smallpox Vaccination Strategies
The ring vaccination strategy has been credited for the smallpox eradication and involves creating a buffer of immunity around a case by vaccinating the contacts and ultimately preventing disease spread. The current mpox epidemic gives the world an opportunity to deploy the same strategy by utilizing proven smallpox vaccines. An epidemiology-based approach that hinges on robust case-finding strategies to identify mpox cases and identification and vaccination of high-risk individuals could potentially build herd immunity in a global population that lacks immunity against Orthopoxviruses. Such a strategy should be supported by dose-sparing approaches given the low stockpiles of these vaccines to ensure that high vaccination coverage [20].
4. Strategies to Ensure Global Equity and Access, Particularly in Endemic and Low-Income Regions
In the short term, there is a need for a clear demonstration of global solidarity to respond to the mpox outbreak. Such solidarity should acknowledge the failures witnessed during the COVID-19 pandemic, where low-resourced countries lacked sufficient vaccines for their populations. A globally coordinated framework to ensure mpox vaccines are available to endemic and low-income countries should be developed. Implementation should be informed by epidemiological need rather than financial capacity. A shift from centralized structures to structures that promote regional coordination and context-driven responses should be supported. This has been demonstrated on the African continent, where the Africa Centers for Disease Control and Prevention (Africa CDC) and WHO Regional Office for Africa (WHO/AFRO) have coordinated the mpox continental preparedness and response to achieve localized and equitable health responses [21].
5. Development of Newer, Cheaper, Safer and More Effective Vaccines: Safer, More Effective Vaccines with Broader Cross-Protection
In the medium to long term, mpox vaccine research and development should be scaled up, particularly in Africa. Vaccine developers should consider expanding their manufacturing footprint in Africa, where more than 70% of health technology requirements are imported. Countries should allocate sufficient research and development funding through several mechanisms such as multilateral financing schemes, assurance of demand to entice private sector investments, strengthened public–private partnerships, and deployment of policies that incentivize local investments in R&D [22]. African countries should be included in global vaccine clinical trials on an equal footing, as currently they contribute a paltry 3% of the trials [23].
6. Risk Communication and Community Engagement to Combat Vaccine Hesitancy and Misinformation
Additionally, investments in vaccine research and development should be supplemented by community engagement and participation in mpox vaccine deployment. This should be achieved through a well-developed and implemented Risk Communication and Community Engagement (RCCE) strategy. The RCCE strategy should be multidisciplinary and informed by the prevailing epidemiological, contextual, social, economic, cultural, and behavioral considerations. The RCCE strategy could leverage the available traditional and digital media channels [24]. Countries should consider conducting research into social behaviors so as to understand human behaviors and inform the integration of risk communication and behavioral insights into the RCCE strategies [24].
7. Strengthen Integrated Surveillance Systems and Conduct Focused Research to Monitor and Track Progress of Herd Immunity
The ability of mpox vaccines to achieve individual and community-level (herd) immunity in various settings needs to be demonstrated and documented. In this regard, studies to assess the efficacy of the candidate mpox vaccines should be supported to provide data to support national rollout of mpox vaccination programs. With successful vaccine rollout, studies to measure the magnitude of any herd effects should become a priority, and this requires a high-quality mpox disease surveillance system. The systems should incorporate new technologies such as pathogen genomics, pathogen genetic sequence data sharing platforms, and advanced technologies like artificial intelligence.
8. Financing
The above strategies need to be supported by sustainable financing mechanisms. In the wake of the current global upheaval, securing domestic financial resources to support vaccine development, research, and vaccination campaigns is critical. Establishing vaccination strategies and programs that enhance health security through self-sustaining financial mechanisms and reduced reliance on external donors is essential.
9. Conclusions
Approximately 50 years since the smallpox vaccination program was halted following the eradication of smallpox, there has been no dedicated program for sustainable immune boosting. The majority of the world’s young people are at risk, with the Mpox outbreak being a warning sign of broader Orthopoxvirus vulnerability. The world urgently needs to restore humanity’s shield against her historically most notorious, indiscriminate, and highly fatal virus family. This is a call to action to rebuild population-level immunity through informed, equitable, and science-based approaches.
Author Contributions
Conceptualization: M.W., H.K.-B., B.K. and W.M.; Methodology: M.W. and H.K.-B.; Software: W.M.; Validation: O.O.O., A.S.G., N.N., N.K., M.O.F., B.K., S.L., Q.L. and C.I.; Resources: M.W., H.K.-B., B.K.; Data curation: H.K.-B. and W.M.; Writing—original draft preparation: M.W., H.K.-B. and W.M.; Writing—review and editing: B.K., O.O.O., A.S.G., N.N., N.K., M.O.F., S.L., Q.L. and C.I.; Visualization: W.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hopkins, D.R. The Greatest Killer: Smallpox in History, with a New Introduction; University of Chicago Press: Chicago, IL, USA, 1983; Available online: https://share.google/rDLPuIsJXi3JlUmNX (accessed on 25 March 2025).
- World Health Organisation (WHO). WHO Director-General Declares the Ongoing Monkeypox Outbreak a Public Health Emergency of International Concern. Published 2022. Available online: https://www.who.int/europe/news/item/23-07-2022-who-director-general-declares-the-ongoing-monkeypox-outbreak-a-public-health-event-of-international-concern (accessed on 26 March 2025).
- World Health Organization (WHO). Mpox (Monkeypox)—Democratic Republic of the Congo. Published 2023. Available online: https://www.who.int/emergencies/disease-outbreak-news/item/2023-DON493 (accessed on 26 March 2025).
- Masirika, L.M.; Udahemuka, J.C.; Schuele, L.; Nieuwenhuijse, D.F.; Ndishimye, P.; Boter, M.; Mbiribindi, J.B.; Kacita, C.; Lang, T.; Gortázar, C.; et al. Epidemiological and genomic evolution of the ongoing outbreak of clade Ib mpox virus in the eastern Democratic Republic of the Congo. Nat. Med. 2025, 31, 1459–1463. [Google Scholar] [CrossRef]
- Katoto, P.D.; Muttamba, W.; Bahizire, E.; Malembaka, E.B.; Bosa, H.K.; Kazadi, D.M.; Lubambo, G.; Siangoli, F.B.; Bakamutumaho, B.; Wayengera, M.; et al. Shifting transmission patterns of human mpox in South Kivu, DR Congo. Lancet Infect. Dis. 2024, 24, e354–e355. [Google Scholar] [CrossRef]
- Colson, P.; Penant, G.; Delerce, J.; Boschi, C.; Wurtz, N.; Bedotto, M.; Branger, S.; Brouqui, P.; Parola, P.; Lagier, J.; et al. Sequencing of monkeypox virus from infected patients reveals viral genomes with APOBEC3—Like editing, gene inactivation, and bacterial agents of skin superinfection. J. Med. Virol. 2023, 95, e28799. [Google Scholar] [CrossRef] [PubMed]
- Luciani, L.; Lapidus, N.; Amroun, A.; Falchi, A.; Souksakhone, C.; Mayxay, M.; Dubot-Pérès, A.; Villarroel, P.M.S.; Diarra, I.; Koita, O.; et al. Orthopoxvirus Seroprevalence and Infection Susceptibility in France, Bolivia, Laos, and Mali. Emerg. Infect. Dis. 2022, 28, 2463–2471. [Google Scholar] [CrossRef]
- Rimoin, A.W.; Mulembakani, P.M.; Johnston, S.C.; Smith, J.O.L.; Kisalu, N.K.; Kinkela, T.L.; Blumberg, S.; Thomassen, H.A.; Pike, B.L.; Fair, J.N.; et al. Major increase in human monkeypox incidence 30 years after smallpox vaccination campaigns cease in the Democratic Republic of Congo. Proc. Natl. Acad. Sci. USA 2010, 107, 16262–16267. [Google Scholar] [CrossRef] [PubMed]
- Taube, J.C.; Rest, E.C.; Lloyd-Smith, J.O.; Bansal, S. The global landscape of smallpox vaccination history and implications for current and future orthopoxvirus susceptibility: A modelling study. Lancet Infect. Dis. 2023, 23, 454–462. [Google Scholar] [CrossRef]
- Simpson, K.; Heymann, D.; Brown, C.S.; Edmunds, W.J.; Elsgaard, J.; Fine, P.; Hochrein, H.; Hoff, N.A.; Green, A.; Ihekweazu, C.; et al. Human monkeypox—After 40 years, an unintended consequence of smallpox eradication. Vaccine 2020, 38, 5077–5081. [Google Scholar] [CrossRef] [PubMed]
- Sagy, Y.W.; Zucker, R.; Hammerman, A.; Markovits, H.; Arieh, N.G.; Abu Ahmad, W.; Battat, E.; Ramot, N.; Carmeli, G.; Mark-Amir, A.; et al. Real-world effectiveness of a single dose of mpox vaccine in males. Nat. Med. 2023, 29, 748–752. [Google Scholar] [CrossRef] [PubMed]
- Bertran, M.; Andrews, N.; Davison, C.; Dugbazah, B.; Boateng, J.; Lunt, R.; Hardstaff, J.; Green, M.; Blomquist, P.; Turner, C.; et al. Effectiveness of one dose of MVA—BN smallpox vaccine against mpox in England using the case-coverage method: An observational study. Lancet Infect. Dis. 2023, 23, 828–835. [Google Scholar] [CrossRef]
- Morino, E.; Mine, S.; Tomita, N.; Uemura, Y.; Shimizu, Y.; Saito, S.; Suzuki, T.; Okumura, N.; Iwasaki, H.; Terada, J.; et al. Mpox Neutralizing Antibody Response to LC16m8 Vaccine in Healthy Adults. NEJM Evid. 2024, 3, EVIDoa2300290. [Google Scholar] [CrossRef]
- Tomita, N.; Terada-Hirashima, J.; Uemura, Y.; Shimizu, Y.; Iwasaki, H.; Yano, R.; Suzuki, T.; Saito, S.; Okumura, N.; Sugiura, W.; et al. An open-label, non-randomized study investigating the safety and efficacy of smallpox vaccine, LC16, as post-exposure prophylaxis for mpox. Hum. Vaccines Immunother. 2023, 19, 2242219. [Google Scholar] [CrossRef]
- Matusali, G.; Petruccioli, E.; Cimini, E.; Colavita, F.; Bettini, A.; Tartaglia, E.; Sbarra, S.; Meschi, S.; Lapa, D.; Francalancia, M.; et al. Evaluation of Cross-Immunity to the Mpox Virus Due to Historic Smallpox Vaccination. Vaccines 2023, 11, 1541. [Google Scholar] [CrossRef]
- Karem, K.L.; Reynolds, M.; Hughes, C.; Braden, Z.; Nigam, P.; Crotty, S.; Glidewell, J.; Ahmed, R.; Amara, R.; Damon, I.K. Monkeypox-Induced Immunity and Failure of Childhood Smallpox Vaccination To Provide Complete Protection. Clin. Vaccine Immunol. 2007, 14, 1318–1327. [Google Scholar] [CrossRef] [PubMed]
- Hammarlund, E.; Lewis, M.W.; Carter, S.V.; Amanna, I.; Hansen, S.G.; Strelow, L.I.; Wong, S.W.; Yoshihara, P.; Hanifin, J.M.; Slifka, M.K. Multiple diagnostic techniques identify previously vaccinated individuals with protective immunity against monkeypox. Nat. Med. 2005, 11, 1005–1011. [Google Scholar] [CrossRef] [PubMed]
- Huhn, G.D.; Bauer, A.M.; Yorita, K.; Graham, M.B.; Sejvar, J.; Likos, A.; Damon, I.K.; Reynolds, M.G.; Kuehnert, M.J. Clinical Characteristics of Human Monkeypox, and Risk Factors for Severe Disease. Clin. Infect. Dis. 2005, 41, 1742–1751. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Johnstone, J.; Loeb, M. Vaccine herd effect. Scand. J. Infect. Dis. 2011, 43, 683–689. [Google Scholar] [CrossRef]
- Dimitrov, D.; Adamson, B.; Matrajt, L. Evaluation of mpox vaccine dose-sparing strategies. PNAS Nexus 2023, 2, pgad095. [Google Scholar] [CrossRef]
- Ndembi, N.; Foláyan, M.O. A regional approach to addressing global health inequities. Lancet 2025, 405, 297–298. [Google Scholar] [CrossRef]
- Ndembi, N.; Karuna, S.; Cowden, J.; Cagigi, A.; Pilorget, A.; Moodley, A.; Ake, J.; Vasan, S.; Michael, N.; Kim, J.H. Accelerating vaccine development in Africa: Lessons from HIV research. Lancet 2025, 405, 1726–1728. [Google Scholar] [CrossRef]
- Ndembi, N.; Mekonen, T.T.; Folayan, M.O.; Dereje, N.; Kruger, A.; Fokam, J.; Temfack, E.; Raji, T.; Nachega, J.; Boum, Y.; et al. Strengthening and expanding capacities in clinical trials: Advancing pandemic prevention, preparedness and response in Africa. Nat. Commun. 2024, 15, 8662. [Google Scholar] [CrossRef]
- Njagi, D.; Nyikuri, M.; Ndembi, N. Integrating social behavioural insights in risk communication and community engagement approaches for better health outcomes in Africa. BMJ Glob. Health 2024, 9, e015548. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).