Hiding in Plain Sight: Genomic Characterization of a Novel Nackednavirus and Evidence of Diverse Adomaviruses in a Hyperpigmented Lesion of a Largemouth Bass (Micropterus nigricans)
Abstract
1. Introduction
2. Materials and Methods
3. Results
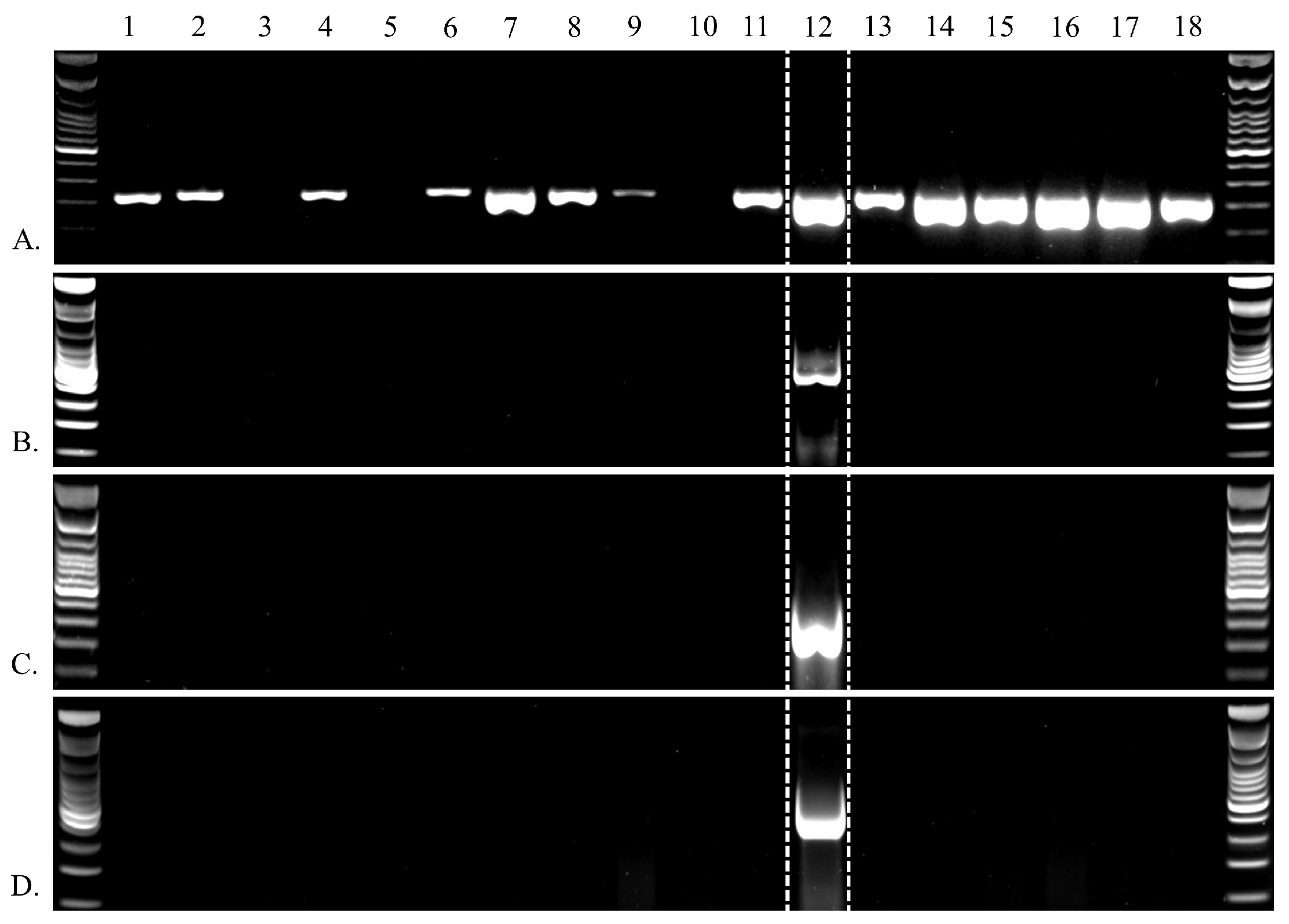
3.1. Genetic Data Availability
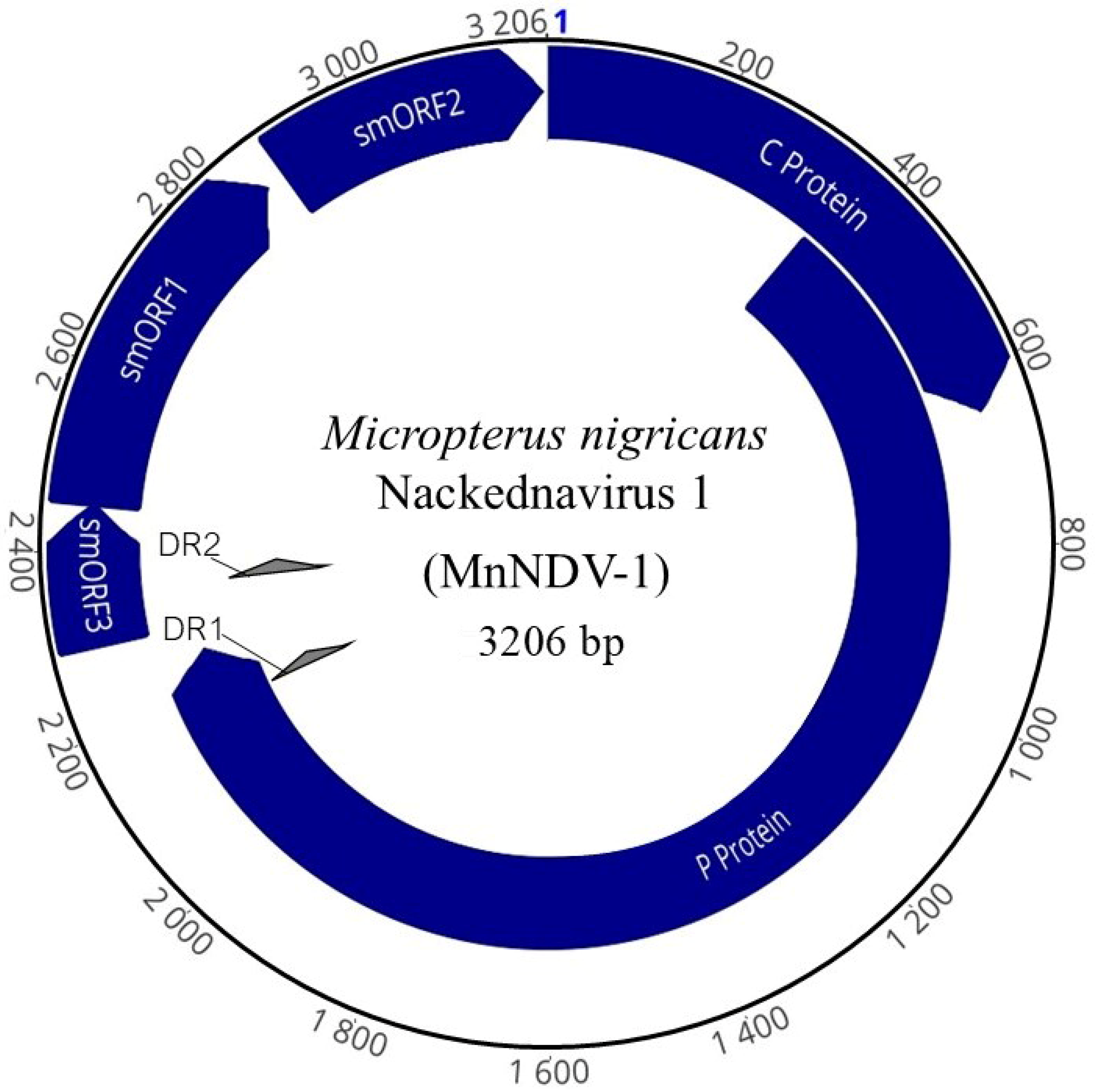
3.2. LMB Nackednavirus Genome Organization
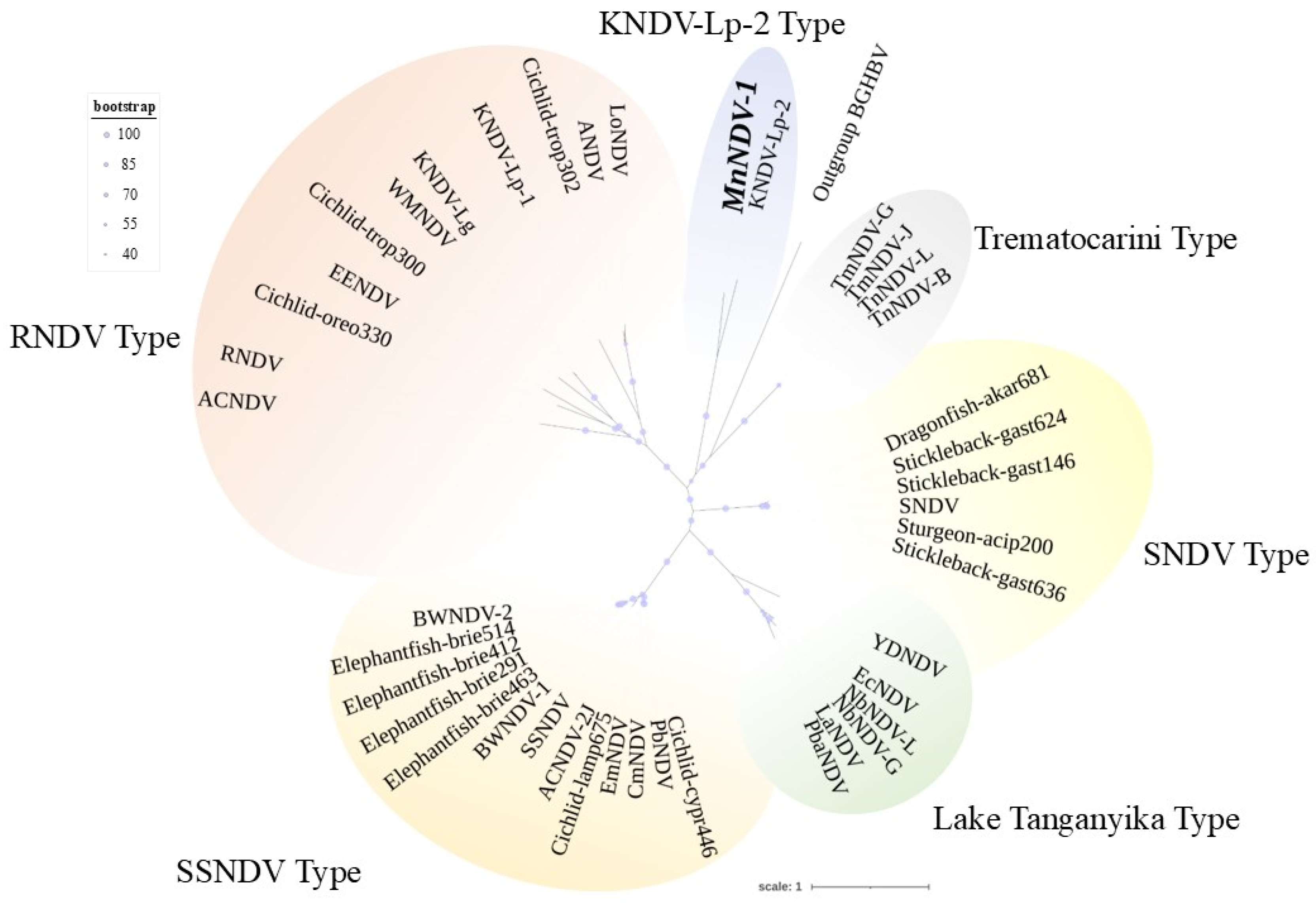
| Accession Number | Host | Tissue Source | Figure Label | % Identity |
|---|---|---|---|---|
| PV448632 | Micropterus nigricans | Fin tissue | MnNDV-1 | - |
| WLN26308 | Pseudosimochromis babaulti | Gill | PbaNDV | 49.6 |
| SRX340853 * | Lucania parva | Pooled organs | KNDVLp2 | 49.0 |
| WLN26317 | Enantiopus melanogenys | Brain | EmNDV | 45.3 |
| WLN26318 | Callochromis macrops | Gill | CmNDV | 43.8 |
| WLN26322 | Lamprologus ocellatus | Gonads | LoNDV | 42.3 |
| OR350361 | Neolamprologus buescheri | Gill | NbNDVG | 39.0 |
| OR350369 | Trematocara nigrifrons | Brain | TnNDVB | 36.7 |
| WLN26321 | Trematocara nigrifrons | Liver | TnNDV-L | 36.7 |
| OR350368 | Trematocara marginatum | Lower pharyngeal jaw | TmNDV-J | 36.0 |
| WLN26320 | Trematocara marginatum | Gill | TmNDV-G | 36.0 |
| SRX340836 | Lucania parva | Pooled organs | KNDV-Lp-1 | 35.6 |
| OR350333 | Lepidiolamprologus attenuatus | Gill | LaNDV | 35.3 |
| ERX240954 | Astatotilapia sp. | Unknown | ANDV | 35.2 |
| C_AA050294 | Brienomyrus brachyistius | Unknown | Elephantfishbrie463 | 35.1 |
| C_AA050263 | Tropheus sp. “Ikola” | Unknown | Cichlidtrop302 | 35.0 |
| C_AA050253 | Paracyprichromis brieni | Unknown | Cichlidcypr446 | 34.9 |
| C_AA050256 | Lamprologus ocellatus | Unknown | Cichlidlamp675 | 34.5 |
| C_AA050291 | Brienomyrus brachyistius | Unknown | Elephantfishbrie291 | 34.4 |
| C_AA050292 | Brienomyrus brachyistius | Unknown | Elephantfishbrie412 | 34.2 |
| SRX553136 | Brienomyrus brachyistius | Muscle | BWNDV1 | 34.2 |
| OR350367 | Paracyprichromis brieni | Brain | PbNDV | 34.1 |
| SRX700630 | Anguilla anguilla | Olfactory epithelium | EENDV | 34.1 |
| C_AA050295 | Brienomyrus brachyistius | Unknown | Elephantfishbrie514 | 33.9 |
| C_AA050270 | Akarotaxis nudiceps | Unknown | Dragonfishakar681 | 33.9 |
| OR350336 | Eretmodus cyanostictus | Lower pharyngeal jaw | EcNDV | 33.8 |
| SRX265393 | Oncorynchus nerka | Pooled organs | SSNDV | 33.7 |
| SRX340220 | Lucania goodei | Pooled organs | KNDV-Lg | 33.6 |
| SRX376926 | Gambusia affinis | Ovary | WMNDV | 33.4 |
| SRX573075 | Brienomyrus brachyistius | Electric organ | BWNDV-2 | 33.4 |
| WLN26316 | Lamprologus ocellatus | Lower pharyngeal jaw | ACNDV-2J | 33.4 |
| AZP02119 | Sebastes nigrocinctus | Brain | RNDV | 33.1 |
| MH158727 | Ophthalmotilapia ventralis | Pooled organs | ACNDV | 33.1 |
| C_AA050351 | Gasterosteus aculeatus | Unknown | Sticklebackgast624 | 32.8 |
| C_AA050259 | Oreochromis sp. (strain BJ-2021) | Unknown | Cichlidoreo330 | 32.7 |
| C_AA050262 | Tropheus sp. ‘Ikola’ | Unknown | Cichlidtrop300 | 32.5 |
| SRX367575 | Nibea albiflora | Unknown | YDNDV | 32.4 |
| C_AA050350 | Gasterosteus aculeatus | Unknown | Sticklebackgast146 | 32.3 |
| C_AA050352 | Gasterosteus aculeatus | Unknown | Sticklebackgast636 | 32.3 |
| C_AA050353 | Acipenser ruthenus x Huso huso | Unknown | Sturgeonacip200 | 32.3 |
| SRX1037831 * | Gasterosteus aculeatus | Unknown | SNDV | 31.3 |
| YP_009259541 | Lepomis macrochirus | Lip | BGHBV | 28.3 |
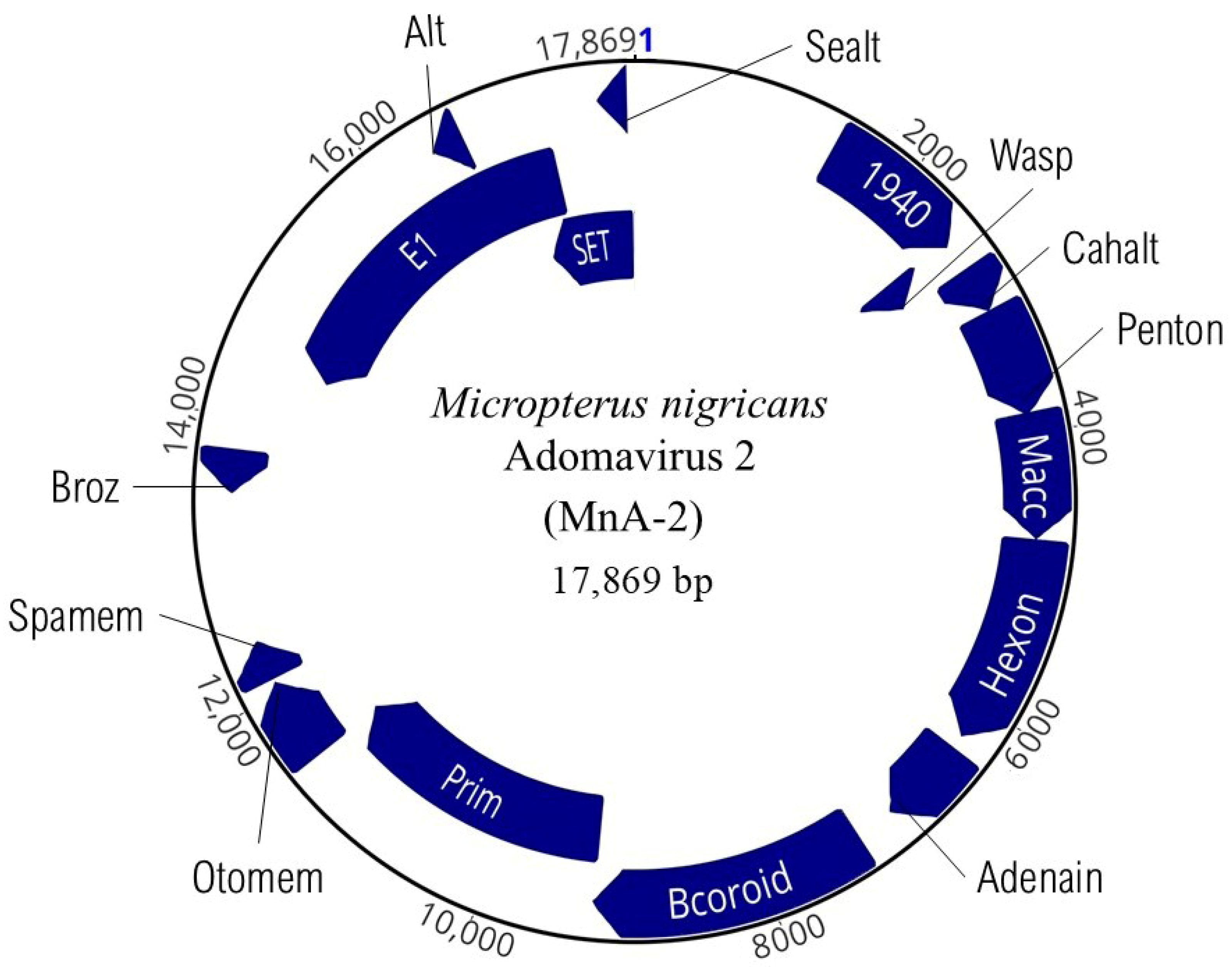
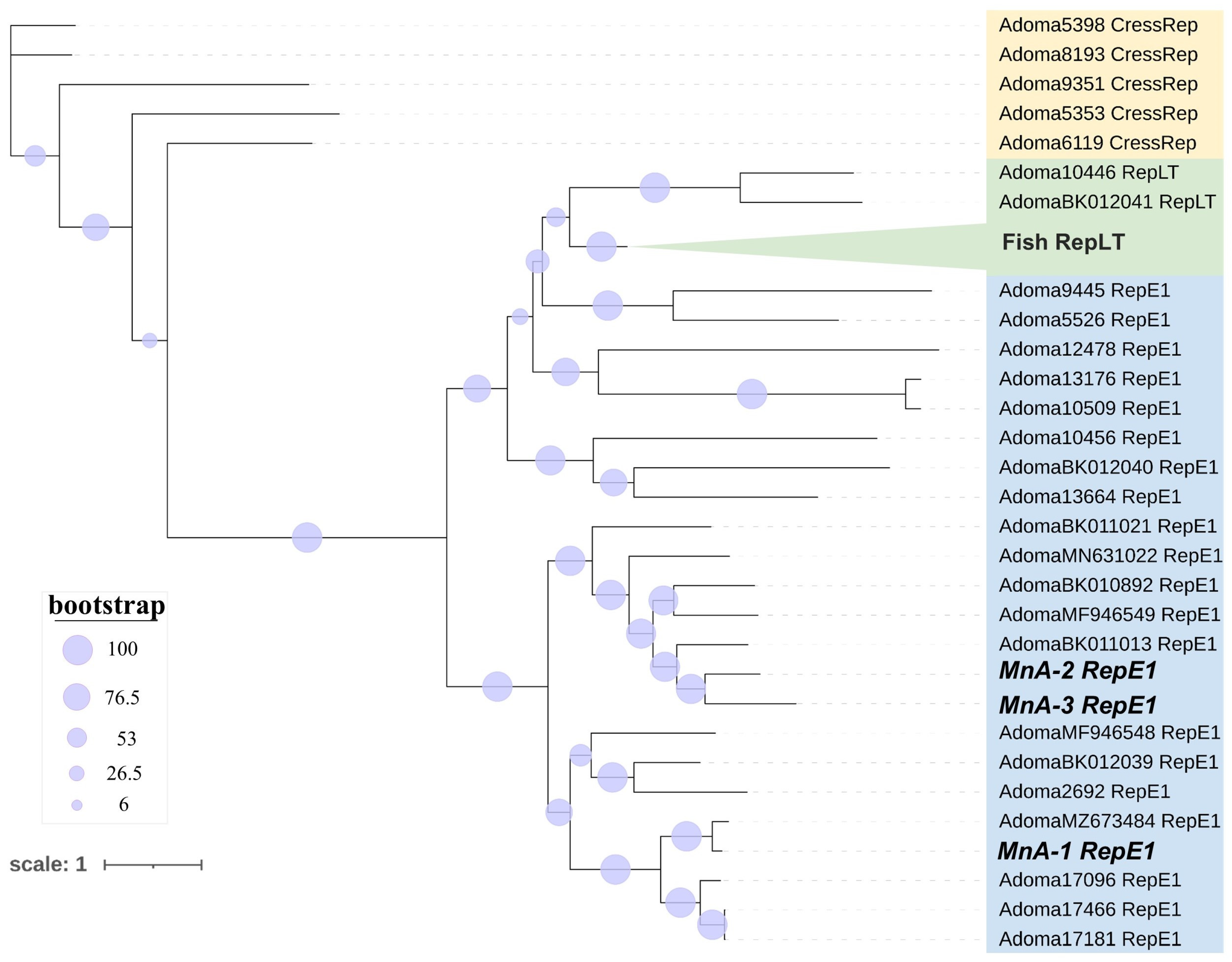
3.3. Adomaviruses
Pairwise Comparisons and Phylogenetic Analysis of ADmV Replicase Proteins
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Long, J.M.; Allen, M.S.; Porak, W.F.; Suski, C.D. A Historical Perspective of Black Bass Management in the United States. In Proceedings of the American Fisheries Society Symposium, Portland, OR, USA, 19 November 2015; Volume 82, pp. 99–122. [Google Scholar]
- Taylor, A.T.; Long, J.M.; Tringali, M.D.; Barthel, B.L. Conservation of Black Bass Diversity: An Emerging Management Paradigm. Fisheries 2019, 44, 20–36. [Google Scholar] [CrossRef]
- Kassler, T.W.; Koppelman, J.B.; Near, T.J.; Dillman, C.B.; Levengood, J.; Swofford, D.L.; VanOrman, J.L.; Claussen, J.E.; Philipp, D.P. Molecular and Morphological Analyses of the Black Basses: Implications for Taxonomy and Conservation. In Proceedings of the American Fisheries Society Symposium, Bethesda, MD, USA, 19 August 2002; Volume 2002, pp. 291–322. [Google Scholar]
- Miranda, L.E.; Bettoli, P.W. Largemouth Bass Natural History. In Largemouth Bass Aquaculture; CABI: Oxford, UK, 2019. [Google Scholar]
- Kim, D.; Taylor, A.T.; Near, T.J. Phylogenomics and Species Delimitation of the Economically Important Black Basses (Micropterus). Sci. Rep. 2022, 12, 9113. [Google Scholar] [CrossRef]
- Philipp, D.P. Genetic Implications of Introducing Florida Largemouth Bass, Micropterus Salmoides Floridanus. Can. J. Fish. Aquat. Sci. 1991, 48, 58–65. [Google Scholar] [CrossRef]
- Barthel, B.; Allen, M.; Porak, W.; Kerns, J. Florida Bass Micropterus Floridanus (LeSueur, 1822). In Black Bass Diversity: Multidisciplinary Science for Conservation; American Fisheries Society, Symposium: Bethesda, MD, USA, 2015; Volume 82, pp. 43–53. [Google Scholar]
- Sammons, S.M.; Dorsey, L.G.; Loftis, C.S.; Chrisman, P.; Scott, M.; Hammonds, J.; Jolley, M.; Hatcher, H.; Odenkirk, J.; Damer, J.; et al. Alabama Bass Alter Reservoir Black Bass Species Assemblages When Introduced Outside Their Native Range. N. Am. J. Fish. Manag. 2023, 43, 384–399. [Google Scholar] [CrossRef]
- Johansen, T.; Besnier, F.; Quintela, M.; Jorde, P.E.; Glover, K.A.; Westgaard, J.-I.; Dahle, G.; Lien, S.; Kent, M.P. Genomic Analysis Reveals Neutral and Adaptive Patterns That Challenge the Current Management Regime for East Atlantic Cod Gadus morhua L. Evol. Appl. 2020, 13, 2673–2688. [Google Scholar] [CrossRef] [PubMed]
- Morrison, W., III; Lohr, J.; Duchen, P.; Wilches, R.; Trujillo, D.; Mair, M.; Renner, S. The Impact of Taxonomic Change on Conservation: Does It Kill, Can It Save, or Is It Just Irrelevant? Biol. Conserv. 2009, 142, 3201–3206. [Google Scholar] [CrossRef]
- MacCrimmon, H.R.; Robbins, W.H. Distribution of the Black Basses in North America. In Black Bass Biology and Management; Sport Fishing Institute: Washington, DC, USA, 1975; pp. 56–66. [Google Scholar]
- Lee, D.S.; Gilbert, C.R.; Hocutt, C.H.; Jenkins, R.E.; McAllister, D.E.; Stauffer, J.R., Jr. Atlas of North American Freshwater Fishes; North Carolina State Museum of natural history: Raleigh, NC, USA, 1980. [Google Scholar]
- Shaw, S. Black Bass Diversity and Conservation: An Overview. In Black Bass Diversity: Multidisciplinary Science for Conservation; American Fisheries Society, Symposium: Bethesda, MD, USA, 2015; Volume 82, pp. 3–8. [Google Scholar]
- Robbins, W.H.; MacCrimmon, H.R. The Blackbass in America and Overseas; Publications Division, Biomanagement and Research Enterprises: Sault Ste. Marie, ON, Canada, 1974. [Google Scholar]
- Brown, T.; Runciman, B.; Pollard, S.; Grant, A. Biological Synopsis of Largemouth Bass (Micropterus salmoides). Can. Manuscr. Rep. Fish. Aquat. Sci. 2009, 2884, 1–26. [Google Scholar]
- USFWS. 2016 National Survey of Fishing, Hunting and Wildlife-Associated Recreation; U.S. Fish & Wildlife Service: Washington, DC, USA, 2018.
- Seguy, L.; Long, J.M. Perceived Ecological Threats and Economic Benefits of Non-Native Black Bass in the United States. Fisheries 2020, 46, 56–65. [Google Scholar] [CrossRef]
- Moffitt, C.M. Reflections: A Photographic History of Fisheries and the American Fisheries Society in North America; American Fisheries Society: Bethesda, MD, USA, 2001. [Google Scholar]
- Tidwell, J.H.; Nickum, J.G. History of Largemouth Bass Production. In Largemouth Bass Aquaculture; CABI: Oxford, UK, 2019. [Google Scholar]
- Swingle, H. History of Warmwater Pond Culture in the United States. In A Century of Fisheries in North America; American Fisheries Society, Special Publication: Bethesda, MD, USA, 1970; Volume 7, pp. 95–105. [Google Scholar]
- Zhou, Y.; Liu, C. Largemouth Bass Production in China. In Largemouth Bass Aquaculture; 5M Publishing Ltd Benchmark House: Sheffield, UK, 2019; pp. 37–47. [Google Scholar]
- Bai, J.; Li, S. Current Status and Development Trend on China Largemouth Bass Industry. Chin. Fish. Econ. 2013, 31, 104–108. [Google Scholar]
- Steeger, T.M.; Grizzle, J.M.; Weathers, K.; Newman, M. Bacterial Diseases and Mortality of Angler-Caught Largemouth Bass Released after Tournaments on Walter F. George Reservoir, Alabama–Georgia. N. Am. J. Fish. Manag. 1994, 14, 435–441. [Google Scholar] [CrossRef]
- Francis-Floyd, R.; Reed, P.; Bolon, B.; Estes, J.; McKinney, S. An Epizootic of Edwardsiella tarda in Largemouth Bass (Micropterus salmoides). J. Wildl. Dis. 1993, 29, 334–336. [Google Scholar] [CrossRef]
- Camus, A.; Griffin, M.; Armwood, A.; Soto, E. A Spontaneous Outbreak of Systemic Edwardsiella piscicida Infection in Largemouth Bass Micropterus salmoides (Lacépède, 1802) in California, USA. J. Fish Dis. 2019, 42, 759–763. [Google Scholar] [CrossRef]
- Grizzle, J.M.; Altinok, I.; Fraser, W.A.; Francis-Floyd, R. First Isolation of Largemouth Bass Virus. Dis. Aquat. Org. 2002, 50, 233–235. [Google Scholar] [CrossRef]
- Chen, Z.-Y.; Gao, X.-C.; Zhang, Q.-Y. Whole-Genome Analysis of a Novel Fish Reovirus (MsReV) Discloses Aquareovirus Genomic Structure Relationship with Host in Saline Environments. Viruses 2015, 7, 4282–4302. [Google Scholar] [CrossRef]
- Sibley, S.D.; Finley, M.A.; Baker, B.B.; Puzach, C.; Armién, A.G.; Giehtbrock, D.; Goldberg, T.L. Novel Reovirus Associated with Epidemic Mortality in Wild Largemouth Bass (Micropterus salmoides). J. Gen. Virol. 2016, 97, 2482–2487. [Google Scholar] [CrossRef]
- Qin, Y.; Zhang, P.; Zhang, M.; Guo, W.; Deng, S.; Liu, H.; Yao, L. Isolation and Identification of a New Strain Micropterus Salmoides Rhabdovirus (MSRV) from Largemouth Bass Micropterus salmoides in China. Aquaculture 2023, 572, 739538. [Google Scholar] [CrossRef]
- Iwanowicz, L.R.; Raines, C.D.; Young, K.T.; Blazer, V.S.; Walsh, H.L.; Smith, G.; Holt, C.; Odenkirk, J.S.; Jones, T.; Hessenauer, J.-M.; et al. Novel Adomaviruses Associated with Blotchy Bass Syndrome in Black Basses. PLoS ONE 2025, 20, 657292. [Google Scholar]
- Xu, Z.; Liao, J.; Zhang, D.; Liu, S.; Zhang, L.; Kang, S.; Xu, L.; Chen, H.; Peng, W.; Zhou, S.; et al. Isolation, Characterization, and Transcriptome Analysis of an ISKNV-like Virus from Largemouth Bass. Viruses 2023, 15, 398. [Google Scholar] [CrossRef] [PubMed]
- Kibenge, F.; Godoy, M. Reoviruses of Aquatic Organisms. In Aquaculture Virology; Elsevier: Amsterdam, The Netherlands, 2016; pp. 205–236. [Google Scholar]
- Hanson, L.A.; Petrie-Hanson, L.; Meals, K.O.; Chinchar, V.G.; Rudis, M. Persistence of Largemouth Bass Virus Infection in a Northern Mississippi Reservoir after a Die-Off. J. Aquat. Anim. Health 2001, 13, 27–34. [Google Scholar] [CrossRef]
- Grizzle, J.M.; Brunner, C.J. Review of Largemouth Bass Virus. Fisheries 2003, 28, 10–14. [Google Scholar] [CrossRef]
- Gao, E.-B.; Chen, G. Micropterus Salmoides Rhabdovirus (MSRV) Infection Induced Apoptosis and Activated Interferon Signaling Pathway in Largemouth Bass Skin Cells. Fish Shellfish Immunol. 2018, 76, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Deng, G.; Bai, J.; Li, S.; Yu, L.; Quan, Y.; Yang, X.; Jiang, X.; Zhu, Z.; Ye, X. A Strain of Siniperca chuatsi Rhabdovirus Causes High Mortality among Cultured Largemouth Bass in South China. J. Aquat. Anim. Health 2013, 25, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Blazer, V.S.; Young, K.T.; Smith, G.D.; Sperry, A.J.; Iwanowicz, L.R. Hyperpigmented Melanistic Skin Lesions of Smallmouth Bass Micropterus Dolomieu from the Chesapeake Bay Watershed. Dis. Aquat. Org. 2020, 139, 199–212. [Google Scholar] [CrossRef]
- Odhiambo, B.; Coxon, T.; Somers, H. Sediment and Phosphorous Fluxes Analysis in Aquia Creek, a Sub-Watershed of the Chesapeake Bay Basin, VA, USA. Water Air Soil Pollut. 2018, 229, 1–15. [Google Scholar] [CrossRef]
- Jastram, J.D. Streamflow, Water Quality, and Aquatic Macroinvertebrates of Selected Streams in Fairfax County, Virginia, 2007-12; US Department of the Interior, US Geological Survey: Reston, VA, USA, 2014.
- Froelich, A.J.; Zenone, C. Maps Showing Geologic Terrane, Drainage Basins, Overburden, and Low Flow of Streams in Fairfax County and Vicinity, Virginia; US Geological Survey: Reston, VA, USA, 1985.
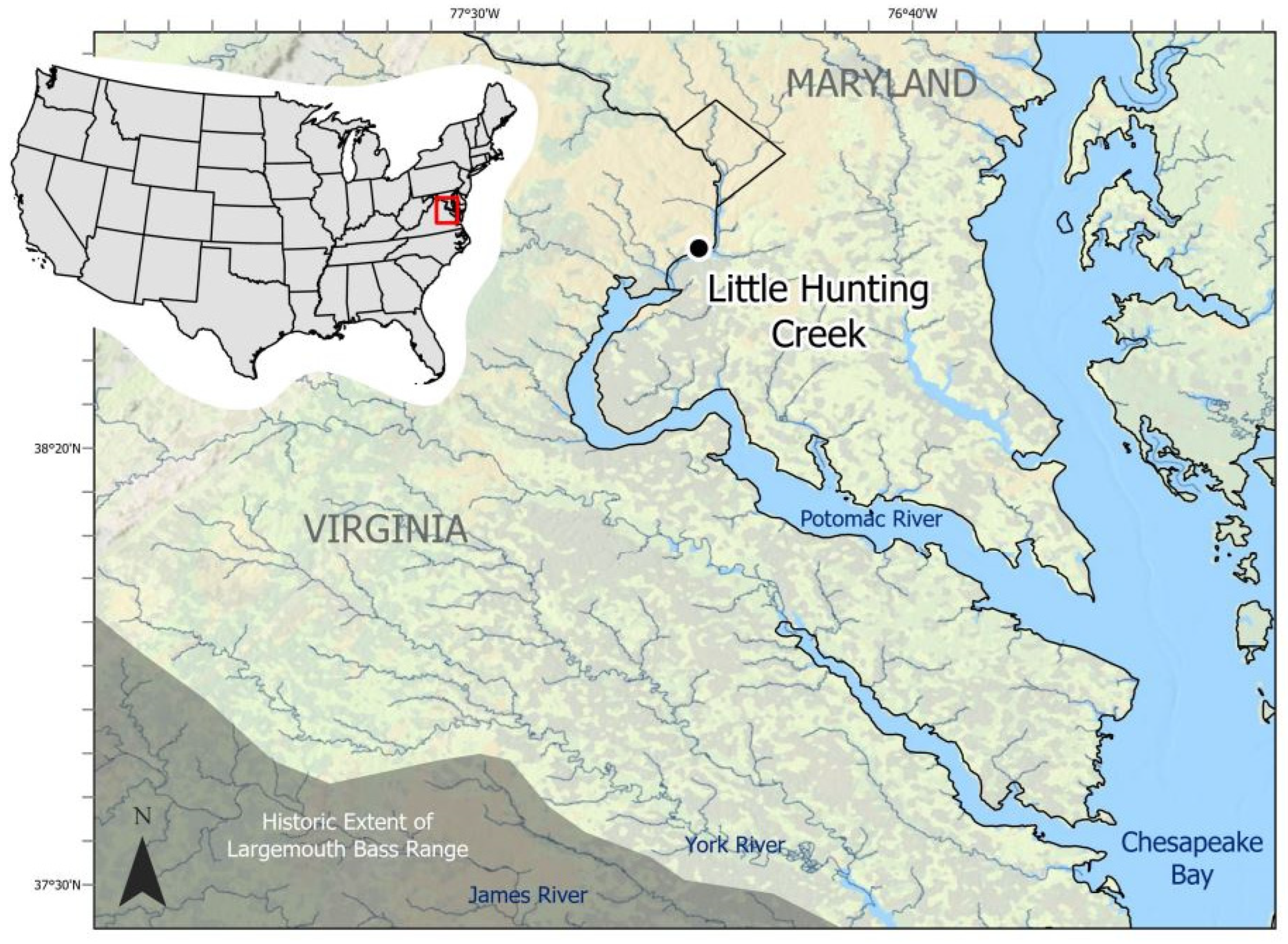
- Frie, S. Little Hunting Creek Watershed Management Plan; Fairfax County Stormwater Planning Division: Fairfax, VA, USA, 2004; p. 212. [Google Scholar]
- Dalzell, R.F., Jr.; Dalzell, L.B. George Washington’s Mount Vernon: At Home in Revolutionary America; Oxford University Press: Oxford, UK, 2000. [Google Scholar]
- Li, D.; Liu, C.-M.; Luo, R.; Sadakane, K.; Lam, T.-W. MEGAHIT: An Ultra-Fast Single-Node Solution for Large and Complex Metagenomics Assembly via Succinct de Bruijn Graph. Bioinformatics 2015, 31, 1674–1676. [Google Scholar] [CrossRef] [PubMed]
- Tisza, M.J.; Belford, A.K.; Dominguez-Huerta, G.; Bolduc, B.; Buck, C.B. Cenote-Taker 2 Democratizes Virus Discovery and Sequence Annotation. Virus Evol. 2021, 7, veaa100. [Google Scholar] [CrossRef]
- Zimmermann, L.; Stephens, A.; Nam, S.-Z.; Rau, D.; Kübler, J.; Lozajic, M.; Gabler, F.; Söding, J.; Lupas, A.N.; Alva, V. A Completely Reimplemented MPI Bioinformatics Toolkit with a New HHpred Server at Its Core. J. Mol. Biol. 2018, 430, 2237–2243. [Google Scholar] [CrossRef]
- Bu, C.; Zheng, X.; Zhao, X.; Xu, T.; Bai, X.; Jia, Y.; Chen, M.; Hao, L.; Xiao, J.; Zhang, Z.; et al. GenBase: A Nucleotide Sequence Database. Genom. Proteom. Bioinform. 2024, 22, qzae047. [Google Scholar] [CrossRef]
- Lauber, C.; Seitz, S.; Mattei, S.; Suh, A.; Beck, J.; Herstein, J.; Börold, J.; Salzburger, W.; Kaderali, L.; Briggs, J.A.; et al. Deciphering the Origin and Evolution of Hepatitis B Viruses by Means of a Family of Non-Enveloped Fish Viruses. Cell Host Microbe 2017, 22, 387–399. [Google Scholar] [CrossRef]
- Costa, V.A.; Ronco, F.; Mifsud, J.C.; Harvey, E.; Salzburger, W.; Holmes, E.C. Host Adaptive Radiation Is Associated with Rapid Virus Diversification and Cross-Species Transmission in African Cichlid Fishes. Curr. Biol. 2024, 34, 1247–1257. [Google Scholar] [CrossRef]
- Ben, H.; Wang, X.; Yang, P.; Li, L.; Liu, P.; Gao, Y.; Wang, Y.; Liu, Y.; Huang, C.; Chen, D. Metagenomic Analysis Uncovers Novel Hepadnaviruses and Nackednaviruses. Sci. Rep. 2025, 15, 24699. [Google Scholar] [CrossRef]
- Welch, N.L.; Yutin, N.; Dill, J.A.; Camus, A.C.; Pang, Y.-Y.S.; Schiller, J.T.; An, P.; Cantalupo, P.G.; Pipas, J.M.; Delwart, E.; et al. Adomaviruses: An Emerging Virus Family Provides Insights into DNA Virus Evolution. BioRxiv 2018. [Google Scholar]
- Koonin, E.V.; Dolja, V.V.; Krupovic, M. Origins and Evolution of Viruses of Eukaryotes: The Ultimate Modularity. Virology 2015, 479, 2–25. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. MUSCLE: Multiple Sequence Alignment with High Accuracy and High Throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef]
- Trifinopoulos, J.; Nguyen, L.-T.; von Haeseler, A.; Minh, B.Q. W-IQ-TREE: A Fast Online Phylogenetic Tool for Maximum Likelihood Analysis. Nucleic Acids Res. 2016, 44, W232–W235. [Google Scholar] [CrossRef] [PubMed]
- Letunic, I.; Bork, P. Interactive Tree Of Life (iTOL) v5: An Online Tool for Phylogenetic Tree Display and Annotation. Nucleic Acids Res. 2021, 49, W293–W296. [Google Scholar] [CrossRef]
- Muhire, B.M.; Varsani, A.; Martin, D.P. SDT: A Virus Classification Tool Based on Pairwise Sequence Alignment and Identity Calculation. PLoS ONE 2014, 9, e108277. [Google Scholar] [CrossRef]
- Ford, C.E.; Dunn, C.D.; Leis, E.M.; Thiel, W.A.; Goldberg, T.L. Five Species of Wild Freshwater Sport Fish in Wisconsin, USA, Reveal Highly Diverse Viromes. Pathogens 2024, 13, 150. [Google Scholar] [CrossRef]
- Iwanowicz, L.R.; Young, K.T.; Adams, C.R.; Blazer, V.S.; Smith, G.D.; Cornman, R.S. Draft Genome Sequence of an Adomavirus Associated with Raised Mucoid Skin Lesions on Smallmouth Bass (Micropterus dolomieu). Microbiol. Resour. Announc. 2020, 9, 14. [Google Scholar] [CrossRef]
- Buck, C.B.; Welch, N.; Belford, A.K.; Varsani, A.; Pastrana, D.V.; Tisza, M.J.; Starrett, G.J. Widespread Horizontal Gene Transfer Among Animal Viruses. bioRxiv 2024, preprint. [Google Scholar] [CrossRef]
- Welch, N.L.; Tisza, M.J.; Starrett, G.J.; Belford, A.K.; Pastrana, D.V.; Pang, Y.-Y.S.; Schiller, J.T.; An, P.; Cantalupo, P.G.; Pipas, J.M.; et al. Identification of Adomavirus Virion Proteins. bioRxiv 2020, 341131. [Google Scholar] [CrossRef]
- Dill, J.A.; Camus, A.C.; Leary, J.H.; Ng, T.F.F. Microscopic and Molecular Evidence of the First Elasmobranch Adomavirus, the Cause of Skin Disease in a Giant Guitarfish, Rhynchobatus djiddensis. mBio 2018, 9, e00185-18. [Google Scholar] [CrossRef]
- Kazlauskas, D.; Varsani, A.; Koonin, E.V.; Krupovic, M. Multiple Origins of Prokaryotic and Eukaryotic Single-Stranded DNA Viruses from Bacterial and Archaeal Plasmids. Nat. Commun. 2019, 10, 3425. [Google Scholar] [CrossRef]
- Hahn, C.M.; Iwanowicz, L.R.; Cornman, R.S.; Conway, C.M.; Winton, J.R.; Blazer, V.S. Characterization of a Novel Hepadnavirus in the White Sucker (Catostomus commersonii) from the Great Lakes Region of the United States. J. Virol. 2015, 89, 11801–11811. [Google Scholar] [CrossRef]
- Magnius, L.; Mason, W.S.; Taylor, J.; Kann, M.; Glebe, D.; Dény, P.; Sureau, C.; Norder, H.; Consortium, I.R. ICTV Virus Taxonomy Profile: Hepadnaviridae. J. Gen. Virol. 2020, 101, 571. [Google Scholar] [CrossRef]
- Buigues, J.; Viñals, A.; Martínez-Recio, R.; Monrós, J.S.; Cuevas, J.M.; Sanjuán, R. Phylogenetic Evidence Supporting the Nonenveloped Nature of Hepadnavirus Ancestors. Proc. Natl. Acad. Sci. USA 2024, 121, e2415631121. [Google Scholar] [CrossRef]
- Pfister, S.; Rabl, J.; Wiegand, T.; Mattei, S.; Malär, A.A.; Lecoq, L.; Seitz, S.; Bartenschlager, R.; Böckmann, A.; Nassal, M.; et al. Structural Conservation of HBV-like Capsid Proteins over Hundreds of Millions of Years despite the Shift from Non-Enveloped to Enveloped Life-Style. Nat. Commun. 2023, 14, 1574. [Google Scholar] [CrossRef]
- Liu, Y.; Maya, S.; Ploss, A. Animal Models of Hepatitis B Virus Infection–Success, Challenges, and Future Directions. Viruses 2021, 13, 777. [Google Scholar] [CrossRef]
- COX, F.E. Concomitant Infections, Parasites and Immune Responses. Parasitology 2001, 122, S23–S38. [Google Scholar] [CrossRef] [PubMed]
- Bakaletz, L.O. Developing Animal Models for Polymicrobial Diseases. Nat. Rev. Microbiol. 2004, 2, 552–568. [Google Scholar] [CrossRef]
- Kotob, M.H.; Menanteau-Ledouble, S.; Kumar, G.; Abdelzaher, M.; El-Matbouli, M. The Impact of Co-Infections on Fish: A Review. Vet. Res. 2017, 47, 98. [Google Scholar] [CrossRef]
- Vonaesch, P.; Anderson, M.; Sansonetti, P.J. Pathogens, Microbiome and the Host: Emergence of the Ecological Koch’s Postulates. FEMS Microbiol. Rev. 2018, 42, 273–292. [Google Scholar] [CrossRef]
- Smith, G.; Blazer, V.; Walsh, H.; Iwanowicz, L.; Starliper, C.; Sperry, A. The Effects of Disease-Related Mortality of Young-of-Year Smallmouth Bass on the Population Characteristics in the Susquehanna River Basin, Pennsylvania and Potential Implications to Conservation of Black Bass Diversity. In Proceedings of the American Fisheries Society Symposium, Portland, OR, USA, 19 November 2015; Volume 82, pp. 319–332. [Google Scholar]
- Arway, J.A.; Smith, G. The Susquehanna River—A Fishery in Decline. Fisheries 2013, 38, 235–236. [Google Scholar] [CrossRef]
- Walsh, H.L.; Blazer, V.S.; Smith, G.D.; Lookenbill, M.; Alvarez, D.A.; Smalling, K.L. Risk Factors Associated with Mortality of Age-0 Smallmouth Bass in the Susquehanna River Basin, Pennsylvania. J. Aquat. Anim. Health 2018, 30, 65–80. [Google Scholar] [CrossRef] [PubMed]
- Keplinger, B.; Rota, C.T. Effects of Poor Recruitment on Riverine Smallmouth Bass Population Dynamics. N. Am. J. Fish. Manag. 2024, 44, 247–261. [Google Scholar] [CrossRef]
- Keplinger, B.; Hedrick, J.; Blazer, V.S. Temporal Trends in Macroscopic Indicators of Fish Health in the South Branch Potomac River. N. Am. J. Fish. Manag. 2022, 42, 277–294. [Google Scholar] [CrossRef]
- Robertson, L.S.; Iwanowicz, L.R.; Marranca, J.M. Identification of Centrarchid Hepcidins and Evidence That 17β-Estradiol Disrupts Constitutive Expression of Hepcidin-1 and Inducible Expression of Hepcidin-2 in Largemouth Bass (Micropterus salmoides). Fish Shellfish Immunol. 2009, 26, 898–907. [Google Scholar] [CrossRef] [PubMed]
- Ripley, J.; Iwanowicz, L.; Blazer, V.; Foran, C. Utilization of Protein Expression Profiles as Indicators of Environmental Impairment of Smallmouth Bass (Micropterus dolomieu) from the Shenandoah River, Virginia, USA. Environ. Toxicol. Chem. 2008, 27, 1756–1767. [Google Scholar] [CrossRef]
- Willacker, J.J.; Eagles-Smith, C.A.; Blazer, V.S. Mercury Bioaccumulation in Freshwater Fishes of the Chesapeake Bay Watershed. Ecotoxicology 2020, 29, 459–484. [Google Scholar] [CrossRef] [PubMed]
- Blazer, V.S.; Gordon, S.; Jones, D.K.; Iwanowicz, L.R.; Walsh, H.L.; Sperry, A.J.; Smalling, K.L. Retrospective Analysis of Estrogenic Endocrine Disruption and Land-Use Influences in the Chesapeake Bay Watershed. Chemosphere 2021, 266, 129009. [Google Scholar] [CrossRef]
- Blazer, V.S.; Iwanowicz, L.R.; Starliper, C.E.; Iwanowicz, D.; Barbash, P.; Hedrick, J.; Reeser, S.; Mullican, J.; Zaugg, S.; Burkhardt, M.; et al. Mortality of Centrarchid Fishes in the Potomac Drainage: Survey Results and Overview of Potential Contributing Factors. J. Aquat. Anim. Health 2010, 22, 190–218. [Google Scholar] [CrossRef] [PubMed]
- Rapport, D.J. Epidemiology and Ecosystem Health: Natural Bridges. Ecosyst. Health 1999, 5, 174–180. [Google Scholar] [CrossRef]
- Vethaak, A.D.; Jol, J.G.; Pieters, J.P. Long-Term Trends in the Prevalence of Cancer and Other Major Diseases among Flatfish in the Southeastern North Sea as Indicators of Changing Ecosystem Health. Environ. Sci. Technol. 2009, 43, 2151–2158. [Google Scholar] [CrossRef]
- Blazer, V.S.; Iwanowicz, L.R.; Iwanowicz, D.D.; Smith, D.R.; Young, J.A.; Hedrick, J.; Foster, S.; Reeser, S. Intersex (Testicular oocytes) in Smallmouth Bass from the Potomac River and Selected Nearby Drainages. J. Aquat. Anim. Health 2007, 19, 242–253. [Google Scholar] [CrossRef]
- Blazer, V.S.; Iwanowicz, L.R.; Henderson, H.; Mazik, P.M.; Jenkins, J.A.; Alvarez, D.A.; Young, J.A. Reproductive Endocrine Disruption in Smallmouth Bass (Micropterus dolomieu) in the Potomac River Basin: Spatial and Temporal Comparisons of Biological Effects. Environ. Monit. Assess. 2012, 184, 4309–4334. [Google Scholar] [CrossRef]
- Kolpin, D.W.; Blazer, V.S.; Gray, J.L.; Focazio, M.J.; Young, J.A.; Alvarez, D.A.; Iwanowicz, L.R.; Foreman, W.T.; Furlong, E.T.; Speiran, G.K.; et al. Chemical Contaminants in Water and Sediment near Fish Nesting Sites in the Potomac River Basin: Determining Potential Exposures to Smallmouth Bass (Micropterus dolomieu). Sci. Total Environ. 2013, 443, 700–716. [Google Scholar] [CrossRef] [PubMed]
- Colin, Y.; Berthe, T.; Molbert, N.; Guigon, E.; Vivant, A.-L.; Alliot, F.; Collin, S.; Goutte, A.; Petit, F. Urbanization Constrains Skin Bacterial Phylogenetic Diversity in Wild Fish Populations and Correlates with the Proliferation of Aeromonads. Microb. Ecol. 2021, 82, 523–536. [Google Scholar] [CrossRef]
- Stephens, W.Z.; Burns, A.R.; Stagaman, K.; Wong, S.; Rawls, J.F.; Guillemin, K.; Bohannan, B.J. The Composition of the Zebrafish Intestinal Microbial Community Varies across Development. ISME J. 2016, 10, 644–654. [Google Scholar] [CrossRef] [PubMed]
- Sullam, K.E.; Essinger, S.D.; Lozupone, C.A.; O’CONNOR, M.P.; Rosen, G.L.; Knight, R.; Kilham, S.S.; Russell, J.A. Environmental and Ecological Factors That Shape the Gut Bacterial Communities of Fish: A Meta-Analysis. Mol. Ecol. 2012, 21, 3363–3378. [Google Scholar] [CrossRef]
- Krotman, Y.; Yergaliyev, T.M.; Shani, R.A.; Avrahami, Y.; Szitenberg, A. Dissecting the Factors Shaping Fish Skin Microbiomes in a Heterogeneous Inland Water System. Microbiome 2020, 8, 9. [Google Scholar] [CrossRef]
- Walsh, C.J.; Roy, A.H.; Feminella, J.W.; Cottingham, P.D.; Groffman, P.M.; Morgan, R.P. The Urban Stream Syndrome: Current Knowledge and the Search for a Cure. J. N. Am. Benthol. Soc. 2005, 24, 706–723. [Google Scholar] [CrossRef]
- Meyer, J.L.; Paul, M.J.; Taulbee, W.K. Stream Ecosystem Function in Urbanizing Landscapes. J. N. Am. Benthol. Soc. 2005, 24, 602–612. [Google Scholar] [CrossRef][Green Version]
- Shameena, S.; Kumar, S.; Kumar, K.; Raman, R. Role of Temperature and Co-Infection in Mediating the Immune Response of Goldfish. Microb. Pathog. 2021, 156, 104896. [Google Scholar] [CrossRef] [PubMed]
- Geoghegan, J.L.; Di Giallonardo, F.; Wille, M.; Ortiz-Baez, A.S.; Costa, V.A.; Ghaly, T.; Mifsud, J.C.; Turnbull, O.M.; Bellwood, D.R.; Williamson, J.E.; et al. Virome Composition in Marine Fish Revealed by Meta-Transcriptomics. Virus Evol. 2021, 7, veab005. [Google Scholar] [CrossRef]
- Boonthai, T.; Loch, T.P.; Yamashita, C.J.; Smith, G.D.; Winters, A.D.; Kiupel, M.; Brenden, T.O.; Faisal, M. Laboratory Investigation into the Role of Largemouth Bass Virus (Ranavirus, Iridoviridae) in Smallmouth Bass Mortality Events in Pennsylvania Rivers. BMC Vet. Res. 2018, 14, 62. [Google Scholar] [CrossRef] [PubMed]
- Buchmann, K. Immune Mechanisms in Fish Skin against Monogeneans—A Model. Folia Parasitol. 1999, 46, 1–9. [Google Scholar]
- Ellis, A. Innate Host Defense Mechanisms of Fish against Viruses and Bacteria. Dev. Comp. Immunol. 2001, 25, 827–839. [Google Scholar] [CrossRef]
- Gilliland, E. Livewell Operating Procedures to Reduce Mortality of Black Bass during Summer Tournaments. In Proceedings of the American Fisheries Society Symposium, Bethesda, MD, USA, 19 August 2002; pp. 477–488. [Google Scholar]
- Keretz, K.R.; Dinken, C.P.; Allen, P.J.; Colvin, M.E.; Schramm, H.L., Jr. The Effect of Water Temperature, Angling Time, and Dissolved Oxygen on the Survival of Largemouth Bass Subjected to Simulated Angling and Tournament Handling Procedures. N. Am. J. Fish. Manag. 2018, 38, 606–622. [Google Scholar] [CrossRef]
- Suski, C.D.; Killen, S.S.; Morrissey, M.B.; Lund, S.G.; Tufts, B.L. Physiological Changes in Largemouth Bass Caused by Live-Release Angling Tournaments in Southeastern Ontario. N. Am. J. Fish. Manag. 2003, 23, 760–769. [Google Scholar] [CrossRef]
- Suski, C.D.; Killen, S.S.; Cooke, S.J.; Kieffer, J.D.; Philipp, D.P.; Tufts, B.L. Physiological Significance of the Weigh-in during Live-Release Angling Tournaments for Largemouth Bass. Trans. Am. Fish. Soc. 2004, 133, 1291–1303. [Google Scholar] [CrossRef]
- Schramm, H.L., Jr.; Walters, A.R.; Grizzle, J.M.; Beck, B.H.; Hanson, L.A.; Rees, S.B. Effects of Live-Well Conditions on Mortality and Largemouth Bass Virus Prevalence in Largemouth Bass Caught during Summer Tournaments. N. Am. J. Fish. Manag. 2006, 26, 812–825. [Google Scholar] [CrossRef]
- Rothlisberger, J.D.; Chadderton, W.L.; McNulty, J.; Lodge, D.M. Aquatic Invasive Species Transport via Trailered Boats: What Is Being Moved, Who Is Moving It, and What Can Be Done. Fisheries 2010, 35, 121–132. [Google Scholar] [CrossRef]
- Kelly, N.E.; Wantola, K.; Weisz, E.; Yan, N.D. Recreational Boats as a Vector of Secondary Spread for Aquatic Invasive Species and Native Crustacean Zooplankton. Biol. Invasions 2013, 15, 509–519. [Google Scholar] [CrossRef]
- Kemp, C.; Van Riper, C.J.; BouFajreldin, L.; Stewart, W.P.; Scheunemann, J.; van den Born, R.J. Connecting Human–Nature Relationships to Environmental Behaviors That Minimize the Spread of Aquatic Invasive Species. Biol. Invasions 2017, 19, 2059–2074. [Google Scholar] [CrossRef]
- Murray, A.G. Using Simple Models to Review the Application and Implications of Different Approaches Used to Simulate Transmission of Pathogens among Aquatic Animals. Prev. Vet. Med. 2009, 88, 167–177. [Google Scholar] [CrossRef]
- Crane, M.; Hyatt, A. Viruses of Fish: An Overview of Significant Pathogens. Viruses 2011, 3, 2025–2046. [Google Scholar] [CrossRef]
- Noga, E.J. Fish Disease: Diagnosis and Treatment; John Wiley & Sons: Hoboken, NJ, USA, 2010. [Google Scholar]
- Arechavala-Lopez, P.; Sanchez-Jerez, P.; Bayle-Sempere, J.T.; Uglem, I.; Mladineo, I. Reared Fish, Farmed Escapees and Wild Fish Stocks—A Triangle of Pathogen Transmission of Concern to Mediterranean Aquaculture Management. Aquac. Environ. Interact. 2013, 3, 153–161. [Google Scholar] [CrossRef]
- Geoghegan, J.L.; Di Giallonardo, F.; Cousins, K.; Shi, M.; Williamson, J.E.; Holmes, E.C. Hidden Diversity and Evolution of Viruses in Market Fish. Virus Evol. 2018, 4, vey031. [Google Scholar] [CrossRef]
- Dong, X.; Li, C.; Wang, Y.; Hu, T.; Zhang, F.; Meng, F.; Gao, M.; Han, X.; Wang, G.; Qin, J.; et al. Diversity and Connectedness of Brine Shrimp Viruses in Global Hypersaline Ecosystems. Sci. China Life Sci. 2024, 67, 188–203. [Google Scholar] [CrossRef]
- Alfonso, P.; Butković, A.; Fernández, R.; Riesgo, A.; Elena, S.F. Unveiling the Hidden Viromes across the Animal Tree of Life: Insights from a Taxonomic Classification Pipeline Applied to Invertebrates of 31 Metazoan Phyla. Msystems 2024, 9, e00124-24. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K.; Zhang, T.; Chen, X.-W.; Xu, Y.; Zhang, R.; Qian, P.-Y. Viruses in Marine Invertebrate Holobionts: Complex Interactions between Phages and Bacterial Symbionts. Annu. Rev. Mar. Sci. 2024, 16, 467–485. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Meng, F.; Zhou, C.; Li, J.; Hu, T.; Wang, Y.; Wang, G.; Luo, J.; Li, X.; Liu, S.; et al. Enormous Diversity of RNA Viruses in Economic Crustaceans. Msystems 2024, 9, e01016-24. [Google Scholar] [CrossRef]
- Richard, J.C.; Blevins, E.; Dunn, C.D.; Leis, E.M.; Goldberg, T.L. Viruses of Freshwater Mussels during Mass Mortality Events in Oregon and Washington, USA. Viruses 2023, 15, 1719. [Google Scholar] [CrossRef]
- Richard, J.C.; Leis, E.M.; Dunn, C.D.; Harris, C.; Agbalog, R.E.; Campbell, L.J.; Knowles, S.; Waller, D.L.; Putnam, J.G.; Goldberg, T.L. Freshwater Mussels Show Elevated Viral Richness and Intensity during a Mortality Event. Viruses 2022, 14, 2603. [Google Scholar] [CrossRef]
- Pao, H.-Y.; Wu, C.-Y.; Wen, C.M. Persistent Development of Adomavirus and Aquareovirus in a Novel Cell Line from Marbled Eel with Petechial Skin Haemorrhage. J. Fish Dis. 2019, 42, 345–355. [Google Scholar] [CrossRef]
- Chinchar, V.; Logue, O.; Antao, A.; Chinchar, G. Channel Catfish Reovirus (CRV) Inhibits Replication of Channel Catfish Herpesvirus (CCV) by Two Distinct Mechanisms: Viral Interference and Induction of an Anti-Viral Factor. Dis. Aquat. Org. 1998, 33, 77–85. [Google Scholar] [CrossRef] [PubMed]
- LaPatra, S.; Lauda, K.; Jones, G. Aquareovirus Interference Mediated Resistance to Infectious Hematopoietic Necrosis Virus. Vet. Res. 1995, 26, 455–459. [Google Scholar]
- López-Bueno, A.; Mavian, C.; Labella, A.M.; Castro, D.; Borrego, J.J.; Alcami, A.; Alejo, A. Concurrence of Iridovirus, Polyomavirus, and a Unique Member of a New Group of Fish Papillomaviruses in Lymphocystis Disease-Affected Gilthead Sea Bream. J. Virol. 2016, 90, 8768–8779. [Google Scholar] [CrossRef] [PubMed]
- Van Doorslaer, K.; Kraberger, S.; Austin, C.; Farkas, K.; Bergeman, M.; Paunil, E.; Davison, W.; Varsani, A. Fish Polyomaviruses Belong to Two Distinct Evolutionary Lineages. J. Gen. Virol. 2018, 99, 567. [Google Scholar] [CrossRef]
- Buck, C.B.; Van Doorslaer, K.; Peretti, A.; Geoghegan, E.M.; Tisza, M.J.; An, P.; Katz, J.P.; Pipas, J.M.; McBride, A.A.; Camus, A.C.; et al. The Ancient Evolutionary History of Polyomaviruses. PLoS Pathog. 2016, 12, e1005574. [Google Scholar] [CrossRef]
- Monier, A.; Claverie, J.-M.; Ogata, H. Horizontal Gene Transfer and Nucleotide Compositional Anomaly in Large DNA Viruses. Bmc Genom. 2007, 8, 456. [Google Scholar] [CrossRef]
- Salzer, J.E.; Greer, J.B.; Groner, M.; MacKenzie, A.; Gregg, J.L.; Hershberger, P. Effects of Temperature on Viral Load, Inclusion Body Formation, and Host Response in Pacific Herring with Viral Erythrocytic Necrosis (VEN). J. Aquat. Anim. Health 2024, 36, 45–56. [Google Scholar] [CrossRef]
- Pham, P.H.; Misk, E.; Papazotos, F.; Jones, G.; Polinski, M.P.; Contador, E.; Russell, S.; Garver, K.A.; Lumsden, J.S.; Bols, N.C. Screening of Fish Cell Lines for Piscine Orthoreovirus-1 (PRV-1) Amplification: Identification of the Non-Supportive PRV-1 Invitrome. Pathogens 2020, 9, 833. [Google Scholar] [CrossRef]
- Dhamotharan, K.; Vendramin, N.; Markussen, T.; Wessel, Ø.; Cuenca, A.; Nyman, I.B.; Olsen, A.B.; Tengs, T.; Krudtaa Dahle, M.; Rimstad, E. Molecular and Antigenic Characterization of Piscine Orthoreovirus (PRV) from Rainbow Trout (Oncorhynchus mykiss). Viruses 2018, 10, 170. [Google Scholar] [CrossRef] [PubMed]
- Meyers, T.R.; Hickey, N. A Perspective: Molecular Detections of New Agents in Finfish—Interpreting Biological Significance for Fish Health Management. J. Aquat. Anim. Health 2022, 34, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Sommers, P.; Chatterjee, A.; Varsani, A.; Trubl, G. Integrating Viral Metagenomics into an Ecological Framework. Annu. Rev. Virol. 2021, 8, 133–158. [Google Scholar] [CrossRef] [PubMed]
- Munang’andu, H.M.; Mugimba, K.K.; Byarugaba, D.K.; Mutoloki, S.; Evensen, Ø. Current Advances on Virus Discovery and Diagnostic Role of Viral Metagenomics in Aquatic Organisms. Front. Microbiol. 2017, 8, 406. [Google Scholar] [CrossRef]
- Shi, M.; Lin, X.-D.; Chen, X.; Tian, J.-H.; Chen, L.-J.; Li, K.; Wang, W.; Eden, J.-S.; Shen, J.-J.; Liu, L.; et al. The Evolutionary History of Vertebrate RNA Viruses. Nature 2018, 556, 197–202. [Google Scholar] [CrossRef]
- Tisza, M.J.; Pastrana, D.V.; Welch, N.L.; Stewart, B.; Peretti, A.; Starrett, G.J.; Pang, Y.-Y.S.; Krishnamurthy, S.R.; Pesavento, P.A.; McDermott, D.H.; et al. Discovery of Several Thousand Highly Diverse Circular DNA Viruses. Elife 2020, 9, e51971. [Google Scholar] [CrossRef]
- Costa, V.A.; Holmes, E.C. Diversity, Evolution, and Emergence of Fish Viruses. J. Virol. 2024, 98, e00118-24. [Google Scholar] [CrossRef]
- Lauber, C.; Seitz, S. Opportunities and Challenges of Data-Driven Virus Discovery. Biomolecules 2022, 12, 1073. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Priyadarshini, P.; Vrati, S. Unraveling the Web of Viroinformatics: Computational Tools and Databases in Virus Research. J. Virol. 2015, 89, 1489–1501. [Google Scholar] [CrossRef] [PubMed]
- Krishnamurthy, S.R.; Wang, D. Origins and Challenges of Viral Dark Matter. Virus Res. 2017, 239, 136–142. [Google Scholar] [CrossRef] [PubMed]



| Primer Bind | Primer Name | Sequence (5′ → 3′) | Target |
|---|---|---|---|
| MnA-1 | |||
| F | Mna1-E01_851F | TGCTCGTGCCCTTAACAGAG | RepE1 (NCBI accession: PV469406) |
| R | Mna1-E01_851R | TCTCTCAGACGGTTCGT | |
| MnA-2 | |||
| F | Mna2_E01_802F | GCAGCTAAATCGCAGACAGC | RepE1 (NCBI accession: PV469407) |
| R | Mna2_E01_802R | TGTTCACTGGCACCTCATCC | |
| MnA-3 | |||
| F | Mna3_E01_699F | ATCTGAAACCCGGAACCGTC | RepE1 (NBCI accession: PV469408) |
| R | Mna3_E01_699R | GATGGGATGCACCAGTGACA | |
| MnNDV-1 | |||
| F | MnNV462F | CCCGAGATTCAACGATGGGT | Polymerase (NCBI Accession: PV448632) |
| R | MnNV462R | GCATGCAAAAAGGGGAGCTC | |
| CDS | Highest Structural Similarity (HHpred) | GC% | Length (bp) | Interval (nt) | pI | Amino Acids | Molecular Weight (Da) |
|---|---|---|---|---|---|---|---|
| MnNDV-1 (3206 bp, 46.5% GC) | |||||||
| C Protein | Core protein HBV | 53.5 | 648 | 1–648 | 10.24 | 215 | 24,343 |
| P Protein | Polymerase protein HBV | 49.5 | 2298 | 353–2257 | 10.17 | 765 | 72,880 |
| smORF 3 | Phage minor head protein GP7 | 45.1 | 162 | 2293–2454 | 9.13 | 53 | 5886 |
| smORF 1 | Uncharacterized protein | 46.7 | 420 | 2451–2870 | 9.78 | 139 | 15,990 |
| smORF 2 | Uncharacterized protein | 41.7 | 312 | 2892–3203 | 5.26 | 103 | 11,958 |
| MnA-2 (17,869 bp, 46.1% GC) | |||||||
| 1940 | Y1940 ATV Protein Acidianus two-tailed virus | 49.6 | 1071 | 1464–2534 | 11.38 | 357 | 39,252 |
| Wasp | Neural Wiskott–Aldrich syndrome protein | 44.7 | 237 | 2476–2712 | 10.55 | 79 | 9051 |
| Cahalt | Dynein intermediate chain, motor protein | 52 | 327 | 2739–3065 | 4.15 | 109 | 12,153 |
| Penton | Signal transduction histidine kinase, adenovirus penton | 46 | 783 | 3079–3861 | 4.73 | 261 | 28,150 |
| Macc | Late L2 mu core protein | 47.5 | 888 | 3851–4738 | 10.31 | 296 | 31,895 |
| Hexon | ATP synthase subunit b (MdA-2 LO6 analog) | 48.2 | 1494 | 4750–6243 | 5.35 | 498 | 55,108 |
| Adenain | Adenain; alpha and beta protein | 46 | 633 | 6353–6985 | 8.37 | 211 | 24,253 |
| Bcoroid | BCOR, BCL-6 co-repressor | 50.3 | 1968 | 7274–9241 | 5.02 | 656 | 71,264 |
| Prim | DNA primase small subunit | 49.9 | 2256 | 9244–11,499 | 5.98 | 751 | 82,566 |
| Otomem | Proton channel, otopetrin, membrane protein | 44.2 | 591 | 11,496–12,086 | 4.67 | 197 | 22,283 |
| Spamem | Serine palmitoyltransferase small subunit A membrane | 36.1 | 291 | 12,114–12,404 | 5.91 | 97 | 11,238 |
| Broz | 3-ketodihydrosphingosine reductase, oxidoreductase | 37.6 | 303 | 13,478–13,780 | 7.52 | 101 | 11,645 |
| RepE1 | Replication protein E1 | 45.8 | 2787 | 14,451–17,237 | 6.05 | 928 | 104,573 |
| Alt | Movement protein TGBp3 | 47.4 | 228 | 16,397–16,624 | 7.9 | 76 | 8592 |
| SET | SET domain Methyltransferase | 45.2 | 897 | 16,973–17,869 | 9.89 | 298 | 34,089 |
| Sealt | Tospovirus NSs protein | 44.4 | 207 | 17,620–17,826 | 8.5 | 69 | 8469 |
| MnA-3 (partial sequence) | |||||||
| Prim | DNA primase small subunit | 51.2 | 2250 | 1578–3827 | 7.22 | 749 | 83,240 |
| Otomem | Structural protein VP2 Pyrobaculum filamentous virus 1 | 48.0 | 483 | 3704–4186 | 5.79 | 161 | 17,277 |
| RepE1 | Replication protein E1 | 47.2 | 2214 | 5685–7808 | 10.79 | 708 | 74,945 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raines, C.; Odenkirk, J.; Isel, M.; Mazik, P.; Biggs, M.; Iwanowicz, L. Hiding in Plain Sight: Genomic Characterization of a Novel Nackednavirus and Evidence of Diverse Adomaviruses in a Hyperpigmented Lesion of a Largemouth Bass (Micropterus nigricans). Viruses 2025, 17, 1173. https://doi.org/10.3390/v17091173
Raines C, Odenkirk J, Isel M, Mazik P, Biggs M, Iwanowicz L. Hiding in Plain Sight: Genomic Characterization of a Novel Nackednavirus and Evidence of Diverse Adomaviruses in a Hyperpigmented Lesion of a Largemouth Bass (Micropterus nigricans). Viruses. 2025; 17(9):1173. https://doi.org/10.3390/v17091173
Chicago/Turabian StyleRaines, Clayton, John Odenkirk, Michael Isel, Patricia Mazik, Morgan Biggs, and Luke Iwanowicz. 2025. "Hiding in Plain Sight: Genomic Characterization of a Novel Nackednavirus and Evidence of Diverse Adomaviruses in a Hyperpigmented Lesion of a Largemouth Bass (Micropterus nigricans)" Viruses 17, no. 9: 1173. https://doi.org/10.3390/v17091173
APA StyleRaines, C., Odenkirk, J., Isel, M., Mazik, P., Biggs, M., & Iwanowicz, L. (2025). Hiding in Plain Sight: Genomic Characterization of a Novel Nackednavirus and Evidence of Diverse Adomaviruses in a Hyperpigmented Lesion of a Largemouth Bass (Micropterus nigricans). Viruses, 17(9), 1173. https://doi.org/10.3390/v17091173







