An Anti-HIV Drug Is Highly Effective Against SARS-CoV-2 In Vitro and Has Potential Benefit for Long COVID Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Biological Activity
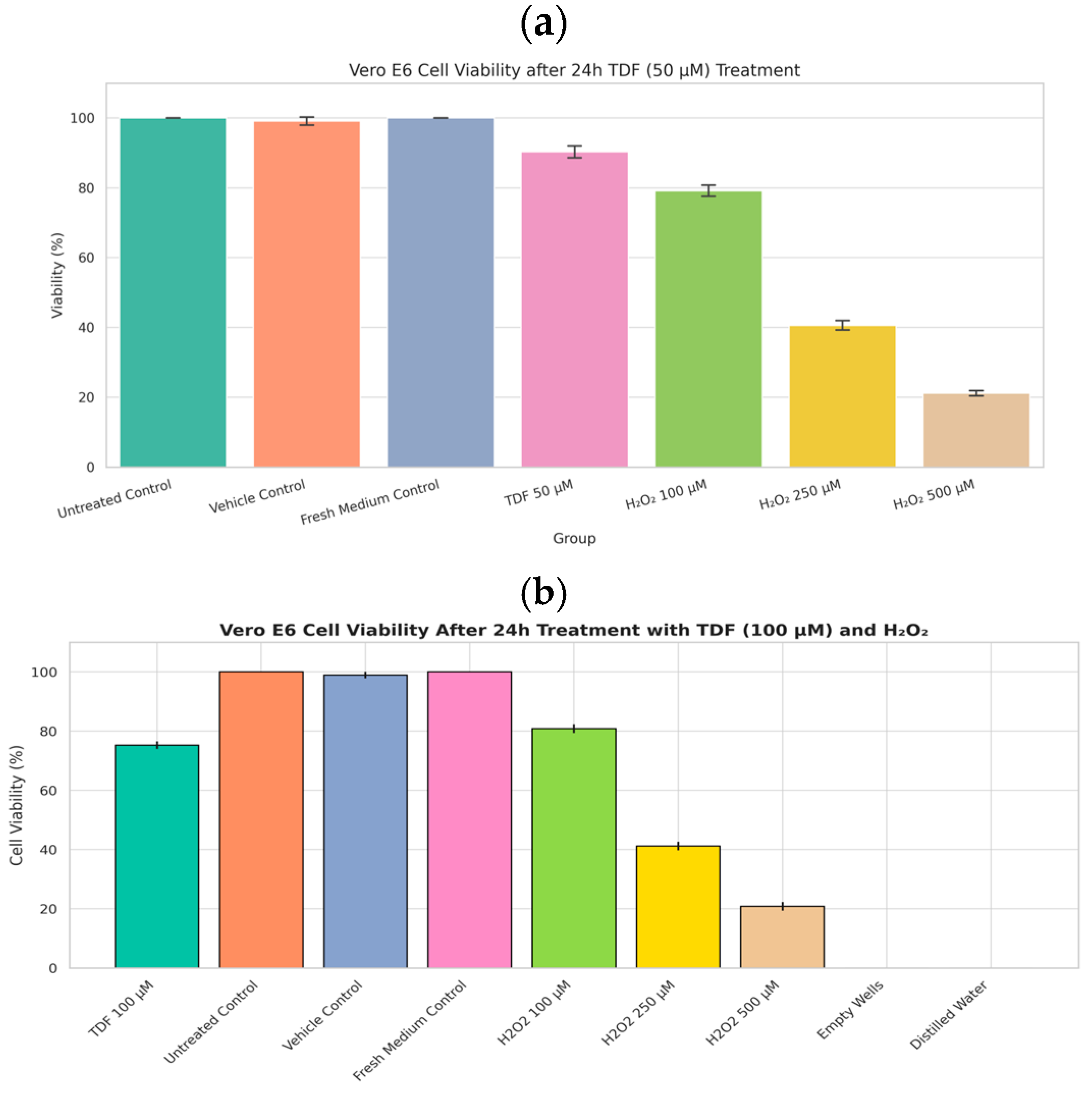
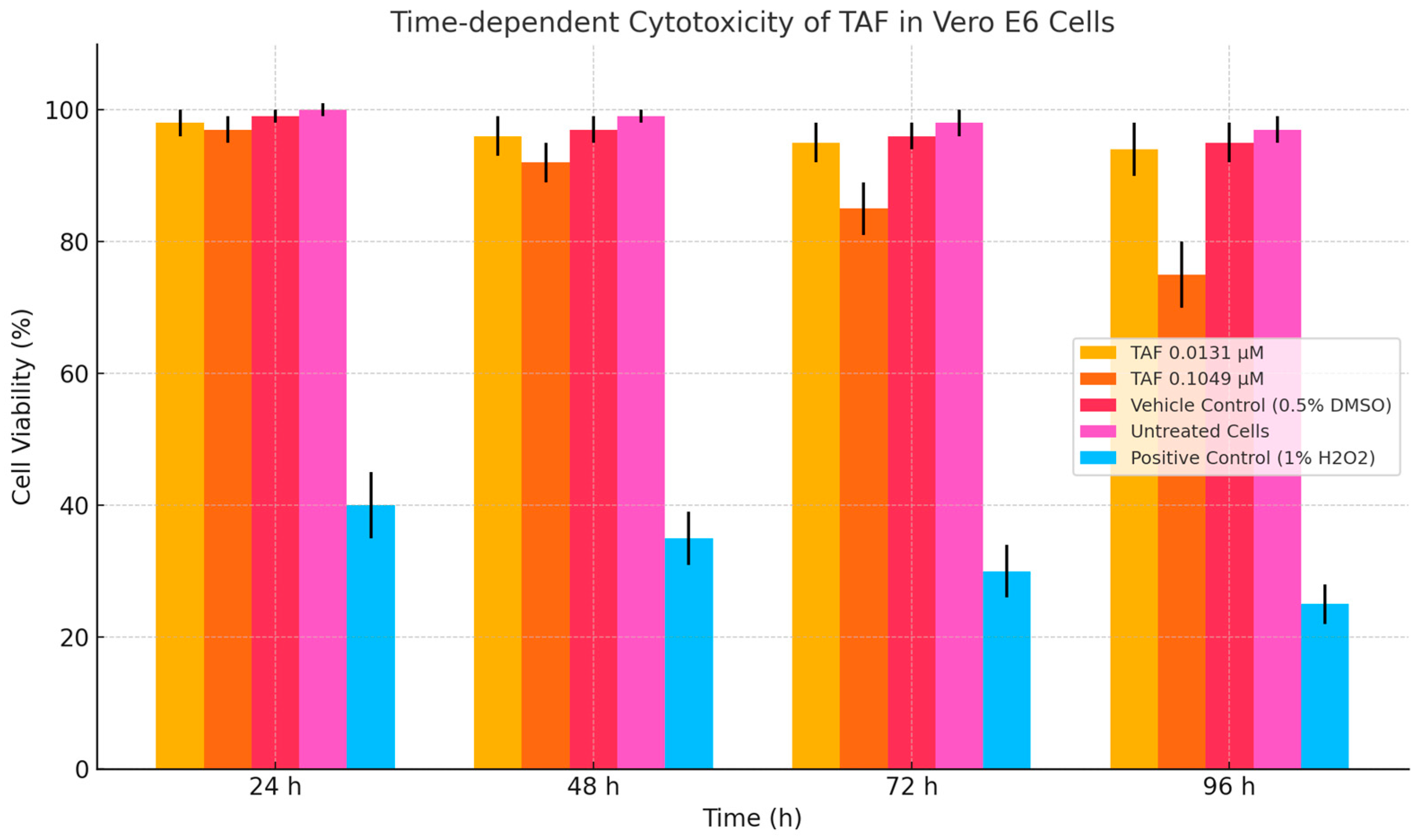
2.1.1. VeroE6 MTT Cytotoxicity Assay
2.1.2. VeroE6 CCK8-Cytotoxicity Assay
2.2. Antiviral Activity
2.2.1. Antiviral Activity TDF
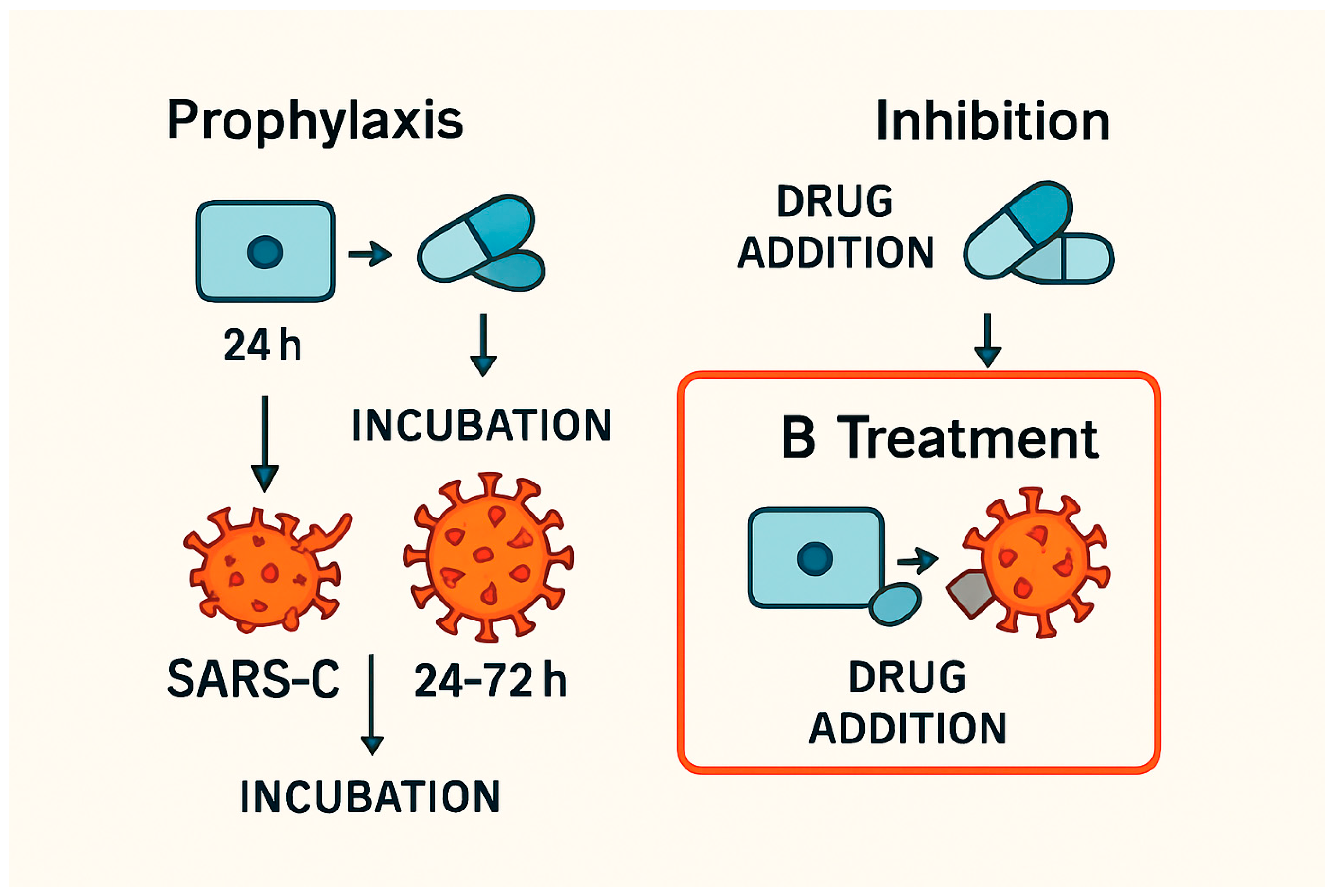
2.2.2. Predicted Antiviral Activity-Treatment and Prophylaxis Mode: TAF
2.2.3. Predicted Antiviral Activity Inhibition Mode: TAF
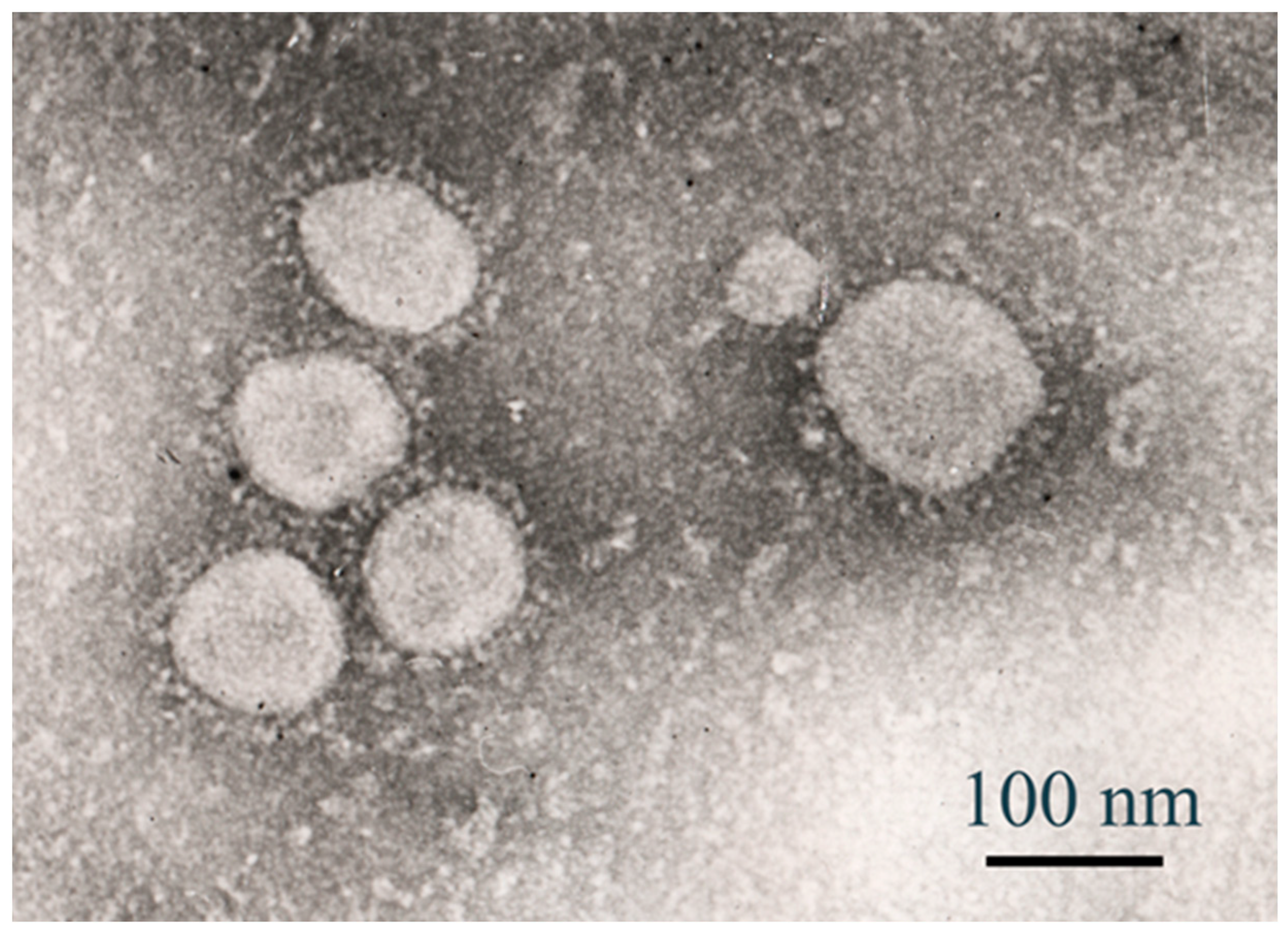
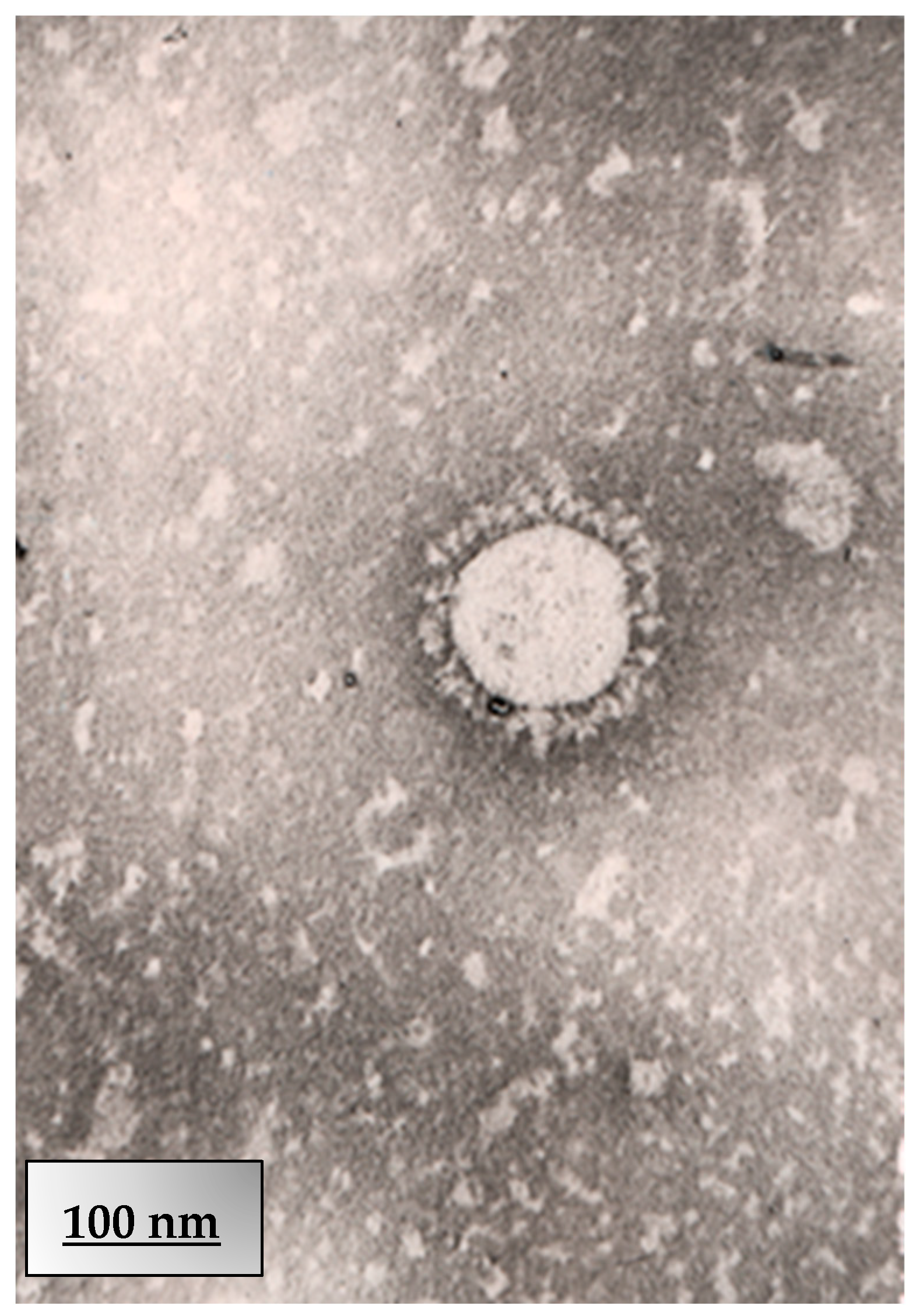
2.3. Electron Microscopy
2.3.1. Cell Culture Infection
2.3.2. Negative Staining (Alternative for Viral Particles)
2.3.3. JEOL JEM-100CX TEM Operation
3. Results and Discussion
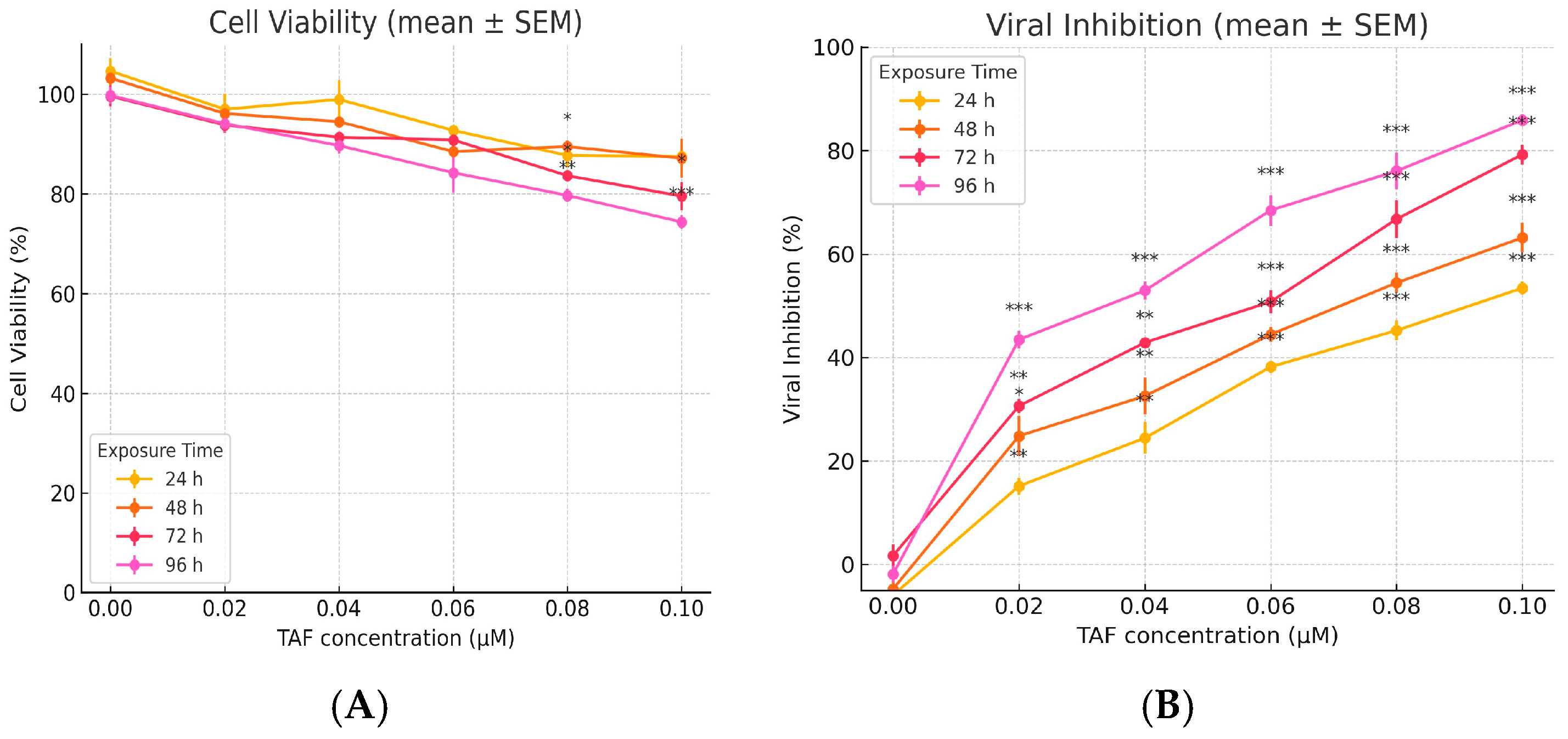
3.1. Cytotoxicity Assay
3.1.1. Tenofovir (TDF)-CCK8
3.1.2. Tenofovir (TAF) MTT
3.2. Antiviral Assay
3.2.1. TDF (25 and 50 µg/mL)
3.2.2. TAF Antiviral Activity (Treatment/Prophylaxis/Inhibition)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jones, K.E.; Patel, N.G.; Levy, M.A.; Storeygard, A.; Balk, D.; Gittleman, J.L.; Daszak, P. Global trends in emerging infectious diseases. Nature 2008, 451, 990–993. [Google Scholar] [CrossRef]
- Weekly Operational Update on COVID-19—30 October 2020. Available online: https://www.who.int/publications/m/item/weekly-operational-update---30-october-2020 (accessed on 1 July 2025).
- Weekly Operational Update on COVID-19—30 November 2020. Available online: https://www.who.int/publications/m/item/weekly-operational-update---30-november-2020 (accessed on 1 July 2025).
- David, M.M.; Anthony, S.F. Emerging Pandemic Diseases: How We Got to COVID-19. Cell 2020, 182, 1077–1092. [Google Scholar] [CrossRef]
- Zhu, N.; Zhang, D.Y.; Wang, W.L.; Li, X.W.; Yang, B.; Song, J.D.; Zhao, X. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef]
- Harvey, W.T.; Carabelli, A.M.; Jackson, B.; Gupta, R.K.; Thomson, E.C.; Harrison, E.M.; Ludden, C.; Reeve, R.; Rambaut, A. SARS-CoV-2 variants, spike mutations and immune escape. Nat. Rev. Microbiol. 2021, 19, 409–424. [Google Scholar] [CrossRef]
- Ashburn, T.T.; Thor, K.B. Drug repositioning: Identifying and developing new uses for existing drugs. Nat. Rev. Drug Discov. 2004, 3, 673–683. [Google Scholar] [CrossRef]
- Pushpakom, S.; Iorio, F.; Eyers, P.A.; Escott, K.J.; Hopper, S.; Wells, A.; Doig, A.; Guilliams, T.; Latimer, J.; McNamee, C.; et al. Drug repurposing: Progress, challenges and recommendations. Nat. Rev. Drug Discov. 2019, 18, 41–58. [Google Scholar] [CrossRef] [PubMed]
- De Clercq, E. Tenofovir alafenamide (TAF) as the successor of tenofovir disoproxil fumarate (TDF). Biochem. Pharmacol. 2016, 119, 1–7. [Google Scholar] [CrossRef]
- Rizzardini, G.; Overton, E.T.; Orkin, C.; Swindells, S.; Arasteh, K.; Górgolas Hernández-Mora, M.; Pokrovsky, V.; Girard, P.M.; Oka, S.; Andrade-Villanueva, J.F.; et al. Long-acting injectable cabotegravir + rilpivirine for HIV maintenance therapy: Week 48 pooled analysis of phase 3 ATLAS and FLAIR trials. J. Acquir. Immune Defic. Syndr. 2020, 85, 498–506. [Google Scholar] [CrossRef] [PubMed]
- Elfiky, A.A. Anti-HCV, nucleotide inhibitors, repurposing against COVID-19. Life Sci. 2020, 248, 117–477. [Google Scholar] [CrossRef]
- De Clercq, E.; Li, G. Approved Antiviral Drugs over the Past 50 Years. Clin. Microbiol. Rev. 2016, 29, 695–747. [Google Scholar] [CrossRef] [PubMed]
- Gallant, J.E.; Daar, E.S.; Raffi, F.; Brinson, C.; Ruane, P.; DeJesus, E.; Deng, H.; Liu, Y.P.; Garner, W.; Huang, J.; et al. Efficacy and safety of tenofovir alafenamide versus tenofovir disoproxil fumarate given as fixed-dose combinations containing emtricitabine as backbones for treatment of HIV-1 infection in virologically suppressed adults: A randomised, double-blind, active-controlled phase 3 trial. Lancet HIV 2016, 3, e158–e165. [Google Scholar] [CrossRef]
- Schloer, S.; Brunotte, L.; Mecate-Zambrano, A.; Zheng, S.; Tang, J.; Ludwig, S.; Rescher, U. Drug synergy of combinatory treatment with remdesivir and the repurposed drugs fluoxetine and itraconazole effectively impairs SARS-CoV-2 infection in vitro. Viruses 2021, 13, 800. [Google Scholar] [CrossRef]
- Sax, P.E.; Wohl, D.; Yin, M.T.; Post, F.; DeJesus, E.; Saag, M.; Pozniak, A.; Thompson, M.; Podzamczer, D.; Molina, J.M.; et al. Tenofovir alafenamide versus tenofovir disoproxil fumarate, coformulated with elvitegravir, cobicistat, and emtricitabine, for initial treatment of HIV-1 infection: Two randomised, double-blind, phase 3, non-inferiority trials. Lancet 2015, 385, 2606–2615. [Google Scholar] [CrossRef]
- Delaney, W.E., 4th; Ray, A.S.; Yang, H.; Qi, X.; Xiong, S.; Zhu, Y.; Miller, M.D. Intracellular metabolism and in vitro activity of tenofovir against hepatitis B virus. Antimicrob. Agents Chemother. 2006, 50, 2471–2477. [Google Scholar] [CrossRef]
- Del Amo, J.; Polo, R.; Moreno, S.; Díaz, A.; Martínez, E.; Arribas, J.R.; Jarrín, I.; Hernán, M.A.; on behalf of the Spanish HIV/COVID-19 Collaboration. Antiretrovirals and risk of COVID-19 diagnosis and hospitalization in HIV-positive persons. Epidemiology 2020, 31, e49–e51. [Google Scholar] [CrossRef]
- Riss, T.L.; Moravec, R.A.; Niles, A.L. Cytotoxicity Testing: Measuring Viable Cells, Dead Cells, and Detecting Mechanism of Cell Death. Methods Mol. Biol. 2015, 1219, 103–114. [Google Scholar] [CrossRef]
- Hadj Hassine, I.; Ben M’hadheb, M.; Menéndez-Arias, L. Lethal Mutagenesis of RNA Viruses and Approved Drugs with Antiviral Mutagenic Activity. Viruses 2022, 14, 841. [Google Scholar] [CrossRef] [PubMed]
- Burashev, Y.; Usserbayev, B.; Kutumbetov, L.; Abduraimov, Y.; Kassenov, M.; Kerimbayev, A.; Myrzakhmetova, B.; Melisbek, A.; Shirinbekov, M.; Khaidarov, S.; et al. Coding Complete Genome Sequence of the SARS-CoV-2 Virus Strain, Variant, B.1.1, Sampled from Kazakhstan. Microbiol. Resour. Announc. 2022, 11, e01114-22. [Google Scholar] [CrossRef]
- Berridge, M.V.; Herst, P.M.; Tan, A.S. Tetrazolium Dyes as Tools in Cell Biology: New Insights into Their Cellular Reduction. Biotechnol. Annu. Rev. 2005, 11, 127–152. [Google Scholar] [CrossRef] [PubMed]
- Tominaga, H.; Ishiyama, M.; Ohseto, F.; Sasamoto, K.; Hamamoto, T.; Suzuki, K.; Watanabe, M. A Water-Soluble Tetrazolium Salt Useful for Colorimetric Cell Viability Assay. Anal. Commun. 1999, 36, 47–50. [Google Scholar] [CrossRef]
- Chaves, O.A.; Sacramento, C.Q.; Ferreira, A.C.; Mattos, M.; Fintelman-Rodrigues, N.; Temerozo, J.R.; Vazquez, L.; Pinto, D.P.; da Silveira, G.P.E.; da Fonseca, L.B.; et al. Atazanavir Is a Competitive Inhibitor of SARS-CoV-2 Mpro, Impairing Variants Replication In Vitro and In Vivo. Pharmaceuticals 2022, 15, 21. [Google Scholar] [CrossRef]
- Ouyang, W.; Yu, Z.; Zhao, X.; Lu, S.; Wang, Z. Aptamers in hematological malignancies and their potential therapeutic implications. Crit. Rev. Oncol. Hematol. 2016, 106, 108–117. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Available online: https://www.who.int/publications/i/item/who-whe-epp-2024.3 (accessed on 1 July 2025).
- Gallant, J.; Brunetta, J.; Crofoot, G.; Benson, P.; Mills, A.; Brinson, C.; Oka, S.; Cheng, A.; Garner, W.; Fordyce, M.; et al. GS-US-292-1249 Study Investigators. Brief Report: Efficacy and Safety of Switching to a Single-Tablet Regimen of Elvitegravir/Cobicistat/Emtricitabine/Tenofovir Alafenamide in HIV-1/Hepatitis B-Coinfected Adults. J. Acquir. Immune Defic. Syndr. 2016, 73, 294–298. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, X.; Jiang, X.M.; Guo, J.; Fu, Z.; Zhou, Z.; Yang, P.; Guo, H.; Guo, X.; Liang, G.; et al. Decreased Inhibition of Ex Vivo SARS-CoV-2 Infection by Hydroxychloroquine and Chloroquine in Human Lung Cells. Int. J. Mol. Sci. 2021, 22, 6023. [Google Scholar] [CrossRef]
- Clososki, G.C.; Soldi, R.A.; Silva, R.M.D.; Guaratini, T.; Lopes, J.N.; Pereira, P.R.; Lopes, J.L.; Santos, T.D.; Martins, R.B.; Costa, C.S.; et al. Tenofovir Disoproxil Fumarate: New Chemical Developments and Encouraging in vitro Biological Results for SARS-CoV-2. J. Braz. Chem. Soc. 2020, 31, 1552–1556. [Google Scholar] [CrossRef]
- Fletcher, C.V.; Podany, A.T.; Thorkelson, A.; Winchester, L.C.; Mykris, T.; Anderson, J.; Jorstad, S.; Baker, J.V.; Schacker, T.W. The Lymphoid Tissue Pharmacokinetics of Tenofovir Disoproxil Fumarate and Tenofovir Alafenamide in HIV-Infected Persons. Clin. Pharmacol. Ther. 2020, 108, 971–975. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, X.; Sacramento, C.Q.; Jockusch, S.; Chaves, O.A.; Tao, C.; Fintelman-Rodrigues, N.; Chien, M.; Temerozo, J.R.; Li, X.; Kumar, S.; et al. Combination of antiviral drugs inhibits SARS-CoV-2 polymerase and exonuclease and demonstrates COVID-19 therapeutic potential in viral cell culture. Commun. Biol. 2022, 5, 154. [Google Scholar] [CrossRef] [PubMed]
- Good, S.S.; Westover, J.; Jung, K.H.; Zhou, X.; Moussa, A.; La Colla, P.; Collu, G.; Canard, B.; Sommadossi, J. AT-527, a Double Prodrug of a Guanosine Nucleotide Analog, Is a Potent Inhibitor of SARS-CoV-2 In Vitro and a Promising Oral Antiviral for Treatment of COVID-19. Antimicrob. Agents Chemother. 2021, 65, 10–128. [Google Scholar] [CrossRef]
- Zanella, I.; Zizioli, D.; Castelli, F.; Quiros-Roldan, E. Tenofovir, Another Inexpensive, Well-Known and Widely Available Old Drug Repurposed for SARS-COV-2 Infection. Pharmaceuticals 2021, 14, 454. [Google Scholar] [CrossRef]
- Huynh, T.; Hu, K.Q. S1335 Tenofovir Disoproxil Fumarate Switching to Tenofovir Alafenamide for Three Years Resulted in Improvement of Hepatic Fibrosis by APRI and FIB-4 Score as Well as Shear Wave Elastography in Patients with Chronic Hepatitis B. Am. J. Gastroenterol. 2022, 117, e961. [Google Scholar] [CrossRef]
- Khater, S.; Kumar, P.; Dasgupta, N.; Das, G.; Ray, S.; Prakash, A. Combining SARS-CoV-2 proofreading exonuclease and RNA-dependent RNA polymerase inhibitors as a strategy to combat COVID-19: A high-throughput in silico screen. Front. Microbiol. 2021, 12, 647–693. [Google Scholar] [CrossRef] [PubMed]
- Yager, J.L.; Brooks, K.M.; Castillo-Mancilla, J.R.; Nemkov, C.; Morrow, M.; Peterson, S.; Ibrahim, M.; Bushman, L.; Kiser, J.J.; MaWhinney, S.; et al. Tenofovir-diphosphate in peripheral blood mononuclear cells during low, medium and high adherence to emtricitabine/tenofovir alafenamide vs. emtricitabine/tenofovir disoproxil fumarate. AIDS 2021, 35, 2481–2487. [Google Scholar] [CrossRef]
- Ruane, P.J.; DeJesus, E.; Berger, D.; Markowitz, M.; Bredeek, U.F.; Callebaut, C.; Zhong, L.; Ramanathan, S.; Rhee, M.S.; Fordyce, M.W.; et al. Antiviral Activity, Safety, and Pharmacokinetics/Pharmacodynamics of Tenofovir Alafenamide as 10-Day Monotherapy in HIV-1–Positive Adults. J. Acquir. Immune Defic. Syndr. 2013, 63, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Rizzardini, G.; Zeroli, C.; Pariani, E.; Degli Antoni, A.; Viganò, O.; Lupo, A.; Carrabba, L. In Vitro Assessment of the Antiviral Activity of Tenofovir against SARS-CoV-2. Int. J. Mol. Sci. 2022, 23, 9093. [Google Scholar] [CrossRef]
- Juhas, M.; Widlake, E.; Teo, J.; Huseby, D.L.; Tyrrell, J.M.; Polikanov, Y.S.; Ercan, O.; Petersson, A.; Cao, S.; Aboklaish, A.F.; et al. In vitro activity of apramycin against multidrug-, carbapenem- and aminoglycoside-resistant Enterobacteriaceae and Acinetobacter baumannii. J. Antimicrob. Chemother. 2019, 74, 944–952. [Google Scholar] [CrossRef]
- Kale, M.A.; Jain, M.V. Drug Discovery and Exploration of Heterocycles for the Development of Anti-HIV Agents, Infectious Disorders. Drug Targets 2025, 25, E18715265290911. [Google Scholar] [CrossRef]
- Yelle, J.; Morisset, R.; Thibodeau, L. Analysis of long-term viral expression in CEM cells persistently infected with non syncytium-inducing HIV-1 strains. Arch. Virol. 1994, 139, 155–172. [Google Scholar] [CrossRef]
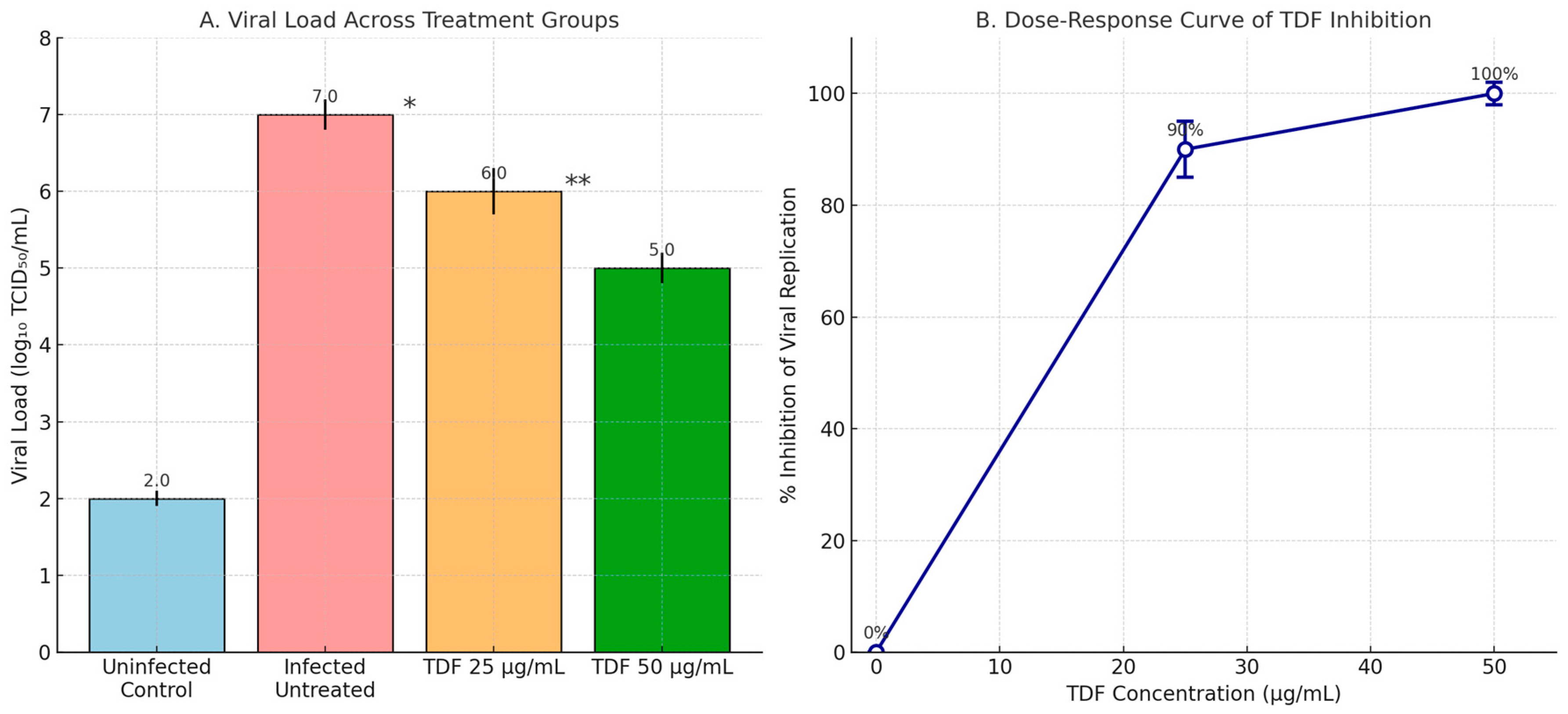



| Table | Predicted Viral Load (log10 TCID50/mL) | % Inhibition (vs. log 7 Control) | Statistical Significance |
|---|---|---|---|
| 6.25 (~13.6 µM) | ~6.5 | ~68% | * p * = 0.032 |
| 12.5 (~27.2 µM) | ~6.2 | ~80% | * p * = 0.005 |
| 25 (~54.5 µM) | ~5.8 | ~87% | * p * < 0.001 |
| 50 (~109 µM) | ~5.0 | ~100% | * p * < 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khaidarov, S.; Hejran, A.B.; Moldakaryzova, A.; Izmailova, S.; Nurgaliyeva, B.; Beisenova, A.; Mustafaeva, A.; Nurzhanova, K.; Belova, Y.; Satbayeva, E.; et al. An Anti-HIV Drug Is Highly Effective Against SARS-CoV-2 In Vitro and Has Potential Benefit for Long COVID Treatment. Viruses 2025, 17, 1170. https://doi.org/10.3390/v17091170
Khaidarov S, Hejran AB, Moldakaryzova A, Izmailova S, Nurgaliyeva B, Beisenova A, Mustafaeva A, Nurzhanova K, Belova Y, Satbayeva E, et al. An Anti-HIV Drug Is Highly Effective Against SARS-CoV-2 In Vitro and Has Potential Benefit for Long COVID Treatment. Viruses. 2025; 17(9):1170. https://doi.org/10.3390/v17091170
Chicago/Turabian StyleKhaidarov, Saken, Abdul Bari Hejran, Aizhan Moldakaryzova, Slu Izmailova, Bayan Nurgaliyeva, Aizhan Beisenova, Aigul Mustafaeva, Kuanysh Nurzhanova, Yelena Belova, Elmira Satbayeva, and et al. 2025. "An Anti-HIV Drug Is Highly Effective Against SARS-CoV-2 In Vitro and Has Potential Benefit for Long COVID Treatment" Viruses 17, no. 9: 1170. https://doi.org/10.3390/v17091170
APA StyleKhaidarov, S., Hejran, A. B., Moldakaryzova, A., Izmailova, S., Nurgaliyeva, B., Beisenova, A., Mustafaeva, A., Nurzhanova, K., Belova, Y., Satbayeva, E., Aidarov, A., Ossikbayeva, S., Kukubassov, Y., Amankulov, J., Goncharova, T., Yeszhan, B., Tulman, E., Tazhibayeva, K. N., Sadykova, A., ... Burashev, Y. (2025). An Anti-HIV Drug Is Highly Effective Against SARS-CoV-2 In Vitro and Has Potential Benefit for Long COVID Treatment. Viruses, 17(9), 1170. https://doi.org/10.3390/v17091170








