HSV-1 Infection in Retinal Pigment Epithelial Cells: A Possible Contribution to Age-Related Macular Degeneration
Abstract
1. Introduction
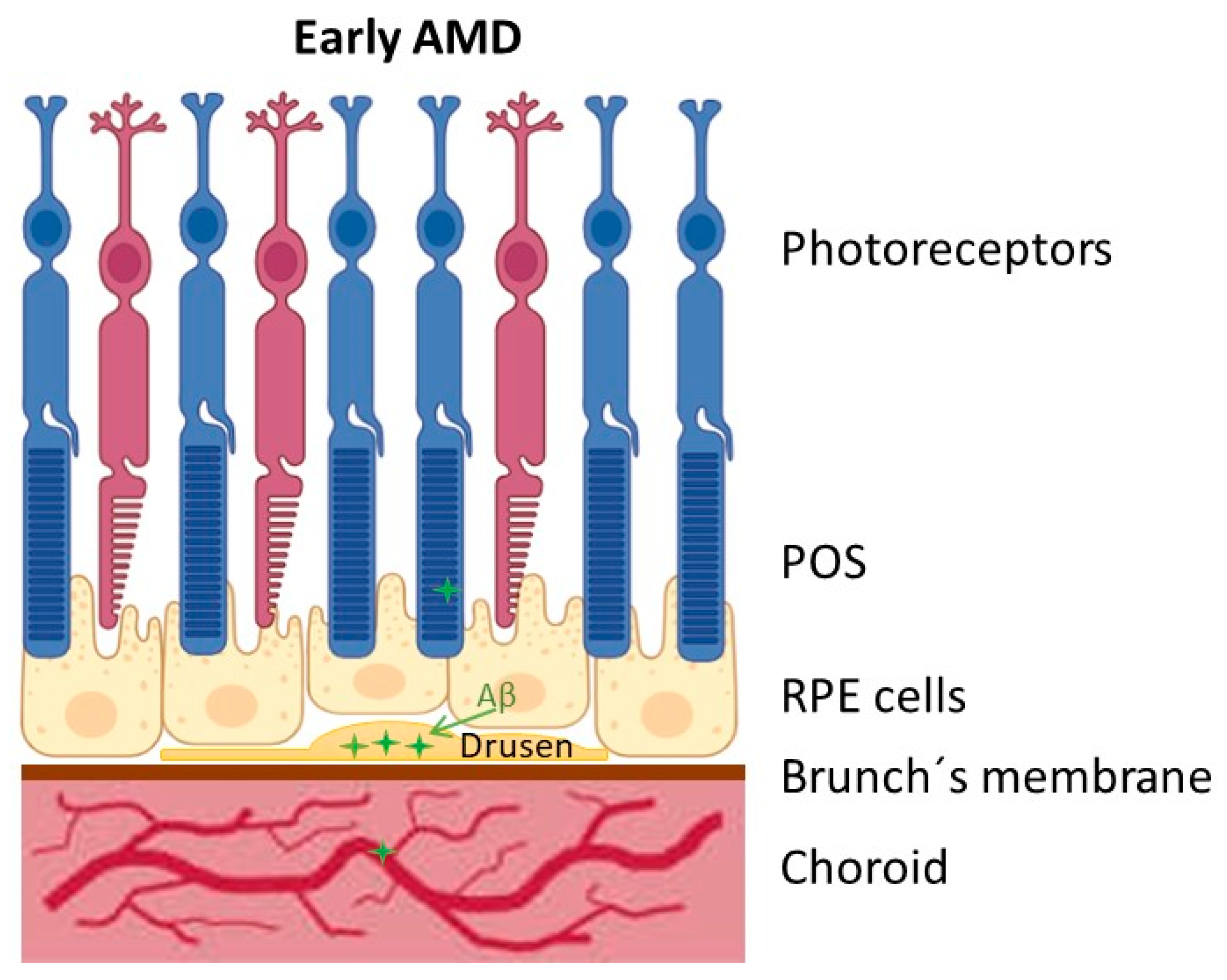
2. Age-Related Macular Degeneration (AMD)
3. Amyloid Beta Peptide (Aβ) in AMD
4. RPE-AMD-HSV-1
5. How HSV-1 Reaches and Infects the RPE
6. Conclusions and Future Perspective
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Aβ | Amyloid-β peptide |
| AD | Alzheimer’s disease |
| AMD | Age-related macular degeneration |
| APP | Amyloid precursor protein |
| CNS | Central nervous system |
| CMV | Cytomegalovirus |
| GA | Geographic atrophy |
| HBV | Hepatitis B virus |
| HCV | Hepatitis C virus |
| HIV | Human immunodeficiency virus |
| HSV-1 | Herpes simplex virus 1 |
| IL | Interleukin |
| RGCs | Retinal ganglion cells |
| POS | Photoreceptor outer segment |
| ROS | Reactive oxygen species |
| RPE | Retinal pigment epithelium |
| VZV | Varicella-zoster virus |
References
- Hampel, H.; Lista, S.; Khachaturian, Z.S. Development of biomarkers to chart all Alzheimer’s disease stages: The royal road to cutting the therapeutic Gordian Knot. Alzheimer’s Dement. 2012, 8, 312–336. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.C.; Lockwood, A.H.; Sonawane, B.R. Neurodegenerative Diseases: An Overview of Environmental Risk Factors. Environ. Health Perspect. 2005, 113, 1250–1256. [Google Scholar] [CrossRef] [PubMed]
- Leblanc, P.; Vorberg, I.M. Viruses in neurodegenerative diseases: More than just suspects in crimes. PLoS Pathog. 2022, 18, e1010670. [Google Scholar] [CrossRef]
- Gokoffski, K.K.; Peng, M.; Alas, B.; Lam, P. Neuro-protection and Neuro-regeneration of the Optic Nerve: Recent Advances and Future Directions. Curr. Opin. Neurol. 2021, 33, 93–105. [Google Scholar] [CrossRef]
- Kamatham, P.T.; Shukla, R.; Khatri, D.K.; Vora, L.K. Pathogenesis, diagnostics, and therapeutics for Alzheimer’s disease: Breaking the memory barrier. Ageing Res. Rev. 2024, 101, 102481. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, Z.; Huang, Y.; Shang, X.; O’Brien, T.J.; Kwan, P.; Ha, J.; Wang, W.; Liu, S.; Zhang, X. Shared genetic aetiology of Alzheimer’s disease and age-related macular degeneration by APOC1 and APOE genes. BMJ Neurol. Open 2024, 6, e000570. [Google Scholar] [CrossRef]
- Jabbehdari, S.; Oganov, A.C.; Rezagholi, F.; Mohammadi, S.; Harandi, H.; Yazdanpanah, G.; Arevalo, J.F. Age-related macular degeneration and neurodegenerative disorders: Shared pathways in complex interactions. Surv. Ophthalmol. 2024, 69, 303–310. [Google Scholar] [CrossRef]
- Butovsky, O.; Rosenzweig, N. Alzheimer’s disease and age-related macular degeneration: Shared and distinct immune mechanisms. Immunity 2025, 58, 1120–1139. [Google Scholar] [CrossRef]
- den Haan, J.; Verbraak, F.D.; Visser, P.J.; Bouwman, F.H. Retinal thickness in Alzheimer’s disease: A systematic review and meta-analysis. Alzheimer’s Dement. 2017, 6, 162–170. [Google Scholar] [CrossRef]
- Mirzaei, N.; Shi, H.; Oviatt, M.; Doustar, J.; Rentsendorj, A.; Fuchs, D.T.; Sheyn, J.; Black, K.L.; Koronyo, Y.; Koronyo-Hamaoui, M. Alzheimer’s Retinopathy: Seeing Disease in the Eyes. Front. Neurosci. 2020, 14, 921. [Google Scholar] [CrossRef] [PubMed]
- Snyder, P.J.; Alber, J.; Alt, C.; Bain, L.J.; Bouma, B.E.; Bouwman, F.H.; DeBuc, D.C.; Campbell, M.C.W.; Carrillo, M.C.; Chew, E.Y.; et al. Retinal imaging in Alzheimer’s and neurodegenerative diseases. Alzheimer’s Dement. 2021, 17, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Ratnayaka, J.A.; Serpell, L.C.; Lotery, A.J. Dementia of the eye: The role of amyloid beta in retinal degeneration. Eye 2015, 29, 1013–1026. [Google Scholar] [CrossRef]
- Wang, L.; Mao, X. Role of Retinal Amyloid-β in Neurodegenerative Diseases: Overlapping Mechanisms and Emerging Clinical Applications. Int. J. Mol. Sci. 2021, 22, 2360. [Google Scholar] [CrossRef]
- De Chiara, G.; Marcocci, M.E.; Sgarbanti, R.; Civitelli, L.; Ripoli, C.; Piacentini, R.; Garaci, E.; Grassi, C.; Palamara, A.T. Infectious agents and neurodegeneration. Mol. Neurobiol. 2012, 46, 614–638. [Google Scholar] [CrossRef] [PubMed]
- Karim, S.; Mirza, Z.; Ansari, S.A.; Rasool, M.; Iqbal, Z.; Sohrab, S.S.; Kamal, M.A.; Abuzenadah, A.M.; Al-Qahtani, M.H. Transcriptomics Study of Neurodegenerative Disease: Emphasis on Synaptic Dysfunction Mechanism in Alzheimer’s Disease. CNS Neurol. Disord. Drug Targets 2014, 13, 1202–1212. [Google Scholar] [CrossRef] [PubMed]
- Mawanda, F.; Wallace, R. Can infections cause Alzheimer’s disease? Epidemiol. Rev. 2013, 35, 161–180. [Google Scholar] [CrossRef]
- Piacentini, R.; De Chiara, G.; Li Puma, D.D.; Ripoli, C.; Marcocci, M.E.; Garaci, E.; Palamara, A.T.; Grassi, C. HSV-1 and Alzheimer’s disease: More than a hypothesis. Front. Pharmacol. 2014, 5, 97. [Google Scholar] [CrossRef]
- Pyles, R.B.; Sawtell, N.M.; Thompson, R.L. Herpes simplex virus type 1 dUTPase mutants are attenuated for neurovirulence, neuroinvasiveness, and reactivation from latency. J. Virol. 1992, 66, 6706–6713. [Google Scholar] [CrossRef]
- Mukhamed’ianova, A.S.; Aznabaev, R.A.; Aznabaeva, L.F. Intraocular infection with herpesviruses and antiviral immunity in patients with age-related macular degeneration. Vestn. Oftalmol. 2008, 124, 39–42. [Google Scholar]
- Slepova, O.S.; Eremeeva, E.A.; Ryabina, M.V.; Sorozhkina, E.S. Role of infection in the pathogenesis of age-related macular degeneration. Vestn. Oftalmol. 2015, 131, 56–59. [Google Scholar] [CrossRef]
- Larsen, P.P.; Dinet, V.; Delcourt, C.; Helmer, C.; Linard, M. Could Infectious Agents Play a Role in the Onset of Age-related Macular Degeneration? A Scoping Review. Ophthalmol. Sci. 2025, 5, 100668. [Google Scholar] [CrossRef]
- Lee, K.S.; Lin, S.; Copland, D.A.; Dick, A.D.; Liu, J. Cellular senescence in the aging retina and developments of senotherapies for age-related macular degeneration. J. Neuroinflamm. 2021, 18, 32. [Google Scholar] [CrossRef]
- Tan, W.; Zou, J.; Yoshida, S.; Jiang, B.; Zhou, Y. The Role of Inflammation in Age-Related Macular Degeneration. Int. J. Biol. Sci. 2020, 2020, 2989–3001. [Google Scholar] [CrossRef] [PubMed]
- Boulton, M.; Dayhaw-Barker, P. The role of the retinal pigment epithelium: Topographical variation and ageing changes. Eye 2001, 15, 384–389. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.F.; Xu, T.H.; Yan, Y.; Zhou, Y.R.; Jiang, Y.; Melcher, K.; Xu, H.E. Amyloid beta: Structure, biology and structure-based therapeutic development. Acta Pharmacol. Sin. 2017, 38, 1205–1235. [Google Scholar] [CrossRef]
- Zhang, H.; Ma, Q.; Zhang, Y.-W.; Xu, H. Proteolytic processing of Alzheimer’s β-amyloid precursor protein. J. Neurochem. Rev. 2012, 120, 9–21. [Google Scholar] [CrossRef]
- Findeis, M.A. The role of amyloid β peptide 42 in Alzheimer’s disease. Pharmacol. Ther. 2007, 116, 266–286. [Google Scholar] [CrossRef]
- Sun, E.; Motolani, A.; Campos, L.; Lu, T. The Pivotal Role of NF-kB in the Pathogenesis and Therapeutics of Alzheimer’s Disease. Int. J. Mol. Sci. 2022, 23, 8972. [Google Scholar] [CrossRef] [PubMed]
- Kuner, P.; Schubenel, R.; Hertel, C. β-Amyloid binds to p75(NTR) and activates NFκB in human neuroblastoma cells. J. Neurosci. Res. 1998, 54, 798–804. [Google Scholar] [CrossRef]
- Sutinen, E.M.; Pirttilä, T.; Anderson, G.; Salminen, A.; Ojala, J. Pro-inflammatory interleukin-18 increases Alzheimer’s disease-associated amyloid-β production in human neuron-like cells. J. Neuroinflamm. 2012, 9, 199. [Google Scholar] [CrossRef]
- Zhao, Y.; Bhattacharjee, S.; Jones, B.M.; Hill, J.; Dua, P.; Lukiw, W.J. Regulation of Neurotropic Signaling by the Inducible, NF-kB-Sensitive miRNA-125b in Alzheimer’s Disease (AD) and in Primary Human Neuronal-Glial (HNG) Cells. Mol. Neurobiol. 2014, 50, 97–106. [Google Scholar] [CrossRef]
- Naaman, E.; Ya’ari, S.; Itzkovich, C.; Safuri, S.; Macsi, F.; Kellerman, L.; Mimouni, M.; Mann, I.; Gazit, E.; Adler-Abramovich, L.; et al. The retinal toxicity profile towards assemblies of Amyloid-β indicate the predominant pathophysiological activity of oligomeric species. Sci. Rep. 2020, 10, 20954. [Google Scholar] [CrossRef]
- Nizari, S.; Carare, R.O.; Hawkes, C.A. Increased Aβ pathology in aged Tg2576 mice born to mothers fed a high fat diet. Sci. Rep. 2016, 6, 21981. [Google Scholar] [CrossRef]
- Gupta, V.K.; Chitranshi, N.; Gupta, V.B.; Golzan, M.; Dheer, Y.; Wall, R.V.; Georgevsky, D.; King, A.E.; Vickers, J.C.; Chung, R.; et al. Amyloid β accumulation and inner retinal degenerative changes in Alzheimer’s disease transgenic mouse. Neurosci. Lett. 2016, 623, 52–56. [Google Scholar] [CrossRef]
- Inyushin, M.; Zayas-Santiago, A.; Rojas, L.; Kucheryavykh, L. Platelet-generated amyloid beta peptides in Alzheimer’s disease and glaucoma. Histol. Histopathol. 2019, 34, 843. [Google Scholar] [CrossRef] [PubMed]
- Dutescu, R.M.; Li, Q.X.; Crowston, J.; Masters, C.L.; Baird, P.N.; Culvenor, J.G. Amyloid precursor protein processing and retinal pathology in mouse models of Alzheimer’s disease. Graefe’s Arch. Clin. Exp. Ophthalmol. 2009, 247, 1213–1221. [Google Scholar] [CrossRef]
- Wang, J.; Zhu, C.; Xu, Y.; Liu, B.; Wang, M.; Wu, K. Development and Expression of Amyloid-β Peptide 42 in Retinal Ganglion Cells in Rats. Anat. Rec. 2011, 294, 1401–1405. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ohno-Matsui, K.; Morita, I. Elevated amyloid β production in senescent retinal pigment epithelium, a possible mechanism of subretinal deposition of amyloid β in age-related macular degeneration. Biochem. Biophys. Res. Commun. 2012, 423, 73–78. [Google Scholar] [CrossRef]
- Johnson, L.V.; Leitner, W.P.; Rivest, A.J.; Staples, M.K.; Radeke, M.J.; Anderson, D.H. The Alzheimer’s A-peptide is deposited at sites of complement activation in pathologic deposits associated with aging age-related macular degeneration. Proc. Natl. Acad. Sci. USA 2002, 99, 11830–11835. [Google Scholar] [CrossRef]
- Anderson, D.H.; Talaga, K.C.; Rivest, A.J.; Staples, M.K.; Radeke, M.J.; Anderson, D.H. Characterization of β amyloid assemblies in drusen: The deposits associated with aging and age-related macular degeneration. Exp. Eye Res. 2004, 78, 243–256. [Google Scholar] [CrossRef] [PubMed]
- Dentchev, T.; Milam, A.H.; M-YLee, V.; Trojanowski, J.Q.; Dunaief, J.L. Amyloid-β isfoundin drusen from some age-related macular degeneration retinas, but not in drusen from normal retinas. Mol. Vis. 2003, 136, 787. [Google Scholar]
- Koronyo, Y.; Biggs, D.; Barron, E.; Boyer, D.S.; Pearlman, J.A.; Au, W.J.; Kile, S.J.; Blanco, A.; Fuchs, D.T.; Ashfaq, A.; et al. Retinal amyloid pathology and proof-of-concept imaging trial in Alzheimer’s disease. J. Clin. Investig. 2017, 2, e93621. [Google Scholar] [CrossRef]
- Shankar, G.M.; Li, S.; Mehta, T.H.; Garcia-Munoz, A.; Shepardson, N.E.; Smith, I.; Brett, F.M.; Farrell, M.A.; Rowan, M.J.; Lemere, C.A.; et al. Amyloid-β protein dimers isolated directly from Alzheimer’s brains impair synaptic plasticity and memory. Nat. Med. 2008, 14, 837–842. [Google Scholar] [CrossRef]
- Ahmad, F.; Deshmukh, N.; Webel, A.; Johnson, S.; Suleiman, A.; Mohan, R.R.; Fraunfelder, F.; Singh, P.K. Viral infections and pathogenesis of glaucoma: A comprehensive review. Clin. Microbiol. Rev. 2023, 36, e00057-23. [Google Scholar] [CrossRef] [PubMed]
- Voigt, V.; Andoniou, C.E.; Schuster, I.S.; Oszmiana, A.; Ong, M.L.; Fleming, P.; Forrester, J.V.; Degli-Esposti, M.A. Cytomegalovirus establishes a latent reservoir and triggers long-lasting inflammation in the eye. PLoS Pathog. 2018, 14, e1007040. [Google Scholar] [CrossRef] [PubMed]
- Fierz, W. Age-Related Macular Degeneration: A Connection between Human Herpes Virus-6A-Induced CD46 Downregulation and Complement Activation? Front. Immunol. 2017, 8, 1314. [Google Scholar] [CrossRef] [PubMed]
- Rowe, A.M.; St Leger, A.J.; Jeon, S.; Dhaliwal, D.K.; Knickelbein, J.E.; Hendricks, R.L. Herpes keratitis. Prog. Retin. Eye Res. 2013, 32, 88–101. [Google Scholar] [CrossRef]
- Smith, J.S.; Robinson, N.J. Age-specific prevalence of infection with herpes simplex virus types 2 and 1: A global review. J. Infect. Dis. 2002, 186, S3–S28. [Google Scholar] [CrossRef]
- Newman, H.; Gooding, C. Viral ocular manifestations: A broad overview. Rev. Med. Virol. 2013, 23, 281–294. [Google Scholar] [CrossRef]
- Valerio, G.S.; Lin, C.C. Ocular manifestations of herpes simplex virus. Curr. Opin. Ophthalmol. 2019, 30, 525–531. [Google Scholar] [CrossRef]
- De Chiara, G.; Piacentini, R.; Fabiani, M.; Mastrodonato, A.; Marcocci, M.E.; Limongi, D.; Napoletani, G.; Protto, V.; Coluccio, P.; Celestino, I.; et al. Recurrent herpes simplex virus-1 infection induces hallmarks of neurodegeneration and cognitive deficits in mice. PLoS Pathog. 2019, 15, e1007617. [Google Scholar] [CrossRef]
- Zheng, M.; Atherton, S.S. Cytokine profiles and inflammatory cells during HSV-1-induced acute retinal necrosis. Investig. Ophthalmol. Vis. Sci. 2005, 46, 1356–1363. [Google Scholar] [CrossRef]
- Valyi-Nagy, T.; Dermody, T.S. Role of oxidative damage in the pathogenesis of viral infections of the nervous system. Histol. Histopathol. 2005, 20, 957–967. [Google Scholar] [CrossRef]
- Palamara, A.T.; Perno, C.F.; Ciriolo, M.R.; Dini, L.; Balestra, E.; D’Agostini, C.; Di Francesco, P.; Favalli, C.; Rotilio, G.; Garaci, E. Evidence for antiviral activity of glutathione: In vitro inhibition of herpes simplex virus type 1 replication. Antivir. Res. 1995, 27, 237–253. [Google Scholar] [CrossRef] [PubMed]
- Nucci, C.; Palamara, A.T.; Ciriolo, M.R.; Nencioni, L.; Savini, P.; D’Agostini, C.; Rotilio, G.; Cerulli, L.; Garaci, E. Imbalance in corneal redox state during herpes simplex virus 1-induced keratitis in rabbits. Effectiveness of exogenous glutathione supply. Exp. Eye Res. 2000, 70, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Kavouras, J.H.; Prandovszky, E.; Valyi-Nagy, K.; Kovacs, S.K.; Tiwari, V.; Kovacs, M.; Shukla, D.; Valyi-Nagy, T. Herpes simplex virus type 1 infection induces oxidative stress and the release of bioactive lipid peroxidation by-products in mouse P19N neural cell cultures. J. Neurovirol. 2007, 13, 416–425. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Sheng, W.S.; Schachtele, S.J.; Lokensgard, J.R. Reactive oxygen species drive herpes simplex virus (HSV)-1-induced proinflammatory cytokine production by murine microglia. J. Neuroinflamm. 2011, 8, 123. [Google Scholar] [CrossRef]
- Cai, Z.; Zhao, B.; Ratka, A. Oxidative stress and β-amyloid protein in Alzheimer’s disease. Neuromolecular Med. 2011, 13, 223–250. [Google Scholar] [CrossRef]
- Guglielmotto, M.; Giliberto, L.; Tamagno, E.; Tabaton, M. Oxidative stress mediates the pathogenic effect of different Alzheimer’s disease risk factors. Front. Aging Neurosci. 2010, 2, 3. [Google Scholar] [CrossRef]
- Afzal, S.; Abdul Manap, A.S.; Attiq, A.; Albokhadaim, I.; Kandeel, M.; Alhojaily, S.M. From imbalance to impairment: The central role of reactive oxygen species in oxidative stress-induced disorders and therapeutic exploration. Front. Pharmacol. 2023, 14, 1269581. [Google Scholar] [CrossRef]
- Chandimali, N.; Bak, S.G.; Park, E.H.; Lim, H.; Won, Y.; Kim, E.; Park, S.; Lee, S.J. Free radicals and their impact on health and antioxidant defenses: A review. Cell Death Discov. 2025, 11, 19. [Google Scholar] [CrossRef]
- Cheignon, C.; Tomas, M.; Bonnefont-Rousselot, D.; Faller, P.; Hureau, C.; Collin, F. Oxidative stress and the amyloid beta peptide in Alzheimer’s disease. Redox Biol. 2017, 14, 450–464. [Google Scholar] [CrossRef]
- Feng, S.; Liu, Y.; Zhou, Y.; Shu, Z.; Cheng, Z.; Brenner, C.; Feng, P. Mechanistic insights into the role of herpes simplex virus 1 in Alzheimer’s disease. Front. Aging Neurosci. 2023, 15, 1245904. [Google Scholar] [CrossRef]
- Santana, S.; Recuero, M.; Bullido, M.J.; Valdivieso, F.; Aldudo, J. Herpes simplex virus type I induces the accumulation of intracellular β-amyloid in autophagic compartments and the inhibition of the non-amyloidogenic pathway in human neuroblastoma cells. Neurobiol. Aging 2012, 33, 430.e19–430.e33. [Google Scholar] [CrossRef]
- Santana, S.; Sastre, I.; Recuero, M.; Bullido, M.J.; Aldudo, J. Oxidative Stress Enhances Neurodegeneration Markers Induced by Herpes Simplex Virus Type 1 Infection in Human Neuroblastoma Cells. PLoS ONE 2013, 8, e75842. [Google Scholar] [CrossRef]
- Fanlo-Ucar, H.; Picón-Pagès, P.; Herrera-Fernández, V.; ILL-Raga, G.; Muñoz, F.J. The Dual Role of Amyloid Beta-Peptide in Oxidative Stress and Inflammation: Unveiling Their Connections in Alzheimer’s Disease Etiopathology. Antioxidants 2024, 13, 1208. [Google Scholar] [CrossRef]
- Piacentini, R.; Civitelli, L.; Ripoli, C.; Marcocci, M.E.; De Chiara, G.; Garaci, E.; Azzena, G.B.; Palamara, A.T.; Grassi, C. HSV-1 promotes Ca2+-mediated APP phosphorylation and Aβ accumulation in rat cortical neurons. Neurobiol. Aging 2011, 32, 2323.e13–2323.e26. [Google Scholar] [CrossRef] [PubMed]
- Marcocci, M.E.; Napoletani, G.; Protto, V.; Kolesova, O.; Piacentini, R.; Li Puma, D.D.; Lomonte, P.; Grassi, C.; Palamara, A.T.; De Chiara, G. Herpes Simplex Virus-1 in the Brain: The Dark Side of a Sneaky Infection. Trends Microbiol. 2020, 28, 808–820. [Google Scholar] [CrossRef] [PubMed]
- Protto, V.; Miteva, M.T.; Iannuzzi, F.; Marcocci, M.E.; Li Puma, D.D.; Piacentini, R.; Belli, M.; Sansone, L.; Pietrantoni, A.; Grassi, C.; et al. HSV-1 infection induces phosphorylated tau propagation among neurons via extracellular vesicles. MBio 2024, 15, e0152224. [Google Scholar] [CrossRef] [PubMed]
- Giuliani, M.; Uboldi, C.; Dellatorre, F.G.; Latour, E.; Ponce, N.M.A.; Stortz, C.A.; Lassalle, V.L.; Ayala-Peña, V.B. Undaria pinnatifida fucoidan extract inhibits activation of the NF-κB signaling pathway by herpes simplex virus type 1 and prevents amyloid-β peptide synthesis in retinal pigment epithelium cells. Arch. Virol. 2025, 170, 27. [Google Scholar] [CrossRef]
- Wozniak, M.; Bell, T.; Dénes, Á.; Falshaw, R.; Itzhaki, R. Anti-HSV1 activity of brown algal polysaccharides and possible relevance to the treatment of Alzheimer’s disease. Int. J. Biol. Macromol. 2015, 74, 530–540. [Google Scholar] [CrossRef] [PubMed]
- Gosztyla, M.L.; Brothers, H.M.; Robinson, S.R. Alzheimer’s Amyloid-β is an Antimicrobial Peptide: A Review of the Evidence. J. Alzheimers Dis. 2018, 62, 1495–1506. [Google Scholar] [CrossRef]
- Eimer, W.A.; Vijaya Kumar, D.K.; Navalpur Shanmugam, N.K.; Rodriguez, A.S.; Mitchell, T.; Washicosky, K.J.; György, B.; Breakefield, X.O.; Tanzi, R.E.; Moir, R.D. Alzheimer’s Disease-Associated β-Amyloid Is Rapidly Seeded by Herpesviridae to Protect against Brain Infection. Neuron 2018, 99, 56–63.e3. [Google Scholar] [CrossRef]
- Dogrammatzis, C.; Waisner, H.; Kalamvoki, M. “Non-Essential” Proteins of HSV-1 with Essential Roles In Vivo: A Comprehensive Review. Viruses 2021, 13, 17. [Google Scholar] [CrossRef]
- Duarte, L.F.; Reyes, A.; Farías, M.A.; Riedel, C.A.; Bueno, S.M.; Kalergis, A.M.; González, P.A. Crosstalk Between Epithelial Cells, Neurons and Immune Mediators in HSV-1 Skin Infection. Front. Immunol. 2021, 12, 662234. [Google Scholar] [CrossRef]
- Thurman, J.M.; Renner, B.; Kunchithapautham, K.; Ferreira, V.P.; Pangburn, M.K.; Ablonczy, Z.; Tomlinson, S.; Holers, V.M.; Rohrer, B. Oxidative Stress Renders Retinal Pigment Epithelial Cells Susceptible to Complement-mediated Injury. J. Biol. Chem. 2009, 284, 16939–16947. [Google Scholar] [CrossRef] [PubMed]
- Ayala-Peña, V.B.; Pilotti, F.; Volonté, Y.; Rotstein, N.P.; Politi, L.E.; German, O.L. Protective effects of retinoid x receptors on retina pigment epithelium cells. Biochim. Et Biophys. Acta (BBA)-Mol. Cell Res. 2016, 1863, 1134–1145. [Google Scholar] [CrossRef]
- Winkler, B.S.; Boulton, M.E.; Gottsch, J.D.; Sternberg, P. Oxidative damage and age-related macular degeneration. Mol. Vis. 1999, 5, 32. [Google Scholar] [PubMed] [PubMed Central]
- Dasari, B.; Prasanthi, J.R.; Marwarha, G.; Singh, B.B.; Ghribi, O. The oxysterol 27-hydroxycholesterol increases β-amyloid and oxidative stress in retinal pigment epithelial cells. BMC Ophthalmol. 2010, 10, 22. [Google Scholar] [CrossRef]
- Dasari, B.; Prasanthi, J.R.; Marwarha, G.; Singh, B.B.; Ghribi, O. Cholesterol-enriched diet causes age-related macular de-generation-like pathology in rabbit retina. BMC Ophthalmol. 2011, 18, 22. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Prasanthi, J.R.P.; Huls, A.; Thomasson, S.; Thompson, A.; Schommer, E.; Ghribi, O. Differential effects of 24-hydroxycholesterol and 27-hydroxycholesterol on beta-amyloid precursor protein levels and processing in human neuroblastoma SH-SY5Y cells. Mol. Neurodegener. 2009, 4, 1. [Google Scholar] [CrossRef]
- Ashok, A.; Singh, N.; Chaudhary, S.; Bellamkonda, V.; Kritikos, A.E.; Wise, A.S.; Rana, N.; McDonald, D.; Ayyagari, R. Retinal Degeneration and Alzheimer’s Disease: An Evolving Link. Int. J. Mol. Sci. 2020, 21, 7290. [Google Scholar] [CrossRef]
- Cheshenko, N.; Liu, W.; Satlin, L.M.; Herold, B.C. Multiple receptor interactions trigger release of membrane and intracellular calcium stores critical for herpes simplex virus entry. Mol. Biol. Cell 2007, 18, 3119–3130. [Google Scholar] [CrossRef]
- Koyama, Y.; Matsuzaki, S.; Gomi, F.; Yamada, K.; Katayama, T.; Sato, K.; Kumada, T.; Fukuda, A.; Matsuda, S.; Tano, Y.; et al. Induction of Amyloid β Accumulation by ER Calcium Disruption and Resultant Upregulation of Angiogenic Factors in ARPE19 Cells. Investig. Ophthalmol. Vis. Sci. 2008, 49, 2376–2383. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Mo, J.; Atherton, S.S. Chapter 15—Cytomegalovirus Blocks Autophagy During Infection of the Retinal Pigment Epithelial Cells: Functional Relationship Between Autophagy Apoptosis. In Autophagy: Cancer, Other Pathologies, Inflammation, Immunity, Infection, and Aging; Hayat, M.A., Ed.; Academic Press: Cambridge, MA, USA, 2016; pp. 267–279. [Google Scholar] [CrossRef]
- Frost, L.S.; Mitchell, C.H.; Boesze-Battaglia, K. Autophagy in the eye: Implications for ocular cell health. Exp. Eye Res. 2014, 124, 56–66. [Google Scholar] [CrossRef]
- Edwards, R.G.; Longnecker, R. Herpesvirus Entry Mediator and Ocular Herpesvirus Infection: More than Meets the Eye. J. Virol. 2017, 91, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Koujah, L.; Suryawanshi, R.K.; Shukla, D. Pathological processes activated by herpes simplex virus-1 (HSV-1) infection in the cornea. Cell. Mol. Life Sci. 2019, 76, 405–419. [Google Scholar] [CrossRef] [PubMed]
- Melchjorsen, J.; Matikainen, S.; Paludan, S.R. Activation and Evasion of Innate Antiviral Immunity by Herpes Simplex Virus. Viruses 2009, 1, 737–759. [Google Scholar] [CrossRef]
- Sen, M.; Eroğul, Ö. Retinoic Acid Neutralizes the Effects of Herpes Simplex Virus Type 1-Infected Cell Protein 0 (ICP0) in Retinal Pigment Epithelial Cells. Cureus 2024, 16, e61089. [Google Scholar] [CrossRef]
- Caproni, A.; Nordi, C.; Fontana, R.; Facchini, M.; Melija, S.; Pappadà, M.; Buratto, M.; Marconi, P. Herpes Simplex Virus ICP27 Protein Inhibits AIM 2-Dependent Inflammasome Influencing Pro-Inflammatory Cytokines Release in Human Pigment Epithelial Cells (hTert-RPE 1). Int. J. Mol. Sci. 2024, 25, 4608. [Google Scholar] [CrossRef]
- Duan, F.; Zeng, W.; Zhang, Y.; Li, D.; Wu, K. Lipopolysaccharide enhances HSV-1 replication and inflammatory factor release in the ARPE-19 cells. Heliyon 2022, 8, e11787. [Google Scholar] [CrossRef]
- Kashiwase, M.; Sata, T.; Yamauchi, Y.; Minoda, H.; Usui, N.; Iwasaki, T.; Kurata, T.; Usui, M. Progressive Outer Retinal Necrosis Caused by Herpes Simplex Virus Type 1 in a Patient with Acquired Immunodeficiency Syndrome. Ophthalmology 2000, 107, 790–794. [Google Scholar] [CrossRef]
- Huemer, H.P.; Larcher, C.; Kirchebner, W.; Klingenschmid, J.; Göttinger, W.; Irschick, E.U. Susceptibility of human retinal pigment epithelial cells to different viruses. Graefe’s Arch. Clin. Exp. Ophthalmol. 1996, 234, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Topp, K.S.; Bisla, K.; Saks, N.D.; Lavailf, J.H. Centripetal transport of Herpes Simplex virus in human retinal pigment epithelial cell in vitro. Neuroscience 1996, 71, 1133–1144. [Google Scholar] [CrossRef] [PubMed]
- Topp, K.S.; Rothman, A.L.; Lavail, J.H. Herpes Virus Infection of RPE and MDCK Cells: Polarity of Infection. Exp. Eye Res. 1997, 64, 343–354. [Google Scholar] [CrossRef]
- Duan, F.; Ni, S.; Nie, Y.; Huang, Q.; Wu, K. Small interfering RNA targeting for infected-cell polypeptide 4 inhibits herpes simplex virus type 1 replication in retinal pigment epithelial cells. Clin. Exp. Ophthalmol. 2012, 40, 195–204. [Google Scholar] [CrossRef]
- Tiwari, V.; Oh, M.-J.; Kovacs, M.; Shukla, S.Y.; Valyi-Nagy, T.; Shukla, D. Role for nectin-1 in herpes simplex virus 1 entry and spread in human retinal pigment epithelial cells. FEBS J. 2008, 275, 5272–5285. [Google Scholar] [CrossRef] [PubMed]
- Politi, L.E.; Adler, R.; Whittum-Hudson, J.A. Differential Sensitivity of Cultured Retinal Neurons and Photoreceptors to Hexes Simplex Infection. Exp. Eye Res. 1987, 44, 923–937. [Google Scholar] [CrossRef]
- Rummelt, V.; Rummelt, C.; Jahn, G.; Wenkel, H.; Sinzger, C.; Mayer, U.M.; Naumann, G.O. Triple retinal infection with Human inmmunodeficiency virus type 1, Cytomegalovirus, and Herpes simplex virus type 1. Ophtalmology 1993, 101, 270–279. [Google Scholar] [CrossRef]
- Ouwendijk, W.J.D.; Dekker, L.J.M.; van den Ham, H.J.; Lenac Rovis, T.; Haefner, E.S.; Jonjic, S.; Haas, J.; Luider, T.M.; Verjans, G.M.G.M. Analysis of Virus and Host Proteomes During Productive HSV-1 and VZV Infection in Human Epithelial Cells. Front. Microbiol. 2020, 11, 1179. [Google Scholar] [CrossRef]
- Rippee-Brooks, M.D.; Wu, W.; Dong, J.; Pappolla, M.; Fang, X.; Bao, X. Viral Infections, Are They a Trigger and Risk Factor of Alzheimer’s Disease? Pathogens 2024, 13, 240. [Google Scholar] [CrossRef]
- Koganti, R.; Yadavalli, T.; Naqvi, R.A.; Shukla, D.; Naqvi, A.R. Pathobiology and treatment of viral keratitis. Exp. Eye Res. 2021, 205, 108483. [Google Scholar] [CrossRef]
- Greenan, E.; Gallagher, S.; Khalil, R.; Murphy, C.C.; Ní Gabhann-Dromgoole, J. Advancing our understanding of corneal herpes simplex virus-1 immune evasion mechanisms and future therapeutics. Viruses 2021, 13, 1856. [Google Scholar] [CrossRef]
- Arii, J.; Hirohata, Y.; Kato, A.; Kawaguchi, Y. Nonmuscle Myosin Heavy Chain IIB Mediates Herpes Simplex Virus 1 Entry. J. Virol. 2015, 89, 1879–1888. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Liang, Q.; Sun, D.; He, Y.; Jiang, J.; Guo, R.; Malla, T.; Hamrah, P.; Liu, X.; Huang, Z.; et al. Nectin-1 and Non-muscle Myosin Heavy Chain-IIB: Major Mediators of Herpes Simplex Virus-1 Entry Into Corneal Nerves. Front. Microbiol. 2022, 13, 830699. [Google Scholar] [CrossRef] [PubMed]
- Toma, H.S.; Murina, A.T.; Areaux, R.G.; Neumann, D.M.; Bhattacharjee, P.S.; Foster, T.P.; Kaufman, H.E.; Hill, J.M. Ocular HSV-1 latency, reactivation and recurrent disease. Semin. Ophthalmol. 2008, 23, 249–273. [Google Scholar] [CrossRef]
- Pepose, J.S.; Kreiger, A.E.; Tomiyasu, U.; Cancilla, P.A.; Foos, R.Y. Immunocytologic Localization of Herpes Simplex Type 1 Viral Antigens in Herpetic Retinitis and Encephalitis in an Adult. Ophthalmology 1985, 92, 160–166. [Google Scholar] [CrossRef]
- Wang, F.; Tang, W.; McGraw, H.M.; Bennett, J.; Enquist, L.W.; Friedman, H.M. Herpes simplex virus type 1 glycoprotein e is required for axonal localization of capsid, tegument, and membrane glycoproteins. J. Virol. 2005, 79, 13362–13372. [Google Scholar] [CrossRef] [PubMed]
- Maertzdorf, J.; Van der Lelij, A.; Baarsma, G.S.; Osterhaus, A.D.; Verjans, G.M. Herpes simplex virus type 1 (HSV-1)--induced retinitis following herpes simplex encephalitis: Indications for brain-to-eye transmission of HSV-1. Ann. Neurol. 2001, 48, 936–939. [Google Scholar] [CrossRef]
- Duker, J.S.; Nielsen, J.C.; Eagle, R.C.; Bosley, T.M.; Granadier, R.; Benson, W.E. Rapidly Progressive Acute Retinal Necrosis Secondary to Herpes Simplex Virus, Type 1. Ophthalmology 1990, 97, 1638–1643. [Google Scholar] [CrossRef]
- Klein, A.; Lefebvre, P. Three consecutive episodes of acute retinal necrosis due to herpes simplex-1 over twelve years following herpetic encephalitis. Ocul. Immunol. Inflamm. 2007, 15, 411–413. [Google Scholar] [CrossRef]
- Matsubara, S.; Atherton, S.S. Spread of HSV-1 to the suprachiasmatic nuclei and retina in T cell depleted BALB/c mice. J. Neuroimmunol. 1997, 80, 165–171. [Google Scholar] [CrossRef]
- DuRaine, G.; Johnson, D.C. Anterograde transport of α-herpesviruses in neuronal axons. Virology 2021, 559, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Pérez De Arcelus, M.; Salinas, A.; Layana, A.G. Manifestaciones retinianas de las enfermedades infecciosas Retinal manifestations of infectious diseases. An. Sist. Sanit. Navar. 2008, 31 (Suppl. S3), 57–68. [Google Scholar] [CrossRef] [PubMed]
- Asghar, M.A.; Tang, S.; Wong, L.P.; Yang, P.; Zhao, Q. Infectious uveitis: A comprehensive systematic review of emerging trends and molecular pathogenesis using network analysis. J. Ophthalmic Inflamm. Infect. 2024, 14, 60. [Google Scholar] [CrossRef]
- Ventura, C.V.; Ventura, L.O. Ophthalmologic Manifestations Associated With Zika Virus Infection. Pediatrics 2018, 141 (Suppl. S2), S161–S166. [Google Scholar] [CrossRef]
- Martínez-Pulgarín, F.D.; Córdoba-Ortega, M.C.; Padilla-Pantoja, D.F. The Eye and the Zika Virus. Current Concepts in Zika Research; IntechOpen: London, UK, 2021. [Google Scholar] [CrossRef]
- Shirane, M.; Yawata, N.; Motooka, D.; Shibata, K.; Khor, S.S.; Omae, Y.; Kaburaki, T.; Yanai, R.; Mashimo, H.; Yamana, S.; et al. Intraocular human cytomegaloviruses of ocular diseases are distinct from those of viremia and are capable of escaping from innate and adaptive immunity by exploiting HLA-E-mediated peripheral and central tolerance. Front. Immunol. 2022, 19, 1008220. [Google Scholar] [CrossRef]
- Maqueda, M.; Mosquera, J.L.; García-Arumí, J.; Veiga, A.; Duarri, A. Repopulation of decellularized retinas with hiPSC-derived retinal pigment epithelial and ocular progenitor cells shows cell engraftment, organization and differentiation. Biomaterials 2021, 276, 121049. [Google Scholar] [CrossRef]
- Park, P.J.; Shukla, D. Role of heparan sulfate in ocular diseases. Exp. Eye Res. 2013, 110, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.M.; Espinosa-Heidmann, D.G.; Legra, J.; Dubovy, S.R.; Sũner, I.J.; Sedmak, D.D.; Dix, R.D.; Cousins, S.W. The association of prior cytomegalovirus infection with neovascular age-related macular degeneration. Am. J. Ophthalmol. 2004, 138, 323–328. [Google Scholar] [CrossRef]
- Protto, V.; Marcocci, M.E.; Miteva, M.T.; Piacentini, R.; Li Puma, D.D.; Grassi, C.; Palamara, A.T.; De Chiara, G. Role of HSV-1 in Alzheimer’s disease pathogenesis: A challenge for novel preventive/therapeutic strategies. Curr. Opin. Pharmacol. 2022, 63, 102200. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ayala-Peña, V.B. HSV-1 Infection in Retinal Pigment Epithelial Cells: A Possible Contribution to Age-Related Macular Degeneration. Viruses 2025, 17, 1056. https://doi.org/10.3390/v17081056
Ayala-Peña VB. HSV-1 Infection in Retinal Pigment Epithelial Cells: A Possible Contribution to Age-Related Macular Degeneration. Viruses. 2025; 17(8):1056. https://doi.org/10.3390/v17081056
Chicago/Turabian StyleAyala-Peña, Victoria Belen. 2025. "HSV-1 Infection in Retinal Pigment Epithelial Cells: A Possible Contribution to Age-Related Macular Degeneration" Viruses 17, no. 8: 1056. https://doi.org/10.3390/v17081056
APA StyleAyala-Peña, V. B. (2025). HSV-1 Infection in Retinal Pigment Epithelial Cells: A Possible Contribution to Age-Related Macular Degeneration. Viruses, 17(8), 1056. https://doi.org/10.3390/v17081056






