Murine Cytomegalovirus and Human Cytomegalovirus Differ in Pyroptosis Induction in Different Cell Types During Productive Replication
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines
2.2. Viruses
2.3. UV Inactivation of MCMV
2.4. Quantitative Real-Time RT-PCR Assay
2.5. Statistical Analysis
3. Results
3.1. Pyroptosis-Associated Transcripts Are Stimulated During Productive Replication of MCMV in Two Different Mouse Cell Types
3.2. Pyroptosis-Associated Transcripts Are Not Stimulated During Productive Replication of HCMV in Two Different Mouse Cell Types
3.3. Pyroptosis-Associated Transcripts Are Not Stimulated in Mouse Fibroblasts Following Inoculation with UV-Inactivated MCMV
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Park, W.; Wei, S.; Kim, B.-S.; Kim, B.; Bae, S.-J.; Chae, C.; Ryu, D.; Ha, K.-T. Diversity and complexity of cell death: A historical review. Exp. Mol. Med. 2023, 55, 1573–1594. [Google Scholar] [CrossRef]
- Cookson, B.T.; Brennan, M.A. Pro-inflammatory programmed cell death. Trends Microbiol. 2001, 9, 113–114. [Google Scholar] [CrossRef]
- Xue, Y.; Tuipulotu, D.E.; Tan, W.H.; Kay, C.; Man, M. Emerging activators and regulators of inflammasomes and pyroptosis. Trends Immunol. 2019, 40, 1035–1052. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Zhao, Y.; Wnag, K.; Shi, X.; Wang, Y.; Huang, H.; Zhuang, Y.; Cai, T.; Wang, F.; Shao, F. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 2015, 526, 660–665. [Google Scholar] [CrossRef]
- Ding, J.; Wang, K.; Liu, W.; She, Y.; Sun, Q.; Shi, J.; Sun, H.; Wang, D.C.; Shao, F. Pore-forming activity and structural autoinhibition of the gasdermin family. Nature 2016, 535, 111–116. [Google Scholar] [CrossRef]
- Fink, S.L.; Cookson, B.T. Caspase-1-dependent pore formation during pyroptosis leads to osmotic lysis of infected host macrophages. Cell Microbiol. 2006, 8, 1812–1825. [Google Scholar] [CrossRef]
- Weber, A.; Wasiliew, P.; Kracht, M. Interleukin-1beta (IL-1β) processing pathway. Sci. Signal. 2010, 3, cm2. [Google Scholar] [CrossRef]
- Lopez-Castejon, G.; Brough, D. Understanding the mechanism of IL-1β secretion. Cytokine Growth Factor Rev. 2011, 22, 189–195. [Google Scholar] [CrossRef]
- Mocarski, E.S. Cytomegalovirus biology viewed through a cell death suppression lens. Viruses 2024, 16, 1820. [Google Scholar] [CrossRef] [PubMed]
- Rao, Z.; Shu, Y.; Yang, P.; Chen, Z.; Xia, Y.; Qiao, C.; Liu, W.; Deng, H.; Li, J.; Ning, P.; et al. Pyroptosis in inflammatory diseases and cancer. Theranostics 2022, 12, 4310–4329. [Google Scholar] [CrossRef] [PubMed]
- Lamsira, H.K.; Sabatini, A.; Ciolfi, S.; Ciccosanti, F.; Sacchi, A.; Piacentini, M.; Nardacci, R. Autophagy and programmed cell death modalities interplay in HIV pathogenesis. Cells 2025, 14, 351. [Google Scholar] [CrossRef] [PubMed]
- Yap, J.K.Y.; Moriyama, M.; Iwasaki, A. Inflammasomes and pyroptosis as therapeutic targets for COVID-19. J. Immunol. 2020, 205, 307–312. [Google Scholar] [CrossRef]
- Yang, M.; So, K.-F.; Lam, W.C.; Lo, A. Novel programmed cell death as therapeutic targets in age-related macular degeneration. Int. J. Mol. Sci. 2020, 21, 7279. [Google Scholar] [CrossRef] [PubMed]
- Newton, F.; Megaw, R. Mechanisms of photoreceptor death in retinitis pigmentosa. Genes 2020, 11, 1120. [Google Scholar] [CrossRef]
- Zhao, W.-J.; Fan, C.-L.; Hu, X.-M.; Ban, X.-X.; Wan, H.; He, Y.; Zhang, Q.; Xiong, K. Regulated cell death of retinal ganglion cells in glaucoma: Molecular insights and therapeutic potentials. Cell Mol. Neurobiol. 2023, 43, 3161–3178. [Google Scholar] [CrossRef] [PubMed]
- Wan, P.; Su, W.; Zhang, Y.; Li, Z.; Den, C.; Li, J.; Jiang, N.; Huang, S.; Long, E.; Zhuo, Y. LncRNA H19 initiates microglial pyroptosis and neuronal cell death in retinal ischemia/reperfusion injury. Cell Death Differ. 2020, 27, 176–191. [Google Scholar] [CrossRef]
- Lee, J.H.; Agarwal, A.; Mahendradas, P.; Lee, C.S.; Gupta, V.; Pavesio, C.E.; Agrawal, R. Viral posterior uveitis. Surv. Ophthalmol. 2017, 62, 404–445. [Google Scholar] [CrossRef]
- Port, A.D.; Orlin, A.; Kiss, S.; Patel, S.; D’Amico, D.J.; Gupta, M.P. Cytomegalovirus retinitis: A review. J. Ocul. Pharmacol. Ther. 2017, 33, 224–234. [Google Scholar] [CrossRef]
- Jabs, D.A. Cytomegalovirus retinitis and the acquired immunodeficiency syndrome—Bench to bedside: LXVII Edward Jackson Memorial Lecture. Am. J. Ophthalmol. 2011, 151, 198–216. [Google Scholar] [CrossRef]
- Carter, J.J.; Alston, C.I.; Oh, J.J.; Duncan, L.-A.M.; Nemeno, J.G.E.; Byfield, S.N.; Dix, R.D. Mechanisms of AIDS-related cytomegalovirus retinitis. Future Virol. 2019, 14, 545–560. [Google Scholar] [CrossRef]
- Oh, J.J.; Carter, J.J.; Dix, R.D. A mouse model that mimics AIDS-related cytomegalovirus retinitis: Insights into pathogenesis. Pathogens 2021, 10, 850. [Google Scholar] [CrossRef] [PubMed]
- Dix, R.D.; Carter, J.J.; Koehler, H.; Guo, H.-Y. New insights into the pathogenesis of experimental cytomegalovirus retinal necrosis with emphasis on inflammasomes and pyroptosis. Pathogens 2025, in press. [Google Scholar]
- Carter, J.J.; Nemeno, J.G.E.; Oh, J.; Houghton, J.E.; Dix, R.D. Atypical cytomegalovirus retinal disease in pyroptosis-deficient mice with murine acquired immunodeficiency syndrome. Exp. Eye Res. 2021, 209, 108651. [Google Scholar] [CrossRef]
- Dix, R.D.; Cray, C.; Cousins, S.W. Mice immunosuppressed by murine retrovirus infection (MAIDS) are susceptible to cytomegalovirus retinitis. Curr. Eye Res. 1994, 13, 587–595. [Google Scholar] [CrossRef] [PubMed]
- Dix, R.D.; Cousins, S.W. Susceptibility to murine cytomegalovirus retinitis during progression of MAIDS: Correlation with intraocular levels of tumor necrosis factor-a and interferon-g. Curr. Eye Res. 2004, 29, 173–180. [Google Scholar] [CrossRef]
- Ho, M. Nonhuman Cytomegaloviruses. In Cytomegalovirus, Biology and Infection, 2nd ed.; Plenum Medical Book Company: New York, NY, USA; London, UK, 1991; pp. 319–326. [Google Scholar]
- Sweet, C. The pathogenicity of cytomegalovirius. FEMS Microbiol. Rev. 1999, 23, 457–482. [Google Scholar] [CrossRef]
- Reddehase, M.J. The immunogenicity of human and murine cytomegaloviruses. Curr. Opin. Immunol. 2000, 12, 390–396. [Google Scholar] [CrossRef]
- McCarthy, M.; Resnick, L.; Taub, F.; Stewart, R.V.; Dix, R.D. Infection of human neural cell aggregate cultures with a clinical isolate of cytomegalovirus. J. Neuropath Exp. Neurol. 1991, 50, 441–450. [Google Scholar] [CrossRef]
- Loh, L.; Hudson, J.B. Immunosuppressive effect of murine cytomegalovirus. Infect. Immun. 1980, 27, 54–60. [Google Scholar] [CrossRef]
- Popkin, D.L.; Watson, M.A.; Karaskov, E.; Dunn, G.P.; Bremner, R.; Virgin, H.W. Murine cytomegalovirus paralyzes macrophages by blocking IFN gamma-induced promoter assembly. Proc. Natl. Acad. Sci. USA 2003, 100, 14309–14314. [Google Scholar] [CrossRef] [PubMed]
- Stinski, M.F. Molecular biology of cytomegaloviruses. In Herpesviriuses; Roizman, B., Ed.; Plenum Press: New York, NY, USA, 1983; pp. 67–113. [Google Scholar]
- Mocarski, E.S., Jr. Cytomegaloviruses and their replication. In Fields Virology, 3rd ed.; Fields, B.N., Knipe, D.M., Howley, P., Eds.; Lippincott-Raven: Philadelphia, PA, USA; New York, NY, USA, 1996; pp. 2447–2492. [Google Scholar]
- Deng, Y.; Agueda-Pinto, A.; Burne, W. No time to die: How cytomegaloviruses suppress apoptosis, necroptosis, and pyroptosis. Viruses 2024, 16, 1272. [Google Scholar] [CrossRef] [PubMed]
- Imre, G. Pyroptosis in health and disease. Am. J. Physiol. Cell Physiol. 2024, 326, C784–C794. [Google Scholar] [CrossRef]
- Ho, M. Murine Cytomegalovirus. In Cytomegalovirus, Biology and Infection, 2nd ed.; Plenum Medical Book Company: New York, NY, USA; London, UK, 1991; pp. 327–353. [Google Scholar]
- Hofman, F.; Hinton, D.R. Tumor necrosis factor-a in the retina of acquired immune deficiency syndrome. Investig. Ophthalmol. Vis. Sci. 1992, 33, 1829–1835. [Google Scholar]
- Chien, H.; Alston, C.I.; Dix, R.D. Suppressor of cytokine signaling (SOCS) 1 and SOCS3 are stimulated within the eye during experimental murine cytomegalovirus retinitis in mice with retrovirus-induced immunosuppression. J. Virol. 2018, 92, e00526-18. [Google Scholar] [CrossRef]
- Dix, R.D. Systemic murine cytomegalovirus infection of mice with retrovirus-induced immunodeficiency (MAIDS) results in ocular infection but not retinitis. Ophthalmic Res. 1998, 30, 295–301. [Google Scholar] [CrossRef]
- Emery, V.C.; Cope, A.V.; Bowen, E.F.; Gor, D.; Griffiths, P.D. The dynamics of human cytomegalovirus replication in vivo. J. Exp. Med. 1999, 190, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Emery, V.C. Viral dynamics during active cytomegalovirus infection and pathology. Intervirology 2000, 42, 405–411. [Google Scholar] [CrossRef]
- Holland, G.N.; Tufail, A.; Jordan, M.C. Cytomegalovirus diseases. In Ocular Infection & Immunity; Pepose, J.S., Holland, G.N., Wilhelmus, K.R., Eds.; Mosby: St. Louis, MO, USA, 1996; pp. 1088–1128. [Google Scholar]
- Alston, C.I.; Dix, R.D. Murine cytomegalovirus infection of mouse macrophages stimulates early expression of suppressor of cytokine signaling (SOCS)1 and SOCS3. PLoS ONE 2017, 12, e0171812. [Google Scholar] [CrossRef] [PubMed]
- McCormick, A.L.; Mocarski, E.S. Cell death pathways controlled by cytomegaloviruses. In Cytomegaloviruses: From Molecular Pathogenesis to Intervention; Reddehase, M.J., Ed.; Caister Scientific Press: Norfolk, UK, 2013; Volume 1, pp. 262–296. [Google Scholar]
- Huang, Y.; Ma, D.; Huang, H.; Lu, Y.; Liao, Y.; Liu, L.; Liu, X.; Fang, F. Interaction between HCMV pUL83 and human AIM2 disrupts the activation of the AIM2 inflammasome. Virol. J. 2017, 14, 34. [Google Scholar] [CrossRef]
- Deng, Y.; Ostermann, E.; Burne, W. A cytomegalovirus inflammsome inhibitor reduces proinflammatory cytokine release and pyroptosis. Nat. Commun. 2024, 15, 786. [Google Scholar] [CrossRef]
- Kalejta, R.F. Tegument proteins of human cytomegalovirus. Microbiol. Mol. Biol. Rev. 2008, 72, 249–265. [Google Scholar] [CrossRef] [PubMed]
- Goodrum, F.; Britt, W.; Mocarski, E.S. Cytomegalovirus. In Fields Virology, 7th ed.; Howley, P.M., Knipe, D.M., Eds.; Lippincott-Wolters Kluwer: Philadelphia, PA, USA, 2022; pp. 389–444. [Google Scholar]
- Morello, C.S.; Cranmer, L.D.; Spector, D.H. In vivo replication, latency, and immunogenicity of murine cytomegalovirus mutants with deletions in the M83 and M84 genes, the putative homologs of human cytomegalovirus pp65 (UL83). J. Virol. 1999, 73, 7678–7693. [Google Scholar] [CrossRef] [PubMed]
- Morello, C.S.; Cranmer, L.D.; Spector, D.H. Suppression of murine cytomegalovirus (MCMV) replication with a DNA vaccine encoding MCMV M84 (a homolog of human cytomegalovirus pp65). J. Virol. 2000, 74, 3696–3708. [Google Scholar] [CrossRef] [PubMed]
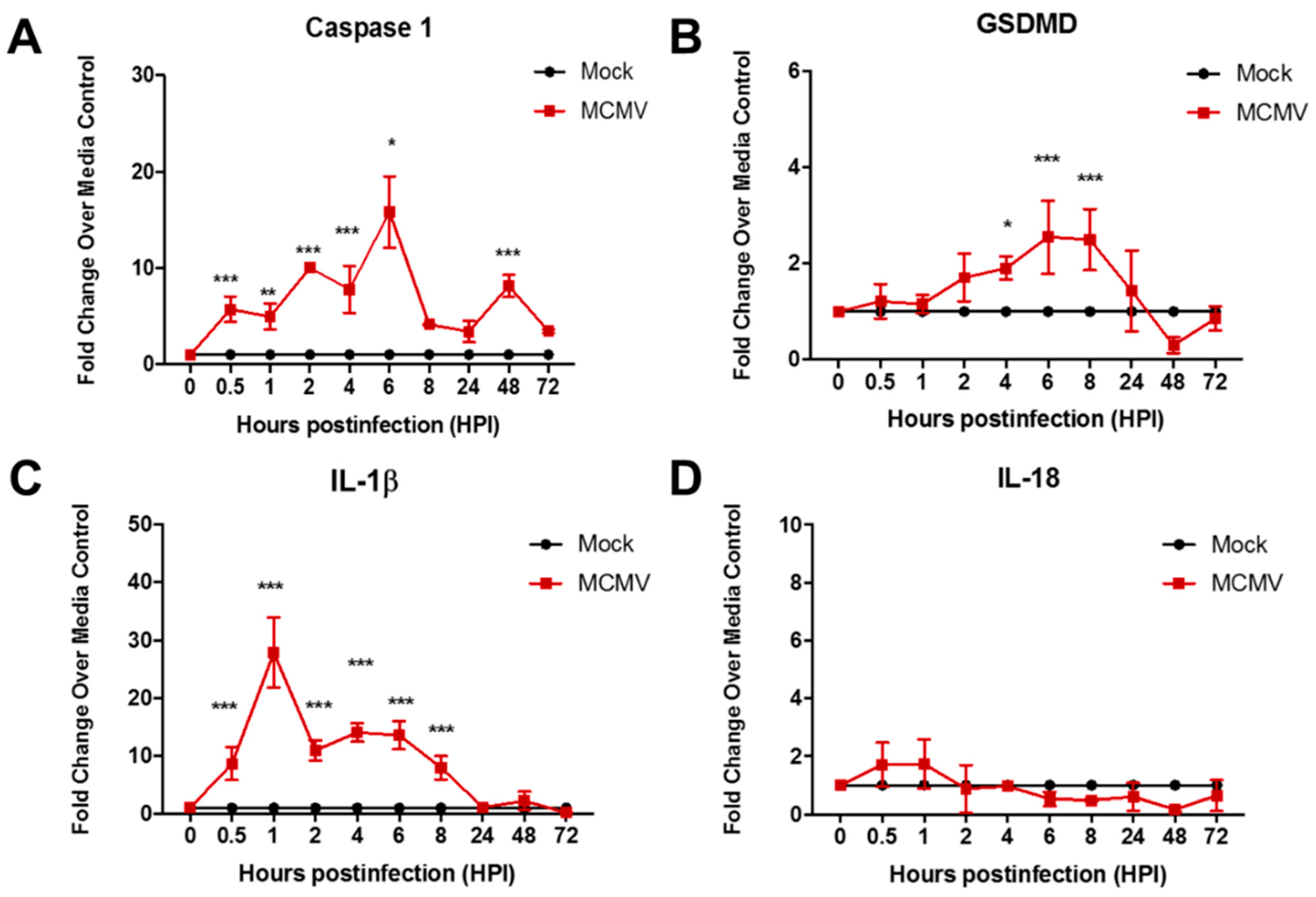
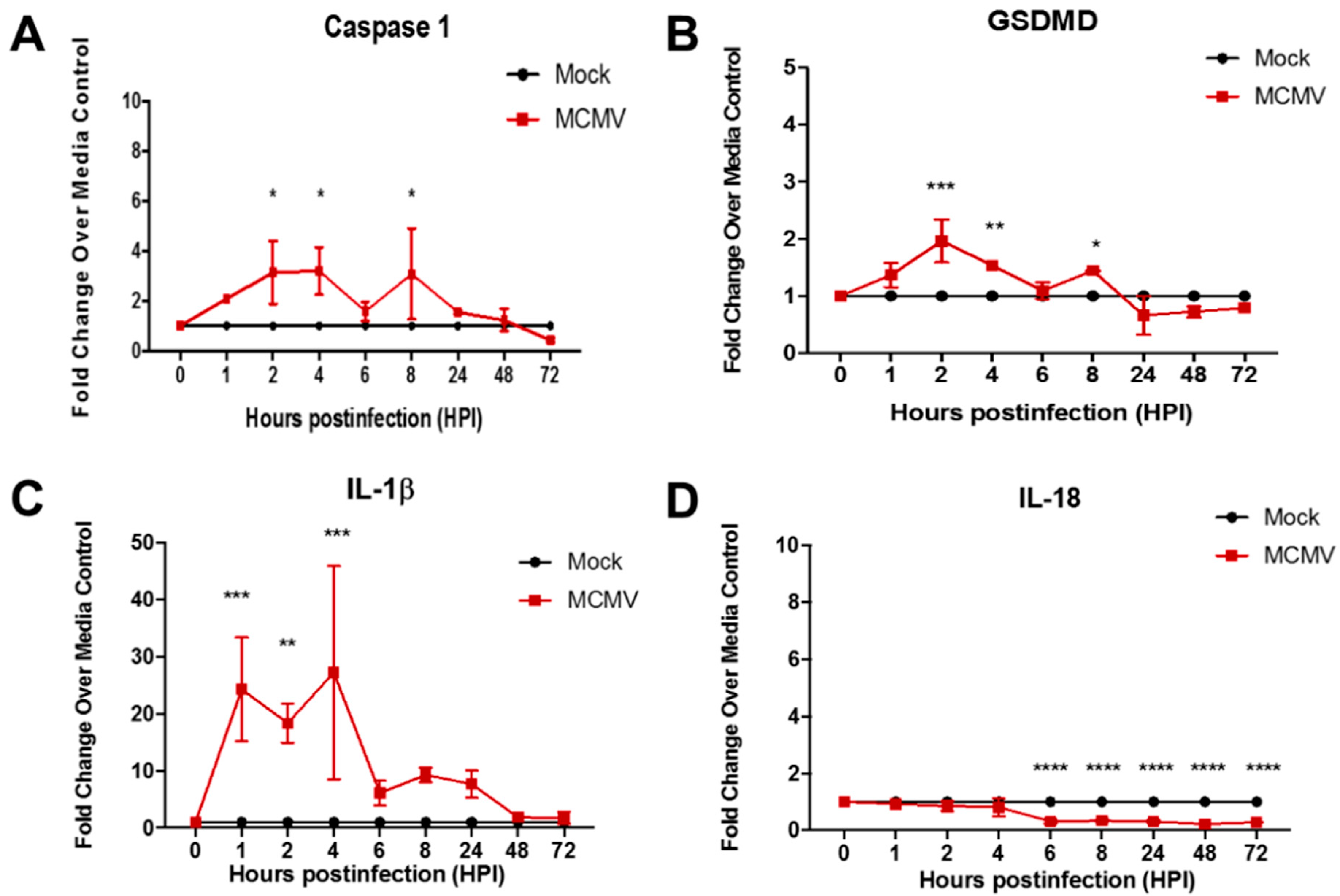

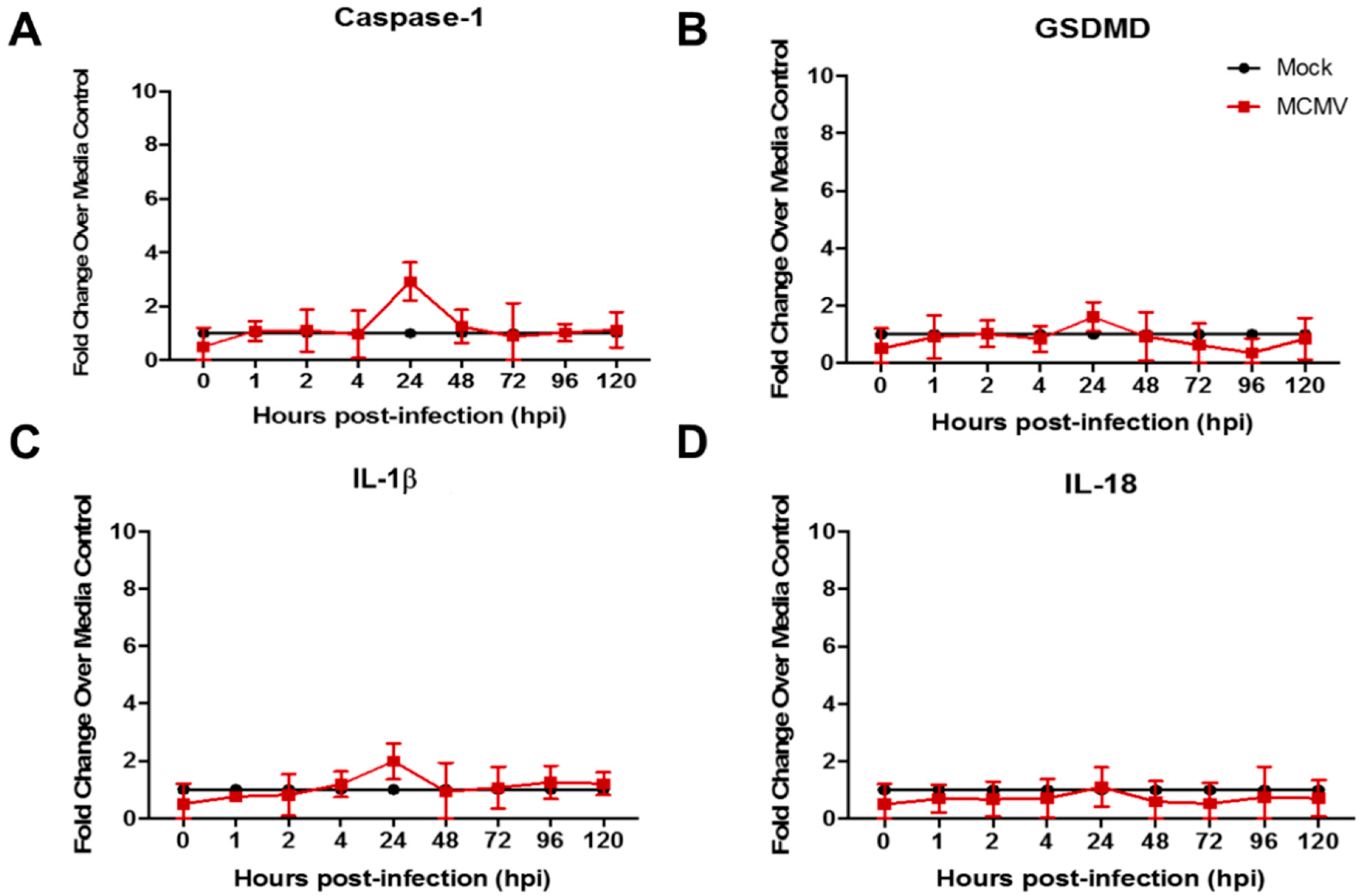

| Signaling Pathway | Consequences | Proinflammatory? | Pathway Suppression via Virus-Encoded Gene a | |
|---|---|---|---|---|
| Extrinsic Apoptosis | TNFR1 TNF Caspase-8 Caspase-3 | Cell lysis Phagocytosis | No | M36 (MCMV) UL36 (HCMV) |
| Canonical Pyroptosis | Inflammasomes Caspase-1 Gasdermin D | Cell lysis Release of IL-1β and IL-18 | Yes | M84 (MCMV) UL83 (HCMV) |
| Necroptosis | RIPK1 RIPK3 MLKL | Cell lysis Release of cellular contents | Yes | M45 (MCMV) |
| UV-inactivated MCMV | MCMV | HCMV | |
|---|---|---|---|
| Cell Type | Mouse fibroblasts Mouse macrophages | Mouse fibroblasts Mouse macrophages | Human fibroblasts ARPE-19 cells |
| Evidence for Pyroptosis Induction | No | Yes | No |
| Stimulation of caspase-1 GSDMD, and IL-1β transcripts |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carter, J.J.; Schneider, D.H.; Hisamuddin, A.M.; Dix, R.D. Murine Cytomegalovirus and Human Cytomegalovirus Differ in Pyroptosis Induction in Different Cell Types During Productive Replication. Viruses 2025, 17, 1106. https://doi.org/10.3390/v17081106
Carter JJ, Schneider DH, Hisamuddin AM, Dix RD. Murine Cytomegalovirus and Human Cytomegalovirus Differ in Pyroptosis Induction in Different Cell Types During Productive Replication. Viruses. 2025; 17(8):1106. https://doi.org/10.3390/v17081106
Chicago/Turabian StyleCarter, Jessica J., Daniel H. Schneider, Arshaan M. Hisamuddin, and Richard D. Dix. 2025. "Murine Cytomegalovirus and Human Cytomegalovirus Differ in Pyroptosis Induction in Different Cell Types During Productive Replication" Viruses 17, no. 8: 1106. https://doi.org/10.3390/v17081106
APA StyleCarter, J. J., Schneider, D. H., Hisamuddin, A. M., & Dix, R. D. (2025). Murine Cytomegalovirus and Human Cytomegalovirus Differ in Pyroptosis Induction in Different Cell Types During Productive Replication. Viruses, 17(8), 1106. https://doi.org/10.3390/v17081106








