Seroprevalence of Equine Influenza Virus Antibodies in Horses from Four Localities in Colombia
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and Laboratory Analysis
2.2. Statistical Analysis
3. Results
3.1. Descriptive Analysis
3.2. Multivariate Analysis
3.3. Distribution of ELISA Results by Clinical Signature and Vaccination History in Horses from Study 1
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singh, R.K.; Dhama, K.; Karthik, K.; Khandia, R.; Munjal, A.; Khurana, S.K.; Chakraborty, S.; Malik, Y.S.; Virmani, N.; Singh, R.; et al. A Comprehensive Review on Equine Influenza Virus: Etiology, Epidemiology, Pathobiology, Advances in Developing Diagnostics, Vaccines, and Control Strategies. Front. Microbiol. 2018, 9, 1941. [Google Scholar] [CrossRef]
- Olguin-Perglione, C.; Barrandeguy, M.E. An Overview of equine influenza in South America. Viruses 2021, 13, 888. [Google Scholar] [CrossRef]
- Karlsson, E.A.; Ciuoderis, K.; Freiden, P.J.; Seufzer, B.; Jones, J.C.; Johnson, J.; Parra, R.; Gongora, A.; Cardenas, D.; Barajas, D. Prevalence and characterization of influenza viruses in diverse species in Los Llanos, Colombia: Prevalence of influenza viruses in Colombia. Emerg. Microbes Infect. 2013, 2, 1–10. [Google Scholar] [CrossRef]
- Olguin-Perglione, C.; Vissani, M.A.; Alamos, F.; Tordoya, M.S.; Barrandeguy, M. Multifocal outbreak of equine influenza in vaccinated horses in Argentina in 2018: Epidemiological aspects and molecular characterisation of the involved virus strains. Equine Vet. J. 2020, 52, 420–427. [Google Scholar] [CrossRef]
- Perglione, C.O.; Gildea, S.; Rimondi, A.; Miño, S.; Vissani, A.; Carossino, M.; Cullinane, A.; Barrandeguy, M. Epidemiological and virological findings during multiple outbreaks of equine influenza in S outh A merica in 2012. Influenza Other Respir. Viruses 2016, 10, 37–46. [Google Scholar] [CrossRef]
- Chambers, T.M. A Brief Introduction to Equine Influenza and Equine Influenza Viruses. In Animal Influenza Virus: Methods and Protocols; Spackman, E., Ed.; Springer: New York, NY, USA, 2020; pp. 355–360. [Google Scholar]
- Diaz-Mendez, A.; Viel, L.; Hewson, J.; Doig, P.; Carman, S.; Chambers, T.; Tiwari, A.; Dewey, C. Surveillance of equine respiratory viruses in Ontario. Can. J. Vet. Res. 2010, 74, 271–278. [Google Scholar]
- Loroño-Pino, M.A.; Farfan-Ale, J.A.; Garcia-Rejon, J.E.; Lin, M.; Rosado-Paredes, E.; Puerto, F.I.; Bates, A.; Root, J.J.; Franklin, A.B.; Sullivan, H.J.; et al. Antibodies to influenza and West Nile viruses in horses in Mexico. Vet. Rec. 2010, 166, 22–23. [Google Scholar] [CrossRef] [PubMed]
- Mena, J.; Brito, B.; Moreira, R.; Tadich, T.; Gonzalez, I.; Cruces, J.; Ortega, R.; van Bakel, H.; Rathnasinghe, R.; Pizarro-Lucero, J.; et al. Reemergence of H3N8 Equine Influenza A virus in Chile, 2018. Transbound. Emerg. Dis. 2018, 65, 1408–1415. [Google Scholar] [CrossRef] [PubMed]
- Moreira, R.; García, A.; Ahumada, C.; Badía, C.; Suárez, P.; Yangari, B.; Aguayo, C.; Herrera, J.; Espejo, G.; Pinto, E. Report of 2018 equine influenza outbreak in Chile. Austral J. Vet. Sci. 2019, 51, 27–31. [Google Scholar] [CrossRef]
- Castro, E.; Perez, R.; Rodriguez, S.; Bassetti, L.; Negro, R.; Vidal, R. Epidemiological and virological findings during an outbreak of equine influenza in Uruguay in 2018. Rev. Sci. Tech. (Int. Off. Epizoot.) 2019, 38, 737–749. [Google Scholar] [CrossRef]
- Daly, J.M.; MacRae, S.; Newton, J.R.; Wattrang, E.; Elton, D.M. Equine influenza: A review of an unpredictable virus. Vet. J. 2011, 189, 7–14. [Google Scholar] [CrossRef]
- Gonzalez-Obando, J.; Zuluaga-Cabrera, A.; Moreno, I.; Úsuga, J.; Ciuderis, K.; Forero, J.E.; Diaz, A.; Rojas-Arbeláez, C.; Hernández-Ortiz, J.P.; Ruiz-Saenz, J. First Molecular Detection and Epidemiological Analysis of Equine Influenza Virus in Two Regions of Colombia, 2020–2023. Viruses 2024, 16, 839. [Google Scholar] [CrossRef]
- Olguin Perglione, C.; Golemba, M.D.; Torres, C.; Barrandeguy, M. Molecular epidemiology and spatio-temporal dynamics of the H3N8 equine influenza virus in South America. Pathogens 2016, 5, 61. [Google Scholar] [CrossRef]
- Van Maanen, C.; Cullinane, A. Equine influenza virus infections: An update. Vet. Q. 2002, 24, 79–94. [Google Scholar] [CrossRef]
- Legrand, L.; Pitel, P.H.; Cullinane, A.; Fortier, G.; Pronost, S. Genetic evolution of equine influenza strains isolated in F rance from 2005 to 2010. Equine Vet. J. 2015, 47, 207–211. [Google Scholar] [CrossRef] [PubMed]
- Oladunni, F.S.; Oseni, S.O.; Martinez-Sobrido, L.; Chambers, T.M. Equine influenza virus and vaccines. Viruses 2021, 13, 1657. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Obando, J.; Forero, J.E.; Zuluaga-Cabrera, A.M.; Ruiz-Saenz, J. Equine influenza virus: An old known enemy in the Americas. Vaccines 2022, 10, 1718. [Google Scholar] [CrossRef] [PubMed]
- Paillot, R.; Marcillaud Pitel, C.; D’Ablon, X.; Pronost, S. Equine Vaccines: How, When and Why? Report of the Vaccinology Session, French Equine Veterinarians Association, 2016, Reims. Vaccines 2017, 5, 46. [Google Scholar] [CrossRef]
- Lee, K.; Pusterla, N.; Barnum, S.M.; Lee, D.H.; Martínez-López, B. Genome-informed characterisation of antigenic drift in the haemagglutinin gene of equine influenza strains circulating in the United States from 2012 to 2017. Transbound. Emerg. Dis. 2022, 69, e52–e63. [Google Scholar] [CrossRef]
- ICA. Resolución 676 del 2015 Colombia. ICA. 2015. Available online: https://Documents/resolucion_676_de_2015_ica_-_instituto_colombiano_agropecuario.aspx#/ (accessed on 13 February 2015).
- Gildea, S.; Lyons, P.; Lyons, R.; Gahan, J.; Garvey, M.; Cullinane, A. Annual booster vaccination and the risk of equine influenza to Thoroughbred racehorses. Equine Vet. J. 2020, 52, 509–515. [Google Scholar] [CrossRef]
- Merck Animal Health. Equilis Prequenza: Technical Sheet/Summary of Product Characteristics; Merck Animal Health: Madison, NJ, USA, 2023; Available online: https://ec.europa.eu/health/documents/community-register/2023/20230726159755/anx_159755_en.pdf (accessed on 24 January 2025).
- Paillot, R.; Hannant, D.; Kydd, J.; Daly, J. Vaccination against equine influenza: Quid novi? Vaccine 2006, 24, 4047–4061. [Google Scholar] [CrossRef]
- Allkofer, A.; Garvey, M.; Ryan, E.; Lyons, R.; Ryan, M.; Lukaseviciute, G.; Walsh, C.; Venner, M.; Cullinane, A. Primary vaccination in foals: A comparison of the serological response to equine influenza and equine herpesvirus vaccines administered concurrently or 2 weeks apart. Arch. Virol. 2021, 166, 571–579. [Google Scholar] [CrossRef]
- Gildea, S.; Arkins, S.; Cullinane, A. A comparative antibody study of the potential susceptibility of Thoroughbred and non-Thoroughbred horse populations in Ireland to equine influenza virus. Influenza Other Respir. Viruses 2010, 4, 363–372. [Google Scholar] [CrossRef]
- Entenfellner, J.; Gahan, J.; Garvey, M.; Walsh, C.; Venner, M.; Cullinane, A. Response of sport horses to different formulations of equine influenza vaccine. Vaccines 2020, 8, 372. [Google Scholar] [CrossRef]
- Woodward, A.L.; Rash, A.S.; Blinman, D.; Bowman, S.; Chambers, T.M.; Daly, J.M.; Damiani, A.; Joseph, S.; Lewis, N.; McCauley, J.W. Development of a surveillance scheme for equine influenza in the UK and characterisation of viruses isolated in Europe, Dubai and the USA from 2010–2012. Vet. Microbiol. 2014, 169, 113–127. [Google Scholar] [CrossRef] [PubMed]
- Cullinane, A.; Newton, J.R. Equine influenza—A global perspective. Vet. Microbiol. 2013, 167, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Colombian Agricultural Institute (ICA). Table of Equine Population by Municipality and Department (2024 National Livestock Census); ICA: Bogotá, Colombia, 2024. Available online: https://www.ica.gov.co/areas/pecuaria/servicios/enfermedades-animales/fiebre-aftosa/tabla-de-poblacion-equina-por-municipio-y-departam.aspx (accessed on 24 January 2025).
- Sabanas, D.S.E. Los Recursos Zoogenéticos y el Desarrollo Sostenible en Sabanas Inundables de Arauca (Colombia) [Internet]. 2014. Available online: https://www.produccion-animal.com.ar/genetica_seleccion_cruzamientos/genetica_en_general/31-Recursos_Zoogneticos.pdf (accessed on 24 January 2025).
- Muzykina, L.; Barrado-Gil, L.; Gonzalez-Bulnes, A.; Crespo-Piazuelo, D.; Cerón, J.J.; Alonso, C.; Montoya, M. Overview of modern commercial kits for laboratory diagnosis of African swine fever and swine influenza A viruses. Viruses 2024, 16, 505. [Google Scholar] [CrossRef] [PubMed]
- Meseko, C.A.; Ehizibolo, D.O.; Nwokike, E.C.; Wungak, Y.S. Serological evidence of equine influenza virus in horse stables in Kaduna, Nigeria. J. Equine Sci. 2016, 27, 99–105. [Google Scholar] [CrossRef]
- Nemoto, M.; Kawanishi, N.; Kambayashi, Y.; Bannai, H.; Yamanaka, T.; Tsujimura, K. Detection of equine influenza virus gene in the air around infected horses. Vet. Microbiol. 2025, 302, 110388. [Google Scholar] [CrossRef]
- Dominguez, M.; Münstermann, S.; De Guindos, I.; Timoney, P. Equine disease events resulting from international horse movements: Systematic review and lessons learned. Equine Vet. J. 2016, 48, 641–653. [Google Scholar] [CrossRef]
- Jiménez, D.; Romero-Zuñiga, J.J.; Dolz, G. Serosurveillance of infectious agents in equines of the Central Valley of Costa Rica. Open Vet. J. 2014, 4, 107–112. [Google Scholar]
- Gildea, S.; Arkins, S.; Cullinane, A. Management and environmental factors involved in equine influenza outbreaks in Ireland 2007–2010. Equine Vet. J. 2011, 43, 608–617. [Google Scholar] [CrossRef] [PubMed]
- Márquez, C.; Escobar, A.; Tadich, T. Características de manejo y conducta en caballos estabulados en el sur de Chile: Estudio preliminar. Arch. De Med. Vet. 2010, 42, 203–207. [Google Scholar] [CrossRef]
- Branda, F.; Yon, D.K.; Albanese, M.; Binetti, E.; Giovanetti, M.; Ciccozzi, A.; Ciccozzi, M.; Scarpa, F.; Ceccarelli, G. Equine Influenza: Epidemiology, Pathogenesis, and Strategies for Prevention and Control. Viruses 2025, 17, 302. [Google Scholar] [CrossRef] [PubMed]
- Schemann, K.; Taylor, M.R.; Toribio, J.-A.; Dhand, N.K. Horse owners’ biosecurity practices following the first equine influenza outbreak in Australia. Prev. Vet. Med. 2011, 102, 304–314. [Google Scholar] [CrossRef]
- Firestone, S.M.; Lewis, F.I.; Schemann, K.; Ward, M.P.; Toribio, J.-A.L.; Taylor, M.R.; Dhand, N.K. Applying Bayesian network modelling to understand the links between on-farm biosecurity practice during the 2007 equine influenza outbreak and horse managers’ perceptions of a subsequent outbreak. Prev. Vet. Med. 2014, 116, 243–251. [Google Scholar] [CrossRef]
- Whitlock, F.; Grewar, J.; Newton, R. An epidemiological overview of the equine influenza epidemic in Great Britain during 2019. Equine Vet. J. 2023, 55, 153–164. [Google Scholar] [CrossRef]
- Crew, C.; Brennan, M.; Ireland, J. Implementation of biosecurity on equestrian premises: A narrative overview. Vet. J. 2023, 292, 105950. [Google Scholar] [CrossRef]
- Lara, A.C.; Fernando, F.S.; Takeuti, K.L.; Bortolozzo, F.P.; Barcellos, D.E.d. Efficacy of disinfectants to inactivate H1N1 influenza A virus isolated from pigs. Pesqui. Veterinária Bras. 2022, 42, e06987. [Google Scholar] [CrossRef]
- Grayson, M.L.; Melvani, S.; Druce, J.; Barr, I.G.; Ballard, S.A.; Johnson, P.D.; Mastorakos, T.; Birch, C. Efficacy of soap and water and alcohol-based hand-rub preparations against live H1N1 influenza virus on the hands of human volunteers. Clin. Infect. Dis. 2009, 48, 285–291. [Google Scholar] [CrossRef]
- Ince, O.; Paksoy, Y.; Sait, A. Risk assessment about effectiveness of biosecurity implementations on horse properties in Turkey. J. Hell. Vet. Med. Soc. 2024, 75, 7397–7406. [Google Scholar] [CrossRef]
- Arthur, R.; Suann, C. Biosecurity and vaccination strategies to minimise the effect of an equine influenza outbreak on racing and breeding. Aust. Vet. J. 2011, 89, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Read, A.; Arzey, K.; Finlaison, D.; Gu, X.; Davis, R.; Ritchie, L.; Kirkland, P. A prospective longitudinal study of naturally infected horses to evaluate the performance characteristics of rapid diagnostic tests for equine influenza virus. Vet. Microbiol. 2012, 156, 246–255. [Google Scholar] [CrossRef] [PubMed]
- Legrand, L.; Pitel, P.H.; Marcillaud-Pitel, C.; Cullinane, A.; Couroucé, A.; Fortier, G.; Freymuth, F.; Pronost, S. Surveillance of equine influenza viruses through the RESPE network in F rance from N ovember 2005 to October 2010. Equine Vet. J. 2013, 45, 776–783. [Google Scholar] [CrossRef] [PubMed]
- Daly, J.; Megid, J.; Langoni, H.; de Nardi Júnior, G.; Ribeiro, M. Retrospective serological survey for influenza in horses from Brazil. Braz. J. Microbiol. 2021, 52, 461–466. [Google Scholar] [CrossRef]
- Lawton, K.; Runk, D.; Hankin, S.; Mendonsa, E.; Hull, D.; Barnum, S.; Pusterla, N. Detection of Selected Equine Respiratory Pathogens in Stall Samples Collected at a Multi-Week Equestrian Show during the Winter Months. Viruses 2023, 15, 2078. [Google Scholar] [CrossRef]
- Reemers, S.; van Bommel, S.; Cao, Q.; Sutton, D.; van de Zande, S. Protection against the new Equine Influenza virus florida clade I outbreak strain provided by a whole inactivated virus vaccine. Vaccines 2020, 8, 784. [Google Scholar] [CrossRef]
- Dilai, M.; Piro, M.; El Harrak, M.; Fougerolle, S.; Dehhaoui, M.; Dikrallah, A.; Legrand, L.; Paillot, R.; Fassi Fihri, O. Impact of mixed equine influenza vaccination on correlate of protection in horses. Vaccines 2018, 6, 71. [Google Scholar] [CrossRef]
- Bambra, W.; Daly, J.; Kendall, N.; Gardner, D.; Brennan, M.; Kydd, J. Equine influenza vaccination as reported by horse owners and factors influencing their decision to vaccinate or not. Prev. Vet. Med. 2020, 180, 105011. [Google Scholar] [CrossRef]
- El-Hage, C.; Savage, C.; Minke, J.; Ficorilli, N.; Watson, J.; Gilkerson, J. Accelerated vaccination schedule provides protective levels of antibody and complete herd immunity to equine influenza. Equine Vet. J. 2013, 45, 235–239. [Google Scholar] [CrossRef]
- Zhang, P.; Sun, Y.; Tan, C.; Ling, M.; Li, X.; Wang, W.; Cong, Y. Preparation and evaluation of virus-like particle vaccine against H3N8 subtype equine influenza. Microb. Pathog. 2021, 157, 104885. [Google Scholar] [CrossRef] [PubMed]
- Colgate, V.A.; Newton, J.R. Equine influenza bi-annual boosters: What does the evidence tell us? Equine Vet J. 2023, 55, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Reemers, S.; Sonnemans, D.; Horspool, L.; van Bommel, S.; Cao, Q.; van de Zande, S. Determining equine influenza virus vaccine efficacy—The specific contribution of strain versus other vaccine attributes. Vaccines 2020, 8, 501. [Google Scholar] [CrossRef] [PubMed]
- Colombian Agricultural Institute (ICA). Resolution 20174 of 2016; ICA: Bogotá, Colombia, 2016. Available online: https://www.ica.gov.co/normatividad/normas-ica/resoluciones-oficinas-nacionales/2016/2016r20174 (accessed on 24 January 2025).
- Landolt, G.A. Equine Influenza Virus. Vet. Clin. N. Am. Equine Pract. 2014, 30, 507–522. [Google Scholar] [CrossRef]
- Nogales, A.; DeDiego, M.L.; Martínez-Sobrido, L. Live attenuated influenza A virus vaccines with modified NS1 proteins for veterinary use. Front. Cell. Infect. Microbiol. 2022, 12, 954811. [Google Scholar] [CrossRef]
- Birch-Machin, I.; Rowan, A.; Pick, J.; Mumford, J.; Binns, M. Expression of the nonstructural protein NS1 of equine influenza A virus: Detection of anti-NS1 antibody in post infection equine sera. J. Virol. Methods 1997, 65, 255–263. [Google Scholar] [CrossRef]
- Ozaki, H.; Sugiura, T.; Sugita, S.; Imagawa, H.; Kida, H. Detection of antibodies to the nonstructural protein (NS1) of influenza A virus allows distinction between vaccinated and infected horses. Vet. Microbiol. 2001, 82, 111–119. [Google Scholar] [CrossRef]
- Castillo-Olivares, J. African horse sickness in Thailand: Challenges of controlling an outbreak by vaccination. Equine Vet. J. 2021, 53, 9. [Google Scholar] [CrossRef]
- Dionísio, L.; Medeiros, F.; Pequito, M.; Faustino-Rocha, A.I. Equine influenza: A comprehensive review from etiology to treatment. Anim. Health Res. Rev. 2021, 22, 56–71. [Google Scholar] [CrossRef]
| Variable | Category | Positive | Total | |
|---|---|---|---|---|
| Study | 1 | 73 | (38.80%) | 188 |
| 2 | 31 | (15.70%) | 197 | |
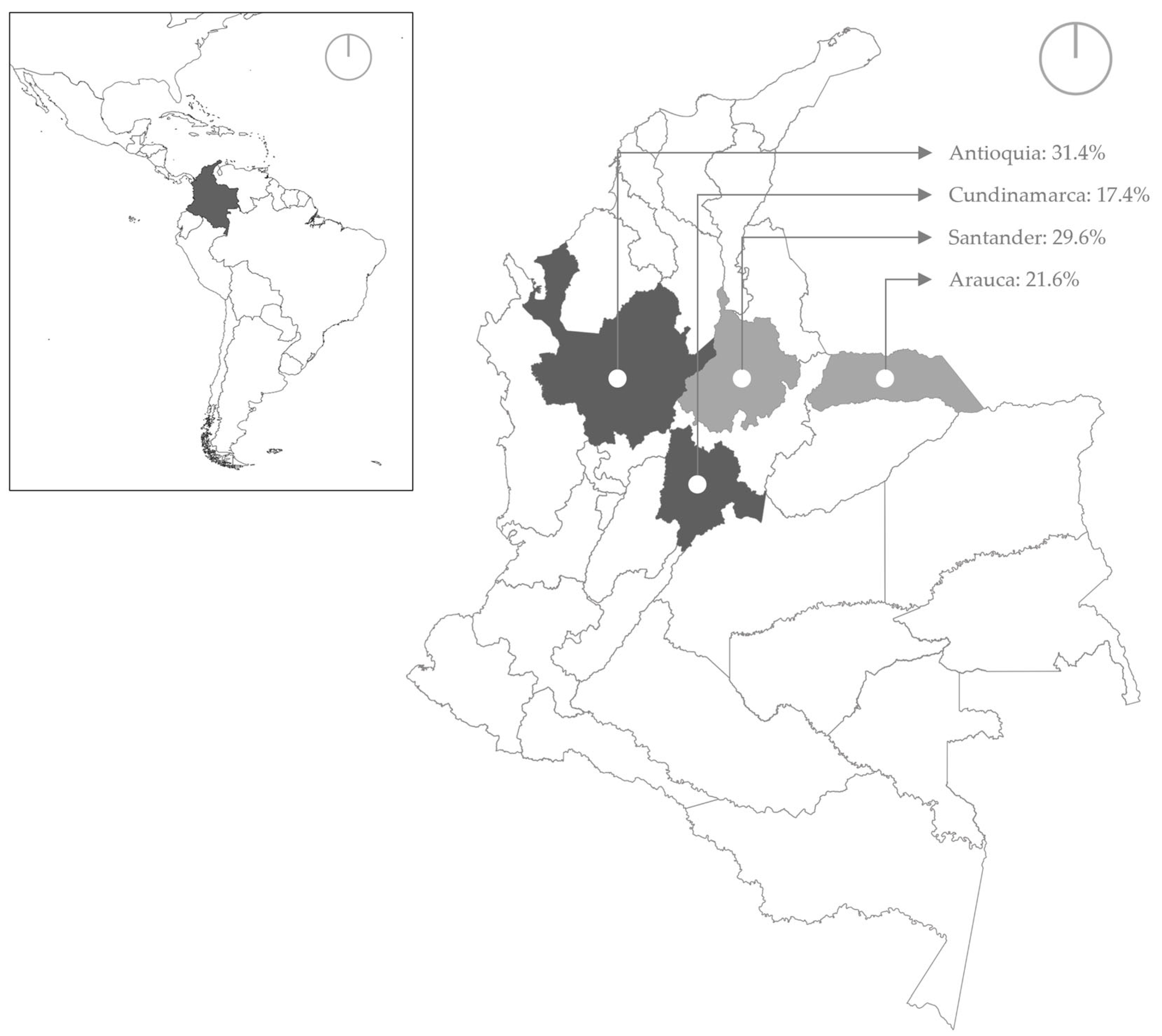
| Locality * | ANT | 42 | (34.70%) | 121 |
| CUN | 31 | (46.20%) | 67 | |
| ARA | 10 | (12.00%) | 83 | |
| SAN | 21 | (18.40%) | 114 | |
| Sex | Male | 40 | (27.80%) | 144 |
| Female | 64 | (26.60%) | 241 | |
| Age | 0–2.5 | 25 | (26.60%) | 94 |
| 2.5–9.9 | 59 | (25.50%) | 231 | |
| <10 | 20 | (33.30%) | 60 | |
| Activity * | Equestrian | 73 | (32.60%) | 224 |
| Others | 31 | (19.30%) | 161 | |
| Shelter * | Stable | 90 | (42.50%) | 212 |
| Open air Shelters | 14 | (8.10%) | 173 | |
| Variables | OR | 95% CI | p-Value | Adjusted OR | 95% CI | p-Value | ||
|---|---|---|---|---|---|---|---|---|
| Shelter | 8.37 | 4.55 | 15.42 | 0.000 | 9.91 | 4.83 | 20.31 | <0.001 * |
| Activity | 2.02 | 1.25 | 3.28 | 0.020 | 0.89 | 0.50 | 1.61 | 0.005 |
| Locality | 0.29 | 0.18 | 0.47 | 0.000 | 0.73 | 0.42 | 1.27 | 0.654 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonzalez-Obando, J.; Jaimes-Dueñez, J.; Zuluaga-Cabrera, A.; Forero, J.E.; Diaz, A.; Rojas-Arbeláez, C.; Ruiz-Saenz, J. Seroprevalence of Equine Influenza Virus Antibodies in Horses from Four Localities in Colombia. Viruses 2025, 17, 999. https://doi.org/10.3390/v17070999
Gonzalez-Obando J, Jaimes-Dueñez J, Zuluaga-Cabrera A, Forero JE, Diaz A, Rojas-Arbeláez C, Ruiz-Saenz J. Seroprevalence of Equine Influenza Virus Antibodies in Horses from Four Localities in Colombia. Viruses. 2025; 17(7):999. https://doi.org/10.3390/v17070999
Chicago/Turabian StyleGonzalez-Obando, Juliana, Jeiczon Jaimes-Dueñez, Angélica Zuluaga-Cabrera, Jorge E. Forero, Andrés Diaz, Carlos Rojas-Arbeláez, and Julian Ruiz-Saenz. 2025. "Seroprevalence of Equine Influenza Virus Antibodies in Horses from Four Localities in Colombia" Viruses 17, no. 7: 999. https://doi.org/10.3390/v17070999
APA StyleGonzalez-Obando, J., Jaimes-Dueñez, J., Zuluaga-Cabrera, A., Forero, J. E., Diaz, A., Rojas-Arbeláez, C., & Ruiz-Saenz, J. (2025). Seroprevalence of Equine Influenza Virus Antibodies in Horses from Four Localities in Colombia. Viruses, 17(7), 999. https://doi.org/10.3390/v17070999







