Characterization of Gene Expression Suppression by Bovine Coronavirus Non-Structural Protein 1
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells
2.2. Plasmids and Plasmid Construction
2.3. Plasmid Transfection, Reporter Assay, and Western Blot Analysis
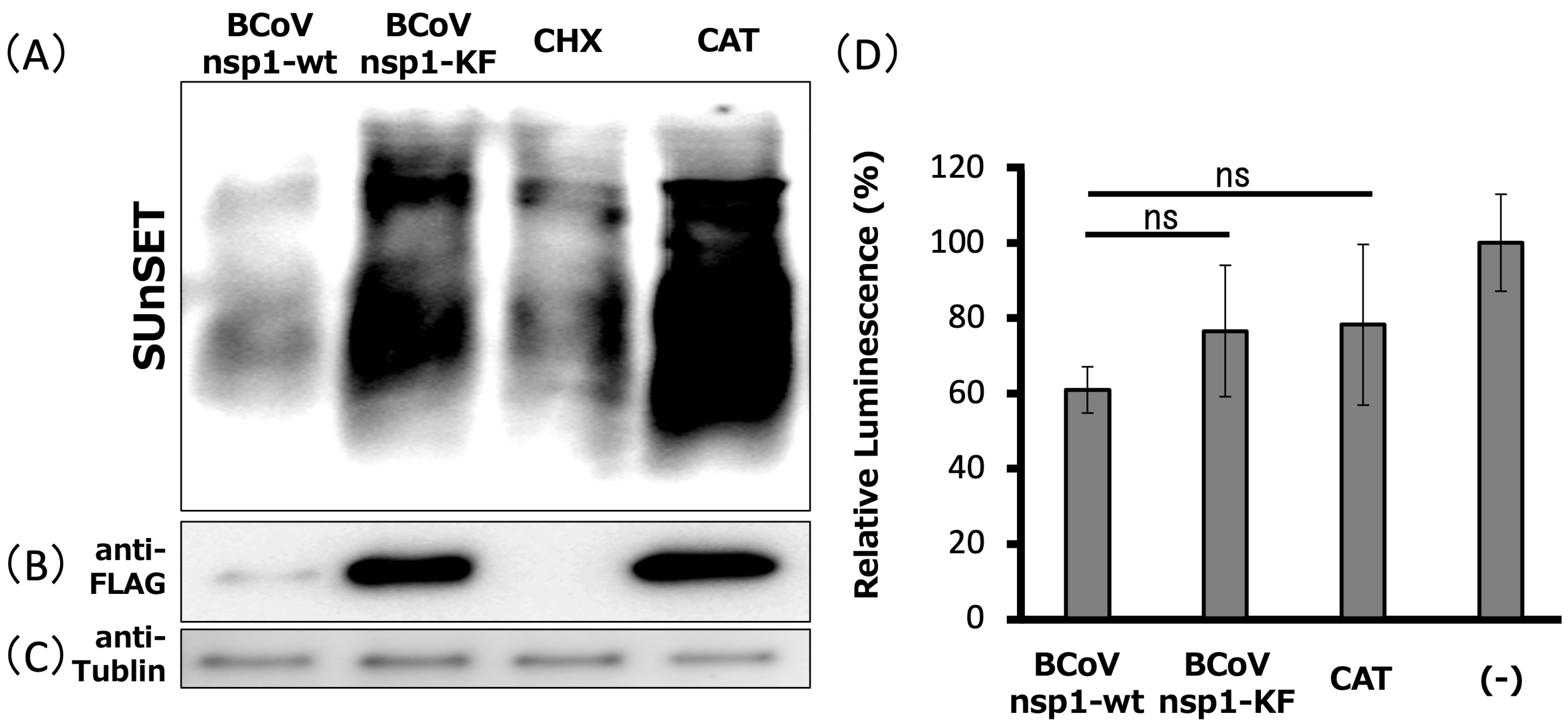
2.4. SUnSET Assay and Viability Measurement
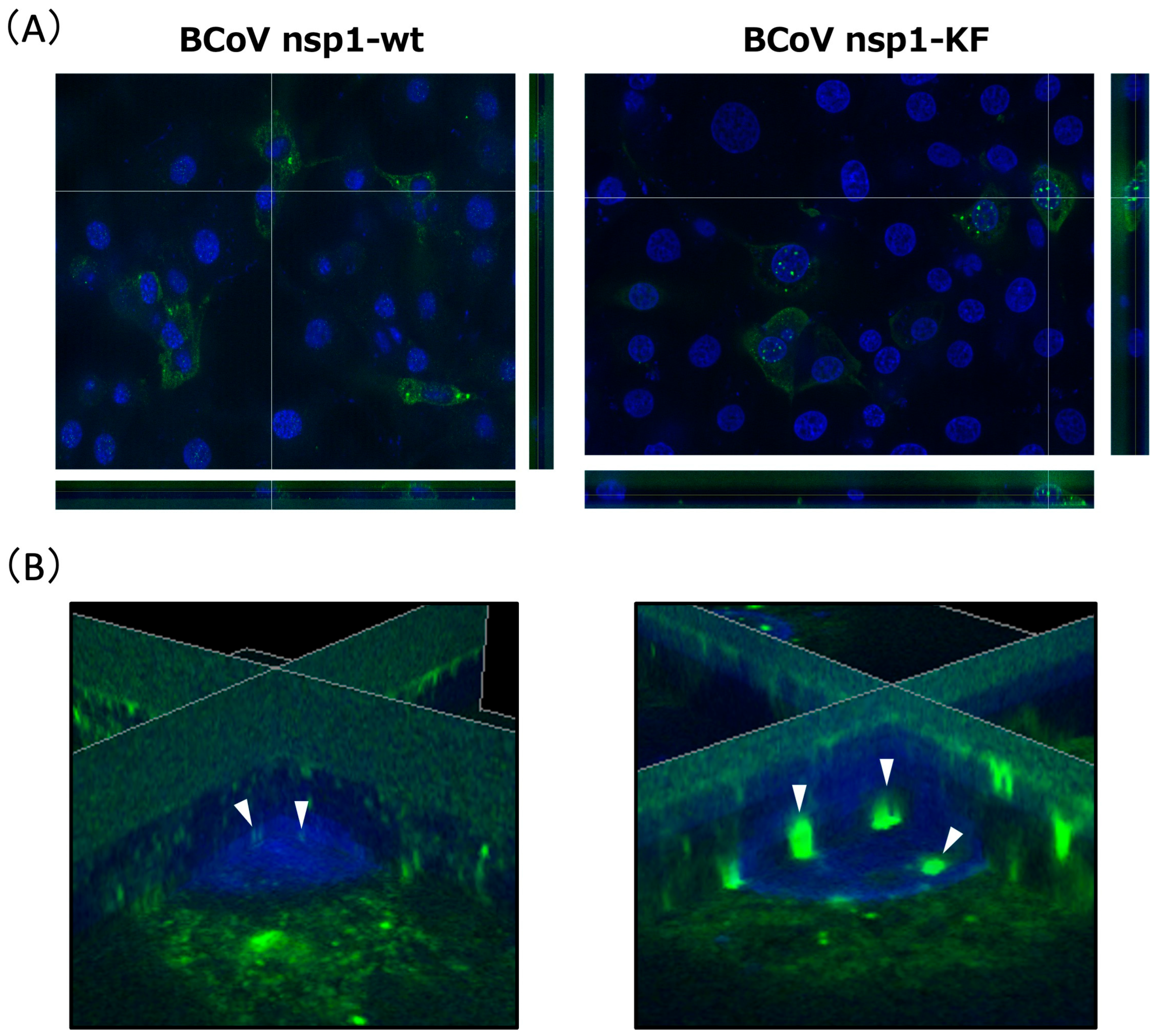
2.5. Immunofluorescence Staining and Confocal Microscopy Analysis
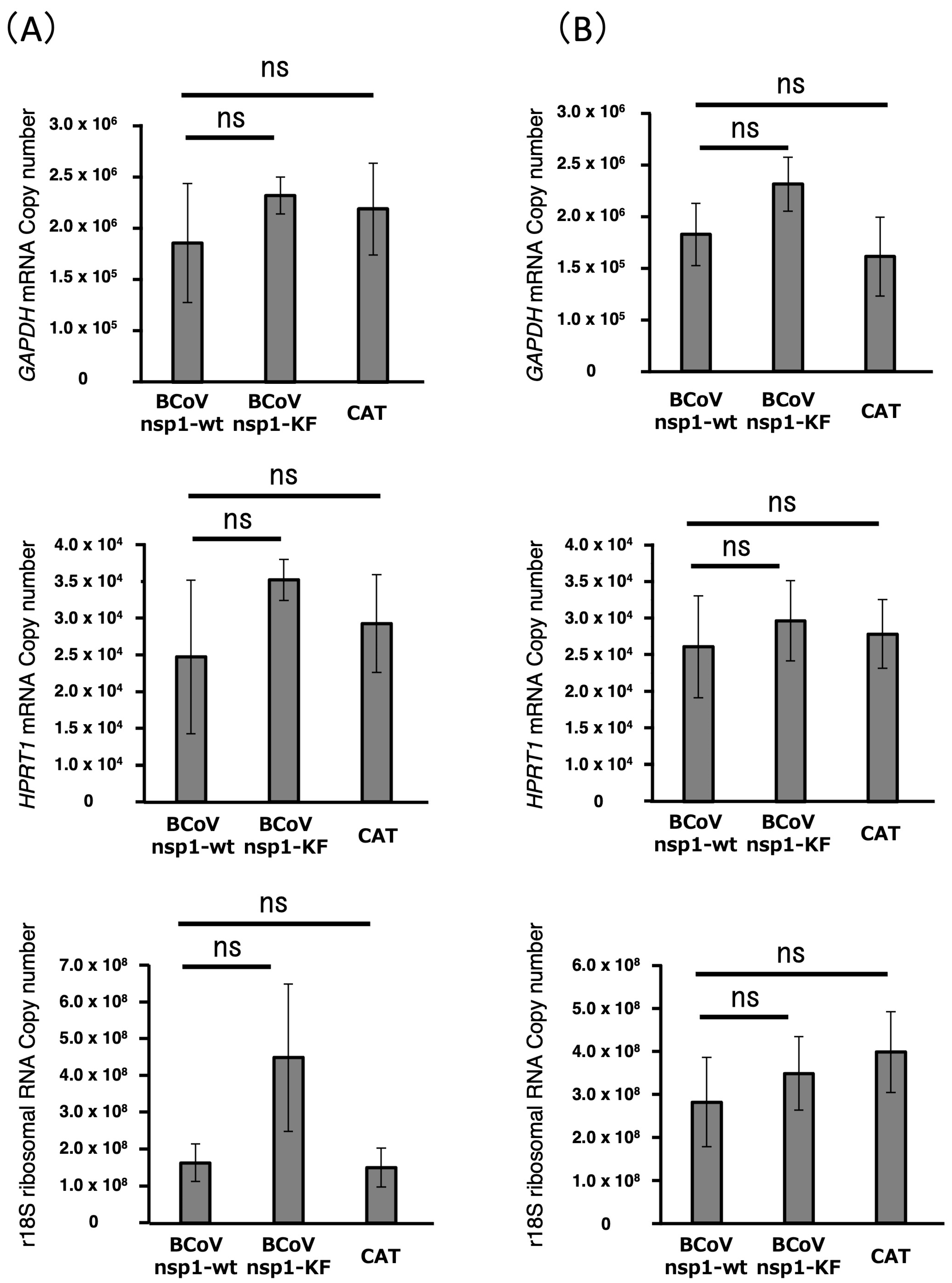
2.6. Total RNA Extraction and qRT-PCR
2.7. Co-Sedimentation Analysis
2.8. Statistical Analysis
3. Results
3.1. Assessment of the Appropriateness of MAC-T Cells in Transfection Experiments
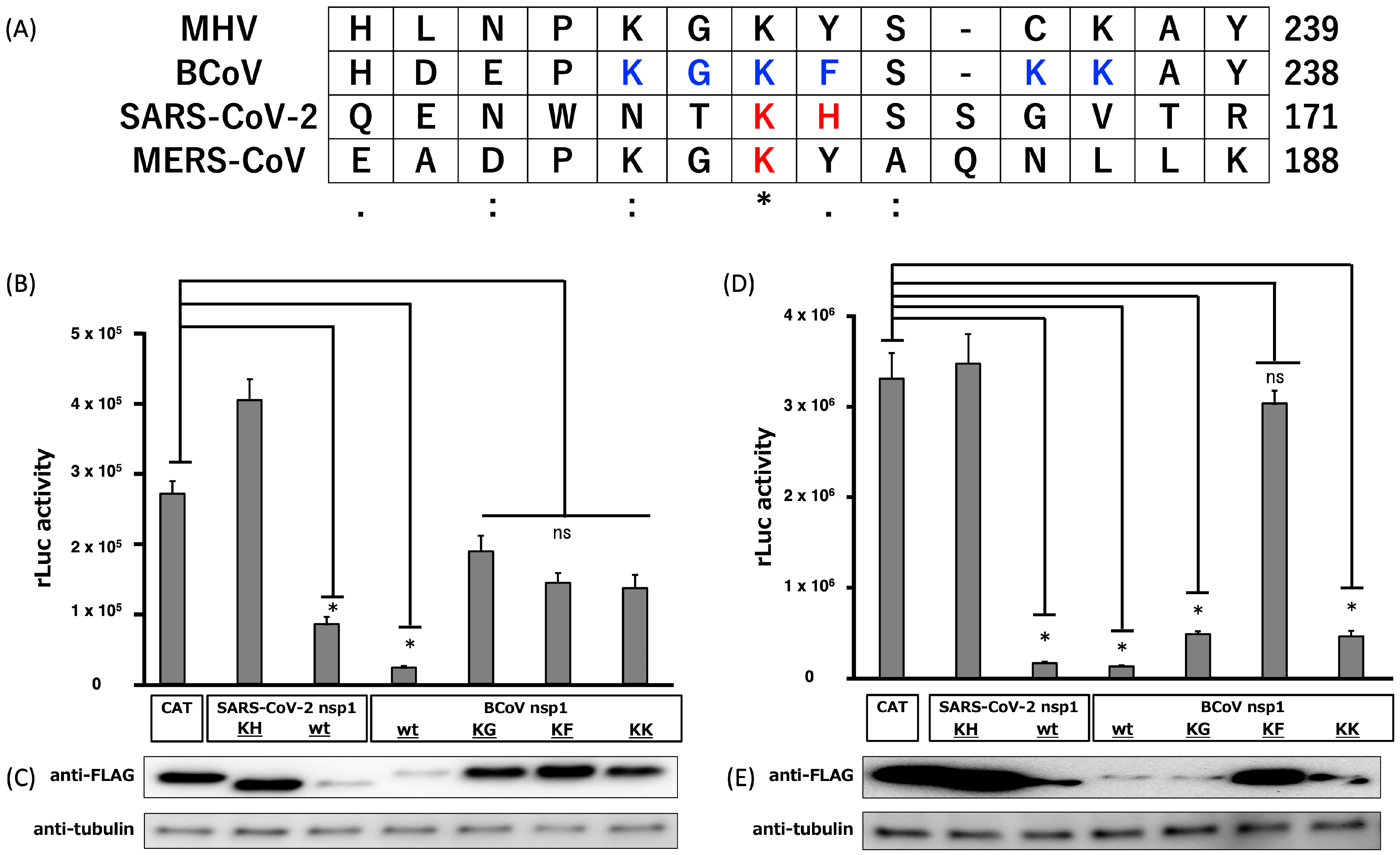
3.2. Suppression of Plasmid-Driven Gene Expression by BCoV nsp1 and Subsequent Identification of Important Amino Acids of BCoV nsp1 for Its Gene Expression Suppression Function
3.3. Expression of BCoV nsp1, but Not BCoV nsp1-KF, Suppresses Host Gene Expression in MAC-T Cells
3.4. Expression Levels of Housekeeping Genes in MAC-T Cells Expressing BCoV nsp1-wt and BCoV nsp1-KF
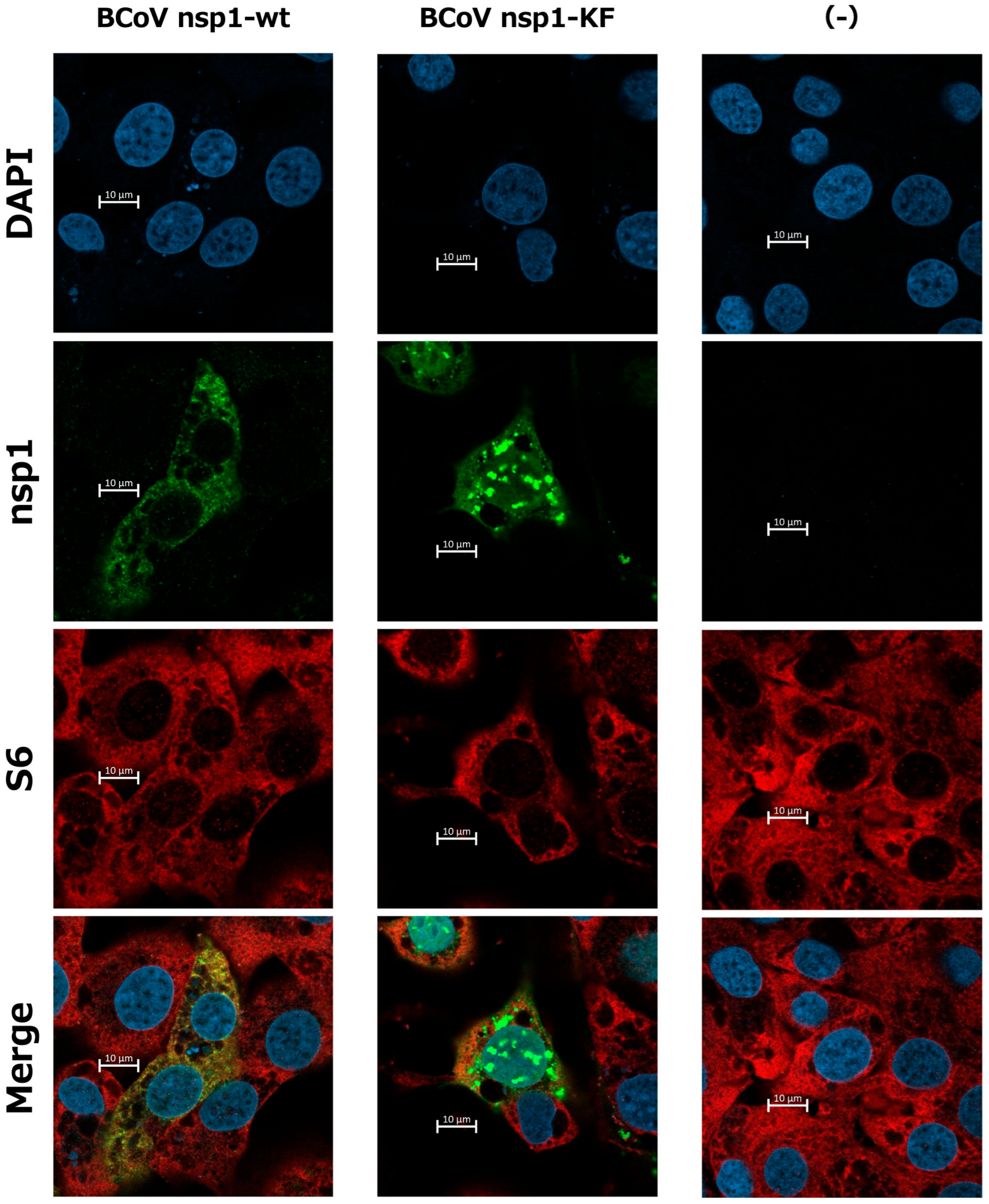
3.5. Subcellular Localization of BCoV nsp1-wt and BCoV nsp1-KF in MAC-T Cells and Colocalization of BCoV nsp1 with Ribosome
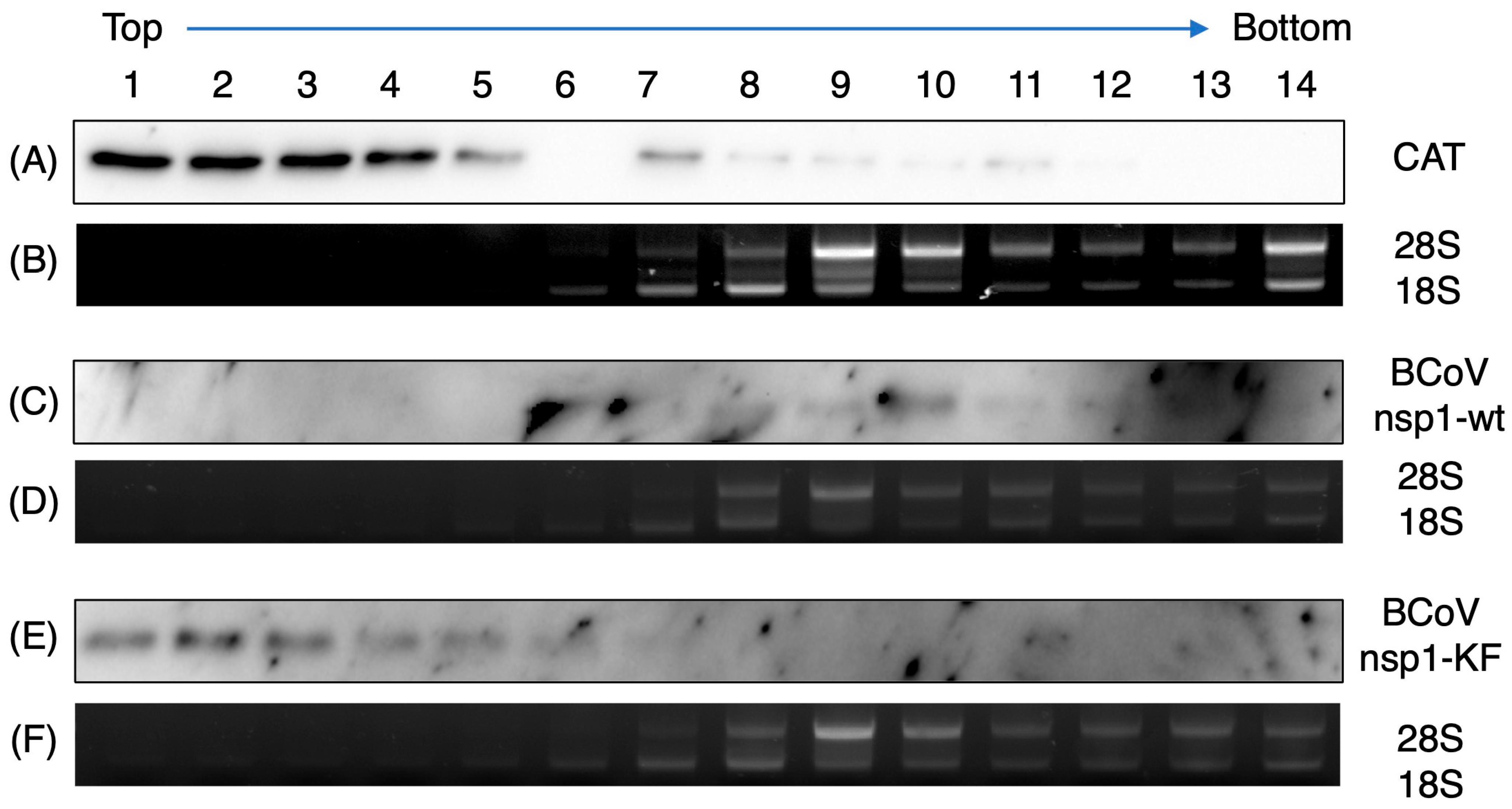
3.6. Co-Sedimentation of BCoV nsp1-wt, Not BCoV nsp1-KF, with the 40S Ribosomal Subunit
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Graaf, D.C.; Vanopdenbosch, E.; Ortega-Mora, L.M.; Abbassi, H.; Peeters, J.E. A Review of the Importance of Cryptosporidiosis in Farm Animals. Int. J. Parasitol. 1999, 29, 1269–1287. [Google Scholar] [CrossRef] [PubMed]
- Hasoksuz, M.; Hoet, A.E.; Loerch, S.C.; Wittum, T.E.; Nielsen, P.R.; Saif, L.J. Detection of Respiratory and Enteric Shedding of Bovine Coronaviruses in Cattle in an Ohio Feedlot. J. Vet. Diagn. Investig. 2002, 14, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Saif, L.J. Bovine Respiratory Coronavirus. Vet. Clin. N. Am.—Food Anim. Pract. 2010, 26, 349. [Google Scholar] [CrossRef] [PubMed]
- Boileau, M.J.; Kapil, S. Bovine Coronavirus Associated Syndromes. Vet. Clin. N. Am.—Food Anim. Pract. 2010, 26, 123. [Google Scholar] [CrossRef]
- Park, G.N.; Choe, S.E.; Song, S.; Kim, K.S.; Shin, J.; An, B.H.; Moon, S.H.; Hyun, B.H.; An, D.J. Characterization and Spike Gene Analysis of a Candidate Attenuated Live Bovine Coronavirus Vaccine. Animals 2024, 14, 389. [Google Scholar] [CrossRef]
- Lin, C.H.; Yang, C.Y.; Wang, M.; Ou, S.C.; Lo, C.Y.; Tsai, T.L.; Wu, H.Y. Effects of Coronavirus Persistence on the Genome Structure and Subsequent Gene Expression, Pathogenicity and Adaptation Capability. Cells 2020, 9, 2322. [Google Scholar] [CrossRef]
- Suzuki, T.; Otake, Y.; Uchimoto, S.; Hasebe, A.; Goto, Y. Genomic Characterization and Phylogenetic Classification of Bovine Coronaviruses through Whole Genome Sequence Analysis. Viruses 2020, 12, 183. [Google Scholar] [CrossRef]
- Hasoksuz, M.; Kayar, A.; Dodurka, T.; Ilgaz, A. Detection of Respiratory and Enteric Shedding of Bovine Coronaviruses in Cattle in Northwestern Turkey. Acta Vet. Hung. 2005, 53, 137–146. [Google Scholar] [CrossRef]
- Zhang, X.; Hasoksuz, M.; Spiro, D.; Halpin, R.; Wang, S.; Vlasova, A.; Janies, D.; Jones, L.R.; Ghedin, E.; Saif, L.J. Quasispecies of Bovine Enteric and Respiratory Coronaviruses Based on Complete Genome Sequences and Genetic Changes after Tissue Culture Adaptation. Virology 2007, 363, 1–10. [Google Scholar] [CrossRef]
- Saif, L.J.; Heckert, R.A.; Miller, K.L.; Tarek, M.M. Cell Culture Propagation of Bovine Coronavirus. J. Tissue Cult. Methods 1988, 11, 139–145. [Google Scholar] [CrossRef]
- Li, Y.; Palomares, R.A.; Liu, M.; Xu, J.; Koo, C.; Granberry, F.; Locke, S.R.; Habing, G.; Saif, L.J.; Wang, L.; et al. Isolation and Characterization of Contemporary Bovine Coronavirus Strains. Viruses 2024, 16, 965. [Google Scholar] [CrossRef] [PubMed]
- Aich, P.; Wilson, H.L.; Kaushik, R.S.; Potter, A.A.; Babiuk, L.A.; Griebel, P. Comparative Analysis of Innate Immune Responses Following Infection of Newborn Calves with Bovine Rotavirus and Bovine Coronavirus. J. Gen. Virol. 2007, 88, 2749–2761. [Google Scholar] [CrossRef] [PubMed]
- David, D.; Storm, N.; Ilan, W.; Sol, A. Characterization of Winter Dysentery Bovine Coronavirus Isolated from Cattle in Israel. Viruses 2021, 13, 1070. [Google Scholar] [CrossRef]
- Socha, W.; Larska, M.; Rola, J.; Bednarek, D. Occurrence of Bovine Coronavirus and Other Major Respiratory Viruses in Cattle in Poland. J. Vet. Res. 2022, 66, 479–486. [Google Scholar] [CrossRef]
- Gunn, L.; Collins, P.J.; O’connell, M.J.; O’shea, H. Phylogenetic Investigation of Enteric Bovine Coronavirus in Ireland Reveals Partitioning between European and Global Strains. Ir. Vet. J. 2015, 68, 31. [Google Scholar] [CrossRef]
- Bok, M.; Miño, S.; Rodriguez, D.; Badaracco, A.; Nuñes, I.; Souza, S.P.; Bilbao, G.; Louge Uriarte, E.; Galarza, R.; Vega, C.; et al. Molecular and Antigenic Characterization of Bovine Coronavirus Circulating in Argentinean Cattle during 1994–2010. Vet. Microbiol. 2015, 181, 221–229. [Google Scholar] [CrossRef]
- Jeong, J.H.; Kim, G.Y.; Yoon, S.S.; Park, S.J.; Kim, Y.J.; Sung, C.M.; Jang, O.J.; Shin, S.S.; Koh, H.B.; Lee, B.J.; et al. Detection and Isolation of Winter Dysentery Bovine Coronavirus Circulated in Korea during 2002–2004. J. Vet. Med. Sci. 2005, 67, 187–189. [Google Scholar] [CrossRef]
- Heckert, R.A.; Saif, L.J.; Mengel, J.P.; Myers, G.W. Mucosal and Systemic Antibody Responses to Bovine Coronavirus Structural Proteins in Experimentally Challenge-Exposed Calves Fed Low or High Amounts of Colostral Antibodies. Am. J. Vet. Res. 1991, 52, 700–708. [Google Scholar] [CrossRef]
- Nakagawa, K.; Kumano, H.; Kitamura, Y.; Kuwata, K.; Tanaka, E.; Fukushi, H. Complete Genome Sequence of Bovine Coronavirus in Blood Diarrhea from Adult Cattle That Died from Winter Dysentery in Japan. Microbiol. Resour. Announc. 2021, 10, e0080721. [Google Scholar] [CrossRef]
- Saif, L.J.; Jung, K. Comparative Pathogenesis of Bovine and Porcine Respiratory Coronaviruses in the Animal Host Species and SARS-CoV-2 in Humans. J. Clin. Microbiol. 2020, 58, e01355-20. [Google Scholar] [CrossRef]
- Snijder, E.J.; Decroly, E.; Ziebuhr, J. The Nonstructural Proteins Directing Coronavirus RNA Synthesis and Processing. Adv. Virus Res. 2016, 96, 59–126. [Google Scholar]
- Hurst-Hess, K.R.; Kuo, L.; Masters, P.S. Dissection of Amino-Terminal Functional Domains of Murine Coronavirus Nonstructural Protein 3. J. Virol. 2015, 89, 6033–6047. [Google Scholar] [CrossRef] [PubMed]
- Graham, R.L.; Sims, A.C.; Brockway, S.M.; Baric, R.S.; Denison, M.R. The Nsp2 Replicase Proteins of Murine Hepatitis Virus and Severe Acute Respiratory Syndrome Coronavirus Are Dispensable for Viral Replication. J. Virol. 2005, 79, 13399–13411. [Google Scholar] [CrossRef] [PubMed]
- Neuman, B.W.; Chamberlain, P.; Bowden, F.; Joseph, J. Atlas of Coronavirus Replicase Structure. Virus Res. 2014, 194, 49–66. [Google Scholar] [CrossRef]
- Chen, C.-J.; Sugiyama, K.; Kubo, H.; Huang, C.; Makino, S. Murine Coronavirus Nonstructural Protein P28 Arrests Cell Cycle in G 0/G 1 Phase. J. Virol. 2004, 78, 10410–10419. [Google Scholar] [CrossRef] [PubMed]
- Wathelet, M.G.; Orr, M.; Frieman, M.B.; Baric, R.S. Severe Acute Respiratory Syndrome Coronavirus Evades Antiviral Signaling: Role of Nsp1 and Rational Design of an Attenuated Strain. J. Virol. 2007, 81, 11620–11633. [Google Scholar] [CrossRef]
- Zhang, K.; Miorin, L.; Makio, T.; Dehghan, I.; Gao, S.; Xie, Y.; Zhong, H.; Esparza, M.; Kehrer, T.; Kumar, A.; et al. Nsp1 Protein of SARS-CoV-2 Disrupts the MRNA Export Machinery to Inhibit Host Gene Expression. Sci. Adv. 2021, 7, eabe7386. [Google Scholar] [CrossRef]
- Narayanan, K.; Huang, C.; Lokugamage, K.; Kamitani, W.; Ikegami, T.; Tseng, C.-T.K.; Makino, S. Severe Acute Respiratory Syndrome Coronavirus Nsp1 Suppresses Host Gene Expression, Including That of Type I Interferon, in Infected Cells. J. Virol. 2008, 82, 4471–4479. [Google Scholar] [CrossRef]
- Lokugamage, K.G.; Narayanan, K.; Nakagawa, K.; Terasaki, K.; Ramirez, S.I.; Tseng, C.-T.K.; Makino, S. Middle East Respiratory Syndrome Coronavirus Nsp1 Inhibits Host Gene Expression by Selectively Targeting MRNAs Transcribed in the Nucleus While Sparing MRNAs of Cytoplasmic Origin. J. Virol. 2015, 89, 10970–10981. [Google Scholar] [CrossRef]
- Nakagawa, K.; Narayanan, K.; Wada, M.; Popov, V.L.; Cajimat, M.; Baric, R.S.; Makino, S. The Endonucleolytic RNA Cleavage Function of Nsp1 of Middle East Respiratory Syndrome Coronavirus Promotes the Production of Infectious Virus Particles in Specific Human Cell Lines. J. Virol. 2018, 92, e01157-18. [Google Scholar] [CrossRef]
- Huang, C.; Lokugamage, K.G.; Rozovics, J.M.; Narayanan, K.; Semler, B.L.; Makino, S. SARS Coronavirus Nsp1 Protein Induces Template-Dependent Endonucleolytic Cleavage of MRNAs: Viral MRNAs Are Resistant to Nsp1-Induced RNA Cleavage. PLoS Pathog. 2011, 7, e1002433. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Wang, G.; Yang, Y.; Shi, J.; Fang, L.; Li, F.; Xiao, S.; Fu, Z.F.; Peng, G. A Conserved Region of Nonstructural Protein 1 from Alphacoronaviruses Inhibits Host Gene Expression and Is Critical for Viral Virulence. J. Biol. Chem. 2019, 294, 13606–13618. [Google Scholar] [CrossRef] [PubMed]
- Züst, R.; Cervantes-Barragán, L.; Kuri, T.; Blakqori, G.; Weber, F.; Ludewig, B.; Thiel, V. Coronavirus Non-Structural Protein 1 Is a Major Pathogenicity Factor: Implications for the Rational Design of Coronavirus Vaccines. PLoS Pathog. 2007, 3, e109. [Google Scholar] [CrossRef]
- Lei, L.; Ying, S.; Baojun, L.; Yi, Y.; Xiang, H.; Wenli, S.; Zounan, S.; Deyin, G.; Qingyu, Z.; Jingmei, L.; et al. Attenuation of Mouse Hepatitis Virus by Deletion of the LLRKxGxKG Region of Nsp1. PLoS ONE 2013, 8, e61166. [Google Scholar] [CrossRef]
- Jimenez-Guardeño, J.M.; Regla-Nava, J.A.; Nieto-Torres, J.L.; DeDiego, M.L.; Castaño-Rodriguez, C.; Fernandez-Delgado, R.; Perlman, S.; Enjuanes, L. Identification of the Mechanisms Causing Reversion to Virulence in an Attenuated SARS-CoV for the Design of a Genetically Stable Vaccine. PLoS Pathog. 2015, 11, e1005215. [Google Scholar] [CrossRef]
- Fisher, T.; Gluck, A.; Narayanan, K.; Kuroda, M.; Nachshon, A.; Hsu, J.C.; Halfmann, P.J.; Yahalom-Ronen, Y.; Tamir, H.; Finkel, Y.; et al. Parsing the Role of NSP1 in SARS-CoV-2 Infection. Cell Rep. 2022, 39, 110954. [Google Scholar] [CrossRef]
- Lokugamage, K.G.; Narayanan, K.; Huang, C.; Makino, S. Severe Acute Respiratory Syndrome Coronavirus Protein Nsp1 Is a Novel Eukaryotic Translation Inhibitor That Represses Multiple Steps of Translation Initiation. J. Virol. 2012, 86, 13598–13608. [Google Scholar] [CrossRef]
- Kamitani, W.; Huang, C.; Narayanan, K.; Lokugamage, K.G.; Makino, S. A Two-Pronged Strategy to Suppress Host Protein Synthesis by SARS Coronavirus Nsp1 Protein. Nat. Struct. Mol. Biol. 2009, 16, 1134–1140. [Google Scholar] [CrossRef]
- Thoms, M.; Buschauer, R.; Ameismeier, M.; Koepke, L.; Denk, T.; Hirschenberger, M.; Kratzat, H.; Hayn, M.; MacKens-Kiani, T.; Cheng, J.; et al. Structural Basis for Translational Shutdown and Immune Evasion by the Nsp1 Protein of SARS-CoV-2. Science (1979) 2020, 369, 1249–1255. [Google Scholar] [CrossRef]
- Kamitani, W.; Narayanan, K.; Huang, C.; Lokugamage, K.; Ikegami, T.; Ito, N.; Kubo, H.; Makino, S. Severe Acute Respiratory Syndrome Coronavirus Nsp1 Protein Suppresses Host Gene Expression by Promoting Host MRNA Degradation. Proc. Natl. Acad. Sci. USA 2006, 103, 12885–12890. [Google Scholar] [CrossRef]
- Huang, C.; Lokugamage, K.G.; Rozovics, J.M.; Narayanan, K.; Semler, B.L.; Makino, S. Alphacoronavirus Transmissible Gastroenteritis Virus Nsp1 Protein Suppresses Protein Translation in Mammalian Cells and in Cell-Free HeLa Cell Extracts but Not in Rabbit Reticulocyte Lysate. J. Virol. 2011, 85, 638–643. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Ye, G.; Deng, F.; Wang, G.; Cui, M.; Fang, L.; Xiao, S.; Fu, Z.F.; Peng, G. Structural Basis for the Inhibition of Host Gene Expression by Porcine Epidemic Diarrhea Virus Nsp1. J. Virol. 2018, 92, e01896-17. [Google Scholar] [CrossRef] [PubMed]
- Huynh, H.T.; Robitaille, G.; Turner, J.D. Establishment of Bovine Mammary Epithelial Cells (MAC-T): An in Vitro Model for Bovine Lactation. Exp. Cell Res. 1991, 197, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Ueno, S.; Amarbayasgalan, S.; Sugiura, Y.; Takahashi, T.; Shimizu, K.; Nakagawa, K.; Kawabata-Iwakawa, R.; Kamitani, W. Eight-Amino-Acid Sequence at the N-Terminus of SARS-CoV-2 Nsp1 Is Involved in Stabilizing Viral Genome Replication. Virology 2024, 595, 110068. [Google Scholar] [CrossRef]
- Wang, G.H.; Liang, C.C.; Li, B.Z.; Du, X.Z.; Zhang, W.Z.; Cheng, G.; Zan, L. Sen Screening and Validation of Reference Genes for QRT-PCR of Bovine Skeletal Muscle-Derived Satellite Cells. Sci. Rep. 2022, 12, 5653. [Google Scholar] [CrossRef]
- Osorio, J.S.; Bionaz, M. Plasmid Transfection in Bovine Cells: Optimization Using a Realtime Monitoring of Green Fluorescent Protein and Effect on Gene Reporter Assay. Gene 2017, 626, 200–208. [Google Scholar] [CrossRef]
- Schmidt, E.K.; Clavarino, G.; Ceppi, M.; Pierre, P. SUnSET, a Nonradioactive Method to Monitor Protein Synthesis. Nat. Methods 2009, 6, 275–277. [Google Scholar] [CrossRef]
- Tohya, Y.; Narayanan, K.; Kamitani, W.; Huang, C.; Lokugamage, K.; Makino, S. Suppression of Host Gene Expression by Nsp1 Proteins of Group 2 Bat Coronaviruses. J. Virol. 2009, 83, 5282–5288. [Google Scholar] [CrossRef]
- Banerjee, A.K.; Blanco, M.R.; Bruce, E.A.; Honson, D.D.; Chen, L.M.; Chow, A.; Bhat, P.; Ollikainen, N.; Quinodoz, S.A.; Loney, C.; et al. SARS-CoV-2 Disrupts Splicing, Translation, and Protein Trafficking to Suppress Host Defenses. Cell 2020, 183, 1325–1339.e21. [Google Scholar] [CrossRef]
- Shen, Z.; Yang, Y.; Yang, S.; Zhang, G.; Xiao, S.; Fu, Z.F.; Peng, G. Structural and Biological Basis of Alphacoronavirus Nsp1 Associated with Host Proliferation and Immune Evasion. Viruses 2020, 12, 812. [Google Scholar] [CrossRef]
- Zhang, Q.; Shi, K.; Yoo, D. Suppression of Type I Interferon Production by Porcine Epidemic Diarrhea Virus and Degradation of CREB-Binding Protein by Nsp1. Virology 2016, 489, 252–268. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Ma, J.; Yoo, D. Inhibition of NF-ΚB Activity by the Porcine Epidemic Diarrhea Virus Nonstructural Protein 1 for Innate Immune Evasion. Virology 2017, 510, 111–126. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shi, H.; Rigolet, P.; Wu, N.; Zhu, L.; Xi, X.G.; Vabret, A.; Wang, X.; Wang, T. Nsp1 Proteins of Group I and SARS Coronaviruses Share Structural and Functional Similarities. Infect. Genet. Evol. 2010, 10, 919–924. [Google Scholar] [CrossRef] [PubMed]
- Maurina, S.F.; O’Sullivan, J.P.; Sharma, G.; Pineda Rodriguez, D.C.; MacFadden, A.; Cendali, F.; Henen, M.A.; Vögeli, B.; Kieft, J.S.; Glasgow, A.; et al. An Evolutionarily Conserved Strategy for Ribosome Binding and Host Translation Inhibition by β-Coronavirus Non-Structural Protein 1. J. Mol. Biol. 2023, 435, 168259. [Google Scholar] [CrossRef]
- Diefenbacher, M.V.; Baric, T.J.; Martinez, D.R.; Baric, R.S.; Catanzaro, N.J.; Sheahan, T.P. A Nano-Luciferase Expressing Human Coronavirus OC43 for Countermeasure Development. Virus Res. 2024, 339, 199286. [Google Scholar] [CrossRef]
- Tardivat, Y.; Sosnowski, P.; Tidu, A.; Westhof, E.; Eriani, G.; Martin, F. SARS-CoV-2 NSP1 Induces MRNA Cleavages on the Ribosome. Nucleic Acids Res. 2023, 51, 8677–8690. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ohkami, T.; Kitashin, I.; Kawashima, R.; Yoshida, A.; Saito, T.; Takashima, Y.; Kamitani, W.; Nakagawa, K. Characterization of Gene Expression Suppression by Bovine Coronavirus Non-Structural Protein 1. Viruses 2025, 17, 978. https://doi.org/10.3390/v17070978
Ohkami T, Kitashin I, Kawashima R, Yoshida A, Saito T, Takashima Y, Kamitani W, Nakagawa K. Characterization of Gene Expression Suppression by Bovine Coronavirus Non-Structural Protein 1. Viruses. 2025; 17(7):978. https://doi.org/10.3390/v17070978
Chicago/Turabian StyleOhkami, Takehiro, Ichika Kitashin, Riko Kawashima, Aimi Yoshida, Taizo Saito, Yasuhiro Takashima, Wataru Kamitani, and Keisuke Nakagawa. 2025. "Characterization of Gene Expression Suppression by Bovine Coronavirus Non-Structural Protein 1" Viruses 17, no. 7: 978. https://doi.org/10.3390/v17070978
APA StyleOhkami, T., Kitashin, I., Kawashima, R., Yoshida, A., Saito, T., Takashima, Y., Kamitani, W., & Nakagawa, K. (2025). Characterization of Gene Expression Suppression by Bovine Coronavirus Non-Structural Protein 1. Viruses, 17(7), 978. https://doi.org/10.3390/v17070978





