Mosquito Species Diversity and Circulation of Mosquito-Borne Viruses in Selected Provinces of Central Vietnam
Abstract
1. Introduction
2. Materials and Methods
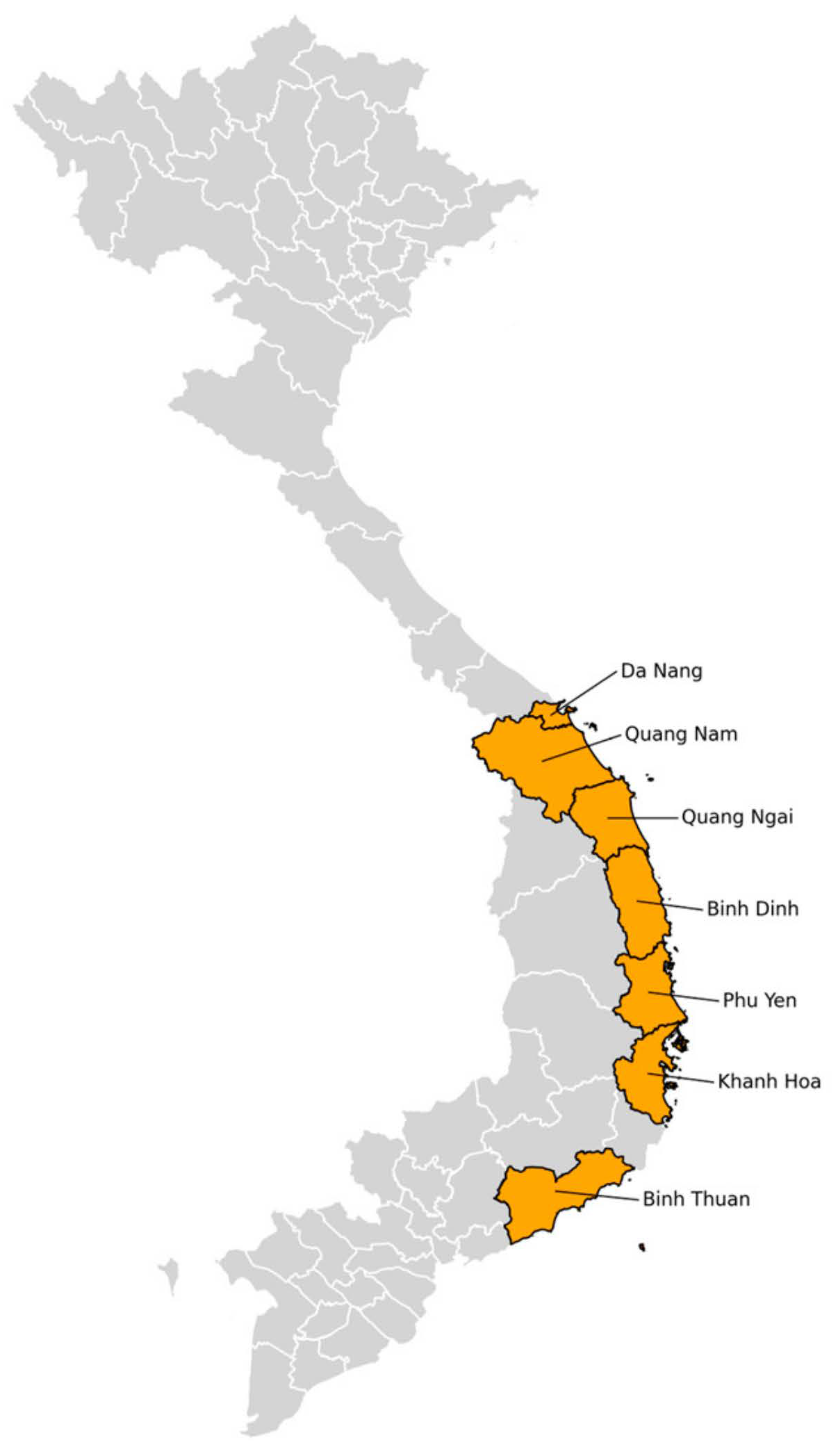
2.1. Field Sampling and Species Determination
2.2. Preparation of Mosquito Samples and RNA Extraction
2.3. Molecular Studies Based on Real-Time PCR
2.4. Spatial Data and Visualization
2.5. Data Aggregation and Plotting
3. Results
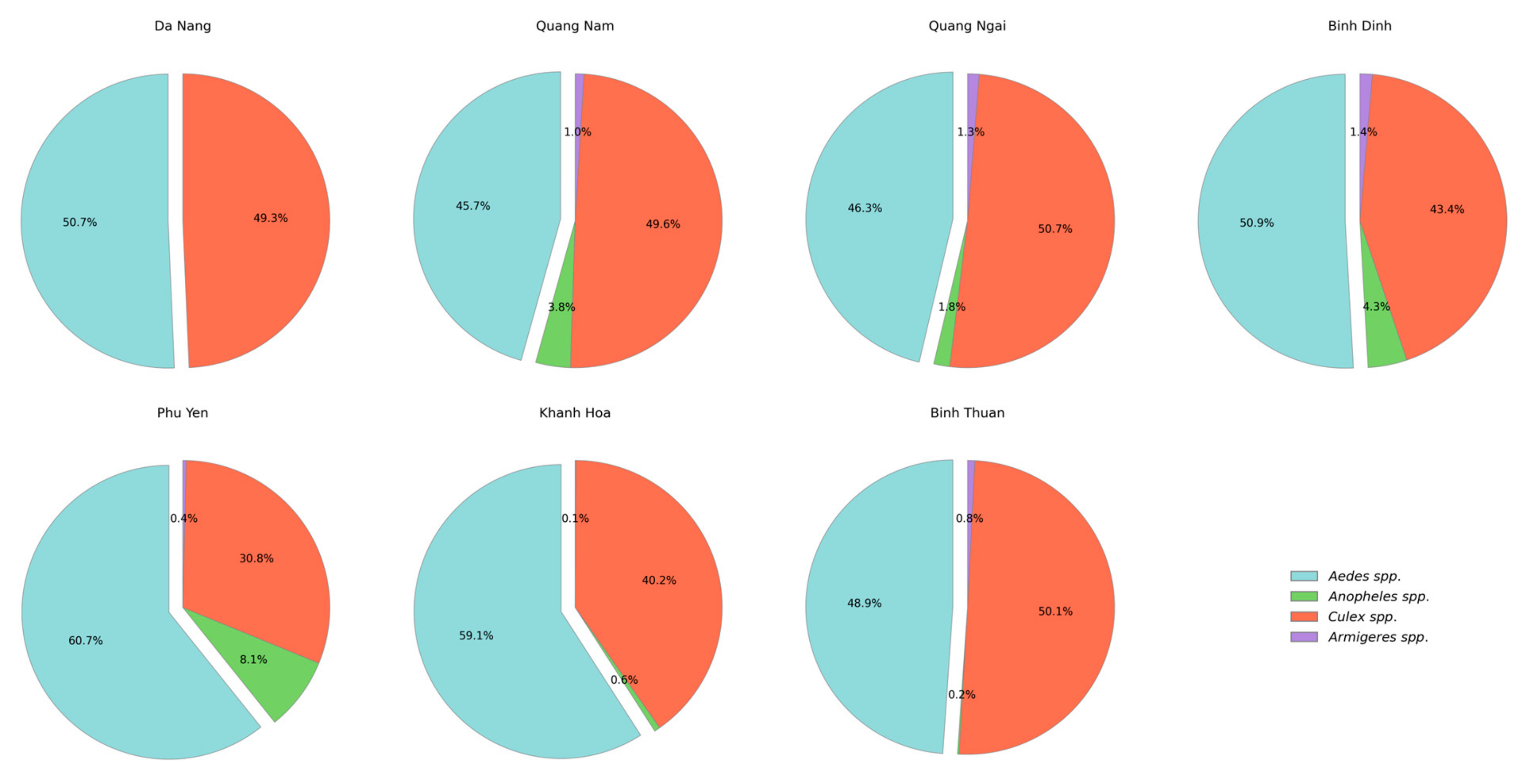
3.1. Species Composition Collected in the Central Region, 2023
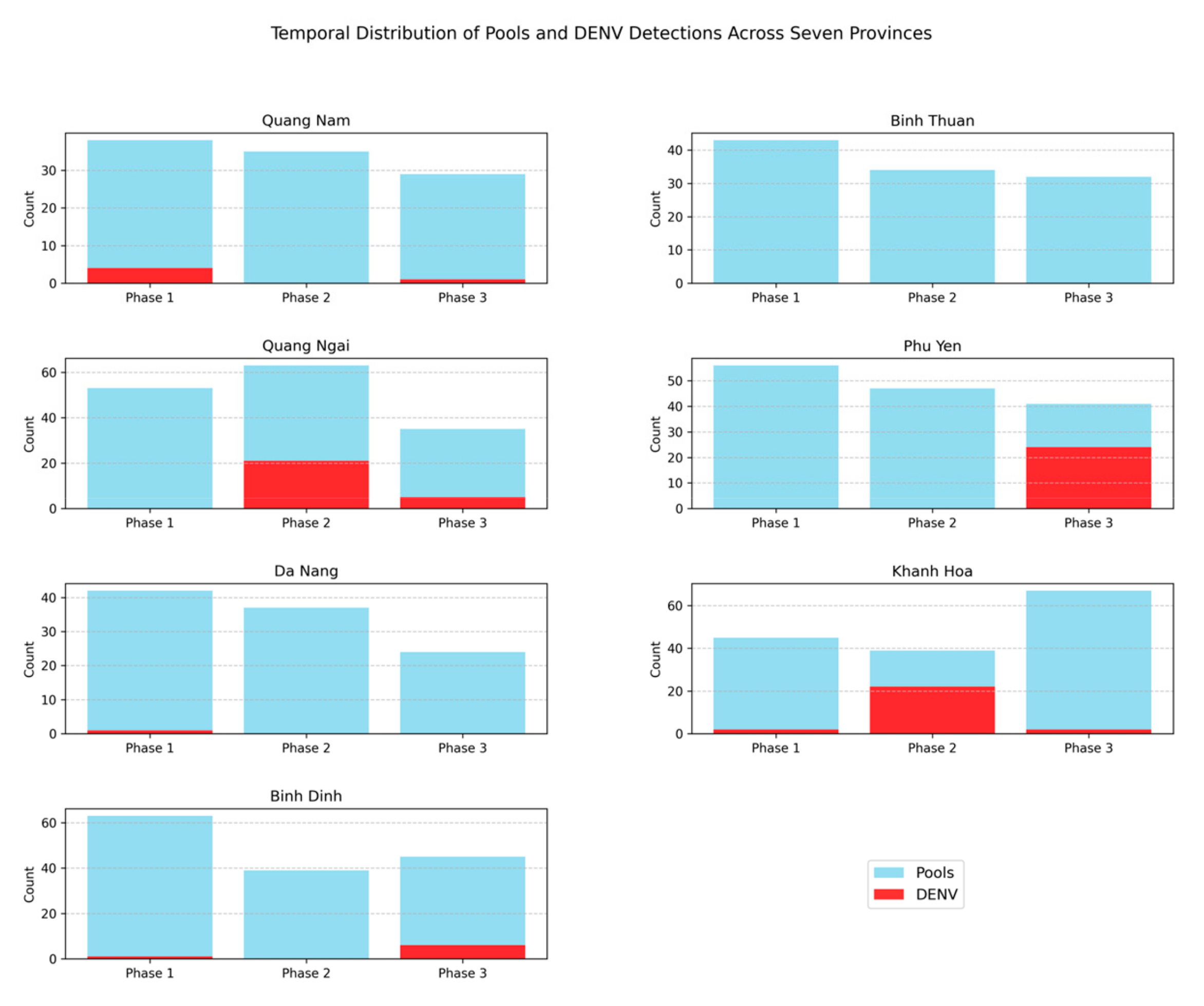
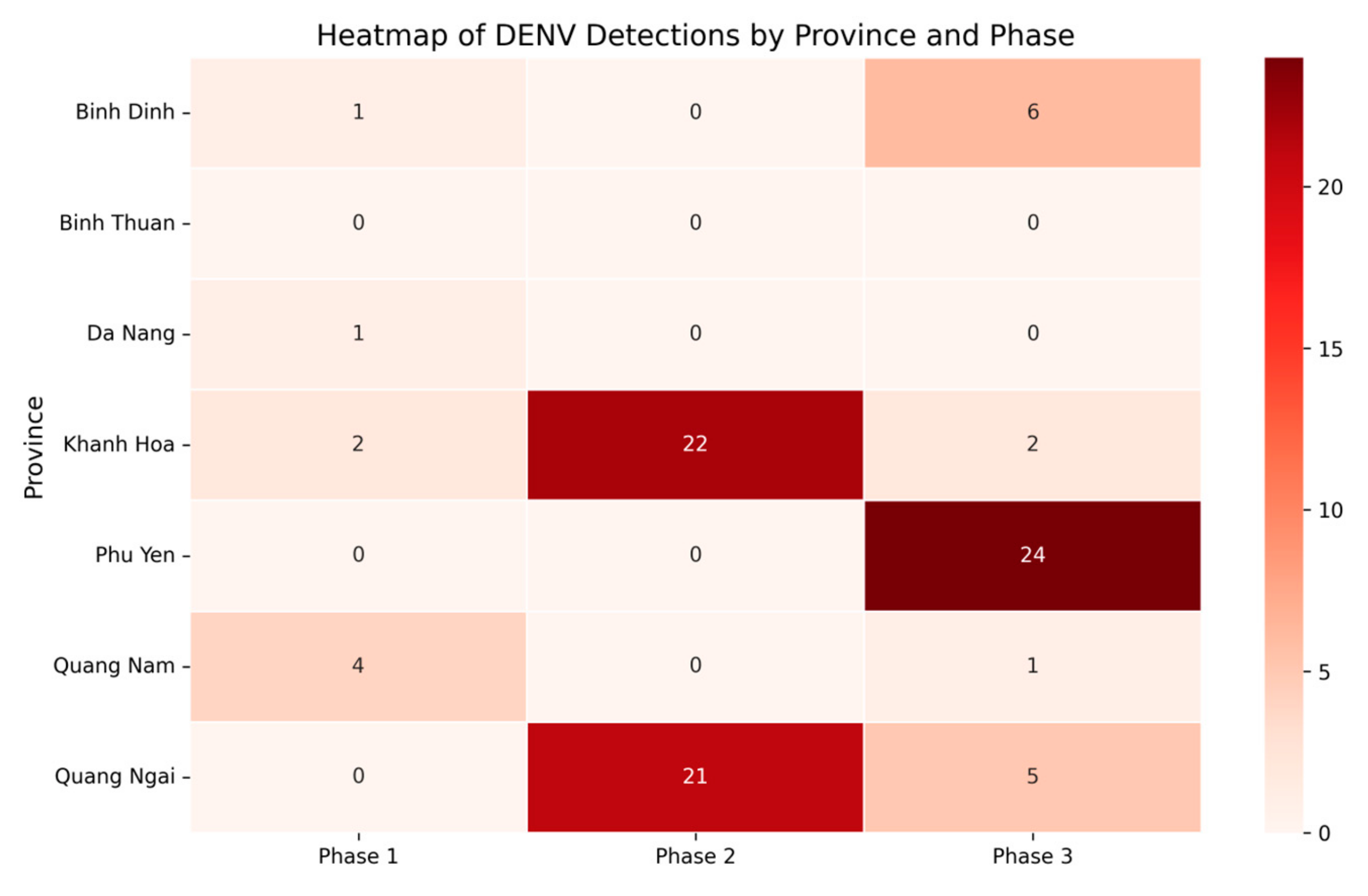
3.2. Prevalence of DENV, ZIKV, and CHIKV in Aedes Mosquitoes
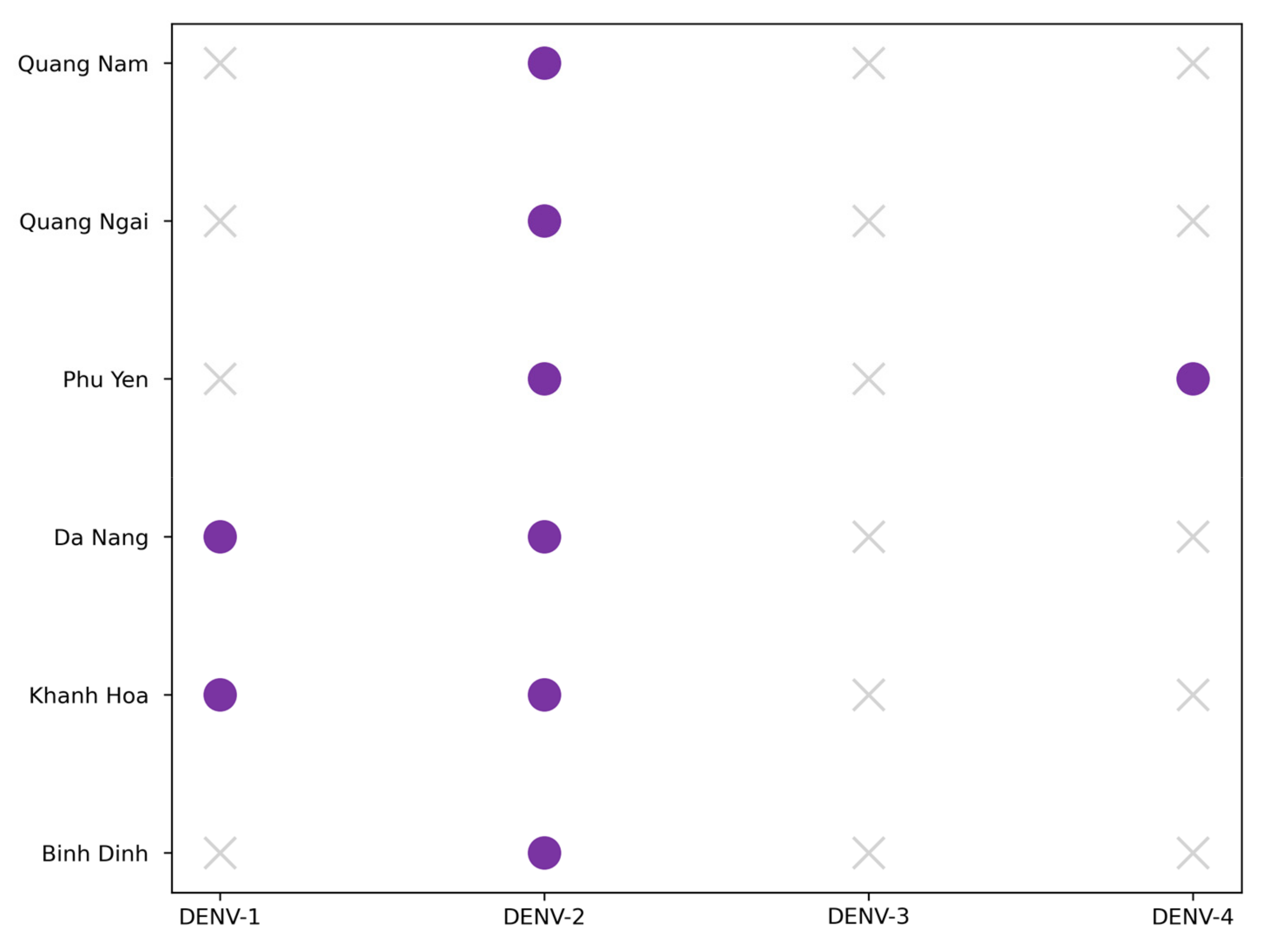
3.3. Dengue Typing
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Weaver, S.C.; Reisen, W.K. Present and future arboviral threats. Antivir. Res. 2010, 85, 328–345. [Google Scholar] [CrossRef] [PubMed]
- Huynh, L.N.; Tran, L.B.; Nguyen, H.S.; Ho, V.H.; Parola, P.; Nguyen, X.Q. Mosquitoes and Mosquito-Borne Diseases in Vietnam. Insects 2022, 13, 1076. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, S.; Gething, P.W.; Brady, O.J.; Messina, J.P.; Farlow, A.W.; Moyes, C.L.; Drake, J.M.; Brownstein, J.S.; Hoen, A.G.; Sankoh, O.; et al. The global distribution and burden of dengue. Nature 2013, 496, 504–507. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Dengue—Global Situation; World Health Organization: Geneva, Switzerland, 2023; Available online: https://www.who.int/emergencies/disease-outbreak-news/item/2023-DON498 (accessed on 15 February 2025).
- Kraemer, M.U.; Sinka, M.E.; Duda, K.A.; Mylne, A.Q.; Shearer, F.M.; Barker, C.M.; Moore, C.G.; Carvalho, R.G.; Coelho, G.E.; Van Bortel, W.; et al. The global distribution of the arbovirus vectors Aedes aegypti and Ae. albopictus. Elife 2015, 4, e08347. [Google Scholar] [CrossRef]
- Main, L.; Sparrow, S.; Weisheimer, A.; Smith, D. Skilful probabilistic medium-range precipitation and temperature forecasts over Vietnam for the development of a future dengue early warning system. Meteorol. Appl. 2024, 31, 105–122. [Google Scholar] [CrossRef]
- Nguyen, L.T.; Le, H.X.; Nguyen, D.T.; Ho, H.Q.; Chuang, T.W. Impact of Climate Variability and Abundance of Mosquitoes on Dengue Transmission in Central Vietnam. Int. J. Environ. Res. Public Health 2020, 17, 2453. [Google Scholar] [CrossRef]
- World Health Organization. Dengue and Severe Dengue; World Health Organization: Geneva, Switzerland, 2023; Available online: https://www.who.int/news-room/fact-sheets/detail/dengue-and-severe-dengue (accessed on 20 February 2025).
- Quang Tran, K.; Hung Pham, V.; Thi Ngoc Tran, T.; Thao Mai, C.; Kieu Anh Pham, T.; Hoang Ngo, T.; Bui Thai Nguyen, H.; Manh Nguyen, C.; Van Duong, H.; Minh Nguyen, P. Dengue virus serotypes and related factors in children with dengue hemorrhagic fever in Southern Vietnam. J. Infect. Dev. Ctries. 2024, 18, 495–500. [Google Scholar] [CrossRef]
- Yamanaka, A.; Imad, H.A.; Phumratanaprapin, W.; Phadungsombat, J.; Konishi, E.; Shioda, T. Antibody-dependent enhancement representing in vitro infective progeny virus titer correlates with the viremia level in dengue patients. Sci. Rep. 2021, 11, 12354. [Google Scholar] [CrossRef]
- Rob, M.A.; Hossain, M.; Sattar, M.A.; Ahmed, I.U.; Chowdhury, A.F.M.N.; Mehedi, H.M.H.; Mohammed, N.; Maruf Ul Quader, M.; Hossain, M.Z.; Rahman, M.; et al. Circulating dengue virus serotypes, demographics, and epidemiology in the 2023 dengue outbreak in Chittagong, Bangladesh. Eur. J. Microbiol. Immunol. 2024, 14, 272–279. [Google Scholar] [CrossRef]
- Musso, D.; Gubler, D.J. Zika Virus. Clin. Microbiol. Rev. 2016, 29, 487–524. [Google Scholar] [CrossRef]
- Musso, D.; Stramer, S.L. AABB Transfusion-Transmitted Diseases Committee; Busch MP.; International Society of Blood Transfusion Working Party on Transfusion-Transmitted Infectious Diseases. Zika virus: A new challenge for blood transfusion. Lancet 2016, 387, 1993–1994. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.; Ortiz, K.; Ansari, A.; Gershwin, M.E. The Zika outbreak of the 21st century. J. Autoimmun. 2016, 68, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Petersen, E.E.; Staples, J.E.; Meaney-Delman, D.; Fischer, M.; Ellington, S.R.; Callaghan, W.M.; Jamieson, D.J. Interim Guidelines for Pregnant Women During a Zika Virus Outbreak--United States. MMWR Morb. Mortal. Wkly. Rep. 2016, 65, 30–33. [Google Scholar] [CrossRef] [PubMed]
- Mlakar, J.; Korva, M.; Tul, N.; Popović, M.; Poljšak-Prijatelj, M.; Mraz, J.; Kolenc, M.; Resman Rus, K.; Vesnaver Vipotnik, T.; Fabjan Vodušek, V.; et al. Zika Virus Associated with Microcephaly. N. Engl. J. Med. 2016, 374, 951–958. [Google Scholar] [CrossRef]
- Desai, S.K.; Hartman, S.D.; Jayarajan, S.; Liu, S.; Gallicano, G.I. Zika Virus (ZIKV): A review of proposed mechanisms of transmission and associated congenital abnormalities. Am. J. Stem Cells 2017, 6, 13–22. [Google Scholar] [PubMed] [PubMed Central]
- Pialoux, G.; Gaüzère, B.A.; Jauréguiberry, S.; Strobel, M. Chikungunya, an epidemic arbovirosis. Lancet Infect. Dis. 2007, 7, 319–327. [Google Scholar] [CrossRef]
- Deller, J.J., Jr.; Russell, P.K. An analysis of fevers of unknown origin in American soldiers in Vietnam. Ann. Intern. Med. 1967, 66, 1129–1143. [Google Scholar] [CrossRef]
- Schuffenecker, I.; Iteman, I.; Michault, A.; Murri, S.; Frangeul, L.; Vaney, M.C.; Lavenir, R.; Pardigon, N.; Reynes, J.M.; Pettinelli, F.; et al. Genome microevolution of chikungunya viruses causing the Indian Ocean outbreak. PLoS Med. 2006, 3, e263. [Google Scholar] [CrossRef]
- Chusri, S.; Siripaitoon, P.; Silpapojakul, K.; Hortiwakul, T.; Charernmak, B.; Chinnawirotpisan, P.; Nisalak, A.; Thaisomboonsuk, B.; Klungthong, C.; Gibbons, R.V.; et al. Kinetics of chikungunya infections during an outbreak in Southern Thailand, 2008–2009. Am. J. Trop. Med. Hyg. 2014, 90, 410–417. [Google Scholar] [CrossRef]
- Kaur, P.; Ponniah, M.; Murhekar, M.V.; Ramachandran, V.; Ramachandran, R.; Raju, H.K.; Perumal, V.; Mishra, A.C.; Gupte, M.D. Chikungunya outbreak, South India, 2006. Emerg. Infect. Dis. 2008, 14, 1623–1625. [Google Scholar] [CrossRef]
- Zanotto, P.M.A.; Leite, L.C.C. The Challenges Imposed by Dengue, Zika, and Chikungunya to Brazil. Front. Immunol. 2018, 9, 1964. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Health Vietnam. Decision No. 3711/q-byt Guidelines for Surveillance and Control of Dengue Fever; issued by Ministry of Health Vietnam on 19 September 2014; Ministry of Health Vietnam: Ba Đình district, Hanoi, 2014. [Google Scholar]
- Chester, J.S.; Harold, G.S. Illustrated Key to Mosquitoes of Vietnam: By Chester J. Stojanovich and Harold George Scott; Communicable Disease Center: Atlanta, GA, USA, 1966. [Google Scholar]
- GADM Database of Global Administrative Areas, Version 4.0; GADM: Dorchester, MA, USA, 2023. Available online: https://gadm.org (accessed on 3 April 2025).
- Santiago, G.A.; Vergne, E.; Quiles, Y.; Cosme, J.; Vazquez, J.; Medina, J.F.; Medina, F.; Colón, C.; Margolis, H.; Muñoz-Jordán, J.L. Analytical and clinical performance of the CDC real time RT-PCR assay for detection and typing of dengue virus. PLoS Negl. Trop. Dis. 2013, 7, e2311, Erratum in PLoS Negl. Trop. Dis. 2013, 7, 3. https://doi.org/10.1371/annotation/ae27d48b-025f-47ce-8427-4af59f821ad7. [Google Scholar] [CrossRef]
- Waggoner, J.J.; Ballesteros, G.; Gresh, L.; Mohamed-Hadley, A.; Tellez, Y.; Sahoo, M.K.; Abeynayake, J.; Balmaseda, A.; Harris, E.; Pinsky, B.A. Clinical evaluation of a single-reaction real-time RT-PCR for pan-dengue and chikungunya virus detection. J. Clin. Virol. 2016, 78, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Van den Bossche, J.; Jordahl, K.; Fleischmann, M.; Richards, M.; McBride, J.; Wasserman, J.; Garcia Badaracco, A.; Snow, A.D.; Ward, B.; Tratner, J.; et al. GeoPandas: Python Tools for Geographic Data, Version 1.0.1; Zenodo: Geneva, Switzerland, 2024. [CrossRef]
- Hunter, J.D. Matplotlib: A 2D Graphics Environment. Comput. Sci. Eng. 2007, 9, 90–95. [Google Scholar] [CrossRef]
- McKinney, W. Data Structures for Statistical Computing in Python. In Proceedings of the 9th Python in Science Conference, Austin, TX, USA, 28 June–3 July 2010; pp. 56–61. [Google Scholar] [CrossRef]
- Waskom, M.L. Seaborn: Statistical Data Visualization. J. Open Source Softw. 2021, 6, 3021. [Google Scholar] [CrossRef]
- World Health Organization. Global Strategy for Dengue Prevention and Control, 2012–2020; World Health Organization: Geneva, Switzerland, 2020; Available online: https://www.who.int/publications/i/item/9789241504034 (accessed on 10 March 2025).
- Nguyen-Tien, T.; Lundkvist, Å.; Lindahl, J. Urban transmission of mosquito-borne flaviviruses—A review of the risk for humans in Vietnam. Infect. Ecol. Epidemiol. 2019, 9, 1660129. [Google Scholar] [CrossRef]
- Tolle, M.A. Mosquito-borne diseases. Curr. Probl. Pediatr. Adolesc. Health Care 2009, 39, 97–140. [Google Scholar] [CrossRef]
- Huber, K.; Le Loan, L.; Hoang, T.H.; Tien, T.K.; Rodhain, F.; Failloux, A.B. Aedes aegypti in south Vietnam: Ecology, genetic structure, vectorial competence and resistance to insecticides. Southeast Asian J. Trop. Med. Public Health 2003, 34, 81–86. [Google Scholar] [PubMed]
- Noorbakhsh, F.; Abdolmohammadi, K.; Fatahi, Y.; Dalili, H.; Rasoolinejad, M.; Rezaei, F.; Salehi-Vaziri, M.; Shafiei-Jandaghi, N.Z.; Gooshki, E.S.; Zaim, M.; et al. Zika Virus Infection, Basic and Clinical Aspects: A Review Article. Iran. J. Public Health 2019, 48, 20–31. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nguyen, T.V.; Ngwe Tun, M.M.; Cao, M.T.; Dao, H.M.; Luong, C.Q.; Huynh, T.K.L.; Nguyen, T.T.T.; Hoang, T.N.D.; Morita, K.; Le, T.Q.M. Serological and Molecular Epidemiology of Chikungunya Virus Infection in Vietnam, 2017–2019. Viruses 2023, 15, 2065. [Google Scholar] [CrossRef]
- Kawada, H.; Higa, Y.; Nguyen, Y.T.; Tran, S.H.; Nguyen, H.T.; Takagi, M. Nationwide investigation of the pyrethroid susceptibility of mosquito larvae collected from used tires in Vietnam. PLoS Negl. Trop. Dis. 2009, 3, e391. [Google Scholar] [CrossRef] [PubMed]
- Garros, C.; Van Nguyen, C.; Trung, H.D.; Van Bortel, W.; Coosemans, M.; Manguin, S. Distribution of Anopheles in Vietnam, with particular attention to malaria vectors of the Anopheles minimus complex. Malar. J. 2008, 7, 11. [Google Scholar] [CrossRef] [PubMed]
- Pham, H.V.; Doan, H.T.; Phan, T.T.; Minh, N.N. Ecological factors associated with dengue fever in a Central Highlands province, Vietnam. BMC Infect. Dis. 2011, 11, 172. [Google Scholar] [CrossRef]
- Phuong, H.L.; de Vries, P.J.; Nga, T.T.; Giao, P.T.; Hung le, Q.; Binh, T.Q.; Nam, N.V.; Nagelkerke, N.; Kager, P.A. Dengue as a cause of acute undifferentiated fever in Vietnam. BMC Infect. Dis. 2006, 6, 123. [Google Scholar] [CrossRef]
- Pham, H.T.; Pham, T.N.T.; Tran, N.H.T.; Ha, Q.D.; Tran, D.K.; Nguyen, N.H.D.; Pham, V.H.; Pham, S.T. Dengue Hemorrhagic Fever in Quang Nam Province (Vietnam) from 2020 to 2022-A Study on Serotypes Distribution and Immunology Factors. Diagnostics 2024, 14, 1772. [Google Scholar] [CrossRef]
- Guzman, M.G.; Alvarez, M.; Halstead, S.B. Secondary infection as a risk factor for dengue hemorrhagic fever/dengue shock syndrome: An historical perspective and role of antibody-dependent enhancement of infection. Arch. Virol. 2013, 158, 1445–1459. [Google Scholar] [CrossRef]
- Katzelnick, L.C.; Gresh, L.; Halloran, M.E.; Mercado, J.C.; Kuan, G.; Gordon, A.; Balmaseda, A.; Harris, E. Antibody-dependent enhancement of severe dengue disease in humans. Science 2017, 358, 929–932. [Google Scholar] [CrossRef]
- Halstead, S.B. Dengue Antibody-Dependent Enhancement: Knowns and Unknowns. Microbiol Spectr. 2014, 2. [Google Scholar] [CrossRef]
- Quyen, N.T.H.; Kien, D.T.H.; Rabaa, M.; Tuan, N.M.; Vi, T.T.; Van Tan, L.; Hung, N.T.; Tuan, H.M.; Van Tram, T.; Le Da Ha, N.; et al. Chikungunya and Zika Virus Cases Detected against a Backdrop of Endemic Dengue Transmission in Vietnam. Am. J. Trop. Med. Hyg. 2017, 97, 146–150. [Google Scholar] [CrossRef]
- Nguyen, C.T.; Moi, M.L.; Le, T.Q.M.; Nguyen, T.T.T.; Vu, T.B.H.; Nguyen, H.T.; Pham, T.T.H.; Le, T.H.T.; Nguyen, L.M.H.; Phu Ly, M.H.; et al. Prevalence of Zika virus neutralizing antibodies in healthy adults in Vietnam during and after the Zika virus epidemic season: A longitudinal population-based survey. BMC Infect. Dis. 2020, 20, 332. [Google Scholar] [CrossRef]
- Quyen, D.L.; Thanh Le, N.; Van Anh, C.T.; Nguyen, N.B.; Hoang, D.V.; Montgomery, J.L.; Kutcher, S.C.; Hoang Le, N.; Hien, N.T.; Hue Kien, D.T.; et al. Epidemiological, Serological, and Virological Features of Dengue in Nha Trang City, Vietnam. Am. J. Trop. Med. Hyg. 2018, 98, 402–409. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popova, M.R.; Sharova, A.A.; Gladkikh, A.S.; Arbuzova, T.V.; Klyuchnikova, E.O.; Sbarzaglia, V.A.; Tsyganova, N.A.; Naydenov, D.D.; Gritseva, A.S.; Ramsay, E.S.; et al. Mosquito Species Diversity and Circulation of Mosquito-Borne Viruses in Selected Provinces of Central Vietnam. Viruses 2025, 17, 905. https://doi.org/10.3390/v17070905
Popova MR, Sharova AA, Gladkikh AS, Arbuzova TV, Klyuchnikova EO, Sbarzaglia VA, Tsyganova NA, Naydenov DD, Gritseva AS, Ramsay ES, et al. Mosquito Species Diversity and Circulation of Mosquito-Borne Viruses in Selected Provinces of Central Vietnam. Viruses. 2025; 17(7):905. https://doi.org/10.3390/v17070905
Chicago/Turabian StylePopova, Margarita R., Alena A. Sharova, Anna S. Gladkikh, Tatiana V. Arbuzova, Ekaterina O. Klyuchnikova, Valeriya A. Sbarzaglia, Nadezhda A. Tsyganova, Dmitry D. Naydenov, Anastasia S. Gritseva, Edward S. Ramsay, and et al. 2025. "Mosquito Species Diversity and Circulation of Mosquito-Borne Viruses in Selected Provinces of Central Vietnam" Viruses 17, no. 7: 905. https://doi.org/10.3390/v17070905
APA StylePopova, M. R., Sharova, A. A., Gladkikh, A. S., Arbuzova, T. V., Klyuchnikova, E. O., Sbarzaglia, V. A., Tsyganova, N. A., Naydenov, D. D., Gritseva, A. S., Ramsay, E. S., Baimova, R. R., Karmokov, I. A., Riabiko, E. G., Tokarevich, N. K., Dong, N. T., Phu, B. T., Phan, V. T., Hung, D. T., Thuc, T. C., & Dedkov, V. G. (2025). Mosquito Species Diversity and Circulation of Mosquito-Borne Viruses in Selected Provinces of Central Vietnam. Viruses, 17(7), 905. https://doi.org/10.3390/v17070905






