Worsening of Controlled Attenuation Parameter and Metabolic Profile After HCV Cure in People with HIV as a Sign of Steatosis
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Individual Characteristics
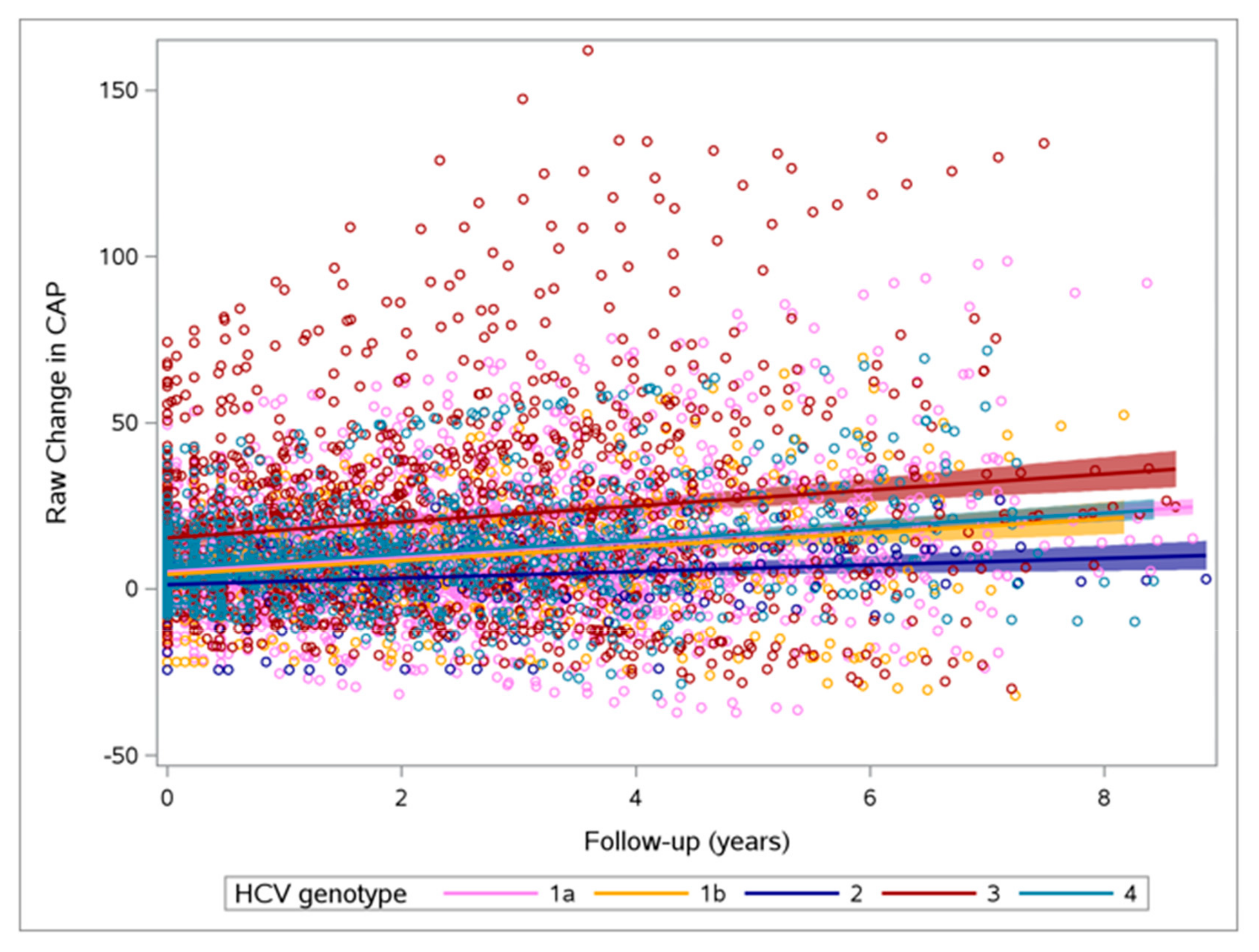
3.2. Changes in CAP and Other Parameters
3.3. Changes in Metabolic and Anthropometric Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- World Health Organizations (WHO) Fact Sheet-Hepatitis, C. Available online: https://www.who.int/news-room/fact-sheets/detail/hepatitis-c (accessed on 9 April 2024).
- Thrift, A.P.; El-Serag, H.B.; Kanwal, F. Global epidemiology and burden of HCV infection and HCV-related disease. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.C.; Cheng, P.N.; Kao, J.H. Systematic review: Chronic viral hepatitis and metabolic derangement. Aliment. Pharmacol. Ther. 2020, 51, 216–230. [Google Scholar] [CrossRef] [PubMed]
- Targett-Adams, P.; Boulant, S.; Douglas, M.W.; McLauchlan, J. Lipid metabolism and HCV infection. Viruses 2010, 2, 1195–1217. [Google Scholar] [CrossRef] [PubMed]
- Albecka, A.; Belouzard, S.; de Beeck, A.O.; Descamps, V.; Goueslain, L.; Bertrand-Michel, J.; Tercé, F.; Duverlie, G.; Rouillé, Y.; Dubuisson, J. Role of low-density lipoprotein receptor in the hepatitis C virus life cycle. Hepatology 2012, 55, 998–1007. [Google Scholar] [CrossRef]
- Sagnelli, E.; Sagnelli, C.; Russo, A.; Pisaturo, M.; Camaioni, C.; Astorri, R.; Coppola, N. Impact of DAA-Based Regimens on HCV-Related Extra-Hepatic Damage: A Narrative Review. Adv. Exp. Med. Biol. 2021, 1323, 115–147. [Google Scholar] [CrossRef]
- Chang, M.L. Metabolic alterations and hepatitis C: From bench to bedside. World J. Gastroenterol. 2016, 22, 1461–1476. [Google Scholar] [CrossRef]
- Syed, G.H.; Amako, Y.; Siddiqui, A. Hepatitis C virus hijacks host lipid metabolism. Trends Endocrinol. Metab. 2010, 21, 33–40. [Google Scholar] [CrossRef] [PubMed Central]
- Clément, S.; Pascarella, S.; Negro, F. Hepatitis C virus infection: Molecular pathways to steatosis, insulin resistance and oxidative stress. Viruses 2009, 1, 126–143. [Google Scholar] [CrossRef] [PubMed Central]
- Hashimoto, S.; Yatsuhashi, H.; Abiru, S.; Yamasaki, K.; Komori, A.; Nagaoka, S.; Saeki, A.; Uchida, S.; Bekki, S.; Kugiyama, Y.; et al. Rapid Increase in Serum Low-Density Lipoprotein Cholesterol Concentration during Hepatitis C Interferon-Free Treatment. PLoS ONE 2016, 11, e0163644. [Google Scholar] [CrossRef]
- Gobran, S.T.; Ancuta, P.; Shoukry, N.H. A Tale of Two Viruses: Immunological Insights Into HCV/HIV Coinfection. Front Immunol. 2021, 12, 726419. [Google Scholar] [CrossRef]
- Hernandez, M.D.; Sherman, K.E. HIV/hepatitis C coinfection natural history and disease progression. Curr. Opin. HIV AIDS. 2011, 6, 478–482. [Google Scholar] [CrossRef] [PubMed]
- Graf, C.; Welzel, T.; Bogdanou, D.; Vermehren, J.; Beckel, A.; Bojunga, J.; Friedrich-Rust, M.; Dietz, J.; Kubesch, A.; Mondorf, A.; et al. Hepatitis C Clearance by Direct-Acting Antivirals Impacts Glucose and Lipid Homeostasis. J. Clin. Med. 2020, 9, 2702. [Google Scholar] [CrossRef] [PubMed]
- Duarte, M.; Tien, P.; Ma, Y.; Noworolski, S.M.; Korn, N.; Price, J.C. Controlled attenuation parameter accurately detects liver steatosis in people with HIV. AIDS 2022, 36, 2147–2152. [Google Scholar] [CrossRef]
- Chromy, D.; Mandorfer, M.; Bucsics, T.; Schwabl, P.; Bauer, D.; Scheiner, B.; Schmidbauer, C.; Lang, G.F.; Szekeres, T.; Ferenci, P.; et al. Prevalence and Predictors of Hepatic Steatosis in Patients with HIV/HCV Coinfection and the Impact of HCV Eradication. AIDS Patient Care STDS 2019, 33, 197–206. [Google Scholar] [CrossRef]
- Chan, A.; Patel, K.; Naggie, S. Genotype 3 Infection: The Last Stand of Hepatitis C Virus. Drugs 2017, 77, 131–144. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Siphepho, P.Y.; Liu, Y.T.; Shabangu, C.S.; Huang, J.F.; Huang, C.F.; Yeh, M.L.; Yu, M.L.; Wang, S.C. The Impact of Steatosis on Chronic Hepatitis C Progression and Response to Antiviral Treatments. Biomedicines 2021, 9, 1491. [Google Scholar] [CrossRef]
- Sheridan, D.A.; Shawa, I.T.; Thomas, E.L.; Felmlee, D.J.; Bridge, S.H.; Neely, D.; Cobbold, J.F.; Holmes, E.; Bassendine, M.F.; Taylor-Robinson, S.D. Infection with the hepatitis C virus causes viral genotype-specific differences in cholesterol metabolism and hepatic steatosis. Sci Rep. 2022, 12, 1–11. [Google Scholar] [CrossRef]
- Gitto, S.; Cicero, A.F.G.; Loggi, E.; Giovannini, M.; Conti, F.; Grandini, E.; Guarneri, V.; Scuteri, A.; Vitale, G.; Cursaro, C.; et al. Worsening of Serum Lipid Profile after Direct Acting Antiviral Treatment. Ann. Hepatol. 2018, 17, 64–75. [Google Scholar] [CrossRef]
- Spaziante, M.; Taliani, G.; Marchetti, G.; Tavelli, A.; Lichtner, M.; Cingolani, A.; Cicalini, S.; Biliotti, E.; Girardi, E.; Antinori, A.; et al. Impact of HCV Eradication on Lipid Metabolism in HIV/HCV Coinfected Patients: Data from ICONA and HepaICONA Foundation Cohort Study. Viruses 2021, 13, 1402. [Google Scholar] [CrossRef]
- Chuaypen, N.; Siripongsakun, S.; Hiranrat, P.; Tanpowpong, N.; Avihingsanon, A.; Tangkijvanich, P. Improvement of liver fibrosis, but not steatosis, after HCV eradication as assessment by MR-based imaging: Role of metabolic derangement and host genetic variants. PLoS ONE 2022, 17, e0269641. [Google Scholar] [CrossRef]
- Patel, A.; Harrison, S.A. Hepatitis C virus infection and nonalcoholic steatohepatitis. Gastroenterol Hepatol 2012, 8, 305–312. [Google Scholar]
- Istvan, E.S.; Deisenhofer, J. Structural mechanism for statin inhibition of HMG-CoA reductase. Science 2001, 292, 1160–1164. [Google Scholar] [CrossRef]
- Villani, R.; Di Cosimo, F.; Romano, A.D.; Sangineto, M.; Serviddio, G. Serum lipid profile in HCV patients treated with direct-acting antivirals: A systematic review and meta-analysis. Sci. Rep. 2021, 11, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kramer, A.S.; Lazzarotto, A.R.; Sprinz, E.; Manfroi, W.C. Metabolic abnormalities, antiretroviral therapy and cardiovascular disease in elderly patients with HIV. Arq Bras Cardiol. 2009, 93, 561–568. [Google Scholar] [CrossRef]
- Jain, R.G.; Furfine, E.S.; Pedneault, L.; White, A.J.; Lenhard, J.M. Metabolic complications associated with antiretroviral therapy. Antivir. Res. 2001, 51, 151–177. [Google Scholar] [CrossRef]
- Chaudhury, C.S.; Sheehan, J.; Chairez, C.; Akoth, E.; Gross, C.; Silk, R.; Kattakuzhy, S.; Rosenthal, E.; Kottilil, S.; Masur, H.; et al. No Improvement in Hemoglobin A1c Following Hepatitis C Viral Clearance in Patients With and Without HIV. J. Infect. Dis. 2017, 217, 47–50. [Google Scholar] [CrossRef]
- Reid, M.; Ma, Y.; Scherzer, R.; Price, J.C.; French, A.L.; Plankey, M.W.; Grunfeld, C.; Tien, P.C. Higher CD163 levels are associated with insulin resistance in hepatitis C virus-infected and HIV-infected adults. AIDS 2017, 31, 385–393. [Google Scholar] [CrossRef]
- Brenchley, J.M.; Price, D.A.; Schacker, T.W.; Asher, T.E.; Silvestri, G.; Rao, S.; Kazzaz, Z.; Bornstein, E.; Lambotte, O.; Altmann, D.; et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat. Med. 2006, 12, 1365–1371. [Google Scholar] [CrossRef]
| 1a (N = 279) | 1b (N = 45) | 2 (N = 23) | 3 (N = 131) | 4 (N = 120) | p Value | |
|---|---|---|---|---|---|---|
| Male sex | 229 (82.1%) | 29 (64.4%) | 20 (87.0%) | 84 (64.1%) | 79 (65.8%) | <0.001 |
| Age | 52.0 [46.2; 55.6] | 52.2 [50.1; 55.0] | 50.8 [44.7; 54.1] | 53.7 [51.1; 56.6] | 54.2 [51.2; 56.3] | <0.001 |
| Risk Factors: | <0.001 | |||||
| Hetero | 18 (6.45%) | 5 (11.1%) | 1 (4.35%) | 14 (10.7%) | 15 (12.5%) | |
| MSM | 104 (37.3%) | 7 (15.6%) | 15 (65.2%) | 10 (7.63%) | 14 (11.7%) | |
| Other | 40 (14.3%) | 8 (17.8%) | 3 (13.0%) | 24 (18.3%) | 18 (15.0%) | |
| Ex-drug Users | 117 (41.9%) | 25 (55.6%) | 4 (17.4%) | 83 (63.4%) | 73 (60.8%) | |
| Years (Yrs) of ART | 17.3 [7.63; 22.6] | 18.4 [14.4; 22.8] | 10.5 [6.31; 21.0] | 19.1 [14.5; 22.3] | 19.4 [13.5; 22.4] | 0.047 |
| Yrs with HIV diagnosis | 24.3 [11.9; 30.8] | 28.5 [19.5; 30.8] | 17.6 [10.7; 25.7] | 28.1 [21.0; 31.3] | 28.0 [20.7; 31.4] | <0.001 |
| Height | 175 [169; 179] | 170 [164; 176] | 174 [169; 180] | 170 [166; 175] | 172 [167; 178] | 0.001 |
| Weight | 69.0 [61.0; 77.0] | 70.0 [60.0; 76.0] | 75.0 [66.5; 80.5] | 68.0 [58.0; 79.0] | 69.5 [58.0; 77.0] | 0.379 |
| Glucose | 85.0 [79.0; 93.0] | 84.5 [78.2; 95.8] | 80.0 [72.0; 87.5] | 83.0 [77.0; 93.0] | 86.0 [77.0; 94.0] | 0.216 |
| Total Cholesterol | 168 [140; 190] | 167 [149; 194] | 191 [152; 210] | 157 [119; 186] | 165 [145; 186] | 0.008 |
| Triglycerides | 98.5 [78.8; 133] | 110 [90.0; 141] | 109 [89.0; 137] | 97.0 [77.0; 130] | 113 [86.0; 150] | 0.093 |
| HDL | 47.0 [38.0; 58.0] | 44.0 [34.5; 59.8] | 49.0 [45.5; 59.5] | 46.0 [38.0; 60.0] | 47.5 [40.0; 66.0] | 0.333 |
| LDL | 106 [83.0; 126] | 111 [84.5; 130] | 137 [94.0; 149] | 92.0 [64.0; 122] | 97.5 [78.2; 121] | 0.013 |
| ALT | 62.0 [42.0; 97.0] | 64.0 [39.0; 106] | 35.0 [29.5; 62.0] | 91.0 [49.0; 159] | 49.0 [34.0; 74.5] | <0.001 |
| AST | 49.0 [35.0; 72.0] | 46.0 [35.0; 91.0] | 31.0 [25.5; 42.0] | 67.0 [45.0; 111] | 41.0 [30.0; 65.0] | <0.001 |
| GGT | 55.5 [31.0; 108] | 67.0 [28.0; 144] | 38.0 [23.0; 47.8] | 54.0 [32.0; 92.0] | 63.0 [34.0; 127] | 0.017 |
| PLT | 202 [158; 250] | 203 [140; 236] | 234 [170; 281] | 174 [136; 221] | 188 [155; 220] | 0.001 |
| Albumin | 41.9 [39.8; 44.1] | 41.3 [38.2; 43.6] | 44.0 [42.1; 45.1] | 41.8 [39.6; 43.6] | 42.8 [40.8; 45.4] | 0.049 |
| HIV RNA ≥ 50 cps/mL | 24 (8.70%) | 6 (13.6%) | 0 (0.00%) | 6 (4.69%) | 10 (8.40%) | 0.197 |
| CD4T | 643 [470; 839] | 624 [365; 878] | 679 [542; 836] | 670 [468; 868] | 712 [518; 878] | 0.696 |
| CD8T | 789 [580; 1109] | 832 [577; 1045] | 843 [636; 1096] | 800 [573; 1121] | 835 [574; 1108] | 0.986 |
| FIB_4 | 1.54 [1.06; 2.37] | 1.64 [1.24; 3.19] | 1.23 [0.88; 1.69] | 2.24 [1.42; 4.16] | 1.71 [1.25; 2.35] | <0.001 |
| LSM | 7.00 [5.00; 11.0] | 8.00 [5.00; 14.0] | 6.00 [4.00; 7.00] | 8.00 [6.00; 12.2] | 7.00 [5.00; 11.2] | 0.017 |
| CAP | 212 [192; 240] | 226 [202; 250] | 211 [188; 245] | 231 [203; 275] | 218 [196; 261] | 0.009 |
| Use of fibrates | 4 (1.43%) | 2 (4.44%) | 2 (8.70%) | 1 (0.76%) | 3 (2.50%) | 0.072 |
| Use of statins | 19 (6.81%) | 4 (8.89%) | 2 (8.70%) | 3 (2.29%) | 14 (11.7%) | 0.036 |
| ART regimens: | 0.006 | |||||
| INSTI | 153 (55.6%) | 22 (50.0%) | 8 (34.8%) | 55 (42.0%) | 52 (43.7%) | |
| INSTI and NNRTI | 15 (5.45%) | 5 (11.4%) | 1 (4.35%) | 5 (3.82%) | 10 (8.40%) | |
| INSTI and PI | 14 (5.09%) | 4 (9.09%) | 1 (4.35%) | 10 (7.63%) | 10 (8.40%) | |
| NNRTI | 41 (14.9%) | 2 (4.55%) | 7 (30.4%) | 12 (9.16%) | 16 (13.4%) | |
| Other | 3 (1.09%) | 0 (0.00%) | 1 (4.35%) | 1 (0.76%) | 3 (2.52%) | |
| PI | 49 (17.8%) | 11 (25.0%) | 5 (21.7%) | 48 (36.6%) | 28 (23.5%) | |
| TAF-based regimen | 76 (27.6%) | 10 (22.7%) | 6 (26.1%) | 25 (19.1%) | 30 (25.2%) | 0.461 |
| Parameter | Overall | GT1a | GT1b | GT2 | GT | GT4 | p Value Difference Between Groups |
|---|---|---|---|---|---|---|---|
| Total Cholesterol | 1.09 (0.56; 1.47) p value = <0.0001 N = 540 | 0.79 (0.12; 1.46) p value = 0.0203 N = 253 | 1.46 (−0.13; 3.05) p value = 0.0728 N = 40 | 0.59 (−1.67; 2.86) p value = 0.6085 N = 21 | 1.9 (0.96; 2.84) p value = <0.0001 N = 120 | 0.75 (−0.3; 1.81) p value = 0.1606 N = 106 | 0.3533 |
| LDL | −0.26 (−1.34; 0.82) p value = 0.6360 N = 376 | −1.5 (−3.04; 0.03) p value = 0.0547 N = 181 | 0.36 (−3.35; 4.06) p value = 0.8503 N = 25 | −0.55 (−5.6; 4.51) p value = 0.8322 N = 15 | 2.84 (0.59; 5.1) p value = 0.0136 N = 86 | 0.47 (−2.16; 3.1) p value = 0.7252 N = 69 | 0.0408 |
| HDL | 1.00 (0.39; 1.61) p value = 0.0013 N = 397 | 0.51 (−0.38; 1.39) p value = 0.2603 N = 189 | 2.02 (0.03; 4) p value = 0.0467 N = 28 | 1.75 (−1.22; 4.73) p value = 0.2482 N = 15 | 1.9 (0.64; 3.16) p value = 0.0031 N = 90 | 0.53 (−0.89; 1.95) p value = 0.4650 N = 75 | 0.3076 |
| Triglycerides | 3.05 (1.87; 4.24) p value = <0.0001 N = 537 | 2.79 (1.03; 4.54) p value = 0.0019 N = 250 | 4.87 (0.91; 8.84) p value = 0.0160 N = 40 | 2.07 (−3.59; 7.73) p value = 0.4731 N = 21 | 1.88 (−0.51; 4.28) p value = 0.1238 N = 120 | 4.41 (1.63; 7.19) p value = 0.0019 N = 106 | 0.5852 |
| Weight | 0.79 (0.59; 0.99)p value = <0.0001 N = 545 | 1.01 (0.72; 1.29) p value = <0.0001 N = 255 | 0.73 (−0.01; 1.47) p value = 0.0529 N = 39 | 0.09 (−0.83; 1.02) p value = 0.8414 N = 22 | 0.43 (0.02; 0.84) p value = 0.0406 N = 118 | 0.88 (0.42; 1.33) p value = 0.0002 N = 111 | 0.1137 |
| Stiffness | −4.28 (−7.16; −1.41) p value = 0.0036 N = 287 | −6.91 (−11.19; −2.62) p value = 0.0017 N = 126 | −2.01 (−13.22; 9.19) p value = 0.7236 N = 19 | −2.25 (−15.49; 11) p value = 0.7384 N = 10 | 0.67 (−5.09; 6.42) p value = 0.8197 N = 70 | −5.5 (−11.95; 0.95) p value = 0.0940 N = 62 | 0.3203 |
| BMI | 0.82 (0.61; 1.03) p value = <0.0001 N = 541 | 1.02 (0.71; 1.32) p value = <0.0001 N = 252 | 0.72 (−0.07; 1.5) p value = 0.0739 N = 39 | 0.09 (−0.89; 1.08) p value = 0.8518 N = 22 | 0.49 (0.05; 0.93) p value = 0.0280 N = 117 | 0.97 (0.48; 1.45) p value = <0.0001 N = 111 | 0.1816 |
| Glucose | 3.40 (3.04; 3.76) p value = <0.0001 N = 574 | 3.36 (2.83; 3.88) p value = <0.0001 N = 268 | 4.14 (2.92; 5.36) p value = <0.0001 N = 42 | 1.38 (0.35; 3.10) p value = 0.1181 N = 23 | 3.43 (2.69; 4.18) p value = <0.0001 N = 126 | 3.62 (2.77; 4.48) p value = <0.0001 N = 115 | 0.1381 |
| CAP | 2.86 (1.76; 3.96) p value = <0.0001 N = 225 | 2.15 (0.52; 3.77) p value = 0.0101 N = 100 | 5.27 (0.63; 9.92) p value = 0.0265 N = 12 | 1.49 (−3.54; 6.52) p value = 0.5576 N = 7 | 1.84 (−0.33; 4) p value = 0.0956 N = 53 | 5.22 (2.85; 7.59) p value = <0.0001 N = 53 | 0.1509 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siribelli, A.; Diotallevi, S.; Galli, L.; Muccini, C.; Morsica, G.; Lolatto, R.; Clemente, T.; Messina, E.; Bertoni, C.; Uberti-Foppa, C.; et al. Worsening of Controlled Attenuation Parameter and Metabolic Profile After HCV Cure in People with HIV as a Sign of Steatosis. Viruses 2025, 17, 906. https://doi.org/10.3390/v17070906
Siribelli A, Diotallevi S, Galli L, Muccini C, Morsica G, Lolatto R, Clemente T, Messina E, Bertoni C, Uberti-Foppa C, et al. Worsening of Controlled Attenuation Parameter and Metabolic Profile After HCV Cure in People with HIV as a Sign of Steatosis. Viruses. 2025; 17(7):906. https://doi.org/10.3390/v17070906
Chicago/Turabian StyleSiribelli, Alessia, Sara Diotallevi, Laura Galli, Camilla Muccini, Giulia Morsica, Riccardo Lolatto, Tommaso Clemente, Emanuela Messina, Costanza Bertoni, Caterina Uberti-Foppa, and et al. 2025. "Worsening of Controlled Attenuation Parameter and Metabolic Profile After HCV Cure in People with HIV as a Sign of Steatosis" Viruses 17, no. 7: 906. https://doi.org/10.3390/v17070906
APA StyleSiribelli, A., Diotallevi, S., Galli, L., Muccini, C., Morsica, G., Lolatto, R., Clemente, T., Messina, E., Bertoni, C., Uberti-Foppa, C., Castagna, A., & Hasson, H. (2025). Worsening of Controlled Attenuation Parameter and Metabolic Profile After HCV Cure in People with HIV as a Sign of Steatosis. Viruses, 17(7), 906. https://doi.org/10.3390/v17070906





