Avian Influenza Surveillance Among Migratory Birds, Poultry, and Humans Around Nansi Lake, China, 2021–2024
Abstract
1. Introduction
2. Materials and Methods
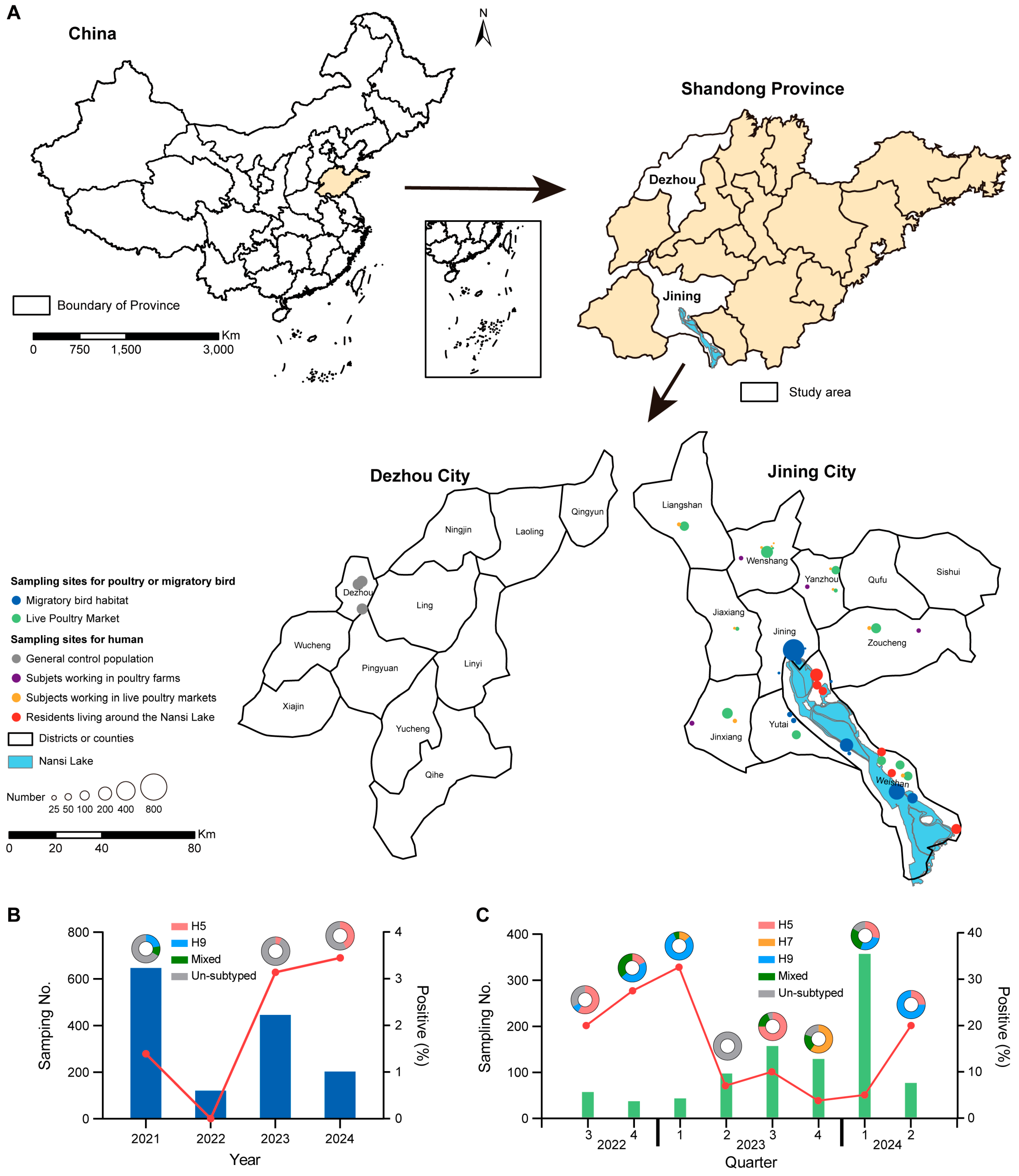
2.1. Study Objects, Sampling, and Data Collection
2.2. Molecular Detection, Isolation, and Sequencing
2.3. Phylogenetic and Reassortment Analysis
2.4. Hemagglutination Inhibition (HI) Assay
2.5. Microneutralization (MN) Assay
2.6. Statistical Analysis
2.7. Biosafety Statement and Ethical Considerations
3. Results
3.1. Prevalence and Distribution of AIVs Among Migratory Birds and Poultry
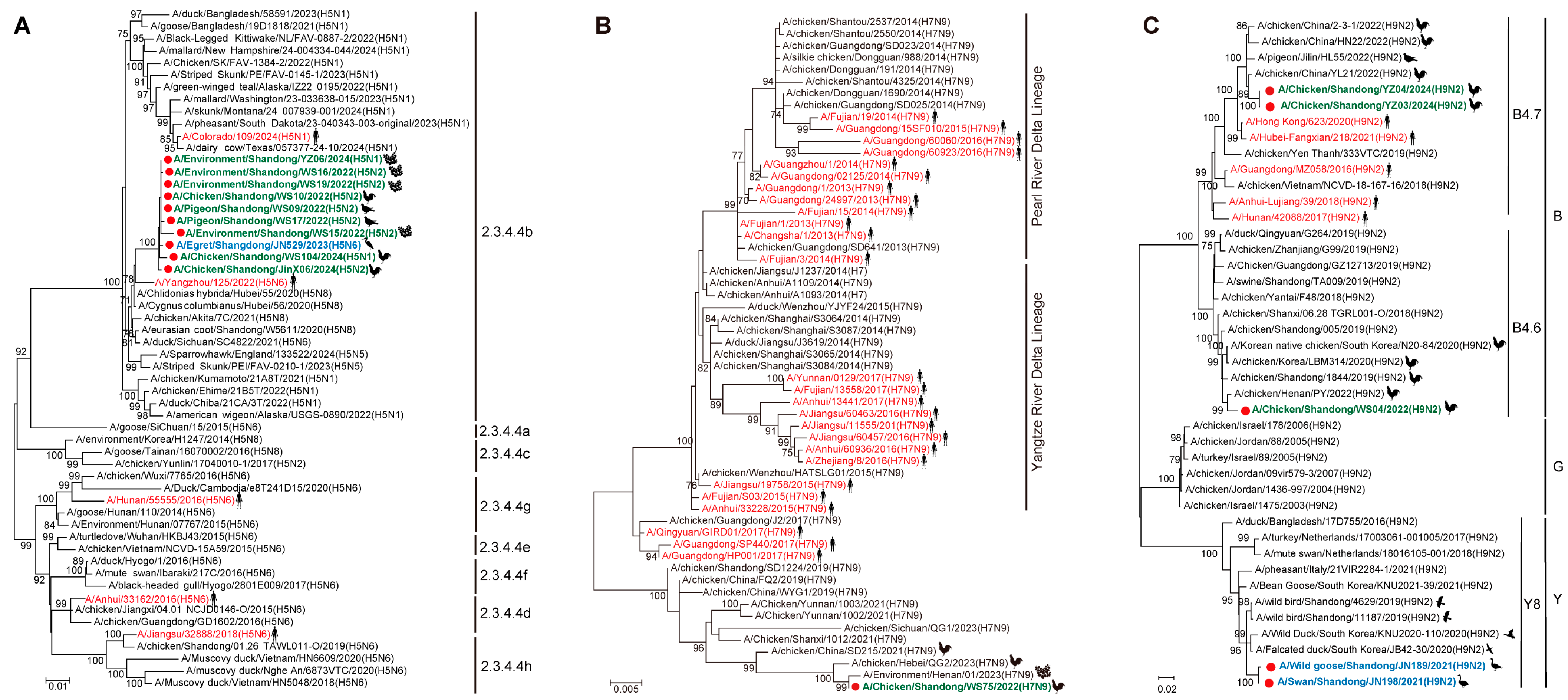
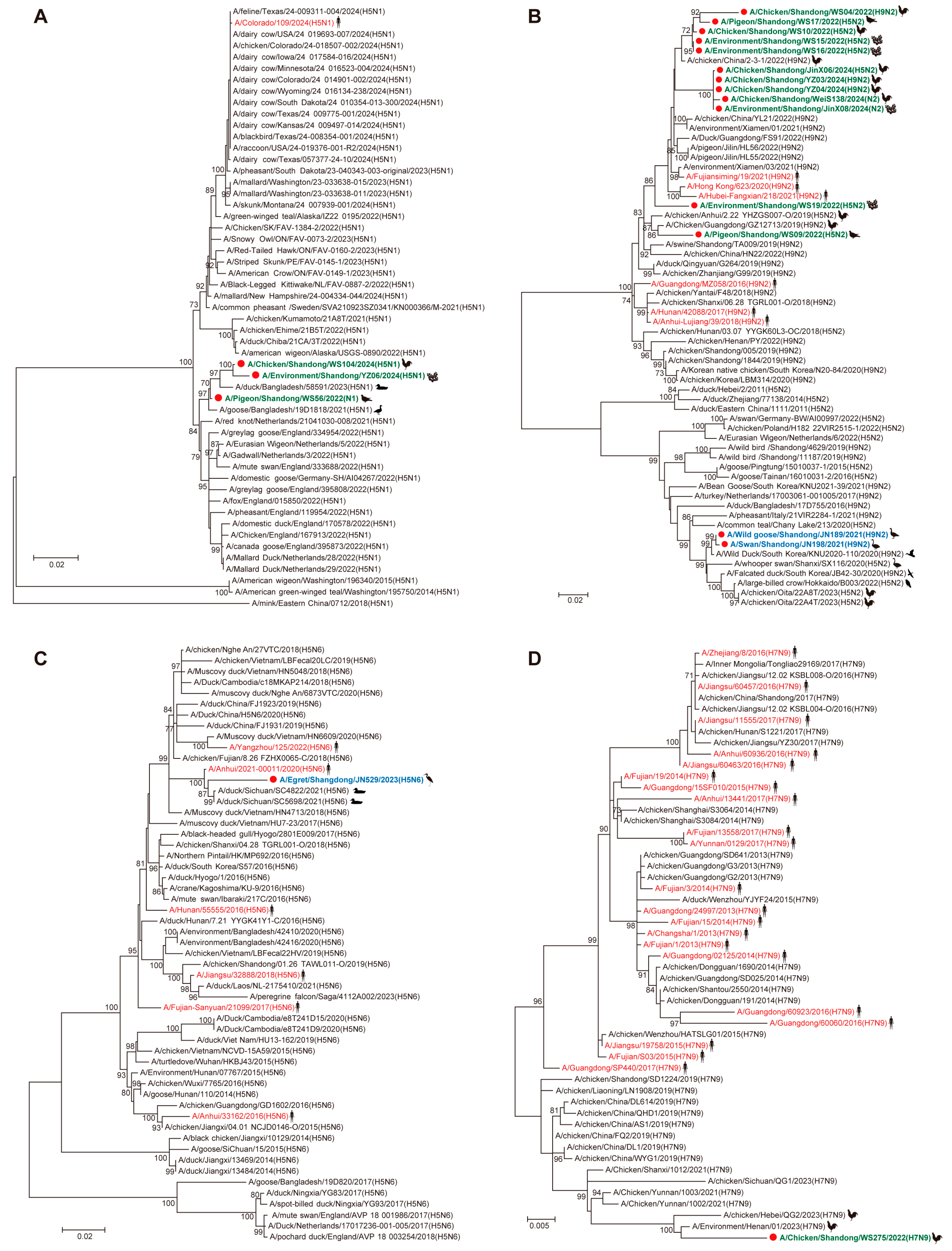
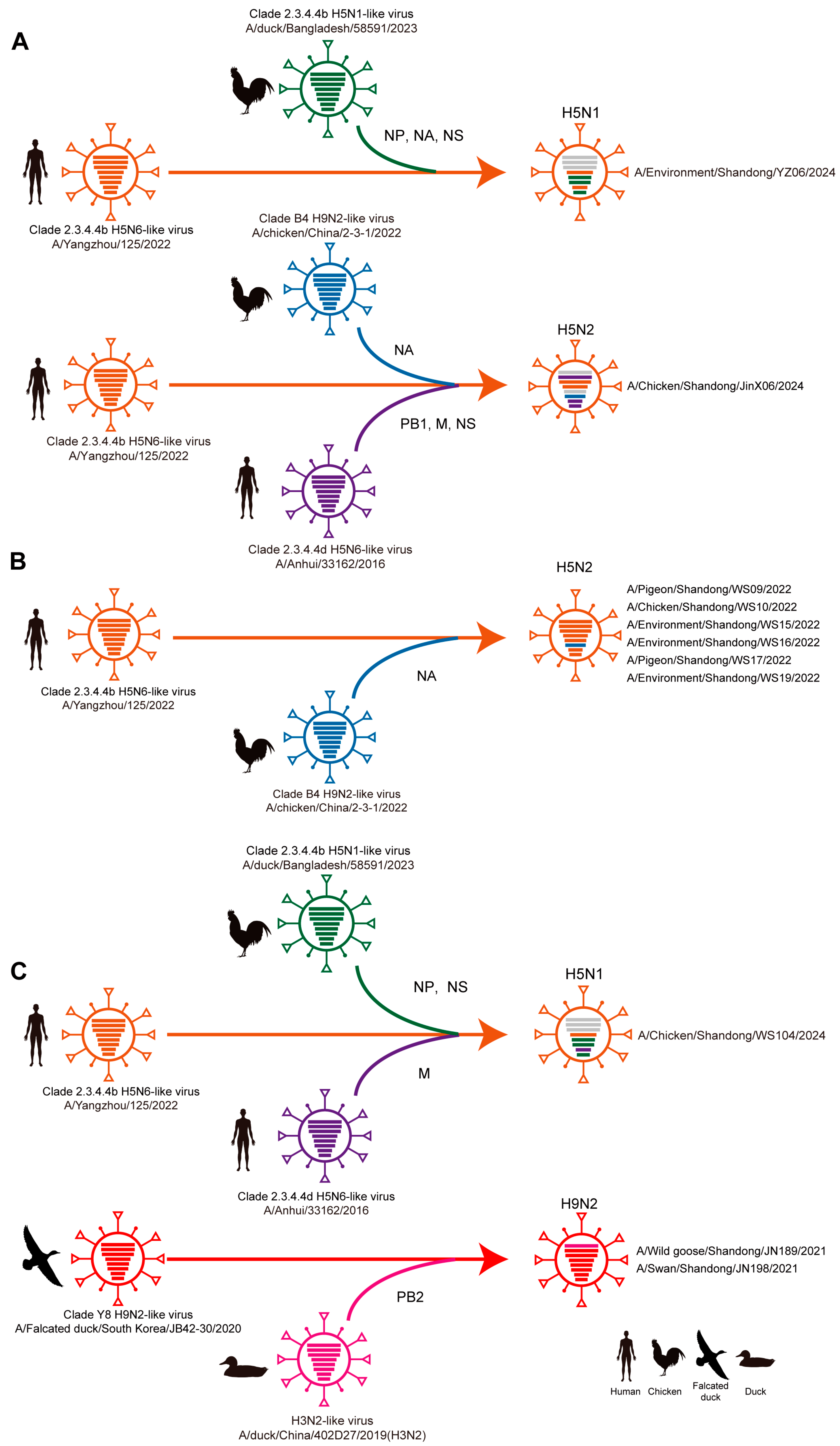
3.2. Phylogenetic and Reassortant Analysis of AIVs Among Migratory Birds and Poultry
3.3. Key Mutations of Identified AIVs Among Migratory Birds and Poultry
3.4. Serological Investigation Among Avian-Exposed and General Population
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Szablewski, C.M.; Iwamoto, C.; Olsen, S.J.; Greene, C.M.; Duca, L.M.; Davis, C.T.; Coggeshall, K.C.; Davis, W.W.; Emukule, G.O.; Gould, P.L.; et al. Reported Global Avian Influenza Detections Among Humans and Animals During 2013–2022: Comprehensive Review and Analysis of Available Surveillance Data. JMIR Public Health Surveill. 2023, 9, e46383. [Google Scholar] [CrossRef]
- Govindaraj, G.; Sridevi, R.; Nandakumar, S.N.; Vineet, R.; Rajeev, P.; Binu, M.K.; Balamurugan, V.; Rahman, H. Economic impacts of avian influenza outbreaks in Kerala, India. Transbound. Emerg. Dis. 2018, 65, e361–e372. [Google Scholar] [CrossRef]
- Kang, M.; Wang, L.F.; Sun, B.W.; Wan, W.B.; Ji, X.; Baele, G.; Bi, Y.H.; Suchard, M.A.; Lai, A.; Zhang, M.; et al. Zoonotic infections by avian influenza virus: Changing global epidemiology, investigation, and control. Lancet Infect. Dis. 2024, 24, e522–e531. [Google Scholar] [CrossRef] [PubMed]
- Fries, A.C.; Nolting, J.M.; Bowman, A.S.; Lin, X.; Halpin, R.A.; Wester, E.; Fedorova, N.; Stockwell, T.B.; Das, S.R.; Dugan, V.G.; et al. Spread and persistence of influenza A viruses in waterfowl hosts in the North American Mississippi migratory flyway. J. Virol. 2015, 89, 5371–5381. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Blagodatski, A.; Trutneva, K.; Glazova, O.; Mityaeva, O.; Shevkova, L.; Kegeles, E.; Onyanov, N.; Fede, K.; Maznina, A.; Khavina, E.; et al. Avian Influenza in Wild Birds and Poultry: Dissemination Pathways, Monitoring Methods, and Virus Ecology. Pathogens 2021, 10, 630. [Google Scholar] [CrossRef]
- Chen, H.; Smith, G.J.D.; Li, K.S.; Wang, J.; Fan, X.H.; Rayner, J.M.; Vijaykrishna, D.; Zhang, J.X.; Zhang, L.J.; Guo, C.T.; et al. Establishment of multiple sublineages of H5N1 influenza virus in Asia: Implications for pandemic control. Proc. Natl. Acad. Sci. USA 2006, 103, 2845–2850. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Smith, G.J.; Zhang, S.Y.; Qin, K.; Wang, J.; Li, K.S.; Webster, R.G.; Peiris, J.S.; Guan, Y. Avian flu: H5N1 virus outbreak in migratory waterfowl. Nature 2005, 436, 191–192. [Google Scholar] [CrossRef]
- Ip, H.S.; Dusek, R.J.; Bodenstein, B.; Torchetti, M.K.; DeBruyn, P.; Mansfield, K.G.; DeLiberto, T.; Sleeman, J.M. High Rates of Detection of Clade 2.3.4.4 Highly Pathogenic Avian Influenza H5 Viruses in Wild Birds in the Pacific Northwest During the Winter of 2014–15. Avian Dis. 2016, 60 (Suppl. S1), 354–358. [Google Scholar] [CrossRef][Green Version]
- Engelsma, M.; Heutink, R.; Harders, F.; Germeraad, E.A.; Beerens, N. Multiple Introductions of Reassorted Highly Pathogenic Avian Influenza H5Nx Viruses Clade 2.3.4.4b Causing Outbreaks in Wild Birds and Poultry in The Netherlands, 2020–2021. Microbiol. Spectr. 2022, 10, e0249921. [Google Scholar] [CrossRef]
- (OIE) WOAH. World Animal Health Information System (OIE-WAHIS). Available online: https://wahis.woah.org/ (accessed on 3 July 2024).
- Burrough, E.R.; Magstadt, D.R.; Petersen, B.; Timmermans, S.J.; Gauger, P.C.; Zhang, J.; Siepker, C.; Mainenti, M.; Li, G.; Thompson, A.C.; et al. Highly Pathogenic Avian Influenza A(H5N1) Clade 2.3.4.4b Virus Infection in Domestic Dairy Cattle and Cats, United States, 2024. Emerg. Infect. Dis. 2024, 30, 1335–1343. [Google Scholar] [CrossRef]
- Uyeki, T.M.; Milton, S.; Abdul Hamid, C.; Reinoso Webb, C.; Presley, S.M.; Shetty, V.; Rollo, S.N.; Martinez, D.L.; Rai, S.; Gonzales, E.R.; et al. Highly Pathogenic Avian Influenza A(H5N1) Virus Infection in a Dairy Farm Worker. N. Engl. J. Med. 2024, 390, 2028–2029. [Google Scholar] [CrossRef]
- Yu, H.; Wu, J.T.; Cowling, B.J.; Liao, Q.; Fang, V.J.; Zhou, S.; Wu, P.; Zhou, H.; Lau, E.H.; Guo, D.; et al. Effect of closure of live poultry markets on poultry-to-person transmission of avian influenza A H7N9 virus: An ecological study. Lancet 2014, 383, 541–548. [Google Scholar] [CrossRef]
- Bi, Y.; Chen, Q.; Wang, Q.; Chen, J.; Jin, T.; Wong, G.; Quan, C.; Liu, J.; Wu, J.; Yin, R.; et al. Genesis, Evolution and Prevalence of H5N6 Avian Influenza Viruses in China. Cell Host Microbe 2016, 20, 810–821. [Google Scholar] [CrossRef]
- Fournie, G.; Guitian, J.; Desvaux, S.; Cuong, V.C.; Dung, D.H.; Pfeiffer, D.U.; Mangtani, P.; Ghani, A.C. Interventions for avian influenza A (H5N1) risk management in live bird market networks. Proc. Natl. Acad. Sci. USA 2013, 110, 9177–9182. [Google Scholar] [CrossRef]
- Wang, Z.; Li, H.; Li, Y.; Wu, Z.; Ai, H.; Zhang, M.; Rong, L.; Blinov, M.L.; Tong, Q.; Liu, L.; et al. Mixed selling of different poultry species facilitates emergence of public-health-threating avian influenza viruses. Emerg. Microbes Infect. 2023, 12, 2214255. [Google Scholar] [CrossRef]
- Philippon, D.A.M.; Wu, P.; Cowling, B.J.; Lau, E.H.Y. Avian Influenza Human Infections at the Human-Animal Interface. J. Infect. Dis. 2020, 222, 528–537. [Google Scholar] [CrossRef]
- Zhang, J.; Ye, H.; Liu, Y.; Liao, M.; Qi, W. Resurgence of H5N6 avian influenza virus in 2021 poses new threat to public health. Lancet Microbe 2022, 3, e558. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Sun, H.; Gao, F.; Luo, K.; Huang, Z.; Tong, Q.; Song, H.; Han, Q.; Liu, J.; Lan, Y.; et al. Human infection of avian influenza A H3N8 virus and the viral origins: A descriptive study. Lancet Microbe 2022, 3, e824–e834. [Google Scholar] [CrossRef]
- Bi, Y.; Li, J.; Shi, W. The time is now: A call to contain H9N2 avian influenza viruses. Lancet Microbe 2022, 3, e804–e805. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, L.; Liao, M.; Qi, W. H9N2 avian influenza viruses: Challenges and the way forward. Lancet Microbe 2023, 4, e70–e71. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Zhang, L.; Kan, X.; Jiang, L.; Yang, J.; Guo, Z.; Ren, Q. Origin and molecular characteristics of a novel 2013 avian influenza A(H6N1) virus causing human infection in Taiwan. Clin. Infect. Dis. 2013, 57, 1367–1368. [Google Scholar] [CrossRef]
- Qi, X.; Qiu, H.; Hao, S.; Zhu, F.; Huang, Y.; Xu, K.; Yu, H.; Wang, D.; Zhou, L.; Dai, Q.; et al. Human Infection with an Avian-Origin Influenza A (H10N3) Virus. N. Engl. J. Med. 2022, 386, 1087–1088. [Google Scholar] [CrossRef]
- Chen, H.; Yuan, H.; Gao, R.; Zhang, J.; Wang, D.; Xiong, Y.; Fan, G.; Yang, F.; Li, X.; Zhou, J.; et al. Clinical and epidemiological characteristics of a fatal case of avian influenza A H10N8 virus infection: A descriptive study. Lancet 2014, 383, 714–721. [Google Scholar] [CrossRef] [PubMed]
- Gomaa, M.R.; Kayed, A.S.; Elabd, M.A.; Zeid, D.A.; Zaki, S.A.; El Rifay, A.S.; Sherif, L.S.; McKenzie, P.P.; Webster, R.G.; Webby, R.J.; et al. Avian influenza A(H5N1) and A(H9N2) seroprevalence and risk factors for infection among Egyptians: A prospective, controlled seroepidemiological study. J. Infect. Dis. 2015, 211, 1399–1407. [Google Scholar] [CrossRef]
- Gomaa, M.R.; El Rifay, A.S.; Abu Zeid, D.; Elabd, M.A.; Elabd, E.; Kandeil, A.; Shama, N.M.A.; Kamel, M.N.; Marouf, M.A.; Barakat, A.; et al. Incidence and Seroprevalence of Avian Influenza in a Cohort of Backyard Poultry Growers, Egypt, August 2015-March 2019. Emerg. Infect. Dis. 2020, 26, 2129–2136. [Google Scholar] [CrossRef] [PubMed]
- Tahir, M.F.; Abbas, M.A.; Ghafoor, T.; Dil, S.; Shahid, M.A.; Bullo, M.M.H.; Ain, Q.U.; Abbas Ranjha, M.; Khan, M.A.; Naseem, M.T. Seroprevalence and risk factors of avian influenza H9 virus among poultry professionals in Rawalpindi, Pakistan. J. Infect. Public Health 2020, 13, 414–417. [Google Scholar] [CrossRef]
- Quan, C.; Wang, Q.; Zhang, J.; Zhao, M.; Dai, Q.; Huang, T.; Zhang, Z.; Mao, S.; Nie, Y.; Liu, J.; et al. Avian Influenza A Viruses among Occupationally Exposed Populations, China, 2014–2016. Emerg. Infect. Dis. 2019, 25, 2215–2225. [Google Scholar] [CrossRef] [PubMed]
- Klim, H.; William, T.; Mellors, J.; Brady, C.; Rajahram, G.S.; Chua, T.H.; Brazal Monzo, H.; John, J.L.; da Costa, K.; Jeffree, M.S.; et al. Serological analysis in humans in Malaysian Borneo suggests prior exposure to H5 avian influenza near migratory shorebird habitats. Nat. Commun. 2024, 15, 8863. [Google Scholar] [CrossRef]
- Hoffmann, E.; Stech, J.; Guan, Y.; Webster, R.G.; Perez, D.R. Universal primer set for the full-length amplification of all influenza A viruses. Arch. Virol. 2001, 146, 2275–2289. [Google Scholar] [CrossRef]
- Zou, S. A practical approach to genetic screening for influenza virus variants. J. Clin. Microbiol. 1997, 35, 2623–2627. [Google Scholar] [CrossRef]
- Fusaro, A.; Pu, J.; Zhou, Y.; Lu, L.; Tassoni, L.; Lan, Y.; Lam, T.T.; Song, Z.; Bahl, J.; Chen, J.; et al. Proposal for a Global Classification and Nomenclature System for A/H9 Influenza Viruses. Emerg. Infect. Dis. 2024, 30, e231176. [Google Scholar] [CrossRef]
- Jimenez-Bluhm, P.; Siegers, J.Y.; Tan, S.; Sharp, B.; Freiden, P.; Johow, M.; Orozco, K.; Ruiz, S.; Baumberger, C.; Galdames, P.; et al. Detection and phylogenetic analysis of highly pathogenic A/H5N1 avian influenza clade 2.3.4.4b virus in Chile, 2022. Emerg. Microbes Infect. 2023, 12, 2220569. [Google Scholar] [CrossRef]
- Wibawa, H.; Wibowo, P.E.; Supriyadi, A.; Lestari, L.; Silaban, J.; Fuadi, A.A.; Fiqri, A.J.; Handayani, R.W.; Irianingsih, S.H.; Fahmia, Z.; et al. Highly Pathogenic Avian Influenza A(H5N1) Virus Clade 2.3.4.4b in Domestic Ducks, Indonesia, 2022. Emerg. Infect. Dis. 2024, 30, 586–590. [Google Scholar] [CrossRef]
- WHO. Manual for the Laboratory Diagnosis and Virological Surveillance of Influenza. 2011. Available online: http://apps.who.int/iris/bitstream/10665/44518/1/9789241548090_eng.pdf (accessed on 18 July 2024).
- Rowe, T.; Abernathy, R.A.; Hu-Primmer, J.; Thompson, W.W.; Lu, X.; Lim, W.; Fukuda, K.; Cox, N.J.; Katz, J.M. Detection of antibody to avian influenza A (H5N1) virus in human serum by using a combination of serologic assays. J. Clin. Microbiol. 1999, 37, 937–943. [Google Scholar] [CrossRef]
- Gao, R.; Gu, M.; Liu, K.; Li, Q.; Li, J.; Shi, L.; Li, X.; Wang, X.; Hu, J.; Liu, X.; et al. T160A mutation-induced deglycosylation at site 158 in hemagglutinin is a critical determinant of the dual receptor binding properties of clade 2.3.4.4 H5NX subtype avian influenza viruses. Vet. Microbiol. 2018, 217, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Teng, Q.; Xu, D.; Shen, W.; Liu, Q.; Rong, G.; Li, X.; Yan, L.; Yang, J.; Chen, H.; Yu, H.; et al. A Single Mutation at Position 190 in Hemagglutinin Enhances Binding Affinity for Human Type Sialic Acid Receptor and Replication of H9N2 Avian Influenza Virus in Mice. J. Virol. 2016, 90, 9806–9825. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Zhang, X.; Gao, W.; Wang, C.; Wang, J.; Sun, H.; Sun, Y.; Guo, L.; Zhang, R.; Chang, K.C.; et al. Prevailing PA Mutation K356R in Avian Influenza H9N2 Virus Increases Mammalian Replication and Pathogenicity. J. Virol. 2016, 90, 8105–8114. [Google Scholar] [CrossRef]
- Fan, S.; Deng, G.; Song, J.; Tian, G.; Suo, Y.; Jiang, Y.; Guan, Y.; Bu, Z.; Kawaoka, Y.; Chen, H. Two amino acid residues in the matrix protein M1 contribute to the virulence difference of H5N1 avian influenza viruses in mice. Virology 2009, 384, 28–32. [Google Scholar] [CrossRef]
- Liu, Q.; Chen, H.; Huang, J.; Chen, Y.; Gu, M.; Wang, X.; Hu, S.; Liu, X.; Liu, X. A nonpathogenic duck-origin H9N2 influenza A virus adapts to high pathogenicity in mice. Arch. Virol. 2014, 159, 2243–2252. [Google Scholar] [CrossRef]
- Jiao, P.; Tian, G.; Li, Y.; Deng, G.; Jiang, Y.; Liu, C.; Liu, W.; Bu, Z.; Kawaoka, Y.; Chen, H. A single-amino-acid substitution in the NS1 protein changes the pathogenicity of H5N1 avian influenza viruses in mice. J. Virol. 2008, 82, 1146–1154. [Google Scholar] [CrossRef]
- Dankar, S.K.; Wang, S.; Ping, J.; Forbes, N.E.; Keleta, L.; Li, Y.; Brown, E.G. Influenza A virus NS1 gene mutations F103L and M106I increase replication and virulence. Virol. J. 2011, 8, 13. [Google Scholar] [CrossRef]
- Tian, H.; Zhou, S.; Dong, L.; Van Boeckel, T.P.; Cui, Y.; Newman, S.H.; Takekawa, J.Y.; Prosser, D.J.; Xiao, X.; Wu, Y.; et al. Avian influenza H5N1 viral and bird migration networks in Asia. Proc. Natl. Acad. Sci. USA 2015, 112, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Gass, J.D., Jr.; Dusek, R.J.; Hall, J.S.; Hallgrimsson, G.T.; Halldorsson, H.P.; Vignisson, S.R.; Ragnarsdottir, S.B.; Jonsson, J.E.; Krauss, S.; Wong, S.S.; et al. Global dissemination of influenza A virus is driven by wild bird migration through arctic and subarctic zones. Mol. Ecol. 2023, 32, 198–213. [Google Scholar] [CrossRef] [PubMed]
- Spackman, E.; McCracken, K.G.; Winker, K.; Swayne, D.E. H7N3 avian influenza virus found in a South American wild duck is related to the Chilean 2002 poultry outbreak, contains genes from equine and North American wild bird lineages, and is adapted to domestic turkeys. J. Virol. 2006, 80, 7760–7764. [Google Scholar] [CrossRef]
- Zhao, T.; Qian, Y.H.; Chen, S.H.; Wang, G.L.; Wu, M.N.; Huang, Y.; Ma, G.Y.; Fang, L.Q.; Gray, G.C.; Lu, B.; et al. Novel H7N2 and H5N6 Avian Influenza A Viruses in Sentinel Chickens: A Sentinel Chicken Surveillance Study. Front. Microbiol. 2016, 7, 1766. [Google Scholar] [CrossRef] [PubMed]
- Xie, R.; Edwards, K.M.; Wille, M.; Wei, X.; Wong, S.S.; Zanin, M.; El-Shesheny, R.; Ducatez, M.; Poon, L.L.M.; Kayali, G.; et al. The episodic resurgence of highly pathogenic avian influenza H5 virus. Nature 2023, 622, 810–817. [Google Scholar] [CrossRef]
- Wan, X.F.; Dong, L.; Lan, Y.; Long, L.P.; Xu, C.; Zou, S.; Li, Z.; Wen, L.; Cai, Z.; Wang, W.; et al. Indications that live poultry markets are a major source of human H5N1 influenza virus infection in China. J. Virol. 2011, 85, 13432–13438. [Google Scholar] [CrossRef]
- Lam, T.T.; Wang, J.; Shen, Y.; Zhou, B.; Duan, L.; Cheung, C.L.; Ma, C.; Lycett, S.J.; Leung, C.Y.; Chen, X.; et al. The genesis and source of the H7N9 influenza viruses causing human infections in China. Nature 2013, 502, 241–244. [Google Scholar] [CrossRef]
- Ma, M.J.; Zhao, T.; Chen, S.H.; Xia, X.; Yang, X.X.; Wang, G.L.; Fang, L.Q.; Ma, G.Y.; Wu, M.N.; Qian, Y.H.; et al. Avian Influenza A Virus Infection among Workers at Live Poultry Markets, China, 2013–2016. Emerg. Infect. Dis. 2018, 24, 1246–1256. [Google Scholar] [CrossRef]
- Peacock, T.; Moncla, L.; Dudas, G.; VanInsberghe, D.; Sukhova, K.; Lloyd-Smith, J.O.; Worobey, M.; Lowen, A.C.; Nelson, M.I. The global H5N1 influenza panzootic in mammals. Nature 2024, 637, 304–313. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, C.; Yuan, Y.; Sun, J.; Lu, L.; Sun, H.; Sun, H.; Chu, D.; Qin, S.; Chen, J.; et al. Novel Avian Influenza Virus (H5N1) Clade 2.3.4.4b Reassortants in Migratory Birds, China. Emerg. Infect. Dis. 2023, 29, 1244–1249. [Google Scholar] [CrossRef]
- Zeng, X.; Tian, G.; Shi, J.; Deng, G.; Li, C.; Chen, H. Vaccination of poultry successfully eliminated human infection with H7N9 virus in China. Sci. China Life Sci. 2018, 61, 1465–1473. [Google Scholar] [CrossRef]
- Pu, J.; Wang, S.; Yin, Y.; Zhang, G.; Carter, R.A.; Wang, J.; Xu, G.; Sun, H.; Wang, M.; Wen, C.; et al. Evolution of the H9N2 influenza genotype that facilitated the genesis of the novel H7N9 virus. Proc. Natl. Acad. Sci. USA 2015, 112, 548–553. [Google Scholar] [CrossRef]
| Group | Subjects Tested No. | HI Titer ≥ 1:40 No. (%) | Odds Ratio (95% CI) a | p-Value a | ||||
|---|---|---|---|---|---|---|---|---|
| Anti-H5N1 | Anti-H5N2 | Anti-H5N6 | Anti-H7N9 | Anti-H9N2 | ||||
| Avian-exposed subjects | 935 | 6 (0.6) | 0 | 5 (0.5) | 0 | 25 (2.7) | 12.9 (2.2–134.1) | 0.003 |
| Residents living around the habitat of migratory birds | 687 | 3 (0.04) | 0 | 1 (0.1) | 0 | 13 (1.9) | 9.1 (1.5–97.2) | 0.022 |
| Workers in poultry farms | 104 | 0 | 0 | 0 | 0 | 8 (7.7) | 39.3 (5.9–436.6) | <0.0001 |
| Workers in live poultry markets | 144 | 3 (2.1) | 0 | 4 (2.8) | 0 | 4 (2.8) | 13.3 (2.2–162.6) | 0.014 |
| General population | 472 | 0 | 0 | 0 | 0 | 1 (0.2) | Reference | Reference |
| Total | 1407 | 6 (0.4) | 0 | 5 (0.4) | 0 | 24 (1.7) | / | / |
| Group | Subjects Tested No. | MN Titer ≥ 1:40 No. (%) | Odds Ratio (95% CI) a | p-Value a | ||||
|---|---|---|---|---|---|---|---|---|
| Anti-H5N1 | Anti-H5N2 | Anti-H5N6 | Anti-H7N9 | Anti-H9N2 | ||||
| Avian-exposed subjects | 935 | 4 (0.4) | 0 | 2 (0.2) | 0 | 20 (2.1) | 10.3 (1.4–76.9) | 0.005 |
| Residents living around the habitat of migratory birds | 687 | 0 | 0 | 0 | 0 | 10 (1.5) | 7.0 (0.9–54.5) | 0.032 |
| Workers in poultry farms | 104 | 0 | 0 | 0 | 0 | 3 (2.9) | 14.0 (1.4–135.9) | 0.003 |
| Workers in live poultry markets | 144 | 4 (2.8) | 0 | 2 (1.4) | 0 | 7 (4.9) | 24.1 (2.9–197.3) | <0.0001 |
| General population | 472 | 0 | 0 | 0 | 0 | 1 (0.2) | Reference | Reference |
| Total | 1407 | 4 (0.3) | 0 | 2 (0.1) | 0 | 21 (1.5) | / | / |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, S.; Liang, Y.-M.; Wang, D.-M.; Shang, C.; Wei, W.-Q.; Zhao, X.-J.; Li, L.-B.; Jiang, W.-G.; Guo, B.-J.; Jiao, B.-Y.; et al. Avian Influenza Surveillance Among Migratory Birds, Poultry, and Humans Around Nansi Lake, China, 2021–2024. Viruses 2025, 17, 1117. https://doi.org/10.3390/v17081117
Zhang S, Liang Y-M, Wang D-M, Shang C, Wei W-Q, Zhao X-J, Li L-B, Jiang W-G, Guo B-J, Jiao B-Y, et al. Avian Influenza Surveillance Among Migratory Birds, Poultry, and Humans Around Nansi Lake, China, 2021–2024. Viruses. 2025; 17(8):1117. https://doi.org/10.3390/v17081117
Chicago/Turabian StyleZhang, Sheng, Yu-Min Liang, Dong-Mei Wang, Chao Shang, Wang-Qian Wei, Xin-Jing Zhao, Li-Bo Li, Wen-Guo Jiang, Bao-Jin Guo, Bo-Yan Jiao, and et al. 2025. "Avian Influenza Surveillance Among Migratory Birds, Poultry, and Humans Around Nansi Lake, China, 2021–2024" Viruses 17, no. 8: 1117. https://doi.org/10.3390/v17081117
APA StyleZhang, S., Liang, Y.-M., Wang, D.-M., Shang, C., Wei, W.-Q., Zhao, X.-J., Li, L.-B., Jiang, W.-G., Guo, B.-J., Jiao, B.-Y., Ma, J., Qiu, Y.-B., Cui, Y.-B., Wang, G.-Q., Chen, J.-J., Xu, Q., Lv, C.-L., Hong, F., Wang, G.-L., & Fang, L.-Q. (2025). Avian Influenza Surveillance Among Migratory Birds, Poultry, and Humans Around Nansi Lake, China, 2021–2024. Viruses, 17(8), 1117. https://doi.org/10.3390/v17081117






