Neurological Manifestation of Canine Distemper Virus: Increased Risk in Young Shih Tzu and Lhasa Apso with Seasonal Prevalence in Autumn †
Abstract
1. Introduction
2. Materials and Methods
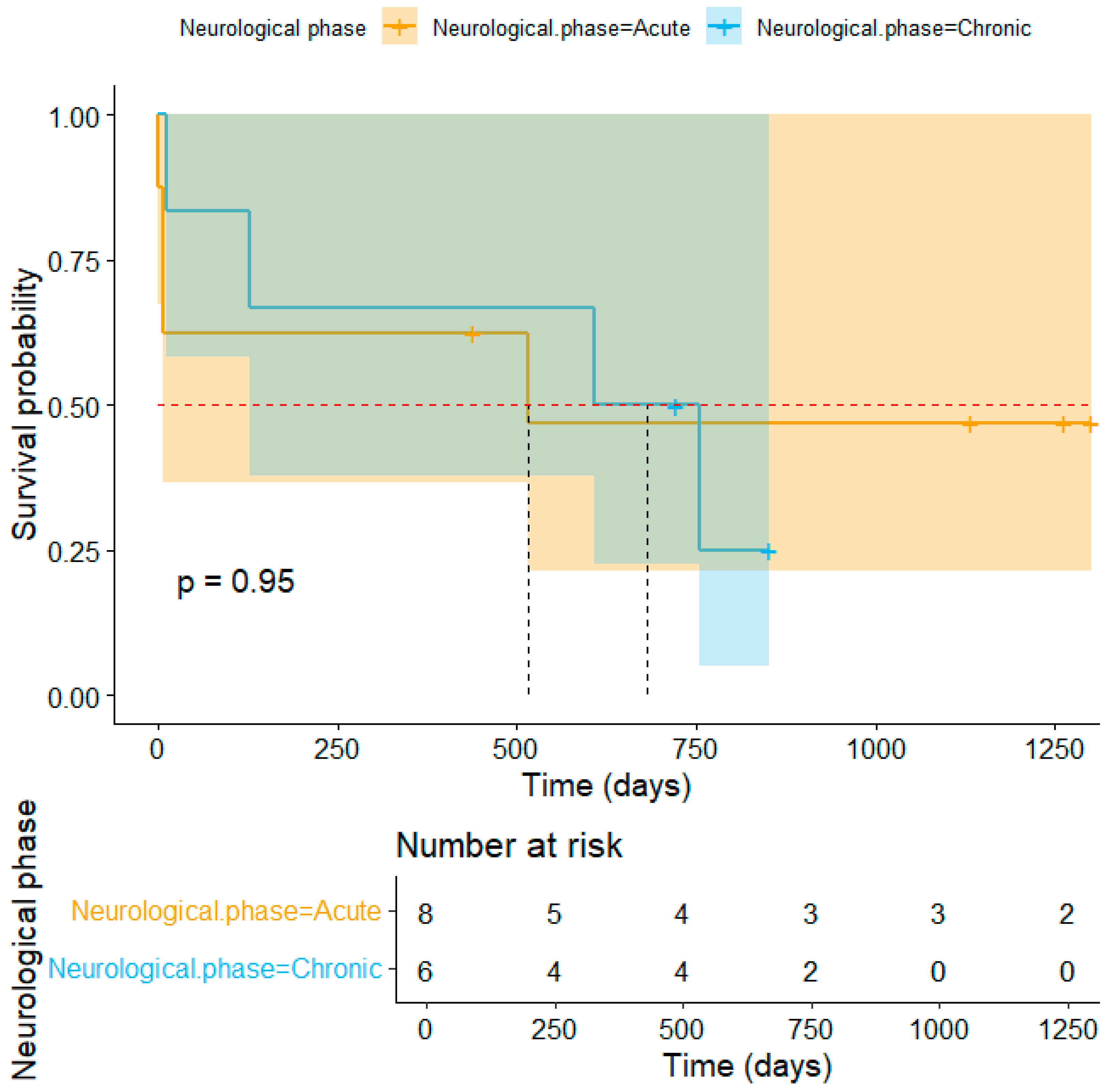
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CDV | Canine distemper virus |
| CG | Control group |
| CNS | Central nervous system |
| DG | Distemper group |
| ELISA | Enzyme-Linked Immunosorbent Assay |
| PCR | Polymerase chain reaction |
| RT-PCR | Reverse transcriptase polymerase chain reaction |
References
- Torres, B.B.G.; Iara, Í.H.N.; Freire, H.I.; Ribeiro, L.S.; Menezes, K.M.; Gonçalves, P.A.; Matta, D.H. Epidemiological Characteristics and Risk Factors Associated with Neurological Manifestation of Canine Distemper Virus. In Proceedings of the ACVIM 2024 Forum, Minneapolis, MN, USA, 6–8 June 2024. [Google Scholar]
- Elia, G.; Camero, M.; Losurdo, M.; Lucente, M.S.; Larocca, V.; Martella, V.; Decaro, N.; Buonavoglia, C. Virological and Serological Findings in Dogs with Naturally Occurring Distemper. J. Virol. Methods 2015, 213, 127–130. [Google Scholar] [CrossRef] [PubMed]
- Costa, V.G.D.; Saivish, M.V.; Rodrigues, R.L.; Lima Silva, R.F.D.; Moreli, M.L.; Krüger, R.H. Molecular and Serological Surveys of Canine Distemper Virus: A Meta-Analysis of Cross-Sectional Studies. PLoS ONE 2019, 14, e0217594. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, T.; Rajeev, R.; Jha, A.; Kumar, S. Canine Distemper: A Fatal Disease Seeking Special Intervention. J. Entomol. Zool. Stud. 2021, 9, 1411–1418. [Google Scholar]
- Santos, C.; Teles, J.; Es, G.; Gil, L.; Vieira, A.; Junior, J.; Silva, C.; Maia, R. Epitope Mapping and a Candidate Vaccine Design from Canine Distemper Virus. Open Vet. J. 2024, 14, 1019. [Google Scholar] [CrossRef]
- Beineke, A.; Puff, C.; Seehusen, F.; Baumgärtner, W. Pathogenesis and Immunopathology of Systemic and Nervous Canine Distemper. Vet. Immunol. Immunopathol. 2009, 127, 1–18. [Google Scholar] [CrossRef]
- Kapil, S.; Yeary, T.J. Canine Distemper Spillover in Domestic Dogs from Urban Wildlife. Vet. Clin. N. Am. Small Anim. Pract. 2011, 41, 1069–1086. [Google Scholar] [CrossRef]
- Martins, B.C.; Torres, B.B.J.; Heinemann, M.B.; Carneiro, R.A.; Melo, E.G. Características epizootiológicas da infecção natural pelo vírus da cinomose canina em Belo Horizonte. Arq. Bras. Med. Vet. Zootec. 2020, 72, 778–786. [Google Scholar] [CrossRef]
- Menezes, K.M.F.; Dábilla, N.; Souza, M.; Damasceno, A.D.; Torres, B.B.J. Identification of a New Polymorphism on the Wild-Type Canine Distemper Virus Genome: Could This Contribute to Vaccine Failures? Braz. J. Microbiol. 2023, 54, 665–678. [Google Scholar] [CrossRef]
- Martella, V.; Elia, G.; Buonavoglia, C. Canine Distemper Virus. Vet. Clin. N. Am. Small Anim. Pract. 2008, 38, 787–797. [Google Scholar] [CrossRef]
- Barber, R.M.; Li, Q.; Levine, J.M.; Ruone, S.J.; Levine, G.J.; Kenny, P.; Tong, S.; Schatzberg, S.J. Screening for Viral Nucleic Acids in the Cerebrospinal Fluid of Dogs With Central Nervous System Inflammation. Front. Vet. Sci. 2022, 9, 850510. [Google Scholar] [CrossRef]
- Sarchahi, A.A.; Arbabi, M.; Mohebalian, H. Detection of Canine Distemper Virus in Cerebrospinal Fluid, Whole Blood and Mucosal Specimens of Dogs with Distemper Using RT-PCR and Immunochromatographic Assays. Vet. Med. Sci. 2022, 8, 1390–1399. [Google Scholar] [CrossRef] [PubMed]
- Dorji, T.; Tenzin, T.; Tenzin, K.; Tshering, D.; Rinzin, K.; Phimpraphai, W.; De Garine-Wichatitsky, M. Seroprevalence and Risk Factors of Canine Distemper Virus in the Pet and Stray Dogs in Haa, Western Bhutan. BMC Vet. Res. 2020, 16, 135. [Google Scholar] [CrossRef] [PubMed]
- Menezes, K.M.F.; Filho, G.D.D.S.; Damasceno, A.D.; Souza, M.; Torres, B.B.J. Epizootiology of Canine Distemper in Naturally Infected Dogs in Goiânia, Brazil. Cienc. Rural 2023, 53, e20220166. [Google Scholar] [CrossRef]
- Dong, B.; Zhang, X.; Wang, J.; Zhang, G.; Li, C.; Wei, L.; Lin, W. A Meta-Analysis of Cross-Sectional Studies on the Frequency and Risk Factors Associated with Canine Morbillivirus Infection in China. Microb. Pathog. 2021, 161, 105258. [Google Scholar] [CrossRef]
- Headley, S.A.; Graça, D.L. Canine Distemper: Epidemiological Findings of 250 Cases. Braz. J. Vet. Res. Anim. Sci. 2000, 37, 136–140. [Google Scholar] [CrossRef]
- Koutinas, A.F.; Polizopoulou, Z.S.; Baumgaertner, W.; Lekkas, S.; Kontos, V. Relation of Clinical Signs to Pathological Changes in 19 Cases of Canine Distemper Encephalomyelitis. J. Comp. Pathol. 2002, 126, 47–56. [Google Scholar] [CrossRef]
- Ariyama, N.; Agüero, B.; Bennett, B.; Urzúa, C.; Berrios, F.; Verdugo, C.; Neira, V. Genetic Characterization of Canine Morbillivirus (Canine Distemper Virus) Field Strains in Dogs, Chile, 2022–2023. Transbound. Emerg. Dis. 2024, 2024, 9993255. [Google Scholar] [CrossRef]
- Allen, C.; Ellis, A.; Liang, R.; Lim, A.; Newbury, S. Prolonged Persistence of Canine Distemper Virus RNA, and Virus Isolation in Naturally Infected Shelter Dogs. PLoS ONE 2023, 18, e0280186. [Google Scholar] [CrossRef]
- Mousafarkhani, F.; Sarchahi, A.A.; Mohebalian, H.; Khoshnegah, J.; Arbabi, M. Prevalence of Canine Distemper in Dogs Referred to Veterinary Hospital of Ferdowsi University of Mashhad Mashhad Iran. Vet. Res. Forum 2023, 14, 153–160. [Google Scholar] [CrossRef]
- Squires, R.A.; Crawford, C.; Marcondes, M.; Whitley, N. 2024 Guidelines for the Vaccination of Dogs and Cats–Compiled by the Vaccination Guidelines Group (VGG) of the World Small Animal Veterinary Association (WSAVA). J. Small Anim. Pract. 2024, 65, 277–316. [Google Scholar] [CrossRef]
- Day, M.J.; Crawford, C.; Marcondes, M.; Squires, R.A. Recommendations on Vaccination for Latin American Small Animal Practitioners: A Report of the WSAVA Vaccination Guidelines Group. J. Small Anim. Pract. 2020, 61, E1–E35. [Google Scholar] [CrossRef] [PubMed]
- Trebbien, R.; Chriel, M.; Struve, T.; Hjulsager, C.K.; Larsen, G.; Larsen, L.E. Wildlife Reservoirs of Canine Distemper Virus Resulted in a Major Outbreak in Danish Farmed Mink (Neovison Vison). PLoS ONE 2014, 9, e85598. [Google Scholar] [CrossRef] [PubMed]
- Oleaga, Á.; Vázquez, C.B.; Royo, L.J.; Barral, T.D.; Bonnaire, D.; Armenteros, J.Á.; Rabanal, B.; Gortázar, C.; Balseiro, A. Canine Distemper Virus in Wildlife in South-Western Europe. Transbound. Emerg. Dis. 2022, 69, e473–e485. [Google Scholar] [CrossRef] [PubMed]
- Van, T.M.; Le, T.Q.; Tran, B.N. Phylogenetic Characterization of the Canine Distemper Virus Isolated from Veterinary Clinics in the Mekong Delta, Vietnam. Vet. World 2023, 16, 1092–1097. [Google Scholar] [CrossRef]
- Parent, S.; Barchi, S.; LeBreton, M.; Casha, S.; Fehlings, M.G. The Impact of Specialized Centers of Care for Spinal Cord Injury on Length of Stay, Complications, and Mortality: A Systematic Review of the Literature. J. Neurotrauma 2011, 28, 1363–1370. [Google Scholar] [CrossRef]
- Berendt, M.; Gredal, H.; Ersbøll, A.K.; Alving, J. Premature Death, Risk Factors, and Life Patterns in Dogs with Epilepsy. Vet. Intern. Med. 2007, 21, 754–759. [Google Scholar] [CrossRef]
- Chang, Y.; Mellor, D.J.; Anderson, T.J. Idiopathic Epilepsy in Dogs: Owners’ Perspectives on Management with Phenobarbitone and/or Potassium Bromide. J. Small Anim. Pract. 2006, 47, 574–581. [Google Scholar] [CrossRef]
- Charalambous, M.; Muñana, K.; Patterson, E.E.; Platt, S.R.; Volk, H.A. ACVIM Consensus Statement on the Management of Status Epilepticus and Cluster Seizures in Dogs and Cats. Vet. Intern. Med. 2024, 38, 19–40. [Google Scholar] [CrossRef]
- Dimopoulou, M.; Engdahl, K.; Ladlow, J.; Andersson, G.; Hedhammar, Å.; Skiöldebrand, E.; Ljungvall, I. The Epidemiology of Upper Respiratory Tract Disorders in a Population of Insured Swedish Dogs (2011–2014), and Its Association to Brachycephaly. Sci. Rep. 2023, 13, 8765. [Google Scholar] [CrossRef]
- Wyllie, S.; Kelman, M.; Ward, M. Epidemiology and Clinical Presentation of Canine Distemper Disease in Dogs and Ferrets in Australia, 2006–2014. Aust. Vet. J. 2016, 94, 215–222. [Google Scholar] [CrossRef]
- Acosta-Jamett, G.; Surot, D.; Cortés, M.; Marambio, V.; Valenzuela, C.; Vallverdu, A.; Ward, M.P. Epidemiology of Canine Distemper and Canine Parvovirus in Domestic Dogs in Urban and Rural Areas of the Araucanía Region in Chile. Vet. Microbiol. 2015, 178, 260–264. [Google Scholar] [CrossRef] [PubMed]
- Headley, S.A.; Amude, A.M.; Alfieri, A.F.; Bracarense, A.P.F.R.L.; Alfieri, A.A. Epidemiological Features and the Neuropathological Manifestations of Canine Distemper Virus-Induced Infections in Brazil: A Review. Semin. Cienc. Agrar. 2012, 33, 1945–1978. [Google Scholar] [CrossRef]
- Silva, M.C.; Fighera, R.A.; Mazzanti, A.; Brum, J.S.; Pierezan, F.; Barros, C.S.L. Neuropatologia da cinomose canina: 70 casos (2005–2008). Pesq. Vet. Bras. 2009, 29, 643–652. [Google Scholar] [CrossRef]
- Pan, Y.; Wang, S.; Li, P.; Yue, F.; Zhang, Y.; Pan, B.; Liu, X. Apoptotic Investigation of Brain Tissue Cells in Dogs Naturally Infected by Canine Distemper Virus. Virol. J. 2021, 18, 165. [Google Scholar] [CrossRef] [PubMed]
- Da Fontoura Budaszewski, R.; Streck, A.F.; Nunes Weber, M.; Maboni Siqueira, F.; Muniz Guedes, R.L.; Wageck Canal, C. Influence of Vaccine Strains on the Evolution of Canine Distemper Virus. Infect. Genet. Evol. 2016, 41, 262–269. [Google Scholar] [CrossRef]


| Risk Factor | DG (n = 17) | CG (n = 343) | p-Value |
|---|---|---|---|
| Breed | Pure = 10 Mixed-breed = 7 | Pure = 201 Mixed-breed = 142 | 0.94338 |
| Gender | Female = 8 Male = 9 | Female = 181 Male = 162 | 0.39279 |
| Neuter status | Yes = 4 No = 13 | Yes = 134 No = 208 NI = 1 | 0.72536 |
| Age | 31 ± 30 months | 68 ± 51 months | 0.00690 ** |
| Weight | 6.4 ± 4.2 Kg | 12.4 ± 11.8 Kg | 0.08683 |
| Breeds | DG (n = 17) | CG (n = 343) | p-Value | LOR | IC-LOR |
|---|---|---|---|---|---|
| Mixed breed | 7 | 142 | >0.05 | −1.38053 | [−1.81932, −0.96728] |
| Shih Tzu | 5 | 37 | 0.00007 *** | 1.53774 | [0.76853, 2.29708] |
| Lhasa Apso | 3 | 6 | 0.000264 *** | 1.76084 | [0.81950, 2.68406] |
| Pinscher | 1 | 15 | >0.05 | −1.38053 | [−1.81932, −0.96728] |
| Australian Cattle dog | 1 | 0 | >0.05 | −1.38053 | [−1.81932, −0.96728] |
| Others | 0 | 143 | >0.05 | −1.38053 | [−1.81932, −0.96728] |
| Types of Signs | Clinical Signs | Animals (n) | Percentage (%) |
|---|---|---|---|
| Neural signs (n = 17) | Paresis/plegia | 12 | 70.59 |
| Altered mental state | 9 | 52.94 | |
| Seizure | 7 | 41.18 | |
| Postural change | 6 | 35.29 | |
| Tremors | 6 | 35.29 | |
| Myoclonus | 6 | 35.29 | |
| Ataxia | 4 | 23.53 | |
| Muscle hypotrophy | 4 | 23.53 | |
| Behavioral change | 3 | 17.65 | |
| Extra-neural signs (n = 16) | Apathy | 9 | 56.25 |
| Dehydration | 7 | 43.75 | |
| Ocular discharge | 7 | 43.75 | |
| Diarrhea and/or Vomiting | 7 | 43.75 | |
| Hyporexia/anorexia | 6 | 37.50 | |
| Hyperthermia | 4 | 25.00 | |
| Hyperkeratosis | 3 | 18.75 | |
| Respiratory signs | 3 | 18.75 | |
| Enamel hypoplasia | 1 | 6.25 | |
| Dermal pustules | 1 | 6.25 |
| Animal | Breed | Vaccination Status | Vaccine Brand | Performed by a Qualified Professional | Neurological Infection Phase | Month of Infection | Outcome |
|---|---|---|---|---|---|---|---|
| 1 | Mix breed | UN | UN | UN | Chronic | October | Dead |
| 2 | Shih Tzu | Up-to-date | Brazilian brand | No | Chronic | September | Dead |
| 3 | Lhasa Apso | Up-to-date | Zoetis | Veterinary | Acute | March | Alive |
| 4 | Mix breed | UN | UN | UN | Chronic | November | Dead |
| 5 | Lhasa Apso | Outdated | UN | UN | Chronic | June | Alive |
| 6 | Shih Tzu | Up-to-date | UN | Veterinary | Chronic | May | Alive |
| 7 | Mix breed | UN | UN | UN | UN | UN | Alive |
| 8 | Mix breed | Outdated | UN | UN | Acute | March | Dead |
| 9 | Shih Tzu | Up-to-date | Grascon | No | Acute | May | Dead |
| 10 | Mix breed | UN | UN | UN | Acute | July | Alive |
| 11 | Australian Cattle Dog | Outdated | UN | UN | UN | August | Alive |
| 12 | Mix breed | Outdated | Zoetis | UN | Acute | October | Alive |
| 13 | Pinscher | Outdated | UN | UN | Acute | February | Dead |
| 14 | Shih Tzu | Up-to-date | UN | UN | Acute | May | Dead |
| 15 | Mix breed | UN | UN | UN | Chronic | May | Dead |
| 16 | Lhasa Apso | Up-to-date | Zoetis | UN | Acute | May | Alive |
| 17 | Shih Tzu | Outdated | Zoetis | UN | Chronic | December | Alive |
| Animal | Breed | Days from Presentation Until Death | Disease Phase at Death | Death Reason | Kind of Death | Sequelae |
|---|---|---|---|---|---|---|
| 1 | Mix breed | 608 | Chronic | Clinical deterioration | Euthanasia | Seizures |
| 2 | Shih Tzu | 128 | Chronic | Unavailable | Natural | Myoclonus and Seizures |
| 4 | Mix breed | 754 | Chronic | Status epilepticus | Natural | Seizures |
| 8 | Mix breed | 6 | Acute | Status epilepticus | Natural | - |
| 9 | Shih Tzu | 7 | Acute | Seizures and clinical deterioration | Euthanasia | - |
| 13 | Pinscher | 1 | Acute | Status epilepticus | Unavailable | - |
| 14 | Shih Tzu | 516 | Chronic | Unavailable | Natural | No |
| 15 | Mix breed | 12 | Acute | Clinical deterioration followed by cardiac arrest | Natural | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Freire, H.L.; Iara, Í.H.N.; Ribeiro, L.S.R.; Gonçalves, P.A.O.; Matta, D.H.; Torres, B.B.J. Neurological Manifestation of Canine Distemper Virus: Increased Risk in Young Shih Tzu and Lhasa Apso with Seasonal Prevalence in Autumn. Viruses 2025, 17, 820. https://doi.org/10.3390/v17060820
Freire HL, Iara ÍHN, Ribeiro LSR, Gonçalves PAO, Matta DH, Torres BBJ. Neurological Manifestation of Canine Distemper Virus: Increased Risk in Young Shih Tzu and Lhasa Apso with Seasonal Prevalence in Autumn. Viruses. 2025; 17(6):820. https://doi.org/10.3390/v17060820
Chicago/Turabian StyleFreire, Heloisa L., Ítalo H. N. Iara, Luana S. R. Ribeiro, Paulo A. O. Gonçalves, David H. Matta, and Bruno B. J. Torres. 2025. "Neurological Manifestation of Canine Distemper Virus: Increased Risk in Young Shih Tzu and Lhasa Apso with Seasonal Prevalence in Autumn" Viruses 17, no. 6: 820. https://doi.org/10.3390/v17060820
APA StyleFreire, H. L., Iara, Í. H. N., Ribeiro, L. S. R., Gonçalves, P. A. O., Matta, D. H., & Torres, B. B. J. (2025). Neurological Manifestation of Canine Distemper Virus: Increased Risk in Young Shih Tzu and Lhasa Apso with Seasonal Prevalence in Autumn. Viruses, 17(6), 820. https://doi.org/10.3390/v17060820








