Role of Extracellular Vesicles in Severe Dengue: Virus–Host Interactions and Biomarker Potential
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Eligibility Criteria
2.3. Bioinformatic Analysis of miRNAs in Dengue
3. Clinical Presentation and Structural Overview of the Dengue Virus
4. Immune Response and the Potential Role of EVs in Dengue Severity
5. Biogenesis of Extracellular Vesicles and Their Role as microRNA Carriers in Dengue Virus Infection
6. Overview of EVs’ Role in Dengue Infection: Transmission Dynamics
7. Extracellular Vesicles as Mediators of Viral Spread and Immune Evasion in Dengue Virus Infection
8. The Role of Extracellular Vesicles in Endothelial Damage and Vascular Hyperpermeability in Dengue
9. Role of Extracellular Vesicle-Derived microRNAs in Dengue Severity
10. Clinical Applications of EVs in Dengue Diagnosis
11. Isolation and Detection of EVs from Dengue Patients
12. EVs as Potential Biomarkers for Dengue Severity: Role of NS1
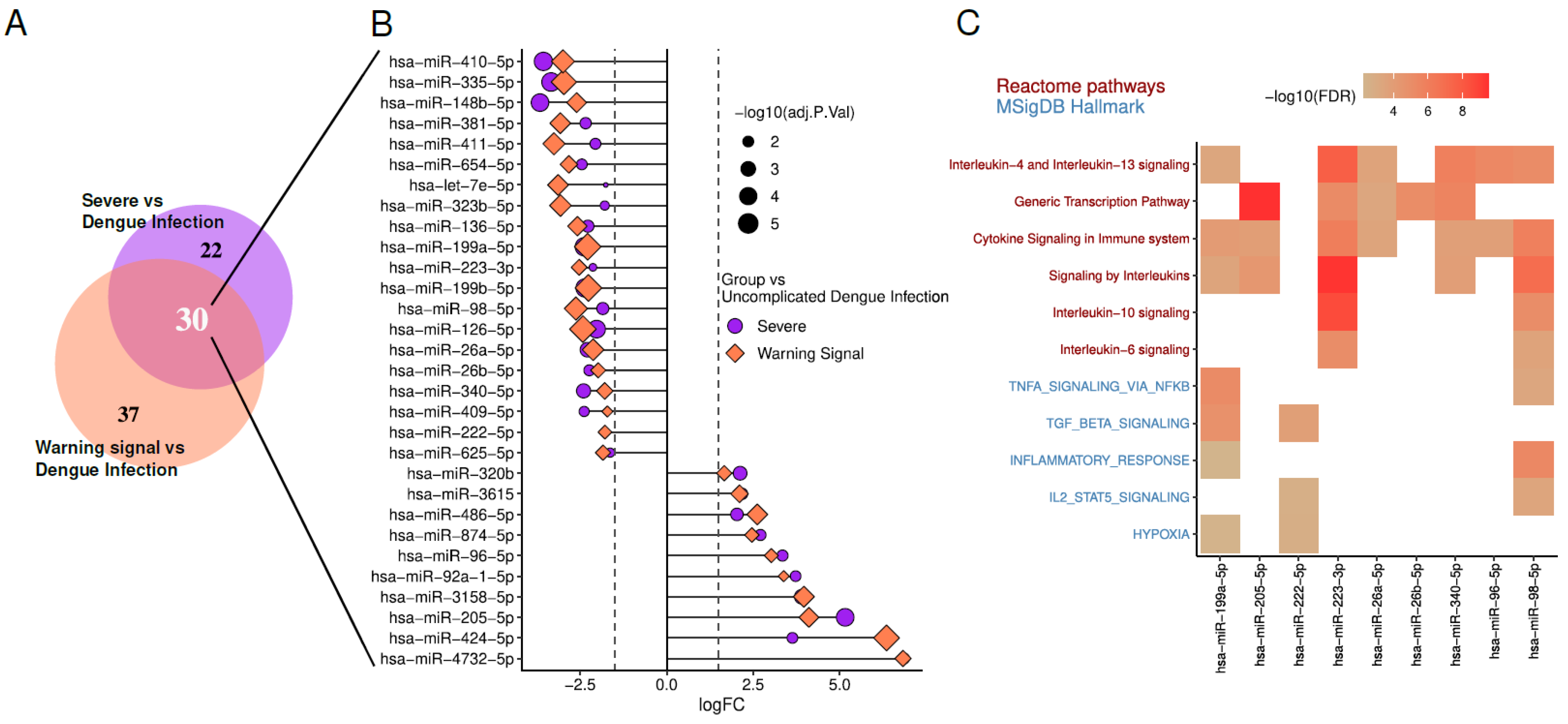
13. Differentially Expressed miRNAs in Dengue Patients and Potential Signaling Pathways Involved
14. Potential Therapeutic Strategies Based on Current Knowledge of EVs
15. Concluding Remarks
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Murugesan, A.; Manoharan, M. Dengue Virus. In Emerging and Reemerging Viral Pathogens; Elsevier: Amsterdam, The Netherlands, 2020; pp. 281–359. ISBN 978-0-12-819400-3. [Google Scholar]
- Ebi, K.L.; Nealon, J. Dengue in a Changing Climate. Environ. Res. 2016, 151, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, S.; Gething, P.W.; Brady, O.J.; Messina, J.P.; Farlow, A.W.; Moyes, C.L.; Drake, J.M.; Brownstein, J.S.; Hoen, A.G.; Sankoh, O.; et al. The Global Distribution and Burden of Dengue. Nature 2013, 496, 504–507. [Google Scholar] [CrossRef] [PubMed]
- Sarker, R.; Roknuzzaman, A.S.M.; Haque, M.A.; Islam, M.R.; Kabir, E.R. Upsurge of Dengue Outbreaks in Several WHO Regions: Public Awareness, Vector Control Activities, and International Collaborations Are Key to Prevent Spread. Health Sci. Rep. 2024, 7, e2034. [Google Scholar] [CrossRef] [PubMed]
- Dengue Multi-Country Grade 3 Outbreak 2024—PAHO/WHO|Pan American Health Organization. Available online: https://www.paho.org/en/topics/dengue/dengue-multi-country-grade-3-outbreak (accessed on 31 December 2024).
- Kularatne, S.A.; Dalugama, C. Dengue Infection: Global Importance, Immunopathology and Management. Clin. Med. 2022, 22, 9–13. [Google Scholar] [CrossRef]
- Singh, R.K.; Tiwari, A.; Satone, P.D.; Priya, T.; Meshram, R.J. Updates in the Management of Dengue Shock Syndrome: A Comprehensive Review. Cureus 2023, 15, e46713. [Google Scholar] [CrossRef]
- Bhatt, P.; Sabeena, S.P.; Varma, M.; Arunkumar, G. Current Understanding of the Pathogenesis of Dengue Virus Infection. Curr. Microbiol. 2021, 78, 17–32. [Google Scholar] [CrossRef]
- Malavige, G.N.; Ogg, G.S. Pathogenesis of Vascular Leak in Dengue Virus Infection. Immunology 2017, 151, 261–269. [Google Scholar] [CrossRef]
- Mishra, R.; Lata, S.; Ali, A.; Banerjea, A.C. Dengue Haemorrhagic Fever: A Job Done via Exosomes? Emerg. Microbes Infect. 2019, 8, 1626–1635. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Ritchie, M.E.; Phipson, B.; Wu, D.; Hu, Y.; Law, C.W.; Shi, W.; Smyth, G.K. Limma Powers Differential Expression Analyses for RNA-Sequencing and Microarray Studies. Nucleic Acids Res. 2015, 43, e47. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023. [Google Scholar]
- Tastsoglou, S.; Skoufos, G.; Miliotis, M.; Karagkouni, D.; Koutsoukos, I.; Karavangeli, A.; Kardaras, F.S.; Hatzigeorgiou, A.G. DIANA-miRPath v4.0: Expanding Target-Based miRNA Functional Analysis in Cell-Type and Tissue Contexts. Nucleic Acids Res. 2023, 51, W154–W159. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, V.D.; Tripathi, I.P.; Tripathi, R.C.; Bharadwaj, S.; Mishra, S.K. Genomics, Proteomics and Evolution of Dengue Virus. Brief. Funct. Genom. 2017, 16, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Guzman, M.G.; Gubler, D.J.; Izquierdo, A.; Martinez, E.; Halstead, S.B. Dengue Infection. Nat. Rev. Dis. Primers 2016, 2, 16055. [Google Scholar] [CrossRef]
- Beltrán-Silva, S.L.; Chacón-Hernández, S.S.; Moreno-Palacios, E.; Pereyra-Molina, J.Á. Clinical and Differential Diagnosis: Dengue, Chikungunya and Zika. Rev. Médica Hosp. Gen. México 2018, 81, 146–153. [Google Scholar] [CrossRef]
- Tejo, A.M.; Hamasaki, D.T.; Menezes, L.M.; Ho, Y.-L. Severe Dengue in the Intensive Care Unit. J. Intensiv. Med. 2024, 4, 16–33. [Google Scholar] [CrossRef] [PubMed]
- Tam, D.T.H.; Clapham, H.; Giger, E.; Kieu, N.T.T.; Nam, N.T.; Hong, D.T.T.; Nuoi, B.T.; Cam, N.T.H.; Quyen, N.T.H.; Turner, H.C.; et al. Burden of Postinfectious Symptoms after Acute Dengue, Vietnam. Emerg. Infect. Dis. 2023, 29, 160–163. [Google Scholar] [CrossRef]
- Kok, B.H.; Lim, H.T.; Lim, C.P.; Lai, N.S.; Leow, C.Y.; Leow, C.H. Dengue Virus Infection—A Review of Pathogenesis, Vaccines, Diagnosis and Therapy. Virus Res. 2023, 324, 199018. [Google Scholar] [CrossRef]
- St. John, A.L.; Rathore, A.P.S. Adaptive Immune Responses to Primary and Secondary Dengue Virus Infections. Nat. Rev. Immunol. 2019, 19, 218–230. [Google Scholar] [CrossRef]
- Lee, M.F.; Voon, G.Z.; Lim, H.X.; Chua, M.L.; Poh, C.L. Innate and Adaptive Immune Evasion by Dengue Virus. Front. Cell. Infect. Microbiol. 2022, 12, 1004608. [Google Scholar] [CrossRef]
- Latanova, A.; Karpov, V.; Starodubova, E. Extracellular Vesicles in Flaviviridae Pathogenesis: Their Roles in Viral Transmission, Immune Evasion, and Inflammation. Int. J. Mol. Sci. 2024, 25, 2144. [Google Scholar] [CrossRef]
- Liu, Y.-J.; Wang, C. A Review of the Regulatory Mechanisms of Extracellular Vesicles-Mediated Intercellular Communication. Cell Commun. Signal. 2023, 21, 77. [Google Scholar] [CrossRef] [PubMed]
- Aloi, N.; Drago, G.; Ruggieri, S.; Cibella, F.; Colombo, P.; Longo, V. Extracellular Vesicles and Immunity: At the Crossroads of Cell Communication. Int. J. Mol. Sci. 2024, 25, 1205. [Google Scholar] [CrossRef]
- Berumen Sánchez, G.; Bunn, K.E.; Pua, H.H.; Rafat, M. Extracellular Vesicles: Mediators of Intercellular Communication in Tissue Injury and Disease. Cell Commun. Signal. 2021, 19, 104. [Google Scholar] [CrossRef] [PubMed]
- Tetta, C.; Ghigo, E.; Silengo, L.; Deregibus, M.C.; Camussi, G. Extracellular Vesicles as an Emerging Mechanism of Cell-to-Cell Communication. Endocrine 2013, 44, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Elmore, S. Apoptosis: A Review of Programmed Cell Death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef]
- Wu, S.; Yang, T.; Ma, M.; Fan, L.; Ren, L.; Liu, G.; Wang, Y.; Cheng, B.; Xia, J.; Hao, Z. Extracellular Vesicles Meet Mitochondria: Potential Roles in Regenerative Medicine. Pharmacol. Res. 2024, 206, 107307. [Google Scholar] [CrossRef]
- Yu, L.; Zhu, G.; Zhang, Z.; Yu, Y.; Zeng, L.; Xu, Z.; Weng, J.; Xia, J.; Li, J.; Pathak, J.L. Apoptotic Bodies: Bioactive Treasure Left behind by the Dying Cells with Robust Diagnostic and Therapeutic Application Potentials. J. Nanobiotechnology 2023, 21, 218. [Google Scholar] [CrossRef]
- Nagata, S.; Sakuragi, T.; Segawa, K. Flippase and Scramblase for Phosphatidylserine Exposure. Curr. Opin. Immunol. 2020, 62, 31–38. [Google Scholar] [CrossRef]
- Clancy, J.W.; Schmidtmann, M.; D’Souza-Schorey, C. The Ins and Outs of Microvesicles. FASEB Bioadv. 2021, 3, 399–406. [Google Scholar] [CrossRef]
- Ramachandran, C.; Patil, R.V.; Combrink, K.; Sharif, N.A.; Srinivas, S.P. Rho-Rho Kinase Pathway in the Actomyosin Contraction and Cell-Matrix Adhesion in Immortalized Human Trabecular Meshwork Cells. Mol. Vis. 2011, 17, 1877–1890. [Google Scholar]
- Sakurada, S.; Takuwa, N.; Sugimoto, N.; Wang, Y.; Seto, M.; Sasaki, Y.; Takuwa, Y. Ca2+-Dependent Activation of Rho and Rho Kinase in Membrane Depolarization-Induced and Receptor Stimulation-Induced Vascular Smooth Muscle Contraction. Circ. Res. 2003, 93, 548–556. [Google Scholar] [CrossRef]
- Lee, Y.J.; Shin, K.J.; Jang, H.-J.; Ryu, J.-S.; Lee, C.Y.; Yoon, J.H.; Seo, J.K.; Park, S.; Lee, S.; Je, A.R.; et al. GPR143 Controls ESCRT-Dependent Exosome Biogenesis and Promotes Cancer Metastasis. Dev. Cell 2023, 58, 320–334.e8. [Google Scholar] [CrossRef] [PubMed]
- Ostrowski, M.; Carmo, N.B.; Krumeich, S.; Fanget, I.; Raposo, G.; Savina, A.; Moita, C.F.; Schauer, K.; Hume, A.N.; Freitas, R.P.; et al. Rab27a and Rab27b Control Different Steps of the Exosome Secretion Pathway. Nat. Cell Biol. 2010, 12, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Villarroya-Beltri, C.; Baixauli, F.; Gutiérrez-Vázquez, C.; Sánchez-Madrid, F.; Mittelbrunn, M. Sorting It out: Regulation of Exosome Loading. Semin. Cancer Biol. 2014, 28, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.A.; Baba, S.K.; Sadida, H.Q.; Marzooqi, S.A.; Jerobin, J.; Altemani, F.H.; Algehainy, N.; Alanazi, M.A.; Abou-Samra, A.-B.; Kumar, R.; et al. Extracellular Vesicles as Tools and Targets in Therapy for Diseases. Signal Transduct. Target. Ther. 2024, 9, 27. [Google Scholar] [CrossRef]
- Yoshikawa, F.S.Y.; Teixeira, F.M.E.; Sato, M.N.; da Oliveira, L.M.S. Delivery of microRNAs by Extracellular Vesicles in Viral Infections: Could the News Be Packaged? Cells 2019, 8, 611. [Google Scholar] [CrossRef]
- Singh, S.; Chen, C.C.; Kim, S.; Singh, A.; Singh, G. Role of Extracellular Vesicle microRNAs and RNA Binding Proteins on Glioblastoma Dynamics and Therapeutics Development. Extracell. Vesicle 2024, 4, 100049. [Google Scholar] [CrossRef]
- Safadi, D.E.; Lebeau, G.; Lagrave, A.; Mélade, J.; Grondin, L.; Rosanaly, S.; Begue, F.; Hoareau, M.; Veeren, B.; Roche, M.; et al. Extracellular Vesicles Are Conveyors of the NS1 Toxin during Dengue Virus and Zika Virus Infection. Viruses 2023, 15, 364. [Google Scholar] [CrossRef]
- Su, Y.; Lin, T.; Liu, C.; Cheng, C.; Han, X.; Jiang, X. microRNAs, the Link Between Dengue Virus and the Host Genome. Front. Microbiol. 2021, 12, 714409. [Google Scholar] [CrossRef]
- Wong, R.R.; Abd-Aziz, N.; Affendi, S.; Poh, C.L. Role of microRNAs in Antiviral Responses to Dengue Infection. J. Biomed. Sci. 2020, 27, 4. [Google Scholar] [CrossRef]
- Kanokudom, S.; Vilaivan, T.; Wikan, N.; Thepparit, C.; Smith, D.R.; Assavalapsakul, W. miR-21 Promotes Dengue Virus Serotype 2 Replication in HepG2 Cells. Antivir. Res. 2017, 142, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Zhang, L.; Zhang, F.; Tang, T.; Zhou, Q.; Feng, C.; Jin, Y.; Wu, Z. Exosome-Mediated miR-146a Transfer Suppresses Type I Interferon Response and Facilitates EV71 Infection. PLoS Pathog. 2017, 13, e1006611. [Google Scholar] [CrossRef]
- Mao, L.; Chen, Y.; Gu, J.; Zhao, Y.; Chen, Q. Roles and Mechanisms of Exosomal microRNAs in Viral Infections. Arch. Virol. 2023, 168, 121. [Google Scholar] [CrossRef]
- de Martins, S.T.; Kuczera, D.; Lötvall, J.; Bordignon, J.; Alves, L.R. Characterization of Dendritic Cell-Derived Extracellular Vesicles During Dengue Virus Infection. Front. Microbiol. 2018, 9, 1792. [Google Scholar] [CrossRef] [PubMed]
- Sorop, A.; Iacob, R.; Iacob, S.; Constantinescu, D.; Chitoiu, L.; Fertig, T.E.; Dinischiotu, A.; Chivu-Economescu, M.; Bacalbasa, N.; Savu, L.; et al. Plasma Small Extracellular Vesicles Derived miR-21-5p and miR-92a-3p as Potential Biomarkers for Hepatocellular Carcinoma Screening. Front. Genet. 2020, 11, 712. [Google Scholar] [CrossRef]
- Sriprapun, M.; Rattanamahaphoom, J.; Sriburin, P.; Chatchen, S.; Limkittikul, K.; Sirivichayakul, C. The Expression of Circulating Hsa-miR-126-3p in Dengue-Infected Thai Pediatric Patients. Pathog. Glob. Health 2023, 117, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Aloia, A.L.; Abraham, A.M.; Bonder, C.S.; Pitson, S.M.; Carr, J.M. Dengue Virus-Induced Inflammation of the Endothelium and the Potential Roles of Sphingosine Kinase-1 and MicroRNAs. Mediat. Inflamm. 2015, 2015, 509306. [Google Scholar] [CrossRef]
- Cloherty, A.P.M.; Rader, A.G.; Patel, K.S.; Eisden, T.-J.T.H.D.; van Piggelen, S.; Schreurs, R.R.C.E.; Ribeiro, C.M.S. Dengue Virus Exploits Autophagy Vesicles and Secretory Pathways to Promote Transmission by Human Dendritic Cells. Front. Immunol. 2024, 15, 1260439. [Google Scholar] [CrossRef]
- Wu, X.; Niu, J.; Shi, Y. Exosomes Target HBV-Host Interactions to Remodel the Hepatic Immune Microenvironment. J. Nanobiotechnology 2024, 22, 315. [Google Scholar] [CrossRef]
- Chapter 3—Pathogenesis of Viral Infections and Diseases. In Fenner’s Veterinary Virology, 4th ed.; MacLachlan, N.J., Dubovi, E.J., Eds.; Academic Press: San Diego, CA, USA, 2011; pp. 43–74. ISBN 978-0-12-375158-4. [Google Scholar]
- Klasse, P.J. The Molecular Basis of Viral Infection; Academic Press: Cambridge, MA, USA, 2015. [Google Scholar]
- Tisoncik, J.R.; Billharz, R.; Burmakina, S.; Belisle, S.E.; Proll, S.C.; Korth, M.J.; García-Sastre, A.; Katze, M.G. The NS1 Protein of Influenza A Virus Suppresses Interferon-Regulated Activation of Antigen-Presentation and Immune-Proteasome Pathways. J. Gen. Virol. 2011, 92, 2093–2104. [Google Scholar] [CrossRef]
- Caobi, A.; Nair, M.; Raymond, A.D. Extracellular Vesicles in the Pathogenesis of Viral Infections in Humans. Viruses 2020, 12, 1200. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Messina, L.; Gutiérrez-Vázquez, C.; Rivas-García, E.; Sánchez-Madrid, F.; de la Fuente, H. Immunomodulatory Role of microRNAs Transferred by Extracellular Vesicles. Biol. Cell 2015, 107, 61–77. [Google Scholar] [CrossRef]
- Torri, A.; Carpi, D.; Bulgheroni, E.; Crosti, M.-C.; Moro, M.; Gruarin, P.; Rossi, R.L.; Rossetti, G.; Di Vizio, D.; Hoxha, M.; et al. Extracellular MicroRNA Signature of Human Helper T Cell Subsets in Health and Autoimmunity. J. Biol. Chem. 2017, 292, 2903–2915. [Google Scholar] [CrossRef] [PubMed]
- Nail, H.M.; Chiu, C.-C.; Leung, C.-H.; Ahmed, M.M.M.; Wang, H.-M.D. Exosomal miRNA-Mediated Intercellular Communications and Immunomodulatory Effects in Tumor Microenvironments. J. Biomed. Sci. 2023, 30, 69. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Sun, J.; Dastgheyb, R.M.; Li, Z. Tumor-Derived Extracellular Vesicles Modulate Innate Immune Responses to Affect Tumor Progression. Front. Immunol. 2022, 13, 1045624. [Google Scholar] [CrossRef]
- Perez-Toledo, M.; Beristain-Covarrubias, N. A New Player in the Game: Platelet-Derived Extracellular Vesicles in Dengue Hemorrhagic Fever. Platelets 2020, 31, 412–414. [Google Scholar] [CrossRef]
- Vedpathak, S.; Sharma, A.; Palkar, S.; Bhatt, V.R.; Patil, V.C.; Kakrani, A.L.; Mishra, A.; Bhosle, D.; Arankalle, V.A.; Shrivastava, S. Platelet Derived Exosomes Disrupt Endothelial Cell Monolayer Integrity and Enhance Vascular Inflammation in Dengue Patients. Front. Immunol. 2024, 14, 1285162. [Google Scholar] [CrossRef]
- Pradhan, A.; Aneja, A.; Ghosh, S.; Devvanshi, H.; Deepika, C.; Sahu, R.; Ross, C.; Kshetrapal, P.; Maitra, A.; Das, S. Association of Exosomal miR-96-5p and miR-146a-5p with the Disease Severity in Dengue Virus Infection. J. Med. Virol. 2023, 95, e28614. [Google Scholar] [CrossRef]
- Kumari, S.; Bandyopadhyay, B.; Singh, A.; Aggarwal, S.; Yadav, A.K.; Vikram, N.K.; Guchhait, P.; Banerjee, A. Extracellular Vesicles Recovered from Plasma of Severe Dengue Patients Induce CD4+ T Cell Suppression through PD-L1/PD-1 Interaction. mBio 2023, 14, e01823-23. [Google Scholar] [CrossRef]
- Mishra, R.; Lahon, A.; Banerjea, A.C. Dengue Virus Degrades USP33–ATF3 Axis via Extracellular Vesicles to Activate Human Microglial Cells. J. Immunol. 2020, 205, 1787–1798. [Google Scholar] [CrossRef]
- Gold, A.S.; Feitosa-Suntheimer, F.; Araujo, R.V.; Hekman, R.M.; Asad, S.; Londono-Renteria, B.; Emili, A.; Colpitts, T.M. Dengue Virus Infection of Aedes Aegypti Alters Extracellular Vesicle Protein Cargo to Enhance Virus Transmission. Int. J. Mol. Sci. 2020, 21, 6609. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Woda, M.; Ennis, F.A.; Libraty, D.H. Dengue Virus Infection Differentially Regulates Endothelial Barrier Function over Time through Type I Interferon Effects. J. Infect. Dis. 2009, 200, 191–201. [Google Scholar] [CrossRef]
- Yacoub, S.; Wertheim, H.; Simmons, C.P.; Screaton, G.; Wills, B. Cardiovascular Manifestations of the Emerging Dengue Pandemic. Nat. Rev. Cardiol. 2014, 11, 335–345. [Google Scholar] [CrossRef]
- Srikiatkhachorn, A.; Kelley, J.F. Endothelial Cells in Dengue Hemorrhagic Fever. Antivir. Res. 2014, 109, 160–170. [Google Scholar] [CrossRef]
- Chatterjee, V.; Yang, X.; Ma, Y.; Wu, M.H.; Yuan, S.Y. Extracellular Vesicles: New Players in Regulating Vascular Barrier Function. Am. J. Physiol. Heart Circ. Physiol. 2020, 319, H1181–H1196. [Google Scholar] [CrossRef]
- Wang, X.; He, B. Insight into Endothelial Cell-Derived Extracellular Vesicles in Cardiovascular Disease: Molecular Mechanisms and Clinical Implications. Pharmacol. Res. 2024, 207, 107309. [Google Scholar] [CrossRef] [PubMed]
- Guo, N.; Wang, Y.; Wen, Z.; Fan, X. Promising Nanotherapeutics of Stem Cell Extracellular Vesicles in Liver Regeneration. Regen. Ther. 2024, 26, 1037–1047. [Google Scholar] [CrossRef] [PubMed]
- Javdani-Mallak, A.; Salahshoori, I. Environmental Pollutants and Exosomes: A New Paradigm in Environmental Health and Disease. Sci. Total. Environ. 2024, 925, 171774. [Google Scholar] [CrossRef]
- Li, Y.; Tan, J.; Miao, Y.; Zhang, Q. MicroRNA in Extracellular Vesicles Regulates Inflammation through Macrophages under Hypoxia. Cell Death Discov. 2021, 7, 285. [Google Scholar] [CrossRef]
- Signorelli, S.S.; Mazzarino, M.C.; Di Pino, L.; Malaponte, G.; Porto, C.; Pennisi, G.; Marchese, G.; Costa, M.P.; Digrandi, D.; Celotta, G.; et al. High Circulating Levels of Cytokines (IL-6 and TNFalpha), Adhesion Molecules (VCAM-1 and ICAM-1) and Selectins in Patients with Peripheral Arterial Disease at Rest and after a Treadmill Test. Vasc. Med. 2003, 8, 15–19. [Google Scholar] [CrossRef]
- Chiaradia, E.; Tancini, B.; Emiliani, C.; Delo, F.; Pellegrino, R.M.; Tognoloni, A.; Urbanelli, L.; Buratta, S. Extracellular Vesicles under Oxidative Stress Conditions: Biological Properties and Physiological Roles. Cells 2021, 10, 1763. [Google Scholar] [CrossRef] [PubMed]
- Tang, F.; Zhao, X.-L.; Xu, L.-Y.; Zhang, J.-N.; Ao, H.; Peng, C. Endothelial Dysfunction: Pathophysiology and Therapeutic Targets for Sepsis-Induced Multiple Organ Dysfunction Syndrome. Biomed. Pharmacother. 2024, 178, 117180. [Google Scholar] [CrossRef] [PubMed]
- Meuren, L.M.; Prestes, E.B.; Papa, M.P.; de Carvalho, L.R.P.; Mustafá, Y.M.; da Costa, L.S.; Da Poian, A.T.; Bozza, M.T.; Arruda, L.B. Infection of Endothelial Cells by Dengue Virus Induces ROS Production by Different Sources Affecting Virus Replication, Cellular Activation, Death and Vascular Permeability. Front. Immunol. 2022, 13, 810376. [Google Scholar] [CrossRef]
- Ferrari, M.; Zevini, A.; Palermo, E.; Muscolini, M.; Alexandridi, M.; Etna, M.P.; Coccia, E.M.; Fernandez-Sesma, A.; Coyne, C.; Zhang, D.D.; et al. Dengue Virus Targets Nrf2 for NS2B3-Mediated Degradation Leading to Enhanced Oxidative Stress and Viral Replication. J. Virol. 2020, 94, e01551-20. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-S.; Rustamov, N.; Roh, Y.-S. The Roles of NFR2-Regulated Oxidative Stress and Mitochondrial Quality Control in Chronic Liver Diseases. Antioxidants 2023, 12, 1928. [Google Scholar] [CrossRef]
- Auber, M.; Svenningsen, P. An Estimate of Extracellular Vesicle Secretion Rates of Human Blood Cells. J. Extracell. Biol. 2022, 1, e46. [Google Scholar] [CrossRef]
- Limothai, U.; Jantarangsi, N.; Suphavejkornkij, N.; Tachaboon, S.; Dinhuzen, J.; Chaisuriyong, W.; Trongkamolchai, S.; Wanpaisitkul, M.; Chulapornsiri, C.; Tiawilai, A.; et al. Discovery and Validation of Circulating miRNAs for the Clinical Prognosis of Severe Dengue. PLoS Neglected Trop. Dis. 2022, 16, e0010836. [Google Scholar] [CrossRef]
- Ouyang, X.; Jiang, X.; Gu, D.; Zhang, Y.; Kong, S.K.; Jiang, C.; Xie, W. Dysregulated Serum MiRNA Profile and Promising Biomarkers in Dengue-Infected Patients. Int. J. Med. Sci. 2016, 13, 195–205. [Google Scholar] [CrossRef]
- Tambyah, P.A.; Ching, C.S.; Sepramaniam, S.; Ali, J.M.; Armugam, A.; Jeyaseelan, K. microRNA Expression in Blood of Dengue Patients. Ann. Clin. Biochem. 2016, 53, 466–476. [Google Scholar] [CrossRef]
- Hapugaswatta, H.; Amarasena, P.; Premaratna, R.; Seneviratne, K.N.; Jayathilaka, N. Differential Expression of microRNA, miR-150 and Enhancer of Zeste Homolog 2 (EZH2) in Peripheral Blood Cells as Early Prognostic Markers of Severe Forms of Dengue. J. Biomed. Sci. 2020, 27, 25. [Google Scholar] [CrossRef]
- Fernández-Hernando, C.; Suárez, Y. MicroRNAs in Endothelial Cell Homeostasis and Vascular Disease. Curr. Opin. Hematol. 2018, 25, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Pena-Philippides, J.C.; Gardiner, A.S.; Caballero-Garrido, E.; Pan, R.; Zhu, Y.; Roitbak, T. Inhibition of MicroRNA-155 Supports Endothelial Tight Junction Integrity Following Oxygen-Glucose Deprivation. J. Am. Heart Assoc. 2018, 7, e009244. [Google Scholar] [CrossRef] [PubMed]
- Punyadee, N.; Mairiang, D.; Thiemmeca, S.; Komoltri, C.; Pan-Ngum, W.; Chomanee, N.; Charngkaew, K.; Tangthawornchaikul, N.; Limpitikul, W.; Vasanawathana, S.; et al. Microparticles Provide a Novel Biomarker to Predict Severe Clinical Outcomes of Dengue Virus Infection. J. Virol. 2015, 89, 1587–1607. [Google Scholar] [CrossRef]
- König, L.; Kasimir-Bauer, S.; Bittner, A.-K.; Hoffmann, O.; Wagner, B.; Santos Manvailer, L.F.; Kimmig, R.; Horn, P.A.; Rebmann, V. Elevated Levels of Extracellular Vesicles Are Associated with Therapy Failure and Disease Progression in Breast Cancer Patients Undergoing Neoadjuvant Chemotherapy. OncoImmunology 2018, 7, e1376153. [Google Scholar] [CrossRef] [PubMed]
- Henao-Agudelo, J.S.; Ayala, S.; Badiel, M.; Zea-Vera, A.F.; Matta Cortes, L. Classical Monocytes-Low Expressing HLA-DR Is Associated with Higher Mortality Rate in SARS-CoV-2+ Young Patients with Severe Pneumonia. Heliyon 2024, 10, e24099. [Google Scholar] [CrossRef]
- Lucien, F.; Gustafson, D.; Lenassi, M.; Li, B.; Teske, J.J.; Boilard, E.; von Hohenberg, K.C.; Falcón-Perez, J.M.; Gualerzi, A.; Reale, A.; et al. MIBlood-EV: Minimal Information to Enhance the Quality and Reproducibility of Blood Extracellular Vesicle Research. J. Extracell. Vesicles 2023, 12, e12385. [Google Scholar] [CrossRef]
- Welsh, J.A.; Goberdhan, D.C.I.; O’Driscoll, L.; Buzas, E.I.; Blenkiron, C.; Bussolati, B.; Cai, H.; Di Vizio, D.; Driedonks, T.A.P.; Erdbrügger, U.; et al. Minimal Information for Studies of Extracellular Vesicles (MISEV2023): From Basic to Advanced Approaches. J. Extracell. Vesicles. 2024, 13, e12404. [Google Scholar] [CrossRef]
- Kowkabany, G.; Bao, Y. Nanoparticle Tracking Analysis: An Effective Tool to Characterize Extracellular Vesicles. Molecules 2024, 29, 4672. [Google Scholar] [CrossRef]
- Asish, P.R.; Dasgupta, S.; Rachel, G.; Bagepally, B.S.; Girish Kumar, C.P. Global Prevalence of Asymptomatic Dengue Infections—A Systematic Review and Meta-Analysis. Int. J. Infect. Dis. 2023, 134, 292–298. [Google Scholar] [CrossRef]
- Welsh, J.A.; Horak, P.; Wilkinson, J.S.; Ford, V.J.; Jones, J.C.; Smith, D.; Holloway, J.A.; Englyst, N.A. FCMPASS Software Aids Extracellular Vesicle Light Scatter Standardization. Cytom. A 2020, 97, 569–581. [Google Scholar] [CrossRef]
- Chen, H.-R.; Chuang, Y.-C.; Lin, Y.-S.; Liu, H.-S.; Liu, C.-C.; Perng, G.-C.; Yeh, T.-M. Dengue Virus Nonstructural Protein 1 Induces Vascular Leakage through Macrophage Migration Inhibitory Factor and Autophagy. PLoS Neglected Trop. Dis. 2016, 10, e0004828. [Google Scholar] [CrossRef]
- Metz, S.W.; Thomas, A.; White, L.; Stoops, M.; Corten, M.; Hannemann, H.; de Silva, A.M. Dengue Virus-like Particles Mimic the Antigenic Properties of the Infectious Dengue Virus Envelope. Virol. J. 2018, 15, 60. [Google Scholar] [CrossRef] [PubMed]
- Saini, J.; Bandyopadhyay, B.; Pandey, A.D.; Ramachandran, V.G.; Das, S.; Sood, V.; Banerjee, A.; Vrati, S. High-Throughput RNA Sequencing Analysis of Plasma Samples Reveals Circulating microRNA Signatures with Biomarker Potential in Dengue Disease Progression. mSystems 2020, 5, e00724-20. [Google Scholar] [CrossRef] [PubMed]
- Yaghoubi, S.; Najminejad, H.; Dabaghian, M.; Karimi, M.H.; Abdollahpour-Alitappeh, M.; Rad, F.; Mahi-Birjand, M.; Mohammadi, S.; Mohseni, F.; Sobhani Lari, M.; et al. How Hypoxia Regulate Exosomes in Ischemic Diseases and Cancer Microenvironment? IUBMB Life 2020, 72, 1286–1305. [Google Scholar] [CrossRef]
- Gan, E.S.; Cheong, W.F.; Chan, K.R.; Ong, E.Z.; Chai, X.; Tan, H.C.; Ghosh, S.; Wenk, M.R.; Ooi, E.E. Hypoxia Enhances Antibody-dependent Dengue Virus Infection. EMBO J. 2017, 36, 1348–1363. [Google Scholar] [CrossRef]
- Roy, S.K.; Bhattacharjee, S. Dengue Virus: Epidemiology, Biology, and Disease Aetiology. Can. J. Microbiol. 2021, 67, 687–702. [Google Scholar] [CrossRef] [PubMed]
- Sahin, M.; Remy, M.M.; Merkler, D.; Pinschewer, D.D. The Janus Kinase Inhibitor Ruxolitinib Prevents Terminal Shock in a Mouse Model of Arenavirus Hemorrhagic Fever. Microorganisms 2021, 9, 564. [Google Scholar] [CrossRef]
- Branche, E.; Tang, W.W.; Viramontes, K.M.; Young, M.P.; Sheets, N.; Joo, Y.; Nguyen, A.-V.T.; Shresta, S. Synergism Between the Tyrosine Kinase Inhibitor Sunitinib and Anti-TNF Antibody Protects Against Lethal Dengue Infection. Antivir. Res. 2018, 158, 1–7. [Google Scholar] [CrossRef]
- Srikiatkhachorn, A.; Mathew, A.; Rothman, A.L. Immune-Mediated Cytokine Storm and Its Role in Severe Dengue. Semin. Immunopathol. 2017, 39, 563–574. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agudelo, J.S.H.; Pereira, G.; Fernandes, C.J.d.C. Role of Extracellular Vesicles in Severe Dengue: Virus–Host Interactions and Biomarker Potential. Viruses 2025, 17, 807. https://doi.org/10.3390/v17060807
Agudelo JSH, Pereira G, Fernandes CJdC. Role of Extracellular Vesicles in Severe Dengue: Virus–Host Interactions and Biomarker Potential. Viruses. 2025; 17(6):807. https://doi.org/10.3390/v17060807
Chicago/Turabian StyleAgudelo, Juan Sebastian Henao, Gabriel Pereira, and Célio Junior da Costa Fernandes. 2025. "Role of Extracellular Vesicles in Severe Dengue: Virus–Host Interactions and Biomarker Potential" Viruses 17, no. 6: 807. https://doi.org/10.3390/v17060807
APA StyleAgudelo, J. S. H., Pereira, G., & Fernandes, C. J. d. C. (2025). Role of Extracellular Vesicles in Severe Dengue: Virus–Host Interactions and Biomarker Potential. Viruses, 17(6), 807. https://doi.org/10.3390/v17060807




