Proteomics Analysis of Peripheral Blood Mononuclear Cells from Patients in Early Dengue Infection Reveals Potential Markers of Subsequent Fluid Leakage
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Setting and Patient Recruitment
2.2. Measurement of Viral Load in Serum
2.3. Measurement of Dengue-Specific IgG in Serum
2.4. PBMC Isolation
2.5. Tandem Mass Tagging
2.6. Liquid Chromatography
2.7. Mass Spectrometry
2.8. Protein Identification
2.9. Proteomic Data Analysis
3. Results
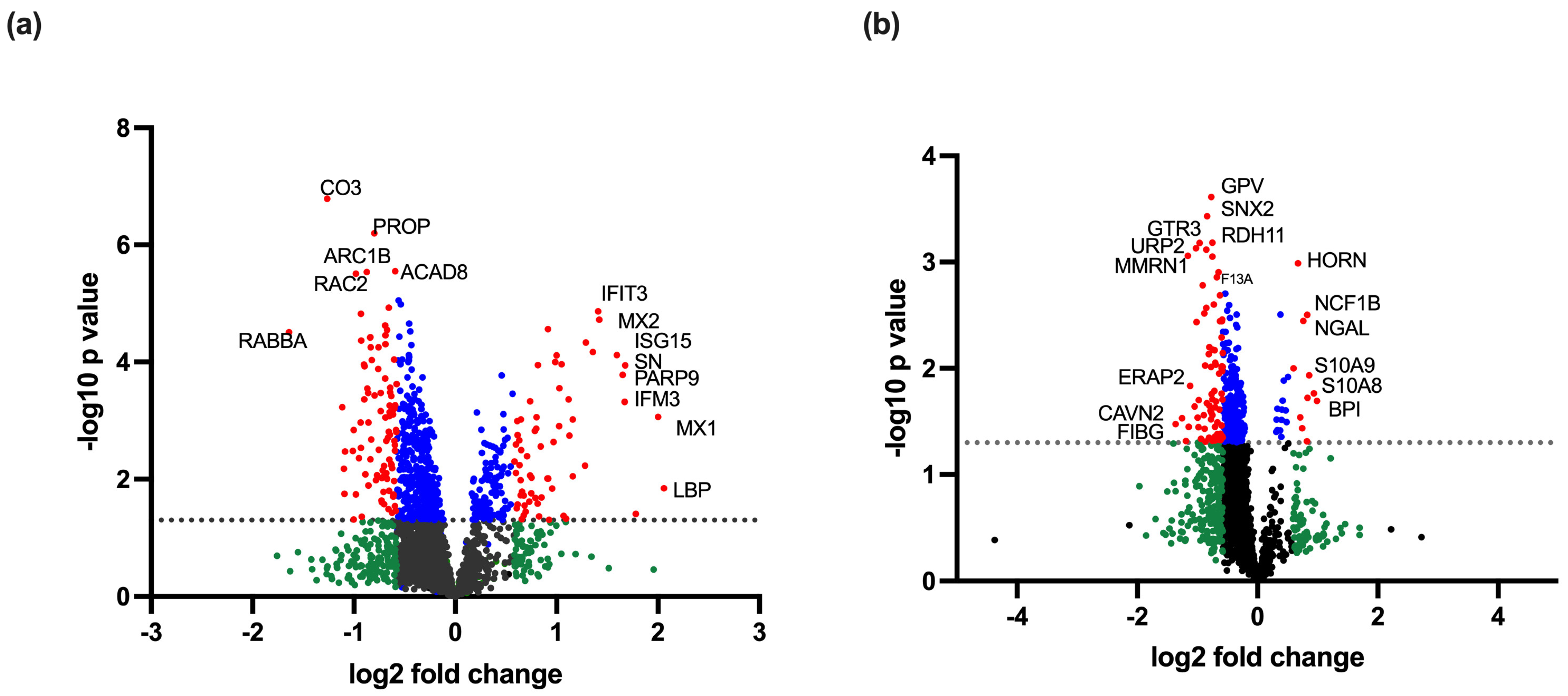
3.1. Proteins Differentially Regulated in Dengue-Infected PBMCs Compared to Healthy Controls
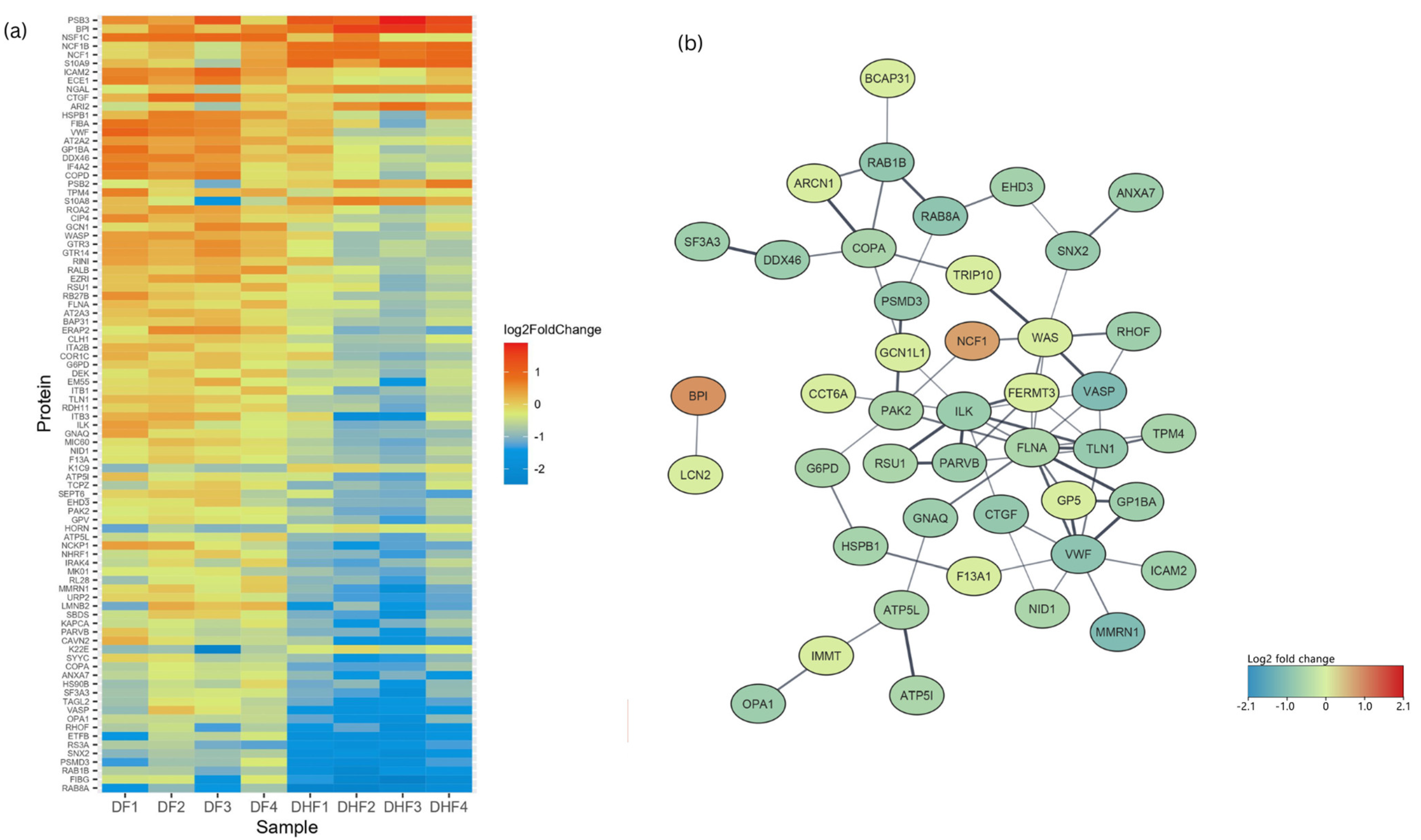
3.2. Proteins Differentially Regulated in DHF Compared to DF
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ALT | Alanine transaminase |
| AST | Aspartate transaminase |
| DENV | Dengue virus infection |
| DF | Dengue fever |
| DHF | Dengue haemorrhagic fever |
| NS1 | Non-structural protein 1 |
| ISG | Interferon-stimulated gene |
| MAPK | Mitogen-activated protein kinase |
| PBMC | Peripheral blood mononuclear cell |
| qRT-PCR | Quantitative reverse transcription polymerase chain reaction |
| ROS | Reactive oxygen species |
| TMT | Tandem mass tag |
References
- Bhatt, S.; Gething, P.W.; Brady, O.J.; Messina, J.P.; Farlow, A.W.; Moyes, C.L.; Drake, J.M.; Brownstein, J.S.; Hoen, A.G.; Sankoh, O.; et al. The global distribution and burden of dengue. Nature 2013, 496, 504–507. [Google Scholar] [CrossRef]
- Beatty, M.E.; Beutels, P.; Meltzer, M.I.; Shepard, D.S.; Hombach, J.; Hutubessy, R.; Dessis, D.; Coudeville, L.; Dervaux, B.; Wichmann, O.; et al. Health economics of dengue: A systematic literature review and expert panel’s assessment. Am. J. Trop. Med. Hyg. 2011, 84, 473–488. [Google Scholar] [CrossRef]
- World Health Organization. Dengue and Severe Dengue. 2024. Available online: https://www.who.int/news-room/fact-sheets/detail/dengue-and-severe-dengue (accessed on 12 February 2023).
- Kalayanarooj, S. Clinical manifestations and management of dengue/DHF/DSS. Trop. Med. Health 2011, 39, 83–87. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Regional Office for South-East Asia. In Comprehensive Guidelines for Prevention and Control of Dengue and Dengue Haemorrhagic Fever; World Health Organization: Geneva, Switzerland, 2011; 196p. [Google Scholar]
- Khan, M.B.; Yang, Z.S.; Lin, C.Y.; Hsu, M.C.; Urbina, A.N.; Assavalapsakul, W.; Wang, W.H.; Chen, Y.H.; Wang, S.F. Dengue overview: An updated systemic review. J. Infect. Public health 2023, 16, 1625–1642. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Dengue Guidelines for Diagnosis, Treatment, Prevention and Control. 2009. Available online: https://www.who.int/publications/i/item/9789241547871 (accessed on 14 April 2023).
- Rodrigo, C.; Sigera, C.; Fernando, D.; Rajapakse, S. Plasma leakage in dengue: A systematic review of prospective observational studies. BMC Infect. Dis. 2021, 21, 1082. [Google Scholar] [CrossRef]
- Barbachano-Guerrero, A.; Endy, T.P.; King, C.A. Dengue virus non-structural protein 1 activates the p38 MAPK pathway to decrease barrier integrity in primary human endothelial cells. J. Gen.Virol. 2020, 101, 484–496. [Google Scholar] [CrossRef]
- Glasner, D.R.; Ratnasiri, K.; Puerta-Guardo, H.; Espinosa, D.A.; Beatty, P.R.; Harris, E. Dengue virus NS1 cytokine-independent vascular leak is dependent on endothelial glycocalyx components. PLoS Pathog. 2017, 13, e1006673. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, P.; Sabeena, S.P.; Varma, M.; Arunkumar, G. Current Understanding of the Pathogenesis of Dengue Virus Infection. Curr. Microbiol. 2021, 78, 17–32. [Google Scholar] [CrossRef]
- Sekaran, S.D.; Ismail, A.A.; Thergarajan, G.; Chandramathi, S.; Rahman, S.K.H.; Mani, R.R.; Jusof, F.F.; Lim, Y.A.L.; Manikam, R. Host immune response against DENV and ZIKV infections. Front. Cell. Infect. Microbiol. 2022, 12, 975222. [Google Scholar] [CrossRef]
- Robinson, M.L.; Glass, D.R.; Duran, V.; Rojas, O.L.A.; Sanz, A.M.; Consuegra, M.; Sahoo, M.K.; Hartmann, F.J.; Bosse, M.; Gelvez, R.M.; et al. Magnitude and Kinetics of the Human Immune Cell Response Associated with Severe Dengue Progression by Single-Cell Proteomics. Sci. Adv. 2023, 9, eade7702. [Google Scholar] [CrossRef]
- Fernandes-Santos, C.; de Azeredo, E.L. Innate Immune Response to Dengue Virus: Toll-like Receptors and Antiviral Response. Viruses 2022, 14, 992. [Google Scholar] [CrossRef]
- King, C.A.; Wegman, A.D.; Endy, T.P. Mobilization and Activation of the Innate Immune Response to Dengue Virus. Front. Cell. Infect. Microbiol. 2020, 10, 574417. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.; Shukla, S.; Pandey, A.D.; Goswami, S.; Bandyopadhyay, B.; Ramachandran, V.; Das, S.; Malhotra, A.; Agarwal, A.; Adhikari, S.; et al. RNA-Seq analysis of peripheral blood mononuclear cells reveals unique transcriptional signatures associated with disease progression in dengue patients. Transl. Res. 2017, 186, 62–78.e9. [Google Scholar] [CrossRef] [PubMed]
- Popper, S.J.; Gordon, A.; Liu, M.; Balmaseda, A.; Harris, E.; Relman, D.A. Temporal Dynamics of the Transcriptional Response to Dengue Virus Infection in Nicaraguan Children. PLoS Negl. Trop. Dis. 2012, 6, e1966. [Google Scholar] [CrossRef] [PubMed]
- Hoang, L.T.; Lynn, D.J.; Henn, M.; Birren, B.W.; Lennon, N.J.; Le, P.T.; Duong, K.T.H.; Nguyen, T.T.H.; Mai, L.N.; Farrar, J.J.; et al. The Early Whole-Blood Transcriptional Signature of Dengue Virus and Features Associated with Progression to Dengue Shock Syndrome in Vietnamese Children and Young Adults. J. Virol. 2010, 84, 12982–12994. [Google Scholar] [CrossRef]
- Jadhav, M.; Nayak, M.; Kumar, S.; Venkatesh, A.; Patel, S.K.; Kumar, V.; Sharma, S.; Samanta, B.; Deb, S.; Karak, A.; et al. Clinical Proteomics and Cytokine Profiling for Dengue Fever Disease Severity Biomarkers. OMICS 2017, 21, 665–677. [Google Scholar] [CrossRef]
- Fragnoud, R.; Flamand, M.; Reynier, F.; Buchy, P.; Duong, V.; Pachot, A.; Paranhos-Baccala, G.; Bedin, F. Differential proteomic analysis of virus-enriched fractions obtained from plasma pools of patients with dengue fever or severe dengue. BMC Infect. Dis. 2015, 15, 518. [Google Scholar] [CrossRef][Green Version]
- Manchala, N.R.; Dungdung, R.; Pilankatta, R. Proteomic analysis reveals the enhancement of human serum apolipoprotein A-1(APO A-1) in individuals infected with multiple dengue virus serotypes. Trop. Med. Int. Health 2017, 22, 1334–1342. [Google Scholar] [CrossRef]
- Han, L.; Ao, X.; Lin, S.; Guan, S.; Zheng, L.; Han, X.; Ye, H. Quantitative Comparative Proteomics Reveal Biomarkers for Dengue Disease Severity. Front. Microbiol. 2019, 10, 2836. [Google Scholar] [CrossRef]
- Metatla, I.; Roger, K.; Chhuon, C.; Ceccacci, S.; Chapelle, M.; Schmit, P.-O.; Demichev, V.; Guerrera, I.C. Neat plasma proteomics: Getting the best out of the worst. Clin. Proteom. 2024, 21, 22. [Google Scholar] [CrossRef]
- Ashburner, M.; Ball, C.A.; Blake, J.A.; Botstein, D.; Butler, H.; Cherry, J.M.; Davis, A.P.; Dolinski, K.; Dwight, S.S.; Eppig, J.T.; et al. Gene Ontology: Tool for the unification of biology. Nat. Genet. 2000, 25, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Fabregat, A.; Jupe, S.; Matthews, L.; Sidiropoulos, K.; Gillespie, M.; Garapati, P.; Haw, R.; Jassal, B.; Korninger, F.; May, B.; et al. The reactome pathway knowledgebase 2022. Nucleic Acids Res. 2022, 50, D687–D692. [Google Scholar]
- Kanehisa, M.; Goto, S. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef]
- Nikolayeva, I.; Bost, P.; Casademont, I.; Duong, V.; Koeth, F.; Prot, M.; Czerwinska, U.; Ly, S.; Bleakley, K.; Cantaert, T.; et al. A blood RNA signature detecting severe disease in young dengue patients at hospital arrival. J. Infect. Dis. 2018, 217, 1690–1698. [Google Scholar] [CrossRef]
- Jose, S.; Jerome, R.; Krishnan, A.; Jagan, O.A.; Li, D.; Menon, V. Differential Expression Patterns of Indoleamine 2,3-Dioxygenase 1 and Other Tryptophan and Arginine Catabolic Pathway Genes in Dengue Correlate with Clinical Severity-Pilot Study Results. Viral Immunol. 2023, 36, 268–281. [Google Scholar] [CrossRef] [PubMed]
- Hapugaswatta, H.; Amarasena, P.; Premaratna, R.; Seneviratne, K.N.; Jayathilaka, N. Differential expression of microRNA, miR-150 and enhancer of zeste homolog 2 (EZH2) in peripheral blood cells as early prognostic markers of severe forms of dengue. J. Biomed. Sci. 2020, 27, 25. [Google Scholar] [CrossRef]
- Robinson, M.; Sweeney, T.E.; Barouch-Bentov, R.; Sahoo, M.K.; Kalesinskas, L.; Vallania, F.; Sanz, A.M.; Ortiz-Lasso, E.; Albornoz, L.L.; Rosso, F.; et al. A 20-Gene Set Predictive of Progression to Severe Dengue. Cell Rep. 2019, 26, 1104–1111.e4. [Google Scholar] [CrossRef]
- Trugilho, M.R.d.O.; Hottz, E.D.; Brunoro, G.V.F.; Teixeira-Ferreira, A.; Carvalho, P.C.; Salazar, G.A.; Zimmerman, G.A.; Bozza, F.A.; Bozza, P.T.; Perales, J. Platelet proteome reveals novel pathways of platelet activation and platelet-mediated immunoregulation in dengue. PLoS Pathog. 2017, 13, e1006385. [Google Scholar] [CrossRef]
- Mirzalieva, O.; Juncker, M.; Schwartzenburg, J.; Desai, S. ISG15 and ISGylation in Human Diseases. Cells 2022, 11, 538. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, W.; Li, J.; Wu, W.; Jiu, Y. The Role of Host Cytoskeleton in Flavivirus Infection. Virol. Sin. 2019, 34, 30–41. [Google Scholar] [CrossRef]
- Suttitheptumrong, A.; Mahutchariyakul, T.; Rawarak, N.; Reamtong, O.; Boonnak, K.; Pattanakitsakul, S.-N. Altered moesin and actin cytoskeleton protein rearrangements affect transendothelial permeability in human endothelial cells upon dengue virus infection and TNF-α treatment. Viruses 2021, 13, 2042. [Google Scholar] [CrossRef] [PubMed]
- Brasier, A.R.; Ju, H.; Garcia, J.; Spratt, H.M.; Victor, S.S.; Forshey, B.M.; Halsey, E.S.; Comach, G.; Sierra, G.; Blair, P.J.; et al. A three-component biomarker panel for prediction of dengue hemorrhagic fever. Am. J. Trop. Med. Hyg. 2012, 86, 341–348. [Google Scholar] [CrossRef]
- Petrich, B.G.; Marchese, P.; Ruggeri, Z.M.; Spiess, S.; Weichert, R.A.; Ye, F.; Tiedt, R.; Skoda, R.C.; Monkley, S.J.; Critchley, D.R.; et al. Talin is required for integrin-mediated platelet function in hemostasis and thrombosis. J. Exp. Med. 2007, 204, 3103–3111. [Google Scholar] [CrossRef]
- De Silva, E.; Hong, F.; Falet, H.; Kim, H. Filamin A in platelets: Bridging the (signaling) gap between the plasma membrane and the actin cytoskeleton. Front. Mol. Biosci. 2022, 9, 1060361. [Google Scholar] [CrossRef] [PubMed]
- Manz, X.D.; Bogaard, H.J.; Aman, J. Regulation of VWF (Von Willebrand Factor) in Inflammatory Thrombosis. Arterioscler. Thromb. Vasc. Biol. 2022, 42, 1307–1320. [Google Scholar] [CrossRef] [PubMed]
- Jamil, M.A.; Singh, S.; El-Maarri, O.; Oldenburg, J.; Biswas, A. Exploring Diverse Coagulation Factor XIII Subunit Expression Datasets: A Bioinformatic Analysis. Int J Mol Sci. 2022, 23, 4725. [Google Scholar] [CrossRef]
- Bagoly, Z.; Katona, É.; Muszbek, L. Factor XIII and inflammatory cells. Thromb Res. 2012, 129 (Suppl. S2), S77–S81. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, H.T.; Tu, H. MAPK signal pathways in the regulation of cell proliferation in mammalian cells. Cell Res. 2002, 12, 9–18. [Google Scholar] [CrossRef]
- Coulthard, L.R.; White, D.E.; Jones, D.L.; McDermott, M.F.; Burchill, S.A. p38MAPK: Stress responses from molecular mechanisms to therapeutics. Trends Mol. Med. 2009, 15, 369–379. [Google Scholar] [CrossRef]
- Zarubin, T.; Han, J. Activation and signaling of the p38 MAP kinase pathway. Cell Res. 2005, 15, 11–18. [Google Scholar] [CrossRef]
- Huerta-Zepeda, A.; Cabello-Gutiérrez, C.; Cime-Castillo, J.; Monroy-Martínez, V.; Manjarrez-Zavala, M.E.; Gutiérrez-Rodríguez, M.; Izaguirre, R.; Ruiz-Ordaz, B.H. Crosstalk between coagulation and inflammation during Dengue virus infection. Thromb. Haemost. 2008, 99, 936–943. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Yip, A.; Seah, P.G.; Blasco, F.; Shi, P.-Y.; Hervé, M. Modulation of inflammation and pathology during dengue virus infection by p38 MAPK inhibitor SB203580. Antivir. Res. 2014, 110, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Pillai, A.B.; Muthuraman, K.R.; Mariappan, V.; Belur, S.S.; Lokesh, S.; Rajendiran, S. Oxidative stress response in the pathogenesis of dengue virus virulence, disease prognosis and therapeutics: An update. Arch. Virol. 2019, 164, 2895–2908. [Google Scholar] [CrossRef]
- Meuren, L.M.; Prestes, E.B.; Papa, M.P.; de Carvalho, L.R.P.; Mustafá, Y.M.; da Costa, L.S.; Da Poian, A.T.; Bozza, M.T.; Arruda, L.B. Infection of endothelial cells by dengue virus induces ROS production by different sources affecting virus replication, cellular activation, death and vascular permeability. Front. Immunol. 2022, 13, 810376. [Google Scholar] [CrossRef]
- Castro, R.; Pinzón, H.S.; Alvis-Guzman, N. A systematic review of observational studies on oxidative/nitrosative stress involvement in dengue pathogenesis. Colomb. Medica 2015, 46, 135–143. [Google Scholar] [CrossRef]
- Hapugaswatta, H.; Wimalasekara, R.L.; Perera, S.S.; Premaratna, R.; Seneviratne, K.N.; Jayathilaka, N. Expression of Nitric Oxide Synthase and Nitric Oxide Levels in Peripheral Blood Cells and Oxidized Low-Density Lipoprotein Levels in Saliva as Early Markers of Severe Dengue. Biomed. Res. Int. 2021, 2021, 6650596. [Google Scholar] [CrossRef] [PubMed]
- Opasawatchai, A.; Amornsupawat, P.; Jiravejchakul, N.; Chan-In, W.; Spoerk, N.J.; Manopwisedjaroen, K.; Singhasivanon, P.; Yingtaweesak, T.; Suraamornkul, S.; Mongkolsapaya, J.; et al. Neutrophil activation and early features of net formation are associated with dengue virus infection in human. Front. Immunol. 2009, 9, 3007. [Google Scholar] [CrossRef]
- Garishah, F.M.; Rother, N.; Riswari, S.F.; Alisjahbana, B.; Overheul, G.J.; van Rij, R.P.; van der Ven, A.; van der Vlag, J.; de Mast, Q. Neutrophil Extracellular Traps in Dengue Are Mainly Generated NOX-Independently. Front. Immunol. 2021, 12, 629167. [Google Scholar] [CrossRef]
- Liu, K.-T.; Liu, Y.-H.; Lin, C.-Y.; Tsai, M.-J.; Hsu, Y.-L.; Yen, M.-C.; Kuo, P.-L. Serum neutrophil gelatinase-associated lipocalin and resistin are associated with dengue infection in adults. BMC Infect. Dis. 2016, 16, 441. [Google Scholar] [CrossRef]



| Clinical Parameter | DF n = 4 | DHF n = 4 | Significance (p-Value) |
|---|---|---|---|
| Age in years, mean (SD) | 25.7 (7.9) | 38.8 (8.8) | 0.07 |
| Males (n, %) | 2 (50) | 2 (50) | 1.0 |
| Laboratory investigations on admission in the febrile phase, mean (SD) | |||
| White cells (×109/L) | 6.2 (3.3) | 5.5 (1.46) | 0.71 |
| Platelets (×109/L) | 189 (82) | 193 (62) | 0.94 |
| Packed cell volume (%) | 39.9 (3.2) | 39.2 (5.1) | 0.82 |
| Highest/lowest values of laboratory investigations during the course of illness, mean (SD) | |||
| Lowest white cells (×109/L) | 2.7 (0.4) | 4.4 (1.5) | 0.07 |
| Lowest platelets (×109/L) * | 70.5 (37.2–157) | 13.5 (12.2–26) | 0.03 |
| Highest packed cell volume (%) | 42.2 (5.2) | 45.2 (7.5) | 0.53 |
| Liver enzymes, median (IQR) | |||
| AST (U/L) | 68.7 (30.4–157) | 65 (51.9–394.2) | 0.56 |
| ALT (U/L) | 65.1 (40.6–154.7) | 54.8 (28.9–339.5) | 0.89 |
| DENV diagnosis, n(%) | |||
| Positive NS1 rapid kit | 4 (100) | 4 (100) | 1.0 |
| Virus detected by qRT-PCR | 4 (100) | 4 (100) | 1.0 |
| Viral load in the acute phase (log10 GE) * | 2.1 (1.5–5.0) | 1.9 (1.4–2.3) | 0.38 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perera, N.; Kumar, A.; Gangadharan, B.; Ranasinghe, D.; Wijewickrama, A.; Malavige, G.N.; Miller, J.L.; Zitzmann, N. Proteomics Analysis of Peripheral Blood Mononuclear Cells from Patients in Early Dengue Infection Reveals Potential Markers of Subsequent Fluid Leakage. Viruses 2025, 17, 805. https://doi.org/10.3390/v17060805
Perera N, Kumar A, Gangadharan B, Ranasinghe D, Wijewickrama A, Malavige GN, Miller JL, Zitzmann N. Proteomics Analysis of Peripheral Blood Mononuclear Cells from Patients in Early Dengue Infection Reveals Potential Markers of Subsequent Fluid Leakage. Viruses. 2025; 17(6):805. https://doi.org/10.3390/v17060805
Chicago/Turabian StylePerera, Nilanka, Abhinav Kumar, Bevin Gangadharan, Diyanath Ranasinghe, Ananda Wijewickrama, Gathsaurie Neelika Malavige, Joanna L. Miller, and Nicole Zitzmann. 2025. "Proteomics Analysis of Peripheral Blood Mononuclear Cells from Patients in Early Dengue Infection Reveals Potential Markers of Subsequent Fluid Leakage" Viruses 17, no. 6: 805. https://doi.org/10.3390/v17060805
APA StylePerera, N., Kumar, A., Gangadharan, B., Ranasinghe, D., Wijewickrama, A., Malavige, G. N., Miller, J. L., & Zitzmann, N. (2025). Proteomics Analysis of Peripheral Blood Mononuclear Cells from Patients in Early Dengue Infection Reveals Potential Markers of Subsequent Fluid Leakage. Viruses, 17(6), 805. https://doi.org/10.3390/v17060805






