Key Laboratory Markers for Early Detection of Severe Dengue
Abstract
1. Introduction
2. Materials and Methods
Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Solomon, T.; Mallewa, M. Dengue and Other Emerging Flaviviruses. J. Infect. 2001, 42, 104–115. [Google Scholar] [CrossRef] [PubMed]
- Halstead, S.B. Dengue. Lancet 2007, 370, 1644–1652. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Dengue: Guidelines for Diagnosis, Treatment, Prevention and Control; World Health Organization: Geneva, Switzerland, 2009; ISBN 9241547871. [Google Scholar]
- Jaenisch, T.; Tam, D.T.H.; Kieu, N.T.T.; Van Ngoc, T.; Nam, N.T.; Van Kinh, N.; Yacoub, S.; Chanpheaktra, N.; Kumar, V.; See, L.L.C. Clinical Evaluation of Dengue and Identification of Risk Factors for Severe Disease: Protocol for a Multicentre Study in 8 Countries. BMC Infect. Dis. 2016, 16, 120. [Google Scholar] [CrossRef]
- Chuang, Y.-C.; Lin, Y.-S.; Liu, C.-C.; Liu, H.-S.; Liao, S.-H.; Shi, M.-D.; Lei, H.-Y.; Yeh, T.-M. Factors Contributing to the Disturbance of Coagulation and Fibrinolysis in Dengue Virus Infection. J. Formos. Med. Assoc. 2013, 112, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, A.C.N.; Montalvão, S.A.L.; Barbosa, K.G.N.; Colella, M.P.; Annichino-Bizzacchi, J.M.; Ozelo, M.C.; De Paula, E.V. Prolonged APTT of Unknown Etiology: A Systematic Evaluation of Causes and Laboratory Resource Use in an Outpatient Hemostasis Academic Unit. Res. Pract. Thromb. Haemost. 2019, 3, 749–757. [Google Scholar] [CrossRef]
- Hassan, J.; Borhany, M.; Abid, M.; Zaidi, U.; Fatima, N.; Shamsi, T. Coagulation Abnormalities in Dengue and Dengue Haemorrhagic Fever Patients. Transfus. Med. 2020, 30, 46–50. [Google Scholar] [CrossRef]
- Dewan, N.; Zuluaga, D.; Osorio, L.; Krienke, M.-E.; Bakker, C.; Kirsch, J. Ultrasound in Dengue: A Scoping Review. Am. J. Trop. Med. Hyg. 2021, 104, 826. [Google Scholar] [CrossRef]
- Ibrahim, M.A.; Hamzah, S.S.; Md Noor, J.; Mohamad, M.I.K.; Mokhtar, M.F.; Isa, M.R.; Abdul Rani, M.F. The Association of Ultrasound Assessment of Gallbladder Wall Thickness with Dengue Fever Severity. Ultrasound J. 2022, 14, 13. [Google Scholar] [CrossRef]
- Prashantha, B.; Varun, S.; Sharat, D.; Murali Mohan, B.V.; Ranganatha, R.; Shivaprasad; Naveen, M. Prophyactic Platelet Transfusion in Stable Dengue Fever Patients: Is It Really Necessary? Indian J. Hematol. Blood Transfus. 2014, 30, 126–129. [Google Scholar] [CrossRef]
- Archuleta, S.; Chia, P.Y.; Wei, Y.; Syed-Omar, S.F.; Low, J.G.; Oh, H.M.; Fisher, D.; Ponnampalavanar, S.S.L.; Wijaya, L.; Kamarulzaman, A. Predictors and Clinical Outcomes of Poor Platelet Recovery in Adult Dengue with Thrombocytopenia: A Multicenter, Prospective Study. Clin. Infect. Dis. 2020, 71, 383–389. [Google Scholar] [CrossRef]
- Lye, D.C.; Lee, V.J.; Sun, Y.; Leo, Y.S. Lack of Efficacy of Prophylactic Platelet Transfusion for Severe Thrombocytopenia in Adults with Acute Uncomplicated Dengue Infection. Clin. Infect. Dis. 2009, 48, 1262–1265. [Google Scholar] [CrossRef]
- Mongkolsapaya, J.; Dejnirattisai, W.; Xu, X.; Vasanawathana, S.; Tangthawornchaikul, N.; Chairunsri, A.; Sawasdivorn, S.; Duangchinda, T.; Dong, T.; Rowland-Jones, S. Original Antigenic Sin and Apoptosis in the Pathogenesis of Dengue Hemorrhagic Fever. Nat. Med. 2003, 9, 921–927. [Google Scholar] [CrossRef]
- Nandwani, S.; Bhakhri, B.K.; Singh, N.; Rai, R.; Singh, D.K. Early Hematological Parameters as Predictors for Outcomes in Children with Dengue in Northern India: A Retrospective Analysis. Rev. Soc. Bras. Med. Trop. 2021, 54, e05192020. [Google Scholar] [CrossRef]
- Arshad, H.; Bashir, M.; Mushtaq, U.S.; Imtiaz, H.; Rajpar, R.; Alam, M.F.; Fatima, S.; Rehman, A.; Abbas, K.; Talpur, A.S. Clinical Characteristics and Symptomatology Associated with Dengue Fever. Cureus 2022, 14, e26677. [Google Scholar] [CrossRef] [PubMed]
- Srisuphanunt, M.; Puttaruk, P.; Kooltheat, N.; Katzenmeier, G.; Wilairatana, P. Prognostic Indicators for the Early Prediction of Severe Dengue Infection: A Retrospective Study in a University Hospital in Thailand. Trop. Med. Infect. Dis. 2022, 7, 162. [Google Scholar] [CrossRef] [PubMed]
- Shabbir, M.; Ameen, F.; Roshan, N.; Israr, M. Nature and Clinical Course of Pleural Effusion in Dengue Fever. Intern. J. Intern. Emerg. Med. 2018, 1, 1006. [Google Scholar]
- Lee, T.-H.; Wong, J.G.X.; Leo, Y.-S.; Thein, T.-L.; Ng, E.-L.; Lee, L.K.; Lye, D.C. Potential Harm of Prophylactic Platelet Transfusion in Adult Dengue Patients. PLoS Negl. Trop. Dis. 2016, 10, e0004576. [Google Scholar] [CrossRef]
- Motla, M.; Manaktala, S.; Gupta, V.; Aggarwal, M.; Bhoi, S.K.; Aggarwal, P.; Goel, A. Sonographic Evidence of Ascites, Pleura-Pericardial Effusion and Gallbladder Wall Edema for Dengue Fever. Prehosp. Disaster Med. 2011, 26, 335–341. [Google Scholar] [CrossRef]
- Dayanand, K.R.; Kavitha, K.; Anilesh, P.S.; Chiranth, N. Correlation of Ultrasound (USG) Findings with Serological Tests in Dengue Fever. J. Evid. Based Med. Healthc. 2016, 3, 371–374. [Google Scholar]
- Adil, B.; Rabbani, A.; Ahmed, S.; Arshad Sr, I.; Khalid, M.A. Gall Bladder Wall Thickening in Dengue Fever–Aid in Labelling Dengue Hemorrhagic Fever and a Marker of Severity. Cureus 2020, 12, e11331. [Google Scholar] [CrossRef]
- Yuan, K.; Chen, Y.; Zhong, M.; Lin, Y.; Liu, L. Risk and Predictive Factors for Severe Dengue Infection: A Systematic Review and Meta-Analysis. PLoS ONE 2022, 17, e0267186. [Google Scholar] [CrossRef] [PubMed]
- Md Sani, S.S.; Han, W.H.; Bujang, M.A.; Ding, H.J.; Ng, K.L.; Amir Shariffuddin, M.A. Evaluation of Creatine Kinase and Liver Enzymes in Identification of Severe Dengue. BMC Infect. Dis. 2017, 17, 505. [Google Scholar] [CrossRef] [PubMed]
- Mallhi, T.H.; Khan, A.H.; Sarriff, A.; Adnan, A.S.; Khan, Y.H. Determinants of Mortality and Prolonged Hospital Stay among Dengue Patients Attending Tertiary Care Hospital: A Cross-Sectional Retrospective Analysis. BMJ Open 2017, 7, e016805. [Google Scholar] [CrossRef]
- Chi, C.-Y.; Sung, T.-C.; Chang, K.; Chien, Y.-W.; Hsu, H.-C.; Tu, Y.-F.; Huang, Y.-T.; Shih, H.-I. Development and Utility of Practical Indicators of Critical Outcomes in Dengue Patients Presenting to Hospital: A Retrospective Cross-Sectional Study. Trop. Med. Infect. Dis. 2023, 8, 188. [Google Scholar] [CrossRef] [PubMed]
- Logia, P.; Selvam, V.; Parasuraman, V.; Renuka, M.K.; Rajagopalan, R.E. Predictors of Clinically Significant Bleeding in Thrombocytopenic Dengue Patients Admitted to Intensive Care Unit: A Retrospective Study. Indian J. Crit. Care Med. 2023, 27, 888. [Google Scholar]
- Thomas, R.S.; Lenin, A.; Sathyendra, S.; Hansdak, S.G.; George, T.; Zachariah, A.; Iyadurai, R.; Belavendra, A.; Geevar, T.; Abraham, A.M. Activated Partial Thromboplastin Time (APTT) as a Screening Test for Predicting Bleeding Manifestations in Adult Patients with Dengue Infection–A Diagnostic Accuracy Study. J. Clin. Infect. Dis. Soc. 2024, 2, 6–11. [Google Scholar] [CrossRef]

| Variable | Platelet Transfusion (n = 102) | No Platelet Transfusion (n = 181) | Bleeding (n = 43) | No Bleeding (n = 240) | Hospital Stay (>6 Days) (n = 82) | Hospital Stay (<6 Days) (n = 201) |
|---|---|---|---|---|---|---|
| Sex (%) | ||||||
| 62 (60.8) | 117 (64.6) | 22 (51.2) | 157 (65.4) | 50 (61) | 129 (64.2) |
| 40 (39.2) | 64 (35.4) | 21 (48.8) | 83 (34.6) | 32 (39) | 72 (35.8) |
| p value | 0.518 a | 0.074 a | 0.612 a | |||
| Age (mean ± SD) | 30.5 ±12.1 | 32.01 ± 14 | 31.7 ± 14 | 31.42 ± 13.3 | 32.52 ± 14.8 | 31.03 ± 12.8 |
| 19 (18.6) | 42 (23.2) | 10 (23.2) | 51 (21.3) | 17 (20.7) | 44 (21.9) |
| 48 (47) | 64 (35.3) | 16 (37.2) | 96 (40) | 30 (36.6) | 82 (40.8) |
| 16 (15.6) | 35 (19.3) | 5 (11.6) | 46 (19.2) | 16 (19.5) | 35 (17.4) |
| 12 (11.7) | 12 (6.6) | 7 (16.3) | 17 (7.1) | 9 (11) | 15 (7.4) |
| 4 (3.9) | 18 (10) | 3 (7) | 19 (7.9) | 3 (3.7) | 19 (9.5) |
| 3 (2.9) | 10 (5.5) | 2 (4.6) | 11 (4.6) | 7 (8.5) | 6 (3) |
| p value | 0.094 a | 0.420 a | 0.173 a | |||
| Comorbidities (%) | ||||||
| Diabetes | 13 (12.7) | 19 (10.5) | 6 (13.9) | 26 (10.8) | 16 (19.5) | 16 (8) |
| Hypothyroidism, | 3 (2.9) | 10 (5.5) | 2 (4.6) | 11 (4.6) | 2 (2.4) | 11 (5.5) |
| systemic | ||||||
| Hypertension | 3 (2.9) | 10 (5.5) | 1 (2.3) | 12 (5) | 6 (7.3) | 7 (3.5) |
| Anemia | 1 (1) | 5 (2.8) | - | 6 (2.5) | 2 (2.4) | 4 (2) |
| Clinical features (%) | ||||||
| Abdominal pain- | 13 (12.7) | 108 (59.7) | - | 121 (50.4) | 28 (34.1) | 93 (46.3) |
| Fluid accumulation | 20 (19.6) | 34 (18.8) | - | 54 (22.5) | 15 (18.3) | 39 (19.4) |
| Rapid decrease in platelets with rise in hematocrit | 16 (15.7) | 7 (3.9) | - | 23 (9.6) | 9 (11) | 15 (7.5) |
| Persistent vomiting | 11 (10.7) | 31 (17.1) | - | 42 (17.5) | 12 (14.6) | 30 (14.9) |
| Radiology findings | ||||||
| Ascites (%) | 42 (41.2) | 22 (12.1) | 16 (37.2) | 48 (20) | 19 (23.2) | 45 (22.4) |
| p value | 0.000 a | 0.013 a | 0.886 a | |||
| Gall bladder wall thickening (%) | 31 (30.4) | 17 (9.4) | 12 (27.9) | 36 (15) | 10 (12.2) | 38 (18.9) |
| p value | 0.000 a | 0.038 a | 0.172 a | |||
| Splenomegaly (%) | 13 (12.7) | 16 (8.8) | 1 (2.3) | 28 (11.7) | 10 (12.2) | 19 (9.4) |
| p value | 0.298 a | 0.063 a | 0.490 a | |||
| Pleural effusion (%) | 26 (25.5) | 19(10.5) | 12 (27.9) | 33 (13.7) | 15 (18.3) | 30 (14.9) |
| p value | 0.001 a | 0.019 a | 0.482 a | |||
| Hepatomegaly (%) | 16 (15.7) | 13 (7.2) | 7 (16.3) | 22 (9.2) | 9 (11) | 20 (9.9) |
| p value | 0.024 a | 0.157 a | 0.796 a | |||
| Blood group (%) | ||||||
| 12 (17.6) | 34 (18.8) | 7 (16.3) | 39 (16.2) | 15 (18.3) | 31 (15.4) |
| 40 (33.3) | 59 (32) | 14 (32.5) | 85 (35.4) | 29 (35.4) | 70 (34.8) |
| 6 (5.9) | 13 (7.7) | 1 (2.3) | 18 (7.5) | 1 (1.2) | 18 (8.9) |
| 44 (43.1) | 75 (41.4) | 21 (48.8) | 98 (40.8) | 37 (45.1) | 82 (40.8) |
| p value | 0.391 a | 0.553 a | 0.124 a | |||
| Rh group (%) | ||||||
| 93 (91.2) | 164 (90.6) | 39 (90.7) | 218 (90.8) | 69 (84.1) | 188 (93.5) |
| 9 (8.8) | 17 (9.4) | 4 (9.3) | 22 (9.2) | 13 (15.8) | 13 (6.5) |
| p value | 0.874 a | 0.977 a | 0.013 a | |||
| Laboratory parameters | ||||||
| (Mean ± SD) WBC (cells/mm3) | 4360.1 ± 2698.1 | 5318.4 ± 3341.1 | 5053.7 ± 3657.8 | 4958.6 ± 3062.9 | 4939.88 ± 3062.3 | 4986.57 ± 3197.4 |
| p value | 0.014 b | 0.856 b | 0.910 b | |||
| PCV (%) | 42.5 ± 6.7 | 39.8 ± 6.3 | 41.2 ± 8 | 40.7 ± 6.3 | 39.8 ± 6 | 41.24 ± 6.7 |
| p value | 0.001 b | 0.641 b | 0.097 b | |||
| HB (g/dL) | 14.4 ± 2.5 | 13.2 ± 2.2 | 13.9 ± 2.7 | 13.5 ± 2.3 | 13.4 + 2.4 | 13.7 ± 2.3 |
| p value | 0.000 b | 0.391 b | 0.358 b | |||
| AST (U/L) | 147.4 ± 288.2 | 100.4 ±101.6 | 125.3 ± 147.7 | 115.97 ± 199 | 97.66 ± 112.6 | 125.4 ± 215.8 |
| p value | 0.048 b | 0.770 b | 0.270 b | |||
| ALT (U/L) | 73.1 ± 106.8 | 68.9 ± 70.4 | 63.6 ± 64.9 | 71.7 ± 88.3 | 57.11 ± 57.2 | 75.92 ± 93.8 |
| p value | 0.693 b | 0.567 b | 0.092 b | |||
| AST2/ALT | 338.5 ± 815.7 | 169.3 ± 189.8 | 274.5 ± 370.4 | 222.4 ± 540 | 188.8 ± 269.3 | 247.3 ± 589.4 |
| p value | 0.008 b | 0.544 b | 0.389 b | |||
| aPTT (sec) | 37.2 ± 8.5 | 32.7 ± 4.2 | 36.4 ± 6 | 34 ± 6.5 | 34 ± 6.3 | 34.5 ± 6.6 |
| p value | 0.000 b | 0.026 b | 0.572 b | |||
| PT (sec) | 14.8 ± 2 | 15 ± 1.8 | 15.2 ± 2.2 | 14.9 ± 1.8 | 15.5 ± 2.3 | 14.7 ± 1.6 |
| p value | 0.410 b | 0.437 b | 0.001 b | |||
| Platelet count (cells/mm3) | ||||||
| (Mean ± SD) | 26,473.5 ± 29,109 | 126,093.92 ± 86,996.4 | 26,179.07 ± 27,351.1 | 101,656.7 ± 88,126.7 | 72,093.9 ± 77,515.6 | 97,570.15 ± 88,623 |
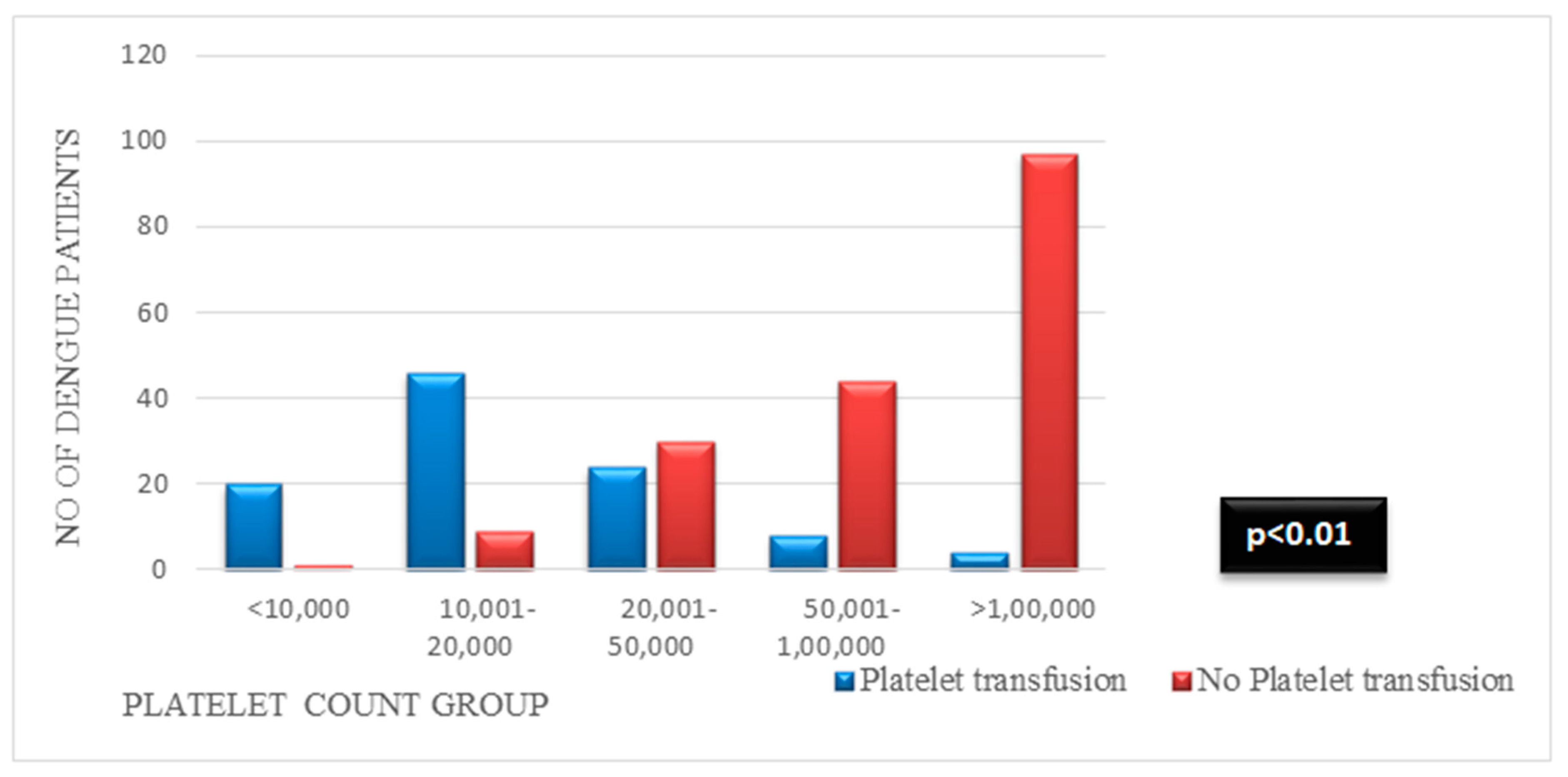
| <10,000 | 20 (19.6) | 1 (0.5) | 7 (16.2) | 14 (5.8) | 11 (13.4) | 10 (5) |
| 10,001–20,000 | 46 (45.1) | 9 (4.9) | 21 (48.9) | 34 (14.2) | 20 (24.4) | 35 (17.4) |
| 20,001–50,000 | 24 (23.5) | 30 (16.6) | 10 (23.2) | 44 (18.3) | 14 (17) | 40 (19.9) |
| 50,001–100,000 | 8 (7.8) | 44 (24.3) | 4 (9.3) | 48 (20) | 14 (17) | 38 (18.9) |
| >100,000 | 4 (3.9) | 97 (53.6) | 1 (2.3) | 100 (41.7) | 23 (28) | 78 (38.8) |
| p value | 0.000 b | 0.000 b | 0.024 b | |||
| Serological test (positive) (%) | ||||||
| NS1 | 93 (91.2)) | 128 (70.7) | 39 (90.7) | 182 (73.8) | 69 (84.1) | 152 (75.6) |
| p value | 0.000 a | 0.030 a | 0.116 a | |||
| IgM | 36 (35.3) | 97 (53.6) | 13 (30.2) | 120 (50) | 31 (37.8) | 102 (50.7) |
| p value | 0.003 a | 0.017 a | 0.048 a | |||
| IgG | 13 (12.7) | 3 (1.6) | 5 (11.6) | 11 (4.5) | 4 (4.9) | 12 (6) |
| p value | 0.000 a | 0.065 a | 0.718 a | |||
| Length of hospital stay days) | (6.5 ± 1.5) | (5.2 ± 2.2) | (6.6 ± 1.7) | (5.5 ± 2.1) | ||
| <6 days | 58 (56.9) | 143 (79) | 25 (58.1) | 176 (73.3) | ||
| >6 days | 44 (43.1)) | 38 (21) | 18 (41.9) | 64 (26.7) | _ | |
| p value | 0.000 a | 0.043 a | ||||
| Parameter | n (%) | Category | Odds Ratio and p Value |
|---|---|---|---|
| NS 1 | 221(78) | Pos | 4.279 (<0.001 c) |
| 62 (21.9) | Neg | ||
| ASCITES | 64 (22.6) | Yes | 5.059 (<0.001 c) |
| 219 (77.4) | No | ||
| GALL BLADDER WALL THICKENING/EDEMA | 48 (17) | Yes | 4.212 (<0.001 c) |
| 235 (83) | No | ||
| PLEURAL EFFUSION | 45 (15.9) | Yes | 2.917 (0.001 c) |
| 238 (84) | No | ||
| WBC (cells/mm3) | 194 (68.5) | <5318 | 2.137 (0.008 c) |
| 89 (31.4) | >5318 | ||
| HB (g/dL) | 111 (39.2) | >14.3 | 2.151 (0.003 c) |
| 172 (60.7) | <14.3 | ||
| AST2/ALT | 127 (45) | >169.3 | 2.431 (<0.001 c) |
| 156 (55.1) | <169.3 | ||
| aPTT (s) | 71 (25) | >37.2 | 5.815 (<0.001 c) |
| 211 (74.5) | <37.2 | ||
| Platelet count (cells/mm3) | 96 (33.9) | <26,473.5 | 26.261 (<0.001 c) |
| 187(66) | >26,473.5 |
| Parameter | n (%) | Category | Odds Ratio and p-Value |
|---|---|---|---|
| aPTT (secs) | 83 (29.3) | >36.4 | 3.074 (0.002 c) |
| 200 (70.6) | <36.4 | ||
| Platelet count (cells/mm3) | 94 (33.2) | <26,179.07 | 8.352 (<0.001 c) |
| 189 (66.8) | >26,179.07 |
| Parameter | n (%) | Category | Odds Ratio and p-Value |
|---|---|---|---|
| PT (secs) | 112 (39.6) | >15.5 | 2.432 (0.001 c) |
| 171 (60.4) | <15.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sivasubramanian, K.; Bharath R, R.; Vajravelu, L.K.; Kumar D, M.; Banerjee, A. Key Laboratory Markers for Early Detection of Severe Dengue. Viruses 2025, 17, 661. https://doi.org/10.3390/v17050661
Sivasubramanian K, Bharath R R, Vajravelu LK, Kumar D M, Banerjee A. Key Laboratory Markers for Early Detection of Severe Dengue. Viruses. 2025; 17(5):661. https://doi.org/10.3390/v17050661
Chicago/Turabian StyleSivasubramanian, Kumar, Raj Bharath R, Leela Kakithakara Vajravelu, Madan Kumar D, and Aritra Banerjee. 2025. "Key Laboratory Markers for Early Detection of Severe Dengue" Viruses 17, no. 5: 661. https://doi.org/10.3390/v17050661
APA StyleSivasubramanian, K., Bharath R, R., Vajravelu, L. K., Kumar D, M., & Banerjee, A. (2025). Key Laboratory Markers for Early Detection of Severe Dengue. Viruses, 17(5), 661. https://doi.org/10.3390/v17050661






