High Prevalence and Genetic Diversity of Human Norovirus Among Children Under 5 Years Old with Acute Gastroenteritis at the Dr. Leonardo Guzmán Regional Hospital, Antofagasta, Chile, 2019
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants and Stool Specimen Preparation
2.2. Viral RNA Extraction
2.3. Detection of Norovirus Genogroups by Real-Time RT-PCR
2.4. Amplification of the B-C Region of the Norovirus Genome by RT-PCR
2.5. Sequencing of Regions B-C of the Norovirus Genome
2.6. Statistics
3. Results
3.1. Epidemiological Data
3.2. Age Distribution of Children with AGE Infected with Norovirus
3.3. Distribution of Hospitalized and Ambulatory Settings of Children with AGE Infected with NoV
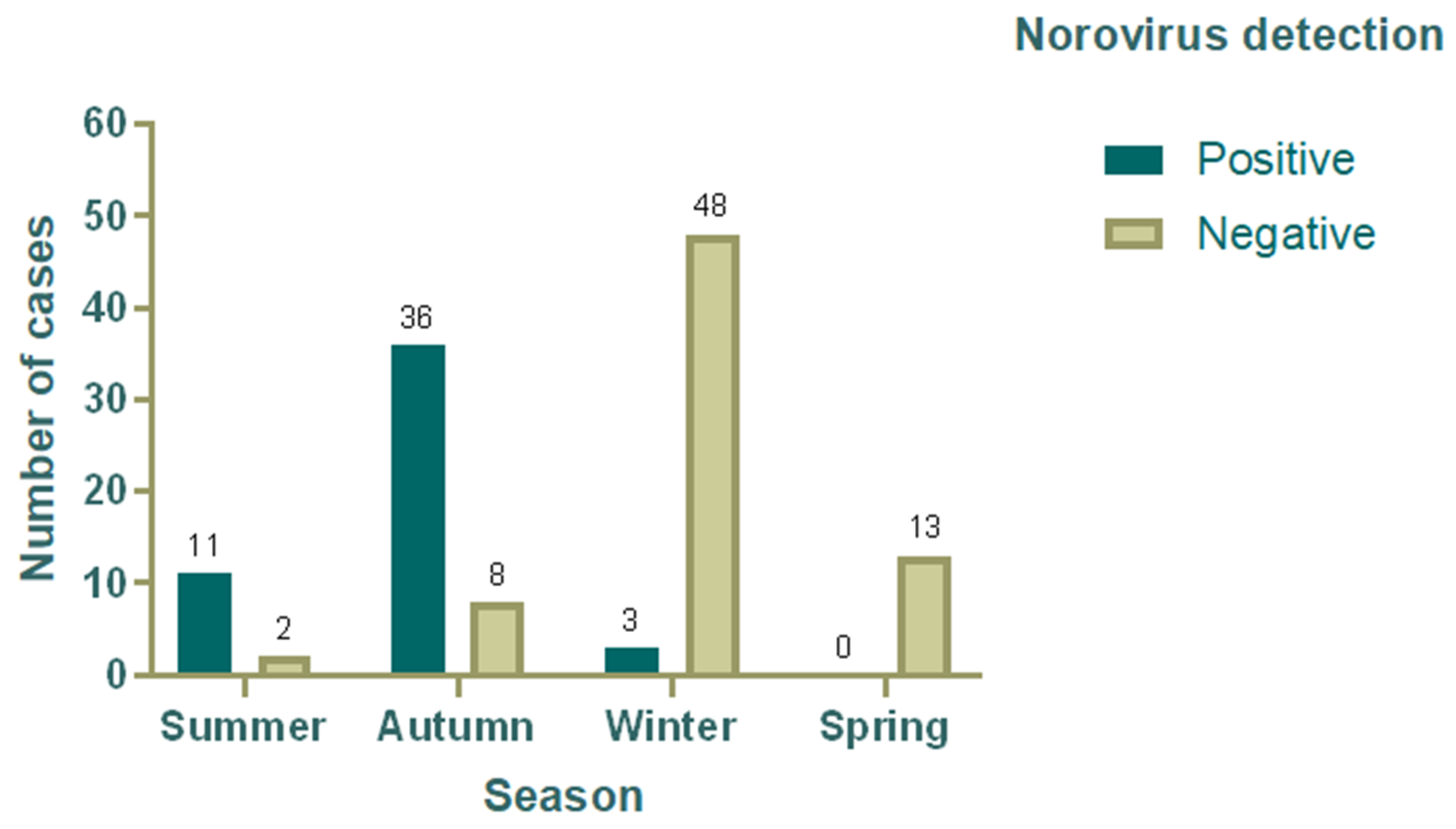
3.4. Seasonal Variation in the Detection Rate of Norovirus
3.5. Norovirus Genotype Distribution
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Disclaimer
References
- World Health Organization. Diarrhoeal Disease. Newsroom 2024. Available online: https://www.who.int/news-room/fact-sheets/detail/diarrhoeal-disease (accessed on 28 March 2025).
- Reymão, T.K.A.; Hernandez, J.D.M.; da Costa, S.T.P.; de Sousa, M.S.; Oliveira, D.d.S.; da Silva, L.D.; Bandeira, R.d.S.; de Lima, I.C.G.; Soares, L.d.S.; Mascarenhas, J.D.P.; et al. Sapoviruses in children with acute gastroenteritis from Manaus, Amazon region, Brazil, 2010–2011. Rev. Inst. Med. Trop. Sao Paulo 2016, 58, 81. [Google Scholar] [CrossRef] [PubMed]
- Burke, R.M.; Mattison, C.P.; Marsh, Z.; Shioda, K.; Donald, J.; Salas, S.B.; Naleway, A.L.; Biggs, C.; Schmidt, M.A.; Hall, A.J. Norovirus and Other Viral Causes of Medically Attended Acute Gastroenteritis across the Age Spectrum: Results from the Medically Attended Acute Gastroenteritis Study in the United States. Clin. Infect. Dis. 2021, 73, E913–E920. [Google Scholar] [CrossRef]
- Hikita, T.; Phan, T.; Okitsu, S.; Hayakawa, S.; Ushijima, H. A Comparative Study of Acute Gastroenteritis Symptoms in Single- versus Multiple-Virus Infections. Int. J. Mol. Sci. 2023, 24, 8364. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.R.; Chae, S.J.; Jung, S.; Choi, W.; Han, M.G.; Yoo, C.K.; Lee, D.Y. Trends in acute viral gastroenteritis among children aged ≤ 5 years through the national surveillance system in South Korea, 2013–2019. J. Med. Virol. 2021, 93, 4875–4882. [Google Scholar] [CrossRef]
- Lee, B. Update on rotavirus vaccine underperformance in low- to middle-income countries and next-generation vaccines. Hum. Vaccines Immunother. 2021, 17, 1787–1802. [Google Scholar] [CrossRef] [PubMed]
- Ben, L.; Baric, R.; Carolina, N.; Kang, C.; College, C.M.; Lee, B. Global Burden of Norovirus and Prospects for Vaccine Development Table of Contents; CDC STACKS: Atlanta, GA, USA, 2015. [Google Scholar]
- Burke, R.M.; Mattison, C.; Pindyck, T.; Dahl, M.; Rudd, J.; Bi, D.; Curns, A.T.; Hall, A.J. The Burden of Norovirus in the United States, as Estimated Based on Administrative Data. Clin. Infect. Dis. 2021, 73, e1–e8. [Google Scholar] [CrossRef]
- Pringle, K.; Lopman, B.; Vega, E.; Vinje, J.; Parashar, U.D.; Hall, A.J. Noroviruses: Epidemiology, immunity and prospects for prevention. Future Microbiol. 2015, 10, 53–67. [Google Scholar] [CrossRef]
- Green, K.Y. Caliciviridae: The Noroviruses. In Fields Virology, 6th ed; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2013; pp. 582–608. [Google Scholar]
- O’Ryan, M.; Riera-Montes, M.; Lopman, B. Norovirus in Latin America: Systematic review and meta-analysis. Pediatr. Infect. Dis. J. 2017, 36, 127–134. [Google Scholar] [CrossRef]
- Lucero, Y.; Matson, D.O.; Ashkenazi, S.; George, S.; O’ryan, M. Norovirus: Facts and reflections from past, present, and future. Viruses 2021, 13, 2399. [Google Scholar] [CrossRef]
- Vinjé, J.; Estes, M.K.; Esteves, P.; Green, K.Y.; Katayama, K.; Knowles, N.J.; L’Homme, Y.; Martella, V.; Vennema, H.; White, P.A. ICTV Virus Taxonomy Profile: Caliciviridae. J. Gen. Virol. 2019, 100, 1469–1470. [Google Scholar] [CrossRef]
- Alhatlani, B.; Vashist, S.; Goodfellow, I. Functions of the 5′ and 3′ ends of calicivirus genomes. Virus Res. 2015, 206, 134–143. [Google Scholar] [CrossRef]
- May, J.; Korba, B.; Medvedev, A.; Viswanathan, P. Enzyme kinetics of the human norovirus protease control virus polyprotein processing order. Virology 2013, 444, 218–224. [Google Scholar] [CrossRef]
- Chhabra, P.; de Graaf, M.; Parra, G.I.; Chan, M.C.W.; Green, K.; Martella, V.; Wang, Q.; White, P.A.; Katayama, K.; Vennema, H.; et al. Updated classification of norovirus genogroups and genotypes. J. Gen. Virol. 2019, 100, 1393–1406. [Google Scholar] [CrossRef]
- Cannon, J.L.; Barclay, L.; Collins, N.R.; Wikswo, M.E.; Castro, C.J.; Magaña, C.; Gregoricus, N.; Marine, R.L. Genetic and Epidemiologic Trends of Norovirus Outbreaks in the United Viruses. J. Clin. Microbiol. 2017, 55, 2208–2221. [Google Scholar] [CrossRef] [PubMed]
- Cannon, J.L.; Bonifacio, J.; Bucardo, F.; Buesa, J.; Bruggink, L.; Chi-Wai Chan, M.; Fumian, T.M.; Giri, S.; Gonzalez, M.D.; Hewitt, J.; et al. Global trends in norovirus genotype distribution among children with acute gastroenteritis. Emerg. Infect. Dis. 2021, 27, 1438–1445. [Google Scholar] [CrossRef]
- Kwok, K.; Niendorf, S.; Lee, N.; Hung, T.N.; Chan, L.Y.; Jacobsen, S.; Nelson, E.A.S.; Leung, T.F.; Lai, R.W.M.; Chan, P.K.S.; et al. Increased detection of emergent recombinant norovirus GII.P16-GII.2 strains in young adults, Hong Kong, China, 2016–2017. Emerg. Infect. Dis. 2017, 23, 1852–1855. [Google Scholar] [CrossRef]
- Zou, W.; Cui, D.; Wang, X.; Guo, H.; Yao, X.; Jin, M.; Huang, Q.; Gao, M.; Wen, X. Clinical characteristics and molecular epidemiology of noroviruses in outpatient children with acute gastroenteritis in huzhou of China. PLoS ONE 2015, 10, e0127596. [Google Scholar] [CrossRef]
- Wang, X.; Wei, Z.; Guo, J.; Cai, J.; Chang, H. Norovirus Activity and Genotypes in Sporadic Acute Diarrhea in Children in Shanghai During 2014–2018. Pediatr. Infect. Dis. J. 2019, 38, 1085–1089. [Google Scholar] [CrossRef]
- Lucero, Y.; Lagomarcino, A.J.; Espinoza, M.; Kawakami, N.; Mamani, N.; Huerta, N.; Del Canto, F.; Farfán, M.; Sawaguchi, Y.; George, S.; et al. Norovirus compared to other relevant etiologies of acute gastroenteritis among families from a semirural county in Chile. Int. J. Infect. Dis. 2020, 101, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Montenegro, S.; Pineda, S.; Enríquez, I.; Enríquez, N.; Rivera, N.; Delgado, C. Detección de norovirus en niños con diarrea adquirida en la comunidad o nosocomial en el hospital guillermo grant benavente de concepción, Chile. Rev. Chil. Infectol. 2014, 31, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Guarines, K.M.; Mendes, R.P.G.; de Magalhães, J.J.F.; Pena, L. Norovirus-associated gastroenteritis, Pernambuco, Northeast Brazil, 2014–2017. J. Med. Virol. 2020, 92, 1093–1101. [Google Scholar] [CrossRef] [PubMed]
- Diez-valcarce, M.; Renee, M.; Lopez, B.; Morales, O.; Sagastume, M.; Cadena, L.; Kaydos-daniels, S.; Jarquin, C.; Mccracken, J.P.; Bryan, J.P.; et al. Prevalence and genetic diversity of viral gastroenteritis viruses in children younger than 5 years of age in Guatemala, 2014–2015. J. Clin. Virol. 2019, 114, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Labayo, H.K.M.; Pajuelo, M.J.; Tohma, K.; Ford-Siltz, L.A.; Gilman, R.H.; Cabrera, L.; Mayta, H.; Sanchez, G.J.; Cornejo, A.T.; Bern, C.; et al. Norovirus-specific immunoglobulin A in breast milk for protection against norovirus-associated diarrhea among infants. EClinicalMedicine 2020, 27, 100561. [Google Scholar] [CrossRef]
- Khodayar-Pardo, P.; Martínez-Costa, C.; Carmona-Vicente, N.M.; Buesa, J. Norovirus GII.4 Antibodies in Breast Milk and Serum Samples Their Role Preventing Virus-Like Particles Binding to Their Receptors. Pediatr. Infect. Dis. J. 2014, 33, 554–559. [Google Scholar] [CrossRef]


| Characteristic | Total Samples (%) | No. of Norovirus Positive Cases (%) | No. of Norovirus Negative Cases (%) |
|---|---|---|---|
| Gender | |||
| Females | 41 | 17 (34) | 24 (33.8) |
| Males | 80 | 33 (66) | 47 (66.2) |
| Age group (months) | |||
| 0 to 12 | 66 | 25 (50) | 41 (57.7) |
| 13 to 24 | 47 | 21 (42) | 26 (36.6) |
| 25 to 36 | 7 | 3 (6) | 4 (5.6) |
| 37 to 48 | 1 | 1 (2) | 0 (0) |
| 49 to 60 | 0 | 0 (0) | 0 (0) |
| Settings | |||
| Hospitalized | 54 (44.6) | 25 (50) | 29 (40.8) |
| Ambulatory | 67 (55.4) | 25 (50) | 42 (59.2) |
| Season | |||
| Summer | 13 | 11 (22) | 2 (2.8) |
| Autumn | 44 | 36 (72) | 8 (11.3) |
| Winter | 51 | 3 (6) | 48 (67.6) |
| Spring | 13 | 0 (0) | 13 (18.3) |
| Total | 121 | 50 (41.3) | 71 (58.7) |
| Age Group (Months) | Hospitalized | Ambulatory | ||
|---|---|---|---|---|
| N°. | % | N°. | % | |
| 0 to 12 | 9 | 36.0 | 16 | 64.0 |
| 13 to 24 | 13 | 52.0 | 8 | 32.0 |
| 25 to 36 | 3 | 12.0 | 0 | 0.0 |
| 37 to 48 | 0 | 0.0 | 1 | 4.0 |
| Total | 25 | 100 | 25 | 100 |
| Genogroups | Polymerase (RdRp) Genotype | Capsid (VP1) Genotype | Frequency (N) | Percentage (%) |
|---|---|---|---|---|
| GI | GI.P3 | GI.3 | 1 | 2 |
| GII | GII.P16 | GII.4 Sydney | 21 | 42 |
| GII.P4 New Orleans | GII.4 Sydney | 27 | 54 | |
| GII.P31 | GII.17 | 1 | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Avellaneda, A.M.; Campillay-Véliz, C.P.; Reyes, D.C.; Herrera, D.; Muñoz, C.A.; Vinjé, J.; Lay, M.K. High Prevalence and Genetic Diversity of Human Norovirus Among Children Under 5 Years Old with Acute Gastroenteritis at the Dr. Leonardo Guzmán Regional Hospital, Antofagasta, Chile, 2019. Viruses 2025, 17, 794. https://doi.org/10.3390/v17060794
Avellaneda AM, Campillay-Véliz CP, Reyes DC, Herrera D, Muñoz CA, Vinjé J, Lay MK. High Prevalence and Genetic Diversity of Human Norovirus Among Children Under 5 Years Old with Acute Gastroenteritis at the Dr. Leonardo Guzmán Regional Hospital, Antofagasta, Chile, 2019. Viruses. 2025; 17(6):794. https://doi.org/10.3390/v17060794
Chicago/Turabian StyleAvellaneda, Andrea M., Claudia P. Campillay-Véliz, Daniela C. Reyes, Daniel Herrera, Christian A. Muñoz, Jan Vinjé, and Margarita K. Lay. 2025. "High Prevalence and Genetic Diversity of Human Norovirus Among Children Under 5 Years Old with Acute Gastroenteritis at the Dr. Leonardo Guzmán Regional Hospital, Antofagasta, Chile, 2019" Viruses 17, no. 6: 794. https://doi.org/10.3390/v17060794
APA StyleAvellaneda, A. M., Campillay-Véliz, C. P., Reyes, D. C., Herrera, D., Muñoz, C. A., Vinjé, J., & Lay, M. K. (2025). High Prevalence and Genetic Diversity of Human Norovirus Among Children Under 5 Years Old with Acute Gastroenteritis at the Dr. Leonardo Guzmán Regional Hospital, Antofagasta, Chile, 2019. Viruses, 17(6), 794. https://doi.org/10.3390/v17060794







