Respiratory Syncytial Virus-Infected Human Mesenchymal Stem Cells Overexpress Toll-like Receptors and Change the Pattern of Distribution of Their Cytoskeleton
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells and Viruses
2.2. Infection of MSC-PL
2.3. qRT-PCR
2.4. Staining and Flow Cytometry Analysis
2.5. Determination of the Distribution of the Cytoskeleton Proteins in RSV-Infected Mesenchymal Stem Cultures
2.6. Statistical Analysis
3. Results
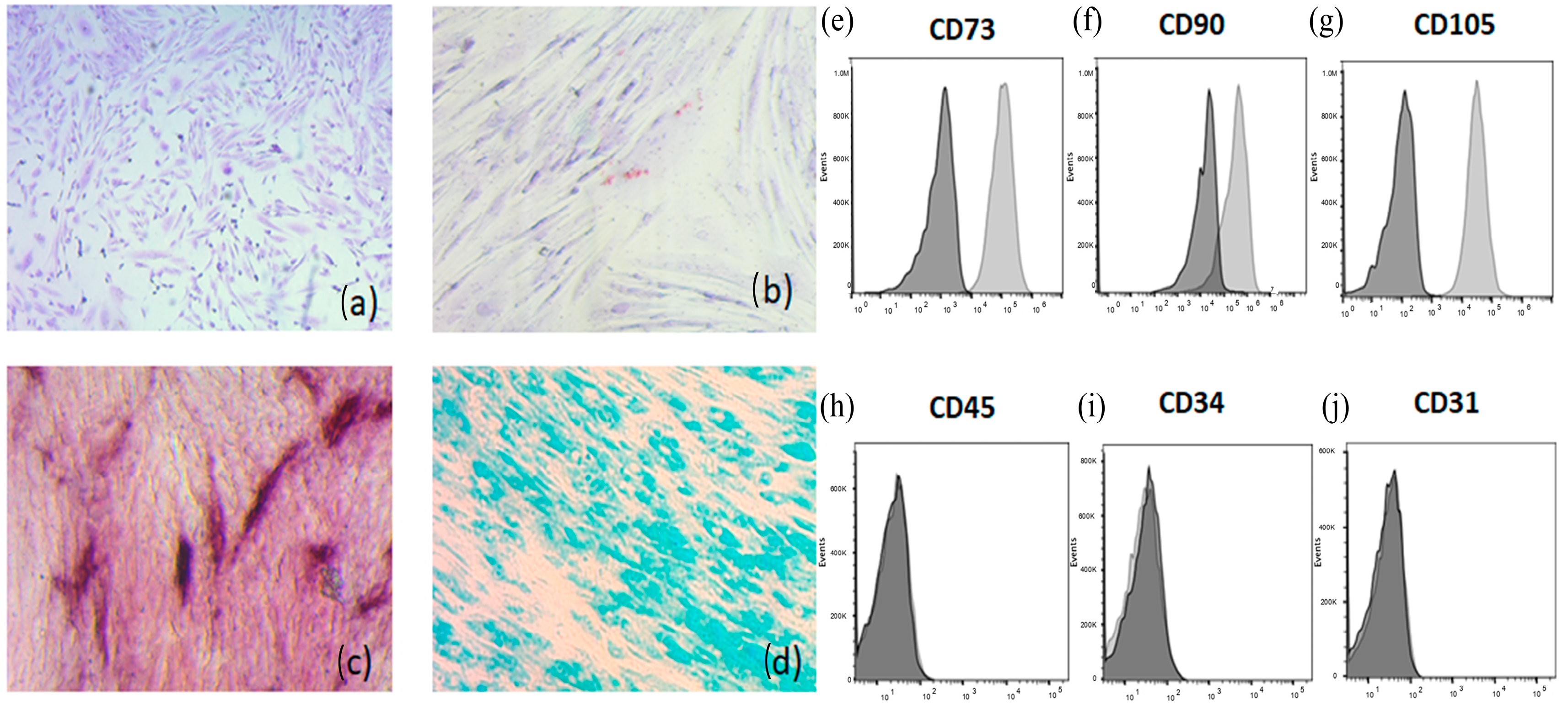
3.1. Characterization of Mesenchymal Stem Cells
3.2. Virus Production in MSC-PL
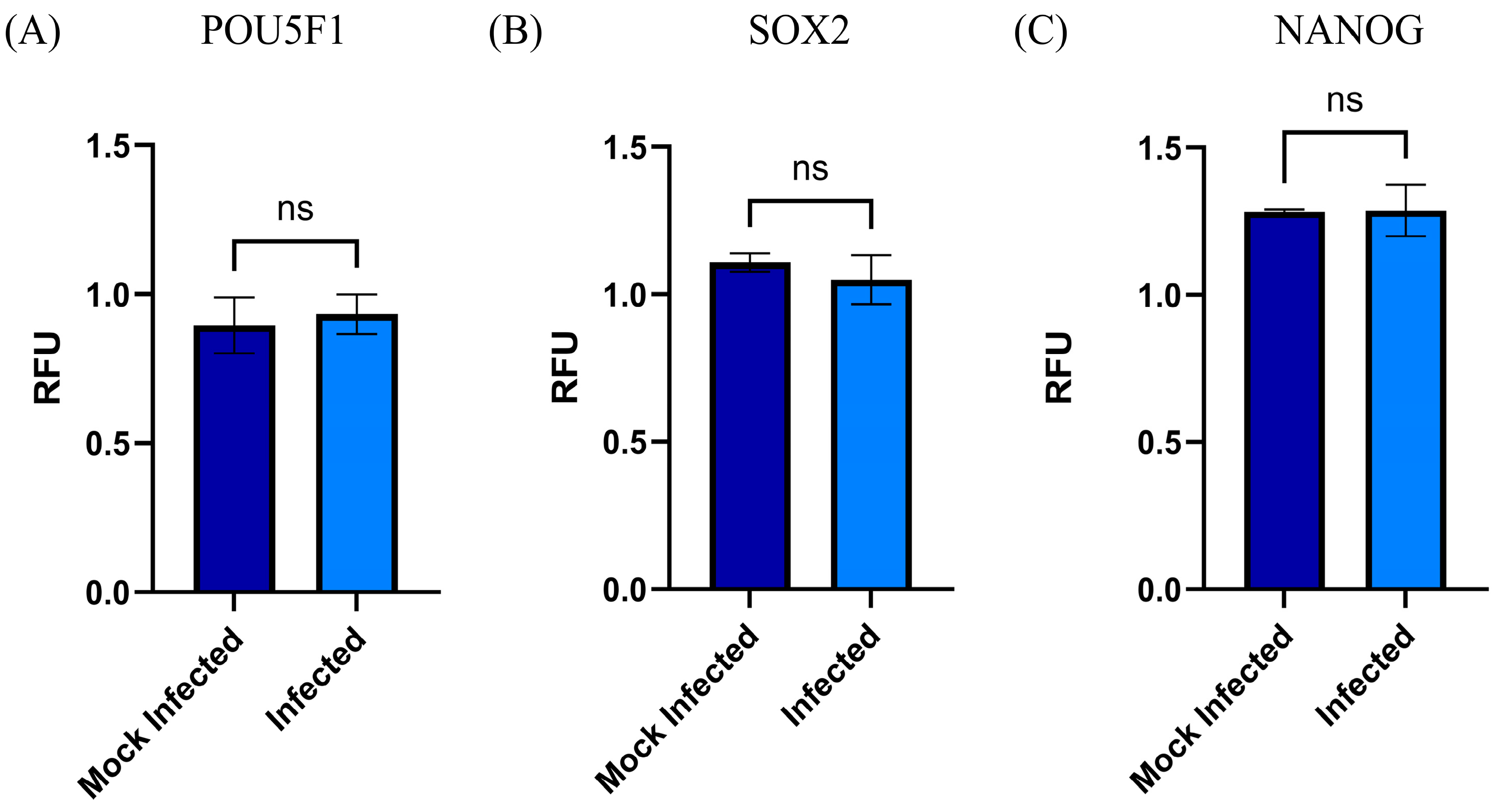
3.3. Changes in the mRNA Expression of Stemness Biomarkers (SOX2, NANOG, and POU5F1) by RSV MSC-PL Infection
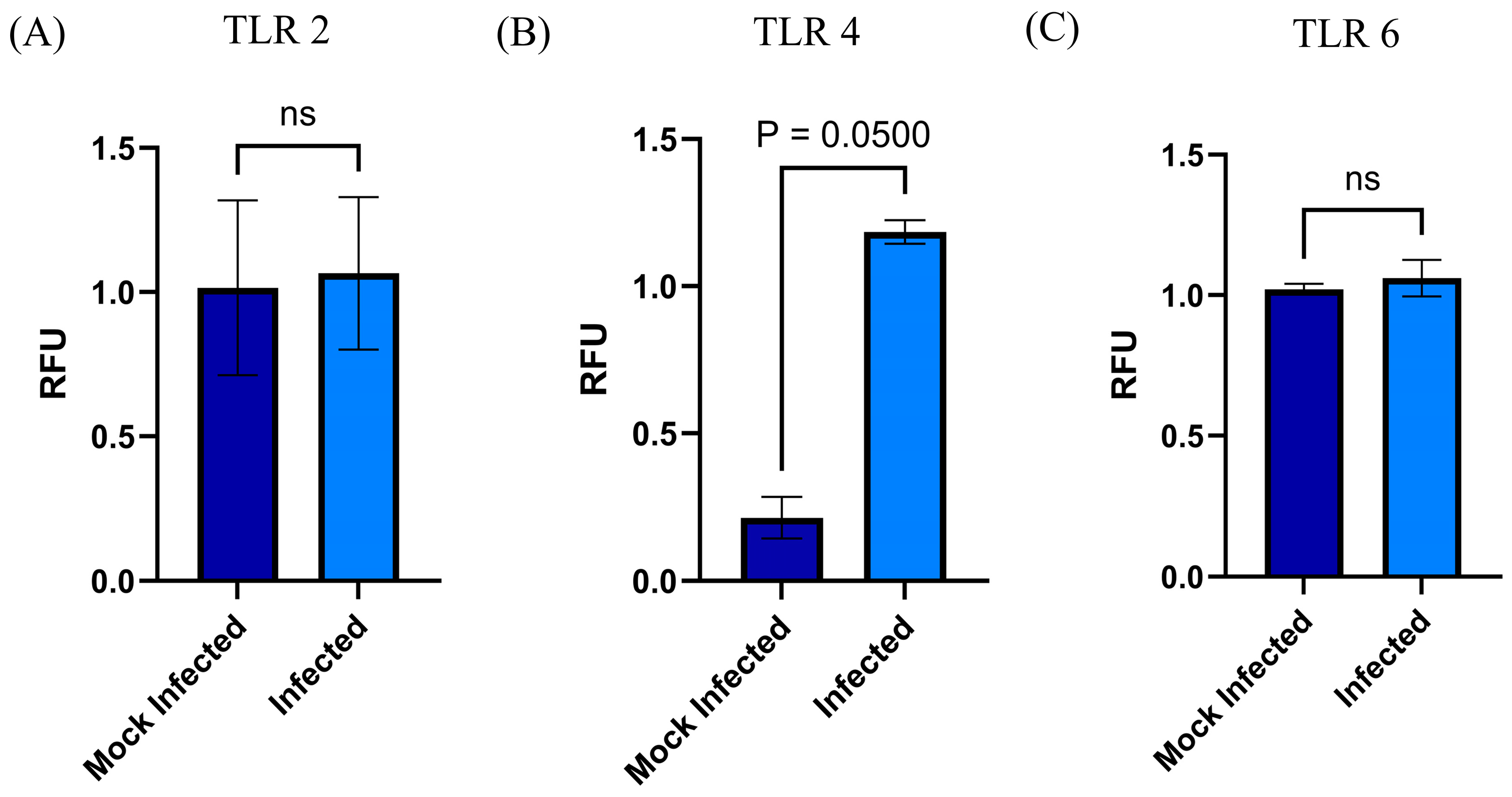
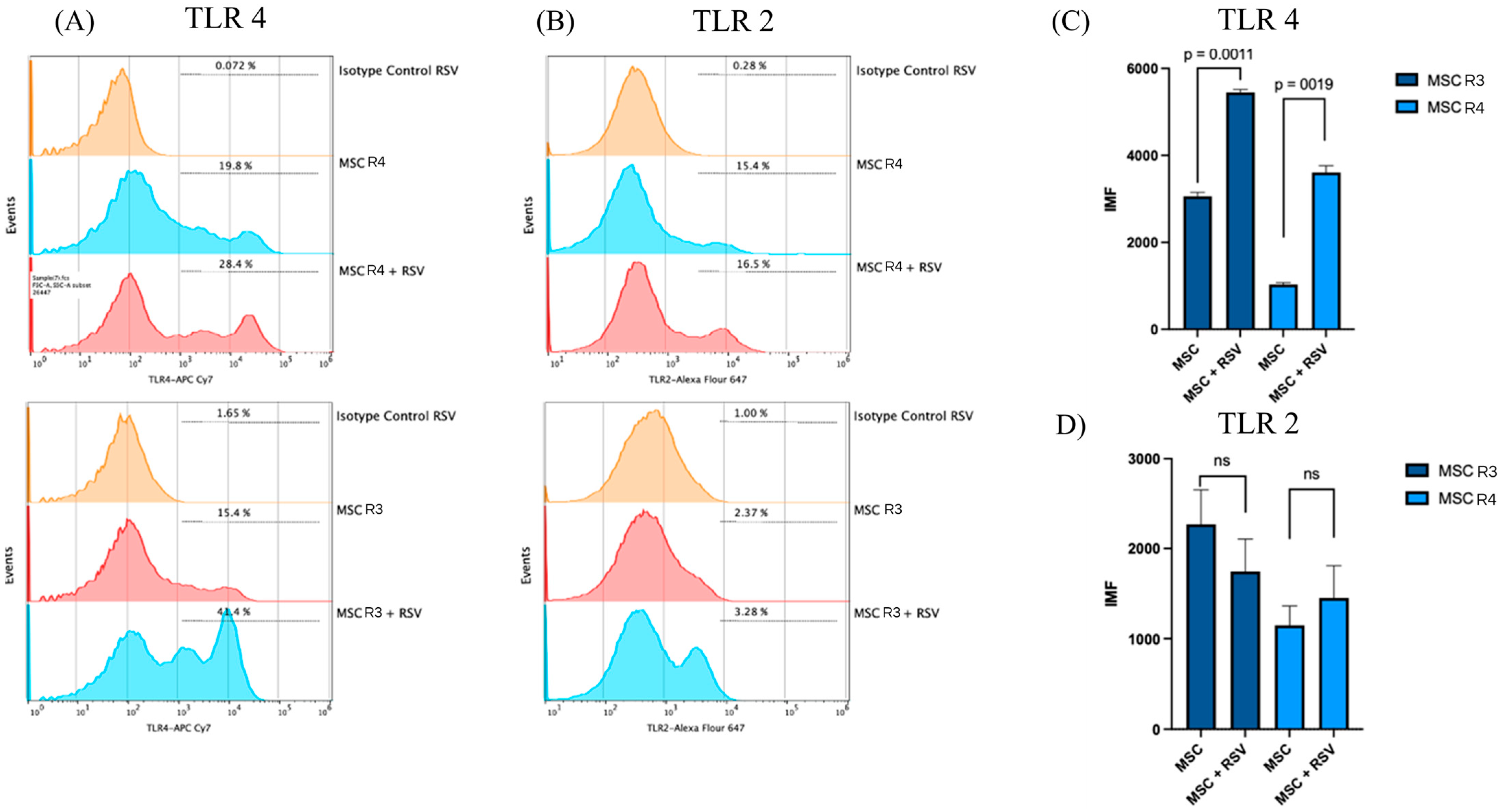
3.4. Changes in mRNA and Surface Expression of TLRs upon Infection by RSV
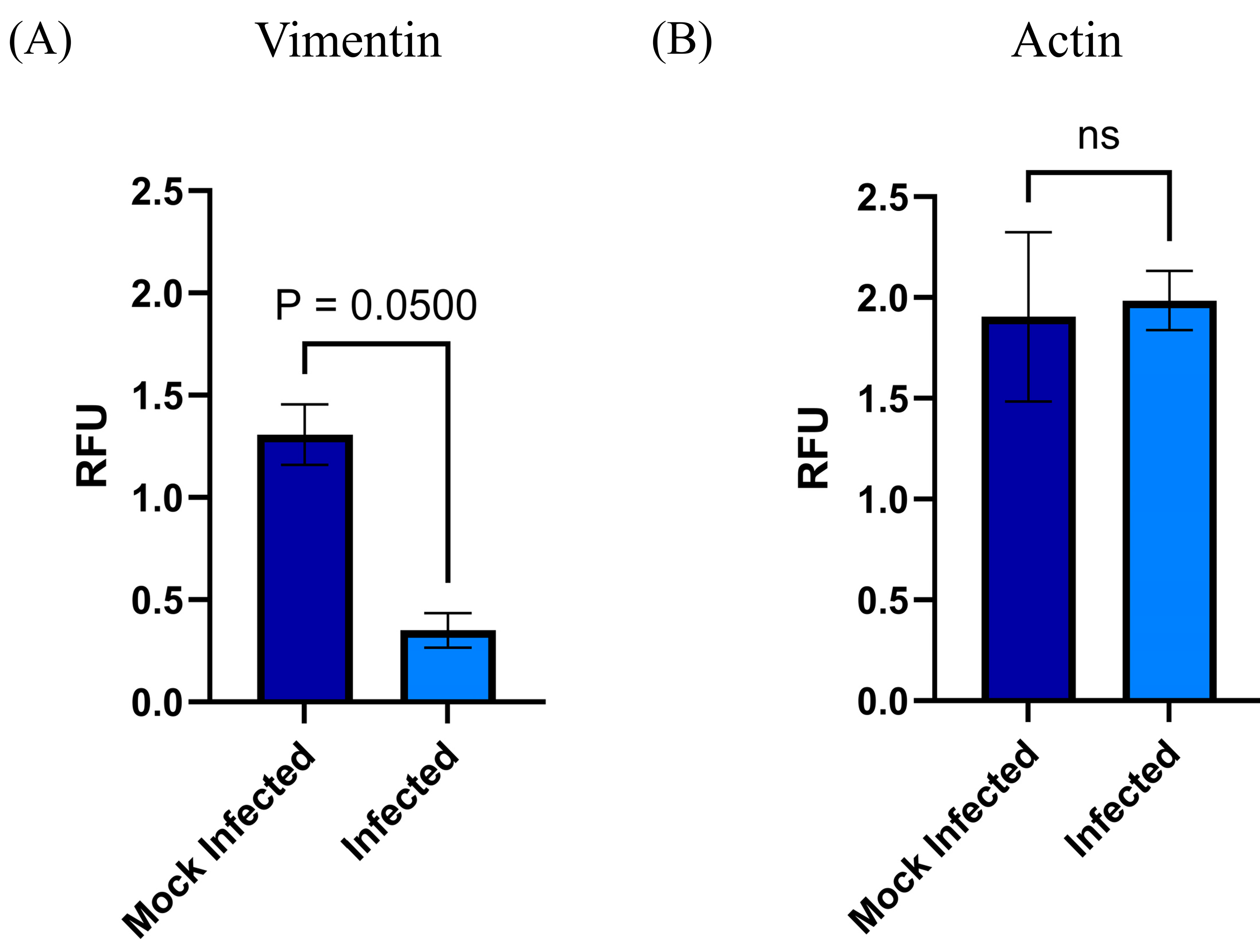
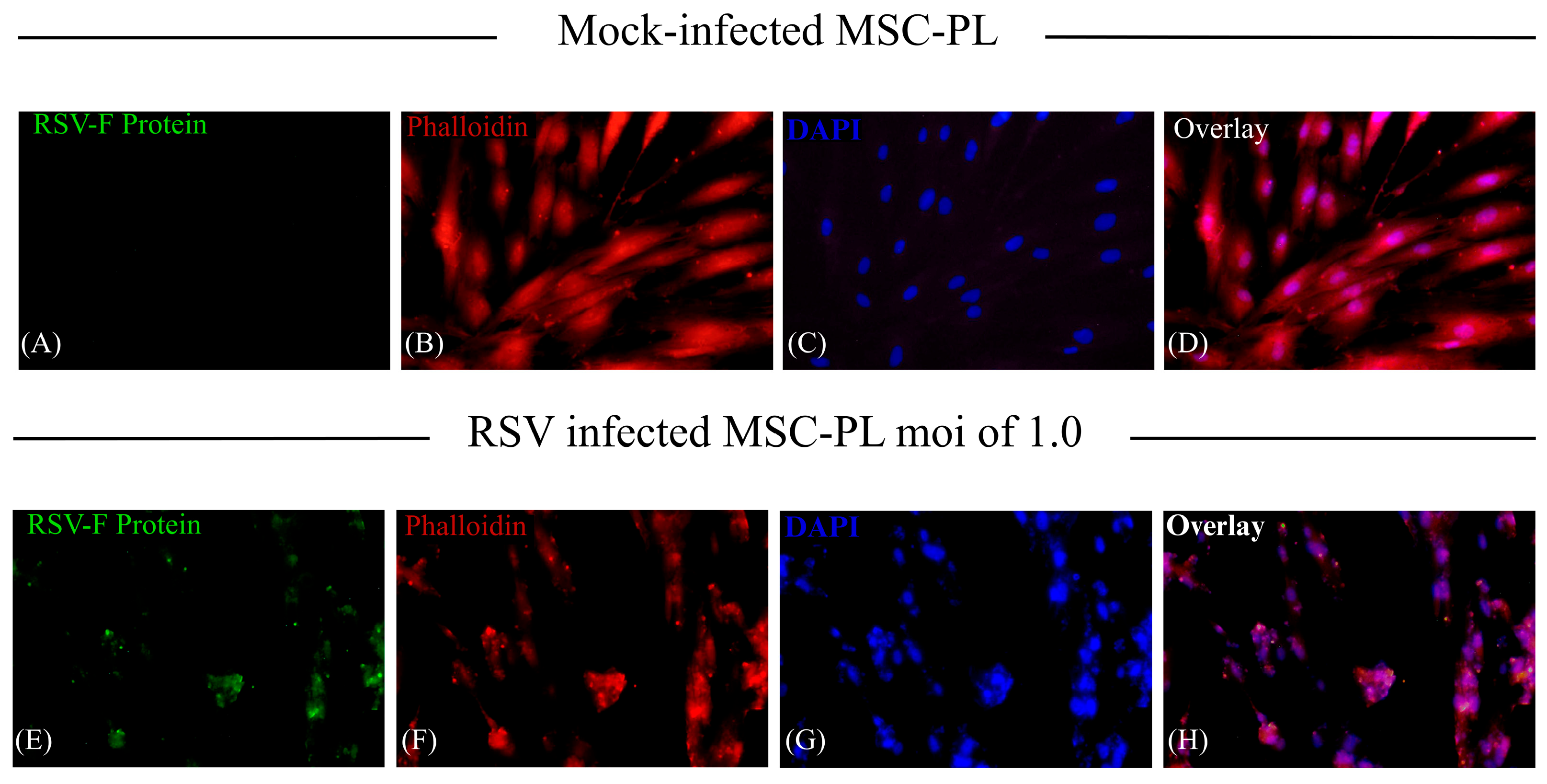
3.5. MSC-PL Cytoskeleton Rearrangement as a Result of the RSV Infection
4. Discussion
- (1)
- The stem cells participate at different levels of biological processes; especially relevant are self-renewal and differentiation. Both activities are regulated by transcription factors that act as molecular rheostats [27]. Thus, sensing self-renewal and poise can induce or suppress the gene expression necessary for differentiation [27]. Among them are SOX2 and POU5F1, which work together to drive the expression of their own genes and many other genes, including NANOG [27]. POU5F1/NANOG/SOX2 is an interconnected regulatory circuit, in which NANOG acts as a direct target for POU5F1/SOX2 binding, which maintains the self-renewal and pluripotency of the stem cells [28]. Recent studies described the nuclear reorganization of transcription factors SOX2 and POU5F1 that play a key role in differentiation and the loss of pluripotency of stem cells by modifying the interaction of these molecules with their chromatin targets [29]. The potentiality and the capacity of differentiation are important events to recover the tissue function. With all this information, our interest is focused on determining whether the RSV infection of MSC-PL modifies the mRNA expression of the interconnected regulatory circuit POU5F1/NANOG/SOX2 and in consequence, alters the potentiality and differentiation capacity. However, our data demonstrated that the RSV infection does not modify the mRNA expression of any of the transcription factors; therefore, the MSC-PL maintains their potentiality, their differentiation capacity, and their stemness markers.
- (2)
- The Toll-like receptors (TLRs) crucially participate in the modulation of the immune response. These receptors function as one of the several groups of PRRs (Pattern Recognition Receptors), which recognize different PAMPs or MAMPs and DAMPs (pathogen/microbe-associated molecular patterns and death/damage-associated molecular patterns) [30]. These receptors are not only expressed by immune cells but they are also expressed in multiple cell types in addition to immune cells, including stem cells [31]. Likewise, the expressed TLRs in different types of MSCs could contribute to different biological activities such as basal motility, self-renewal, differentiation, and immunomodulation [31], according to the contribution of TLRs in biological processes previously described for the MSCs. First, we confirmed that the MSC-PL expresses TLRs 2, 4, and 6 because, notably, the TLR expression profile varies within different mesenchymal stromal cell populations. Second, we hypothesized that RSV infection of MSC-PL modifies the expression of the mRNA and surface molecule expression of TLRs 2, 4, and 6. Our data show that the viral infection induces an overexpression of both mRNA and surface molecule expression of TLR4. These data are in agreement with previous reports that indicated that RSV increased TLR4 [32,33]. This increment is associated with the fact that the virus uses this molecule as a viral entry receptor. Another study demonstrated changes in the expression of TLR4 in stem cells of the apical papilla, in which the increase in TLR4 was related to decrease in cell proliferation while increasing differentiation [31]. These data are crucial because the authors describe a function other than immune response mediators developed by TLRs. Finally, our results confirmed that RSV infection of MSC-PL does not generate changes in TLR 2 and 6, but suggests that the increment of TLR4 might be associated with intrinsic biological processes of mesenchymal cells, like self-renewal and differentiation.
- (3)
- RSV is known to interact with the cytoskeleton in several ways and at various stages throughout its replication cycle. In addition to transcriptional regulation, the cytoskeleton is also likely to be involved in the directional movement of RSV components, and it has a role in virion morphogenesis and cell fusion [34]. Different studies point out the interaction and transport of viral proteins with cytoskeleton filaments; they report the interaction of the viral proteins, Fusion protein (F), Matrix protein (M), and Ribonucleoprotein complex (RNP), with actin filaments to transport virion components to assembly and budding from the infected cells [35]. Moreover, the viral infection induces the upregulation of five genes associated with the cytoskeleton [36]. With the previous information, we decided to study how the RSV infection modifies the expression of the cytoskeleton in the MSC-PL. Our results demonstrate that viral infection does not affect the mRNA expression of actin; however, it was evident that there is a significant change of the morphology of the MSC-PL in which, associated with a redistribution of the actin filaments, the cells lose their fibroblastoid structure and they form a cellular agglomerate. Furthermore, vimentin plays an important role in cell migration, contraction, proliferation, protein synthesis, gene expression, cell apoptosis, and mechanical force transmission, among which participation in cell migration is an important prerequisite for tissue damage repair and inflammation control [37,38]. Contrary to the results with actin, the RSV infection induces a downregulation of the mRNA expression of vimentin. This result suggests that the process of differentiation might be modified, including the rate of MSC growth, according to the study, which states that mice with vimentin gene knockout had slower MSC growth than wild-type mice, and their differentiation status also differed [39].
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ARI | Acute respiratory tract infection |
| RSV | Human respiratory syncytial virus |
| MSC | Mesenchymal stem cell |
| MSC-PL | Placental mesenchymal stem cell |
| TLRs | Toll-like receptors |
| HLA-DR | DR human leukocyte antigen |
| PL | Human placental |
| SOX2 | Sex-determining region Y-box 2 |
| NANOG | Nanog homeobox |
| POU5F1 | POU domain, class 5, transcription factor 1 |
| qRT-PCR | Real-time quantitative reverse transcription polymerase chain reaction |
| PAMPs | Pathogen-associated molecular patterns |
| MAMPs | Microbe-associated molecular patterns |
| DAMPs | Damage-associated molecular patterns |
| F | Viral Fusion protein |
| M | Viral Matrix protein |
| RNP | Viral Ribonucleoprotein complex |
References
- Respiratory Syncytial Virus (RSV) Disease. Available online: https://www.who.int/teams/health-product-policy-and-standards/standards-and-specifications/norms-and-standards/vaccine-standardization/respiratory-syncytial-virus-disease (accessed on 10 December 2024).
- Lu, Y.; Wang, S.; Zhang, L.; Xu, C.; Bian, C.; Wang, Z.; Ma, Y.; Wang, K.; Ma, L.; Meng, C.; et al. Epidemiology of human respiratory viruses in children with acute respiratory tract infections in Jinan. J. Immunol. Res. 2013, 2013, 210490. [Google Scholar] [CrossRef] [PubMed]
- Respiratory Syncytial Virus Infection (RSV). Available online: https://www.cdc.gov/rsv/about/index.html (accessed on 10 December 2024).
- Yang, J.; Hillson, E.; Mauskopf, J.; Copley-Merriman, C.; Shinde, V.; Stoddard, J. The epidemiology of medically attended respiratory syncytial virus in older adults in the United States: A systematic review. PLoS ONE 2017, 12, e0182321. [Google Scholar]
- Informe Semanal de la COVID-19, Influenza y Otros Virus Respiratorios. 2024. Available online: https://www.gob.mx/cms/uploads/attachment/file/960833/ER_SE48_2024.pdf (accessed on 28 February 2025).
- Brügger, M.; Démoulins, T.; Barut, G.T.; Zumkehr, B.; Oliveira Esteves, B.I.; Mehinagic, K.; Haas, Q.; Schögler, A.; Rameix-Welti, M.-A.; Eléouët, J.-F.; et al. Pulmonary mesenchymal stem cells are engaged in distinct steps of host response to respiratory syncytial virus infection. PLoS Pathog. 2021, 17, e1009789. [Google Scholar] [CrossRef] [PubMed]
- Zarate, K.; Ambriz, X.; Ambrosio, J.R.; Tirado, R. In vitro Pneumovirus and Paramixovirus infection is modulated by the passage of mesenchymal stem cells. SCIREA J. Clin. Med. 2022, 7, 167–179. [Google Scholar] [CrossRef]
- Maleki, M.; Ghanbarvand, F.; Behvarz, M.R.; Ejtemaei, M.; Ghadirkhomi, E. Comparison of Mesenchymal Stem Cell Markers in Multiple Human Adult Stem Cells. Int. J. Stem Cells 2014, 7, 118–126. [Google Scholar] [CrossRef]
- Sharov, A.A.; Masui, S.; Sharova, L.V. Identification of Pou5f1, Sox2, and Nanog downstream target genes with statistical confidence by applying a novel algorithm to time course microarray and genome-wide chromatin immunoprecipitation data. BMC Genom. 2008, 9, 269. [Google Scholar] [CrossRef]
- Sallustio, F.; Curci, C.; Stasi, A.; De Palma, G.; Divella, C.; Gramignoli, R.; Castellano, G.; Gallone, A.; Gesualdo, L. Role of Toll-Like Receptors in Actuating Stem/Progenitor Cell Repair Mechanisms: Different Functions in Different Cells. Stem Cells Int. 2019, 1, 6795845. [Google Scholar] [CrossRef]
- Lechler, T. Adherens Junctions and Stem Cells. In Adherens Junctions: From Molecular Mechanisms to Tissue Development and Disease; Harris, T., Ed.; Springer Netherlands: Dordrecht, The Netherlands, 2012; Volume 60, pp. 359–377. ISBN 978-94-007-4186-7. [Google Scholar]
- Li, L.; Xie, T. Stem Cell Niche: Structure and Function. Annu. Rev. Cell Dev. Biol. 2005, 21, 605–631. [Google Scholar] [CrossRef]
- Ohlstein, B.; Kai, T.; Decotto, E.; Spradling, A. The stem cell niche: Theme and variations. Curr. Opin. Cell Biol. 2004, 16, 693–699. [Google Scholar] [CrossRef]
- Ma, S.; Xie, N.; Li, W.; Yuan, B.; Shi, Y.; Wang, Y. Immunobiology of mesenchymal stem cells. Cell Death Differ. 2004, 20, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Su, J.; Roberts, A.I.; Shou, P.; Rabson, A.B.; Ren, G. How mesenchymal stem cells interact with tissue immune responses. Trends Immunol. 2012, 33, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.L.; Liu, K.D.; Li, F.C.; Jiang, X.M.; Jiang, L.; Li, H.L. Human mesenchymal stem cells increases expression of a-tubulin and angiopoietin 1 and 2 in focal cerebral ischemia and reperfusion. Curr. Neurovasc. Res. 2013, 10, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, S.; Scotton, C.J.; McNulty, K.; Nye, E.; Stamp, G.; Laurent, G.; Bonnet, D.; Janes, S.M. Bone marrow stem cells expressing keratinocyte growth factor via an inducible lentivirus protects against bleomycin-induced pulmonary fibrosis. PLoS ONE 2009, 4, e8013. [Google Scholar] [CrossRef]
- Hung, S.P.; Yang, M.H.; Tseng, K.F.; Lee, O.K. Hypoxia-induced secretion of TGF-beta 1 in mesenchymal stem cell promotes breast cancer cell progression. Cell Transplant. 2013, 22, 1869–1882. [Google Scholar] [CrossRef]
- Bohmwald, K.; Gálvez, N.M.S.; Canedo-Marroquín, G.; Pizarro-Ortega, M.S.; Andrade-Parra, C.; Gómez-Santander, F.; Kalergis, A.M. Contribution of Cytokines to Tissue Damage During Human Respiratory Syncytial Virus Infection. Front. Immunol. 2013, 10, 452. [Google Scholar] [CrossRef] [PubMed]
- Galvan, M.A.; Cabello, C.; Mejia, F.; Valle, L.; Valencia, E.; Manjarrez, M.E. Parainfluenza virus type 1 induces epithelial IL-8 production via p38-MAPK signalling. J. Immunol. Res. 2014, 2014, 515984. [Google Scholar]
- Payment, P.; Trudel, M. Isolation and identification of viruses. In Methods and Techiniques in Virology; Payment, P., Trudel, M., Eds.; Dekker, Inc.: New York, NY, USA, 1993; pp. 32–33. ISBN 0-8247-9101-0. [Google Scholar]
- Perezbusta, L.N.; Tirado, R.; Ambrosio, J.R. Respiratory infections and coinfections: Geographical and population patterns. Gac. Méd. Méx. 2020, 156, 265–272. [Google Scholar] [CrossRef]
- Montesinos, J.J.; Flores, E.; Castillo, S.; Flores, P.; Hernández, E.; Fajardo, G.; Orozco, S.; Mayani, H. Human mesenchymal stromal cells from adult and neonatal sources: Comparative analysis of their morphology, immunophenotype, differentiation patterns and neural protein expression. Cytotherapy 2009, 11, 163–176. [Google Scholar] [CrossRef]
- Contini, P.; Murdaca, G.; Puppo, F.; Negrini, S. HLA-G Expressing Immune Cells in Immune Mediated Diseases. Front. Immunol. 2020, 11, 1613. [Google Scholar]
- Negrini, S.; Contini, P.; Murdaca, G.; Puppo, F. HLA-G in Allergy: Does It Play an Immunoregulatory Role? Front. Immunol. 2022, 12, 789684. [Google Scholar] [CrossRef]
- LeMaoult, J.; Yan, W.-H. Editorial: The Biological and Clinical Aspects of HLA-G. Front. Immunol. 2021, 12, 649344. [Google Scholar] [CrossRef] [PubMed]
- Rizzino, A.; Wuebben, E.L. Sox2/Oct4: A delicately balanced partnership in pluripotent stem cells and embryogenesis. Biochim. Biophys. Acta 2016, 1859, 780–791. [Google Scholar] [CrossRef] [PubMed]
- Niharika, S.; Mansee, T.; Jigna, P.; Biswaranjan, S. SOX2, OCT4 and NANOG: The core embryonic stem cell pluripotency regulators in oral carcinogenesis. J. Oral Maxillofac. Pathol. 2020, 24, 368–373. [Google Scholar]
- Verneri, P.; Vazquez, C.; Oses, C.; Stortz, M.; Guberman, A.; Levi, V. Dynamical reorganization of the pluripotency transcription factors Oct4 and Sox2 during early differentiation of embryonic stem cells. Sci. Rep. 2020, 10, 5195. [Google Scholar] [CrossRef]
- Kumar, V. Toll-like Receptors in Health and Disease; Springer: Cham, Switzerland, 2022; pp. 1–7. ISBN 978-3-031-06511-8. [Google Scholar]
- Takimoto, K.; Widbiller, M.; Diogenes, A. Expression of Toll-like Receptors in Stem Cells of the Apical Papilla and Its Implication for Regenerative Endodontics. Cells 2023, 12, 2502. [Google Scholar] [CrossRef]
- Griffiths, C.; Drews, S.J.; Marchant, D.J. Respiratory Syncytial Virus: Infection, Detection, and New Options for Prevention and Treatment. Clin. Microbiol. Rev. 2017, 30, 277–319. [Google Scholar] [CrossRef]
- Kurt-Jones, E.A.; Popova, L.; Kwinn, L.; Haynes, L.M.; Jones, L.P.; Tripp, R.A.; Walsh, E.E.; Freeman, M.W.; Golenbock, D.T.; Anderson, L.J.; et al. Pattern recognition receptors TLR4 and CD14 mediate response to respiratory syncytial virus. Nat. Immunol. 2000, 1, 398–401. [Google Scholar] [CrossRef]
- Shahriari, S.; Gordon, J.; Ghildyal, R. Host cytoskeleton in respiratory syncytial virus assembly and budding. Virol. J. 2016, 13, 161. [Google Scholar] [CrossRef]
- Shahriari, S.; Wei, K.-j.; Ghildyal, R. Respiratory Syncytial Virus Matrix (M) Protein Interacts with Actin In Vitro and in Cell Culture. Viruses 2018, 10, 535. [Google Scholar] [CrossRef]
- Martínez, I.; Lombardía, L.; García-Barreno, B.; Domínguez, O.; Melero, J.A. Distinct gene subsets are induced at different time points after human respiratory syncytial virus infection of A549 cells. J. Gen. Virol. 2007, 88, 570–581. [Google Scholar] [CrossRef]
- Rogel, M.R.; Soni, P.N.; Troken, J.R.; Sitikov, A.; Trejo, H.E.; Ridge, K.M. Vimentin is sufficient and required for wound repair and remodeling in alveolar epithelial cells. FASEB J. 2011, 25, 3873–3883. [Google Scholar] [CrossRef] [PubMed]
- Bear, M.D.; Liu, T.; Abualkhair, S.; Ghamloush, M.A.; Hill, N.S.; Preston, I.; Fanburg, B.L.; Kayyali, U.S.; Toksoz, D. Alpha-Catulin Co-Localizes With Vimentin Intermediate Filaments and Functions in Pulmonary Vascular Endothelial Cell Migration via ROCK. J. Cell. Physiol. 2016, 231, 934–943. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Boxiang, D.; Zhang, Y.; Congyou, W.; Wang, X.; Zhang, X.; Wang, L. Vimentin-Rab7a Pathway Mediates the Migration of MSCs and Lead to Therapeutic Effects on ARDS. Stem Cells Int. 2021, 2021, 9992381. [Google Scholar] [CrossRef] [PubMed]
| POU5F1 | Forward primer 5′AGTAGTCCCTTCGCAAGCCC3′ Reverse primer 5′CATCGGAGTTGCTCTCCACC3′ | 386 pb |
| NANOG | Forward primer 5′GTCTCGTATTTGCTGCATCG3′ Reverse primer 5′ATGCACGTAAGTGCCCTTTT3′ | 481 pb |
| SOX2 | Forward primer 5′AACCAGCGCATGGACAGTTA3′ Reverse primer 5′GACTTGACCACCGAACCCAT′3′ | 278 pb |
| TLR4 | Forward primer 5′GAATGCTAAGGTTGCCGCTT3′ Reverse primer 5′TTAGGAACCACCTCCACGC3′ | 297 pb |
| TLR2 | Forward primer 5′GAGTTCTCCCAGTGTTTGGT3′ Reverse primer 5′TGTTGGAAACTCGAGGCAGA3′ | 161 pb |
| TLR6 | Forward primer 5′TAGAGGGCTGGCCTGATTCT3′ Reverse primer 5′TGAGAGCCTTCAGCTTGTGG3′ | 609 pb |
| F-RSV | Forward primer 5′ATGAACAGTTTAACATTACCAAGTGA3′ Reverse primer 5′CCACGATTTTTATTGGATGCTG 3′ | 193 pb |
| Vimentin | Forward primer 5′GGACCAGCTAACCAACGACA3′ Reverse primer 5′GCAGCTCCTGGATTTCCTCT3′ | 254 pb |
| Actin | Forward primer 5′GAGCAGTGGCTGAAGGTGAT3′ Reverse primer 5′CAATCCACCTGCCCATGTCT3′ | 589 pb |
| mActb | Forward primer 5′GACTTTGTACATTGTTTTG3′ Reverse primer 5′TGCACTTTTATTGGTCTCA3′ | 382 pb |
| hGAPDH | Forward primer 5′CCATTCTTCCACCTTTGATGCT3′ Reverse primer 5′TGTTGCTGTAGCCATATTCATTGT3′ | 87 pb |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosales Velázquez, C.A.; Chavéz Gómez, L.G.; Félix Espinosa, C.A.; Moreno-Eutimio, M.A.; Montesinos, J.J.; Fajardo-Orduña, G.R.; Tirado Mendoza, R. Respiratory Syncytial Virus-Infected Human Mesenchymal Stem Cells Overexpress Toll-like Receptors and Change the Pattern of Distribution of Their Cytoskeleton. Viruses 2025, 17, 763. https://doi.org/10.3390/v17060763
Rosales Velázquez CA, Chavéz Gómez LG, Félix Espinosa CA, Moreno-Eutimio MA, Montesinos JJ, Fajardo-Orduña GR, Tirado Mendoza R. Respiratory Syncytial Virus-Infected Human Mesenchymal Stem Cells Overexpress Toll-like Receptors and Change the Pattern of Distribution of Their Cytoskeleton. Viruses. 2025; 17(6):763. https://doi.org/10.3390/v17060763
Chicago/Turabian StyleRosales Velázquez, César Alexis, Laura Guadalupe Chavéz Gómez, Carlos Arturo Félix Espinosa, Mario Adan Moreno-Eutimio, Juan José Montesinos, Guadalupe R. Fajardo-Orduña, and Rocio Tirado Mendoza. 2025. "Respiratory Syncytial Virus-Infected Human Mesenchymal Stem Cells Overexpress Toll-like Receptors and Change the Pattern of Distribution of Their Cytoskeleton" Viruses 17, no. 6: 763. https://doi.org/10.3390/v17060763
APA StyleRosales Velázquez, C. A., Chavéz Gómez, L. G., Félix Espinosa, C. A., Moreno-Eutimio, M. A., Montesinos, J. J., Fajardo-Orduña, G. R., & Tirado Mendoza, R. (2025). Respiratory Syncytial Virus-Infected Human Mesenchymal Stem Cells Overexpress Toll-like Receptors and Change the Pattern of Distribution of Their Cytoskeleton. Viruses, 17(6), 763. https://doi.org/10.3390/v17060763







