Biosecurity and Vaccines for Emerging Aquatic Animal RNA Viruses
Abstract
1. Introduction
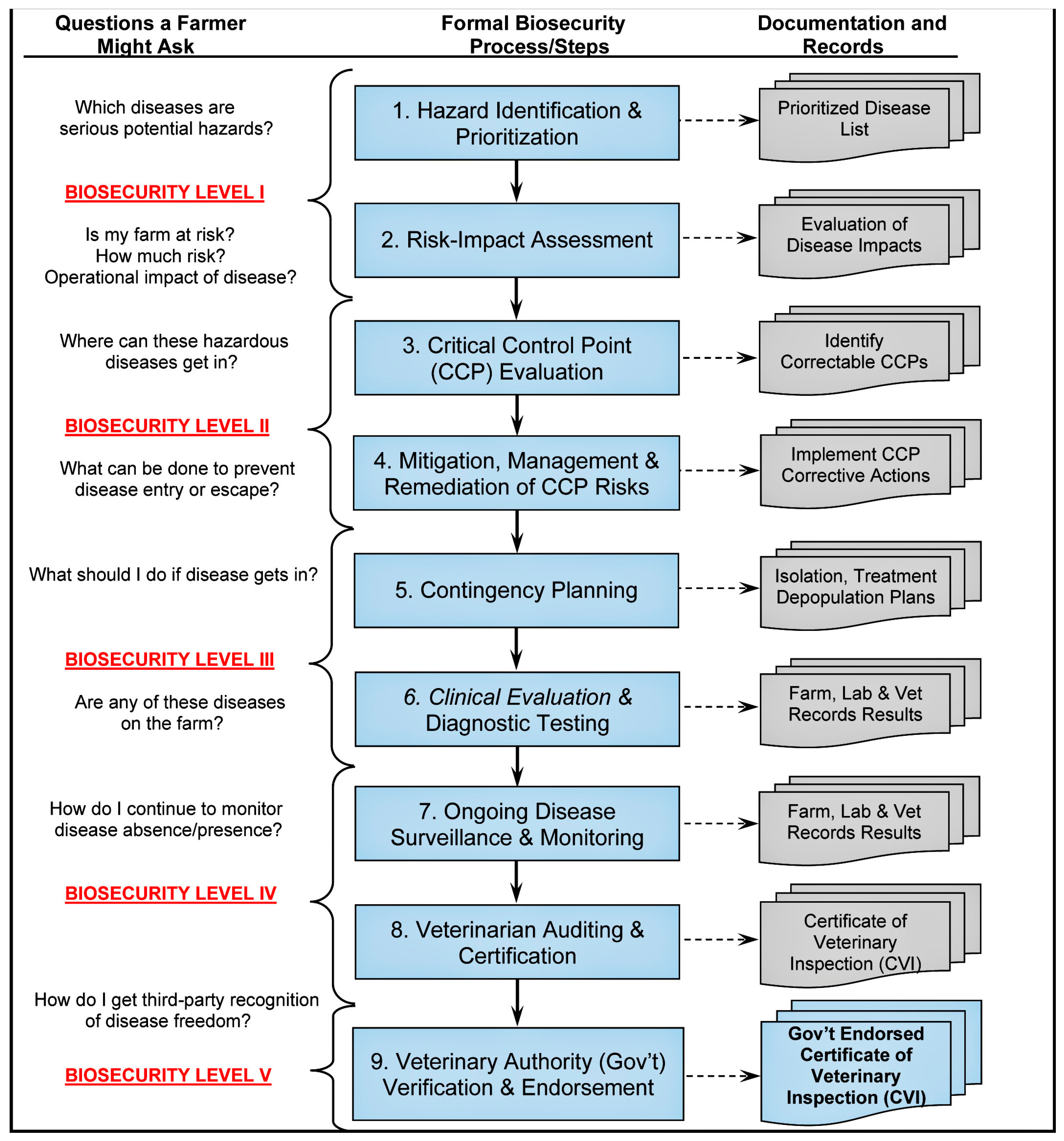
2. Biosecurity Strategies for Emerging RNA Viruses in Aquatic Animals
3. Emerging RNA Viruses in Aquatic Animals
3.1. Fish and Crustaceans
3.1.1. Novirhabdovirus (IHNV and VHSV)
3.1.2. Spring Viremia of Carp Virus (SVCV)
3.1.3. HPR-Deleted or HPR0 Infectious Salmon Anemia Virus (ISAV)
3.1.4. Salmonid Alphavirus (SAV)
3.1.5. Tilapia Lake Virus (TiLV)
3.1.6. Yellow Head Virus (YHV)
3.1.7. Taura Syndrome Virus (TSV)
3.1.8. Infectious Myonecrosis Virus (IMNV)
3.1.9. Macrobrachium Rosenbergii Nodavirus (MrNV)
3.2. Marine Mammals (Wildlife)
3.2.1. Morbilliviruses
3.2.2. Influenza a Virus
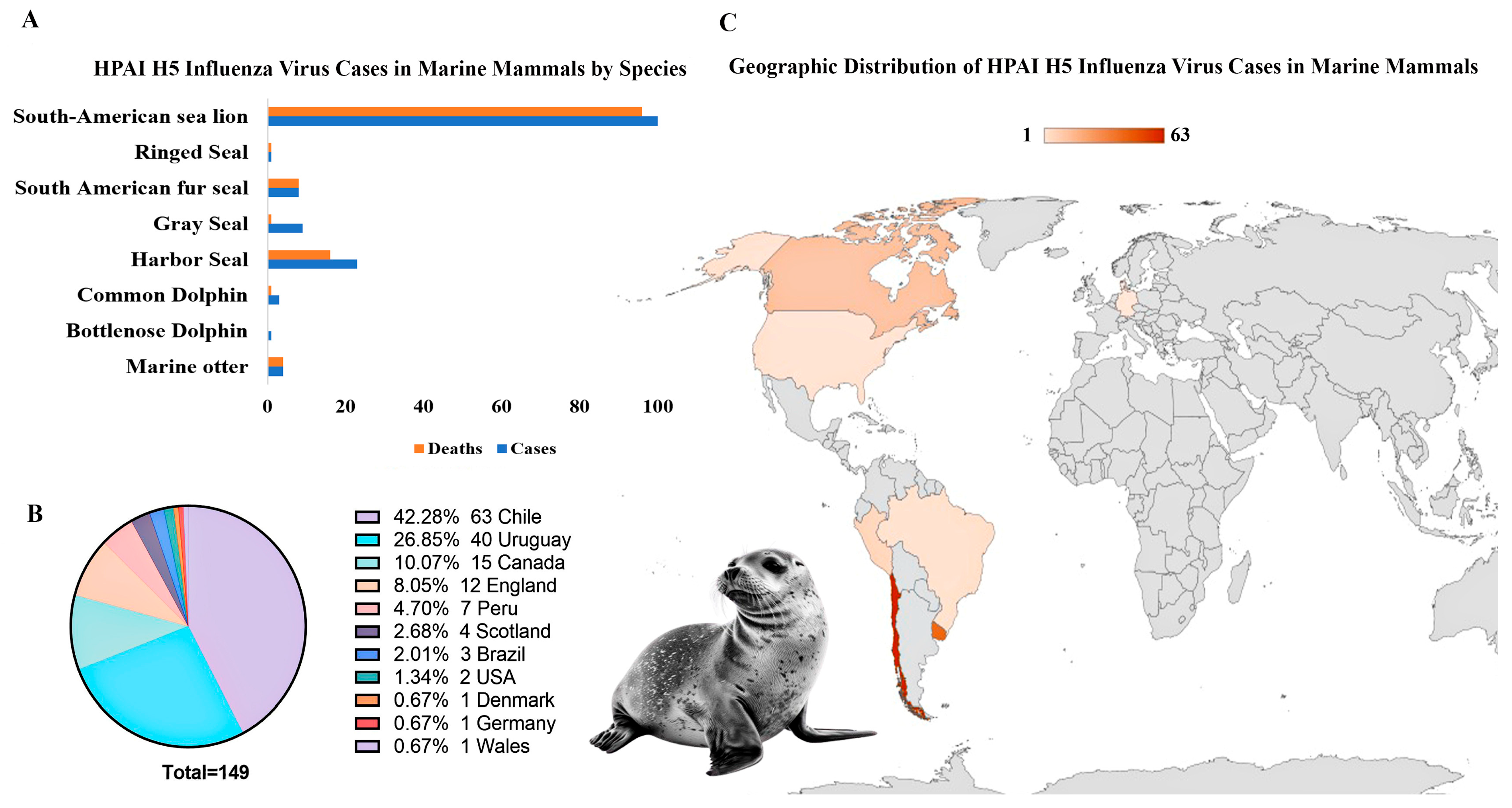
3.2.3. Caliciviruses
4. Vaccines for Emerging RNA Viruses in Aquatic Animals
4.1. Current Vaccine Approaches
4.1.1. Whole Pathogen Vaccines
4.1.2. Subunit Vaccines
4.1.3. Nucleic Acid Vaccines
4.1.4. Live Vector Vaccines
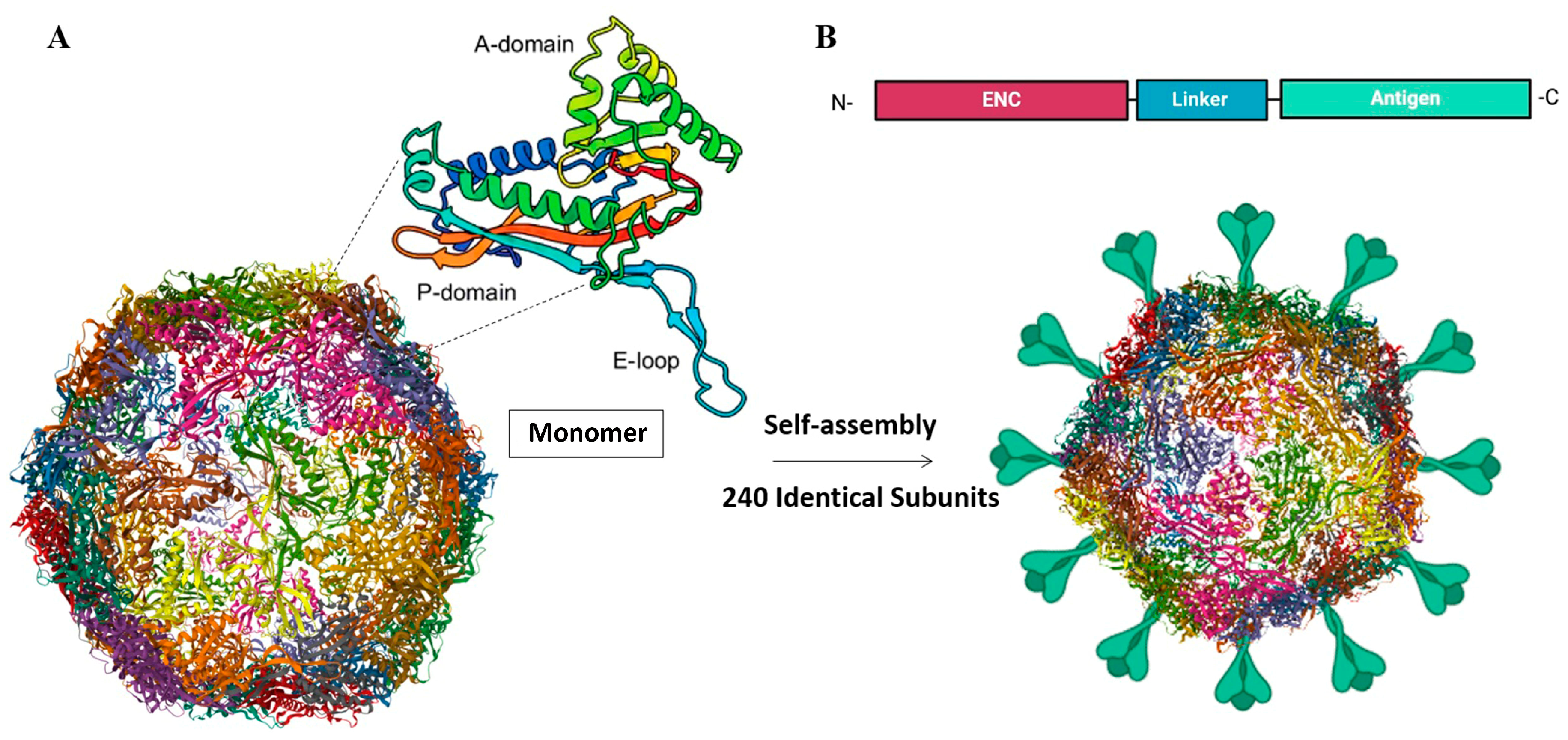
4.2. Self-Assembling Protein Nanocages as Novel Vaccine Platform for Aquatic Animal Viruses
5. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Saiz, J.C. Vaccines against RNA Viruses. Vaccines 2020, 8, 479. [Google Scholar] [CrossRef] [PubMed]
- Ahmadivand, S.; Weidmann, M.; El-Matbouli, M.; Rahmati-Holasoo, H. Low Pathogenic Strain of Infectious Pancreatic Necrosis Virus (IPNV) Associated with Recent Outbreaks in Iranian Trout Farms. Pathogens 2020, 9, 782. [Google Scholar] [CrossRef] [PubMed]
- Mugimba, K.K.; Byarugaba, D.K.; Mutoloki, S.; Evensen, Ø.; Munang’andu, H.M. Challenges and Solutions to Viral Diseases of Finfish in Marine Aquaculture. Pathogens 2021, 10, 673. [Google Scholar] [CrossRef] [PubMed]
- Dadar, M.; Dhama, K.; Vakharia, V.N.; Hoseinifar, S.H.; Karthik, K.; Tiwari, R.; Joshi, S.K. Advances in Aquaculture Vaccines Against Fish Pathogens: Global Status and Current Trends. Rev. Fish. Sci. Aquac. 2017, 25, 184–217. [Google Scholar] [CrossRef]
- Ahmadivand, S.; Palić, D.; Weidmann, M. Molecular epidemiology of Novirhabdoviruses emerging in Iranian trout farms. Viruses 2021, 13, 448. [Google Scholar] [CrossRef]
- Fajardo, C.; De Donato, M.; Macedo, M.; Charoonnart, P.; Saksmerprome, V.; Yang, L.; Purton, S.; Mancera, J.M.; Costas, B. RNA Interference Applied to Crustacean Aquaculture. Biomolecules 2024, 14, 1358. [Google Scholar] [CrossRef]
- World Animal Health Information System (WAHIS). World Organisation for Animal Health (WOAH). Available online: https://wahis.woah.org/ (accessed on 20 April 2025).
- Soltani, M.; Zamani, M.; Taheri-Mirghaed, A.; Ahmadivand, S.; Mohamdian, S.; Abdi, K.; Soltani, E. Incidence and genetic analysis of white spot syndrome virus (WSSV) in farmed shrimps (P. indicus and L. vannamei) in Iran. Bull. Eur. Assoc. Fish Pathol. 2018, 38, 24–34. [Google Scholar]
- Ahmadivand, S.; Soltani, K.; Shokrpoor, S.; Rahmati-Holasoo, H.; El-Matbouli, M.; Taheri-Mirghaed, A. Cyprinid herpesvirus 3 (CyHV-3) transmission and outbreaks in Iran: Detection and characterization in farmed common carp. Microb. Pathog. 2020, 149, 104321. [Google Scholar] [CrossRef]
- Rahmati-Holasoo, H.; Ahmadivand, S.; Marandi, A.; Shokrpoor, S.; Palić, D.; Jahangard, A. Identification and characterization of lymphocystis disease virus (LCDV) from Indian glassy fish (Parambassis ranga Hamilton, 1822) in Iran. Aquac. Int. 2022, 30, 2593–2602. [Google Scholar] [CrossRef]
- Rahmati-Holasoo, H.; Ahmadivand, S.; Shokrpoor, S.; El-Matbouli, M. Detection of Carp pox virus (CyHV-1) from koi (Cyprinus carpio L.) in Iran; clinico-pathological and molecular characterization. Mol. Cell. Probes 2020, 54, 101668. [Google Scholar] [CrossRef]
- Vigil, K.; Wu, H.; Aw, T.G. A Systematic Review on Global Zoonotic Virus-Associated Mortality Events in Marine Mammals. One Health 2024, 19, 100872. [Google Scholar] [CrossRef] [PubMed]
- Ahmadivand, S.; Krpetic, Z.; Martínez, M.M.; García-Ordoñez, M.; Roher, N.; Palić, D. Self-Assembling Ferritin Nanoplatform for the Development of Infectious Hematopoietic Necrosis Virus Vaccine. Front. Immunol. 2024, 15, 1346512. [Google Scholar] [CrossRef]
- Kim, Y.S.; Son, A.; Kim, J.; Kwon, S.B.; Kim, M.H.; Kim, P. Chaperna-mediated assembly of ferritin-based Middle East respiratory syndrome-coronavirus nanoparticles. Front. Immunol. 2018, 9, 1093. [Google Scholar] [CrossRef] [PubMed]
- Ahmadivand, S.; Fux, R.; Palić, D. Ferritin Vaccine Platform for Animal and Zoonotic Viruses. Vaccines 2024, 12, 1112. [Google Scholar] [CrossRef] [PubMed]
- Palić, D.; Scarfe, A.D.; Walster, C.I. A Standardized Approach for Meeting National and International Aquaculture Biosecurity Requirements for Preventing, Controlling, and Eradicating Infectious Diseases. J. Appl. Aquac. 2015, 27, 185–219. [Google Scholar] [CrossRef]
- Palić, D.; Scarfe, A.D. Biosecurity in Aquaculture: Practical Veterinary Approaches for Aquatic Animal Disease Prevention, Control and Potential Eradication. Chapter 19. In Biosecurity in Animal Production and Veterinary Medicine (First Edition); Dewolf, J., Van Immersel, F., Eds.; ACCO Uitgeverij: Leuven, Belgium, 2018; pp. 497–520. ISBN 9789463443784. [Google Scholar]
- Scarfe, A.D.; Palić, D. Chapter 3—Aquaculture Biosecurity: Practical Approach to Prevent, Control, and Eradicate Diseases; Kibenge, F.S.B., Powell, M.D., Eds.; Aquaculture Health Management, Academic Press: Cambridge, MA, USA, 2020; pp. 75–116. [Google Scholar]
- Brummett, R.E.; Adolfo, A.; Kibenge, F.; Forster, J.; Burgos, J.M.; Ibarra, R.; Hilaire, S.S.; Chamberlain, G.C.; Lightner, D.V.; Khoa, L.V.; et al. Reducing Disease Risk in Aquaculture; World Bank Report No. 88257-GLB/Agriculture and Environmental Services Discussion Paper No. 9; World Bank: Washington, DC, USA, 2014; p. 97. [Google Scholar]
- FAO. Development of a Regional Aquatic Biosecurity Strategy for the Southern African Development Community (SADC); FAO Fisheries and Aquaculture Circular No. C1149; FAO: Rome, Italy, 2018; 344p. [Google Scholar]
- Håstein, T.; Binde, M.; Hine, M.; Johnsen, S.; Lillehaug, A.; Olesen, N.J.; Purvis, N.; Scarfe, A.D.; Wright, B. National biosecurity approaches, plans and programmes in response to diseases in farmed aquatic animals: Evolution, effectiveness and the way forward. Rev. Sci. Tech. 2008, 27, 125–145. [Google Scholar]
- World Organisation for Animal Health (WOAH). Aquatic Animals. Available online: https://www.woah.org/en/what-we-do/animal-health-and-welfare/aquatic-animals/ (accessed on 26 April 2025).
- Kurath, G.; Winton, J. Fish Rhabdoviruses. In Encyclopedia of Virology, 3rd ed.; Mahy, B.W.J., van Regenmortel, M.H.V., Eds.; Academic Press: Oxford, UK, 2008; pp. 221–222. [Google Scholar]
- Ammayappan, A.; LaPatra, S.E.; Vakharia, V.N. Molecular characterization of the virulent infectious hematopoietic necrosis virus (IHNV) strain 220-90. Virus Res. 2011, 159, 160–166. [Google Scholar] [CrossRef]
- Biacchesi, S.; Brémont, M. Vaccination against Viral Hemorrhagic Septicemia and Infectious Hematopoietic Necrosis. Fish Vaccin. 2014, 12, 289–302. [Google Scholar]
- World Organisation for Animal Health (WOAH). Infection with Viral Haemorrhagic Septicaemia Virus. In Manual of Diagnostic Tests for Aquatic Animals; WOAH: Paris, France, 2021; Available online: https://www.woah.org/fileadmin/Home/eng/Health_standards/aahm/current/2.3.10_VHS.pdf (accessed on 9 February 2025).
- Ahmadivand, S.; Soltani, M.; Mardani, K.; Shokrpoor, S.; Rahmati-Holasoo, H.; Mokhtari, A.; Hasanzadeh, R. Isolation and identification of viral hemorrhagic septicemia virus (VHSV) from farmed rainbow trout (Oncorhynchus mykiss) in Iran. Acta Trop. 2016, 156, 30–36. [Google Scholar] [CrossRef]
- Ahmadivand, S.; Soltani, M.; Mardani, K.; Shokrpoor, S.; Hassanzadeh, R.; Rahmati-Holasoo, H.; Meshkini, S. Infectious hematopoietic necrosis virus (IHNV) outbreak in farmed rainbow trout in Iran: Viral isolation, pathological findings, molecular confirmation, and genetic analysis. Virus Res. 2017, 229, 17–23. [Google Scholar] [CrossRef]
- Skall, H.F.; Olesen, N.J.; Mellergaard, S. Viral haemorrhagic septicaemia virus in marine fish and its implications for fish farming–A review. J. Fish Dis. 2005, 28, 509–529. [Google Scholar] [CrossRef]
- Bootland, L.M.; Leong, J.C. Infectious haematopoietic necrosis virus. In Fish Diseases and Disorders, Vol 3, Viral, Bacterial, and Fungal Infections, 2nd ed.; Woo, P.T.K., Bruno, D.W., Eds.; CAB International: Wallingford, UK, 2011; pp. 66–109. [Google Scholar]
- EU Reference Laboratory for Fish and Crustacean Diseases. Report on Survey and Diagnosis of Fish Diseases in Europe 2021. Available online: https://www.eurl-fish-crustacean.eu/-/media/sites/eurl-fish-crustacean/fish/survey-and-diagnosis/report-on-survey-and-diagnosis-of-fish-diseases-in-europe-2021.pdf (accessed on 30 January 2025).
- Smail, D.A.; Snow, M. Viral Haemorrhagic Septicaemia. In Fish Diseases and Disorders, Volume 3: Viral, Bacterial and Fungal Infections, 2nd ed.; Woo, P.T.K., Bruno, D.W., Eds.; CABI: Wallingford, UK, 2011; pp. 110–142. [Google Scholar]
- Evensen, Ø.; Leong, J.A. DNA vaccines against viral diseases of farmed fish. Fish Shellfish Immunol. 2013, 35, 1751–1758. [Google Scholar] [CrossRef]
- WOAH. Spring Viremia of Carp (SVC). Manual of Diagnostic Tests for Aquatic Animals; World Organisation for Animal Health: Paris, France, 2021; pp. 1–18. Available online: https://www.woah.org/fileadmin/Home/fr/Health_standards/aahm/current/2.3.09_SVC.pdf (accessed on 2 February 2025).
- Ahne, W.; Bjorklund, H.V.; Essbauer, S.; Fijan, N.; Kurath, G.; Winton, J.R. Spring viremia of carp (SVC). Dis. Aquat. Org. 2002, 52, 261–272. [Google Scholar] [CrossRef]
- Ashraf, U.; Lu, Y.; Lin, L.; Yuan, J.; Wang, M.; Liu, X. Spring viraemia of carp virus: Recent advances. J. Gen. Virol. 2016, 97, 1037–1051. [Google Scholar] [CrossRef]
- Dikkeboom, A.L.; Radi, C.; Toohey-Kurth, K.; Marcquenski, S.; Engel, M.; Goodwin, A.E.; Way, K.; Stone, D.M.; Longshaw, C. First Report of Spring Viremia of Carp Virus (SVCV) in Wild Common Carp in North America. J. Aquat. Anim. Health 2004, 16, 169–178. [Google Scholar] [CrossRef]
- Zhang, N.Z.; Zhang, L.F.; Jiang, Y.N.; Zhang, T.; Xia, C. Molecular analysis of spring viraemia of carp virus in China: A fatal aquatic viral disease that might spread in East Asian. PLoS ONE 2009, 4, e6337. [Google Scholar] [CrossRef]
- Emmenegger, E.J.; Bueren, E.K.; Jia, P.; Hendrix, N.; Liu, H. Comparative virulence of spring viremia of carp virus (SVCV) genotypes in two koi varieties. Dis. Aquat. Org. 2022, 148, 95–112. [Google Scholar] [CrossRef]
- FAO. FishStat: Global Aquaculture Production 1950–2022; FishStatJ.; FAO: Rome, Italy, 2024; Available online: www.fao.org/fishery/en/statistics/software/fishstatj (accessed on 29 March 2025).
- Dixon, P.F. Virus diseases of cyprinids. In Fish Diseases, Vol. 1; Eiras, J.C., Segner, H., Wahli, T., Kapoor, B.G., Eds.; Science Publishers: Enfield, NH, USA, 2008; pp. 87–184. [Google Scholar]
- El-Matbouli, M.; Soliman, H. Transmission of cyprinid herpesvirus-3 (CyHV-3) from goldfish to naïve common carp by cohabitation. Res. Vet. Sci. 2011, 90, 536–539. [Google Scholar] [CrossRef]
- Kanellos, T.; Sylvester, I.D.; D’Mello, F.; Howard, C.R.; Mackie, A.; Dixon, P.F.; Chang, K.C.; Ramstad, A.; Midtlyng, P.J.; Russell, P.H. DNA vaccination can protect Cyprinus Carpio against spring viraemia of carp virus. Vaccine 2006, 24, 4927–4933. [Google Scholar] [CrossRef] [PubMed]
- Embregts, C.W.E.; Rigaudeau, D.; Veselý, T.; Pokorová, D.; Lorenzen, N.; Petit, J.; Houel, A.; Dauber, M.; Schütze, H.; Boudinot, P.; et al. Intramuscular DNA Vaccination of Juvenile Carp against Spring Viremia of Carp Virus Induces Full Protection and Establishes a Virus-Specific B and T Cell Response. Front. Immunol. 2017, 8, 1340. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhang, P.Q.; Guo, S.; Zhao, Z.; Wang, G.X.; Zhu, B. Dual-Targeting Polymer Nanoparticles Efficiently Deliver DNA Vaccine and Induce Robust Prophylactic Immunity against Spring Viremia of Carp Virus Infection. Microbiol. Spectr. 2022, 10, e0308522. [Google Scholar] [CrossRef]
- World Organisation for Animal Health (WOAH). Infection with HPR-Deleted or HPR0 Infectious Salmon Anaemia Virus. In Manual of Diagnostic Tests for Aquatic Animals; WOAH: Paris, France, 2022; Available online: https://www.woah.org/fileadmin/Home/eng/Health_standards/aahm/current/2.3.04_ISA.pdf (accessed on 20 May 2025).
- Mjaaland, S.; Hungnes, O.; Teig, A.; Dannevig, B.H.; Thorud, K.; Rimstad, E. Polymorphism in the Infectious Salmon Anemia Virus Hemagglutinin Gene: Importance and Possible Implications for Evolution and Ecology of Infectious Salmon Anemia Disease. Virology 2002, 302, 379–391. [Google Scholar] [CrossRef]
- Rimstad, E.; Dale, O.B.; Dannevig, B.H.; Falk, K. Infectious Salmon Anaemia. In Fish Diseases and Disorders, Volume 3: Viral, Bacterial and Fungal Infections; Woo, P.T.K., Bruno, D., Eds.; CAB International: Oxfordshire, UK, 2011; pp. 143–165. [Google Scholar]
- Christiansen, D.H.; Østergaard, P.S.; Snow, M.; Dale, O.B.; Falk, K. A Low-Pathogenic Variant of Infectious Salmon Anemia Virus (ISAV1-HPR0) is Highly Prevalent and Causes a Non-Clinical Transient Infection in Farmed Atlantic Salmon (Salmo salar L.) in the Faroe Islands. J. Gen. Virol. 2011, 92, 909–918. [Google Scholar] [CrossRef]
- Kibenge, F.S.B.; Kibenge, M.J.T. Orthomyxoviruses of Fish. In Aquaculture Virology; Kibenge, F.S.B., Godoy, M.G., Eds.; Academic Press: Cambridge, MA, USA, 2016; pp. 299–326. [Google Scholar]
- Xiao, L.; Lin, H.; Yang, M.; Chen, S.; An, W.; Wu, X.; Liu, H.; Li, D.; Yan, Y.; Hu, J.; et al. The First Instance of HPR-Deleted ISAV Detection in Eviscerated, Fresh Salmon at a Chinese Entry-Exit Port. Aquaculture 2018, 485, 220–224. [Google Scholar] [CrossRef]
- Ramírez, R.; Marshall, S.H. Identification and Isolation of Infective Filamentous Particles in Infectious Salmon Anemia Virus (ISAV). Microb. Pathog. 2018, 117, 219–224. [Google Scholar] [CrossRef]
- Lyngstad, T.M.; Hjortaas, M.J.; Kristoffersen, A.B.; Markussen, T.; Karlsen, E.T.; Jonassen, C.M.; Jansen, P.A. Use of Molecular Epidemiology to Trace Transmission Pathways for Infectious Salmon Anaemia Virus (ISAV) in Norwegian Salmon Farming. Epidemics 2011, 3, 1–11. [Google Scholar] [CrossRef]
- Batts, W.N.; LaPatra, S.E.; Katona, R.; Leis, E.; Ng, T.F.F.; Brieuc, M.S.O.; Breyta, R.B.; Purcell, M.K.; Conway, C.M.; Waltzek, T.B.; et al. Molecular Characterization of a Novel Orthomyxovirus from Rainbow and Steelhead Trout (Oncorhynchus mykiss). Virus Res. 2017, 230, 38–49. [Google Scholar] [CrossRef]
- Fringuelli, E.; Rowley, H.M.; Wilson, J.C.; Hunter, R.; Rodger, H.; Graham, D.A. Phylogenetic analyses and molecular epidemiology of European salmonid alphaviruses (SAV) based on partial E2 and nsP3 gene nucleotide sequences. J. Fish Dis. 2008, 31, 811–823. [Google Scholar] [CrossRef]
- Forrester, N.L.; Palacios, G.; Tesh, R.B.; Savji, N.; Guzman, H.; Sherman, M.; Weaver, S.C.; Lipkin, W.I. Genome-scale phylogeny of the alphavirus genus suggests a marine origin. J. Virol. 2012, 86, 2729–2738. [Google Scholar] [CrossRef] [PubMed]
- La Linn, M.; Gardner, J.; Warrilow, D.; Darnell, G.A.; McMahon, C.R.; Field, I.; Hyatt, A.D.; Slade, R.W.; Suhrbier, A. Arbovirus of marine mammals: A new alphavirus isolated from the elephant seal louse, Lepidophthirus macrorhini. J. Virol. 2001, 75, 4103–4109. [Google Scholar] [CrossRef]
- McCleary, S.J.; Giltrap, M.; Henshilwood, K.; Ruane, N.M. Detection of salmonid alphavirus RNA in Celtic and Irish Sea flatfish. Dis. Aquat. Org. 2014, 109, 1–7. [Google Scholar] [CrossRef]
- Snow, M.; Black, I.; McIntosh, R.; Baretto, E.; Wallace, I.S.; Bruno, D.W. Detection of salmonid alphavirus RNA in wild marine fish: Implications for the origin of salmon pancreas disease in aquaculture. Dis. Aquat. Org. 2010, 91, 177–188. [Google Scholar] [CrossRef]
- World Organisation for Animal Health (WOAH). Salmonid Alphavirus (SAV) Standard. Available online: https://www.woah.org/fileadmin/Home/fr/Health_standards/aahm/current/2.3.08_SAV.pdf (accessed on 30 January 2025).
- Kurath, G.; Winton, J. Complex dynamics at the interface between wild and domestic viruses of finfish. Curr. Opin. Virol. 2011, 1, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Graham, D.A.; Wilson, C.; Jewhurst, H.; Rowley, H. Cultural Characteristics of Salmonid Alphaviruses—Influences of Cell Line and Temperature. J. Fish Dis. 2008, 31, 859–868. [Google Scholar] [CrossRef]
- WOAH. Infection with Tilapia Lake Virus (TiLV); Updated 18 August 2022. Available online: https://www.woah.org/app/uploads/2022/10/a-woah-tilv-disease-card-2022.pdf (accessed on 22 January 2025).
- Koonin, E.V.; Krupovic, M.; Surachetpong, W.; Wolf, Y.I.; Kuhn, J.H. ICTV Virus Taxonomy Profile: Amnoonviridae 2023. J. Gen. Virol. 2023, 104, 001903. [Google Scholar] [CrossRef]
- Jaemwimol, P.; Rawiwan, P.; Tattiyapong, P.; Saengnual, P.; Kamlangee, A.; Surachetpong, W. Susceptibility of Important Warm-Water Fish Species to Tilapia Lake Virus (TiLV) Infection. Aquaculture 2018, 497, 462–468. [Google Scholar] [CrossRef]
- Kembou-Ringert, J.E.; Steinhagen, D.; Readman, J.; Daly, J.M.; Adamek, M. Tilapia Lake Virus Vaccine Development: A Review on the Recent Advances. Vaccines 2023, 11, 251. [Google Scholar] [CrossRef]
- Eyngor, M.; Zamostiano, R.; Kembou Tsofack, J.E.; Berkowitz, A.; Bercovier, H.; Tinman, S.; Lev, M.; Hurvitz, A.; Galeotti, M.; Bacharach, E.; et al. Identification of a Novel RNA Virus Lethal to Tilapia. J. Clin. Microbiol. 2014, 52, 4137–4146. [Google Scholar] [CrossRef]
- Dong, H.T.; Senapin, S.; Gangnonngiw, W.; Nguyen, V.V.; Rodkhum, C.; Debnath, P.P.; Delamare-Deboutteville, J.; Mohan, C.V. Experimental Infection Reveals Transmission of Tilapia Lake Virus (TiLV) from Tilapia Broodstock to Their Reproductive Organs and Fertilized Eggs. Aquaculture 2020, 515, 734541. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Q.; Zeng, W.; Yin, J.; Li, Y.; Ren, Y.; Shi, C.; Bergmann, S.M.; Zhu, X. Establishment and Characterization of a Cell Line from Tilapia Brain for Detection of Tilapia Lake Virus. J. Fish Dis. 2018, 41, 1803–1809. [Google Scholar] [CrossRef] [PubMed]
- Boonyaratpalin, S.; Supamataya, K.; Kasornchandra, J.; Direkbusarakom, S.; Aekpanithanpong, U.; Chantanachookhin, C. Non-occluded baculo-like virus, the causative agent of yellow-head disease in the black tiger shrimp Penaeus monodon. Fish Pathol. 1993, 28, 103–109. [Google Scholar] [CrossRef]
- Senapin, S.; Thaowbut, Y.; Gangnonngiw, W.; Chuchird, N.; Sriurairatana, S.; Flegel, T.W. Impact of yellow head virus outbreaks in the whiteleg shrimp, Penaeus vannamei (Boone), in Thailand. J. Fish Dis. 2010, 33, 421–430. [Google Scholar] [CrossRef]
- Flegel, T.W.; Boonyaratpalin, S.; Withyachumnarnkul, B. Current status of research on yellow-head virus and white-spot virus in Thailand. In Diseases in Asian Aquaculture III; Flegel, T.W., MacRae, I.H., Eds.; Fish Health Section, Asian Fisheries Society: Manila, Philippines, 1997; pp. 285–296. [Google Scholar]
- Wongteerasupaya, C.; Tongcheua, W.; Boonsaeng, V.; Panyim, S.; Tassanakajon, A.; Withyachumnarnkul, B.; Flegel, T.W. Detection of yellow-head virus of Penaeus monodon by RT-PCR amplification. Dis. Aquat. Org. 1997, 31, 181–186. [Google Scholar] [CrossRef]
- Mohr, P.G.; Moody, N.J.; Hoad, J.; Williams, L.M.; Bowater, R.O.; Cummins, D.M.; Cowley, J.A.; StJ Crane, M. New yellow head virus genotype (YHV7) in giant tiger shrimp Penaeus monodon indigenous to northern Australia. Dis. Aquat. Org. 2015, 115, 263–268. [Google Scholar] [CrossRef]
- Walker, P.J.; Cowley, J.A.; Dong, X.; Huang, J.; Moody, N.; Ziebuhr, J.; Consortium, I.R. ICTV virus taxonomy profile: Roniviridae. J. Gen. Virol. 2021, 102, jgv001514. [Google Scholar] [CrossRef]
- Wijegoonawardane, P.K.M.; Cowley, J.A.; Phan, T.; Hodgson, R.A.J.; Nielsen, L.; Kiatpathomchai, W.; Walker, P.J. Genetic diversity in the yellow head nidovirus complex. Virology 2008, 380, 213–225. [Google Scholar] [CrossRef]
- Chantanachookin, C.; Boonyaratpalin, S.; Kasornchandra, J.; Direkbusarakom, S.; Aekpanithanpong, U.; Supamattaya, K.; Sriurairatana, S.; Flegel, T.W. Histology and Ultrastructure Reveal a New Granulosis-Like Virus in Penaeus monodon Affected by Yellow-Head Disease. Dis. Aquat. Org. 1993, 17, 145–157. [Google Scholar] [CrossRef]
- Spann, K.M.; Donaldson, R.A.; Cowley, J.A.; Walker, P.J. Differences in the susceptibility of some penaeid prawn species to gill-associated virus (GAV) infection. Dis. Aquat. Org. 2000, 42, 221–225. [Google Scholar] [CrossRef]
- Cowley, J.A.; Hall, M.R.; Cadogan, L.C.; Spann, K.M.; Walker, P.J. Vertical transmission of gill-associated virus (GAV) in the black tiger prawn Penaeus monodon. Dis. Aquat. Org. 2002, 50, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Tirasophon, W.; Roshorm, Y.; Panyim, S. Silencing of yellow head virus replication in penaeid shrimp cells by dsRNA. Biochem. Biophys. Res. Commun. 2005, 334, 102–107. [Google Scholar] [CrossRef]
- Gangnonngiw, W.; Kanthong, N. Failed shrimp vaccination attempt with yellow head virus (YHV) attenuated in an immortal insect cell line. Fish Shellfish Immunol. Rep. 2023, 4, 100084. [Google Scholar] [CrossRef]
- WOAH. Chapter 2.2.7: Infection with Taura Syndrome Virus. Manual of Diagnostic Tests for Aquatic Animals. 2023. Available online: https://www.woah.org/fileadmin/Home/fr/Health_standards/aahm/current/2.2.07_TS.pdf (accessed on 5 February 2025).
- Chong, R.S.-M. Chapter 13—Taura Syndrome Virus Disease. In Aquaculture Pathophysiology, Volume II: Crustacean and Molluscan Diseases; Kibenge, F.S.B., Baldisserotto, B., Chong, R.S.-M., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 95–97. [Google Scholar]
- Robles-Sikisaka, R.; Hasson, K.W.; Garcia, D.K.; Brovont, K.; Cleveland, K.; Kimpel, K.R.; Dhar, A.K. Genetic variation and immunohistochemical differences among geographical isolates of Taura syndrome virus of penaeid shrimp. J. Gen. Virol. 2002, 83, 3123–3130. [Google Scholar] [CrossRef]
- Nielsen, L.; Sang-Oum, W.; Cheevadhanarak, S.; Flegel, T.W. Taura syndrome virus (TSV) in Thailand and its relationship to TSV in China and the Americas. Dis. Aquat. Org. 2005, 63, 101–106. [Google Scholar] [CrossRef]
- Lightner, D.V. (Ed.) A Handbook of Shrimp Pathology and Diagnostic Procedures for Diseases of Cultured Penaeid Shrimp; World Aquaculture Society: Baton Rouge, LA, USA, 1996; 304p. [Google Scholar]
- Lightner, D.V.; Redman, R.M.; Arce, S.; Moss, S.M. Specific pathogen-free shrimp stocks in shrimp farming facilities as a novel method for disease control in crustaceans. In Shellfish Safety and Quality; Shumway, S., Rodrick, G., Eds.; Woodhead Publishers: London, UK, 2009; pp. 384–424. [Google Scholar]
- Hasson, K.W.; Lightner, D.V.; Poulos, B.T.; Redman, R.M.; White, B.L.; Brock, J.A.; Bonami, J.R. Taura syndrome in Penaeus vannamei: Demonstration of a viral etiology. Dis. Aquat. Org. 1995, 23, 115–126. [Google Scholar] [CrossRef]
- Chen, S.N.; Chang, P.S.; Kou, G.H. Infection route and eradication of Penaeus monodon baculovirus (MBV) in larval giant tiger prawns, Penaeus monodon. In Diseases of Cultured Penaeid Shrimp in Asia and the United States; Fulks, W., Main, K.L., Eds.; Oceanic Institute: Honolulu, HI, USA, 1992; pp. 177–184. [Google Scholar]
- Vanpatten, K.A.; Nunan, L.M.; Lightner, D.V. Seabirds as potential vectors of penaeid shrimp viruses and the development of a surrogate laboratory model utilizing domestic chickens. Aquaculture 2004, 241, 31–46. [Google Scholar] [CrossRef]
- Prochaska, J.; Poompuang, S.; Koonawootrittriron, S.; Sukhavachana, S.; Na-Nakorn, U. Evaluation of a commercial SPF Litopenaeus vannamei shrimp breeding program: Resistance to Infectious Myonecrosis Virus (IMNV), Taura Syndrome Virus (TSV), and White Spot Syndrome Virus (WSSV) from laboratory challenges. Aquaculture 2022, 554, 738145. [Google Scholar] [CrossRef]
- World Organisation for Animal Health (WOAH). Infection with Infectious Myonecrosis Virus. In Manual of Diagnostic Tests for Aquatic Animals; WOAH: Paris, France, 2023; Available online: https://www.woah.org/fileadmin/Home/eng/Health_standards/aahm/current/2.2.05_IMN.pdf (accessed on 20 May 2025).
- Lee, D.; Yu, Y.-B.; Choi, J.-H.; Jo, A.-H.; Hong, S.-M.; Kang, J.-C.; Kim, J.-H. Viral Shrimp Diseases Listed by the OIE: A Review. Viruses 2022, 14, 585. [Google Scholar] [CrossRef]
- Prasad, K.P.; Shyam, K.U.; Banu, H.; Jeena, K.; Krishnan, R. Infectious Myonecrosis Virus (IMNV)–An Alarming Viral Pathogen to Penaeid Shrimps. Aquaculture 2017, 477, 99–105. [Google Scholar] [CrossRef]
- Poulos, B.T.; Lightner, D.V. Detection of Infectious Myonecrosis Virus (IMNV) of Penaeid Shrimp by Reverse-Transcriptase Polymerase Chain Reaction (RT-PCR). Dis. Aquat. Org. 2006, 73, 69–72. [Google Scholar] [CrossRef] [PubMed]
- Sahul Hameed, A.S.; Abdul Majeed, S.; Vimal, S.; Madan, N.; Rajkumar, T.; Santhoshkumar, S.; Sivakumar, S. Studies on the Occurrence of Infectious Myonecrosis Virus in Pond-Reared Litopenaeus vannamei (Boone, 1931) in India. J. Fish Dis. 2017, 40, 1823–1830. [Google Scholar] [CrossRef]
- Lightner, D.V. Virus Diseases of Farmed Shrimp in the Western Hemisphere (the Americas): A Review. J. Invertebr. Pathol. 2011, 106, 110–130. [Google Scholar] [CrossRef]
- Jha, R.K.; Babikian, H.; Kristina; Srisombat, S. Managing Infectious Myonecrosis Virus (IMNV) in Vannamei Shrimp Culture: Learning by Doing. Int. J. Fish. Aquat. Stud. 2021, 9, 385–391. [Google Scholar] [CrossRef]
- Da Silva, S.M.B.C.; Lavander, H.D.; De Sanatana, L.U.N.A.M.M.; Da Silva, A.O.M.E.; Galvez, A.O.; Coimbra, M.R.M. Artemia franciscana as a Vector for Infectious Myonecrosis Virus (IMNV) to Litopenaeus vannamei Juvenile. J. Invertebr. Pathol. 2015, 126, 1–5. [Google Scholar] [CrossRef]
- Sahul Hameed, A.S.; Bonami, J.R. White Tail Disease of Freshwater Prawn, Macrobrachium rosenbergii. Indian J. Virol. 2012, 23, 134–140. [Google Scholar] [CrossRef]
- Bonami, J.R.; Shi, Z.; Qian, D.; Sri Widada, J. White tail disease of the giant freshwater prawn, Macrobrachium rosenbergii: Separation of the associated virions and characterization of MrNV as a new type of nodavirus. J. Fish Dis. 2005, 28, 23–31. [Google Scholar] [CrossRef]
- Arcier, J.-M.; Herman, F.; Lightner, D.V.; Redman, R.M.; Mari, J.; Bonami, J.-R. A viral disease associated with mortalities in hatchery-reared postlarvae of the giant freshwater prawn Macrobrachium rosenbergii. Dis. Aquat. Org. 1999, 38, 177–181. [Google Scholar] [CrossRef]
- WOAH. Manual of Diagnostic Tests for Aquatic Animals. Chapter 2.2.7, Infection with Macrobrachium rosenbergii Nodavirus (White Tail Disease). 2024. Available online: https://www.woah.org/fileadmin/Home/fr/Health_standards/aahm/current/2.2.07._MrNv_WTD.pdf (accessed on 9 February 2025).
- Gangnonngiwa, W.; Bunnontae, M.; Phiwsaiya, K.; Senapin, S.; Dhar, A.K. In experimental challenge with infectious clones of Macrobrachium rosenbergii nodavirus (MrNV) and extra small virus (XSV), MrNV alone can cause mortality in freshwater prawn (Macrobrachium rosenbergii). Virology 2020, 540, 30–37. [Google Scholar] [CrossRef]
- Murwantoko, M.; Bimantara, A.; Roosmanto, R.; Kawaichi, M. Macrobrachium rosenbergii nodavirus infection in a giant freshwater prawn hatchery in Indonesia. Springerplus 2016, 5, 1729. [Google Scholar] [CrossRef]
- Sahul Hameed, A.S.; Yoganandhan, K.; Sri Widada, J.; Bonami, J.R. Experimental transmission and tissue tropism of Macrobrachium rosenbergii nodavirus (MrNV) and its associated small virus (XSV). Dis. Aquat. Org. 2004, 62, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Sri Widada, J.; Durand, S.; Cambournac, I.; Qian, D.; Shi, Z.; Dejonghe, E.; Richard, V.; Bonami, J.R. Genome-based detection methods of Macrobrachium rosenbergii nodavirus, a pathogen of the giant freshwater prawn, Macrobrachium rosenbergii: Dot-blot, in situ hybridization and RT-PCR. J. Fish Dis. 2003, 26, 583–590. [Google Scholar] [CrossRef]
- Farook, M.A.; Sundar Raj, N.; Madan, N.; Vimal, S.; Abdul Majeed, S.; Taju, G.; Rajkumar, T.; Santhoshkumar, S.; Sivakumar, S.; Sahul Hameed, A.S. Immunomodulatory effect of recombinant Macrobrachium rosenbergii nodavirus capsid protein (r-MCP) against white tail disease of giant freshwater prawn, Macrobrachium rosenbergii (de Man, 1879). Aquaculture 2014, 433, 395–403. [Google Scholar] [CrossRef]
- Jo, W.K.; Osterhaus, A.D.; Ludlow, M. Transmission of morbilliviruses within and among marine mammal species. Curr. Opin. Virol. 2018, 28, 133–141. [Google Scholar]
- Wellehan, J.; Cortes-Hinojosa, G. Marine Mammal Viruses. In Fowler’s Zoo and Wild Animal Medicine Current Therapy, Volume 9; National Institutes of Health: Bethesda, MD, USA, 2019; pp. 597–602. [Google Scholar]
- Padalino, I.; Di Guardo, G.; Carbone, A.; Troiano, P.; Parisi, A.; Galante, D.; Cafiero, M.A.; Caruso, M.; Palazzo, L.; Guarino, L.; et al. Dolphin morbillivirus in Eurasian otters, Italy. Emerg. Infect. Dis. 2019, 25, 372–374. [Google Scholar] [CrossRef]
- Petrella, A.; Mazzariol, S.; Padalino, I.; Di Francesco, G.; Casalone, C.; Grattarola, C.; Di Guardo, G.; Smoglica, C.; Centelleghe, C.; Gili, C. Cetacean morbillivirus and Toxoplasma gondii co-infection in Mediterranean monk seal pup, Italy. Emerg. Infect. Dis. 2021, 27, 1237–1239. [Google Scholar]
- Kennedy, J.M.; Earle, J.A.P.; Omar, S.; Abdullah, H.; Nielsen, O.; Roelke-Parker, M.E.; Cosby, S.L. Canine and Phocine Distemper Viruses: Global Spread and Genetic Basis of Jumping Species Barriers. Viruses 2019, 11, 944. [Google Scholar] [CrossRef]
- Cornwell, H.; Thompson, H.; McCandlish, I.; Macartney, L.; Nash, A. Encephalitis in dogs associated with a batch of canine distemper (Rockborn) vaccine. Vet. Rec. 1988, 122, 54–59. [Google Scholar] [CrossRef]
- VanWormer, E.; Mazet, J.A.K.; Hall, A.; Gill, V.A.; Boveng, P.L.; London, J.M.; Gelatt, T.; Fadely, B.S.; Lander, M.E.; Sterling, J.; et al. Viral emergence in marine mammals in the North Pacific may be linked to Arctic sea ice reduction. Sci. Rep. 2019, 9, 15569. [Google Scholar] [CrossRef]
- Stone, B.M.; Blyde, D.J.; Saliki, J.T.; Morton, J.M. Morbillivirus infection in live stranded, injured, trapped, and captive cetaceans in southeastern Queensland and northern New South Wales, Australia. J. Wildl. Dis. 2012, 48, 47–55. [Google Scholar] [CrossRef]
- Morris, S.E.; Zelner, J.L.; Fauquier, D.A.; Rowles, T.K.; Rosel, P.E.; Gulland, F.; Grenfell, B.T. Partially Observed Epidemics in Wildlife Hosts: Modelling an Outbreak of Dolphin Morbillivirus in the Northwestern Atlantic, June 2013–2014. J. R. Soc. Interface 2015, 12, 20150676. [Google Scholar] [CrossRef] [PubMed]
- Klepac, P.; Pomeroy, L.W.; Bjornstad, O.N.; Kuiken, T.; Osterhaus, A.D.; Rijks, J.M. Stage-Structured Transmission of Phocine Distemper Virus in the Dutch 2002 Outbreak. Proc. Biol. Sci. 2009, 276, 2469–2476. [Google Scholar] [CrossRef] [PubMed]
- Bossart, G.D.; Reif, J.S.; Schaefer, A.; Goldstein, J.; Fair, P.A.; Salikii, J.T. Morbillivirus infection in free-ranging Atlantic bottlenose dolphins (Tursiops truncatus) from the southeastern United States: Seroepidemiologic and pathologic evidence of subclinical infection. Vet. Microbiol. 2010, 143, 160–166. [Google Scholar] [CrossRef]
- Vargas-Castro, I.; Peletto, S.; Mattioda, V.; Goria, M.; Serracca, L.; Varello, K.; Sánchez-Vizcaíno, J.M.; Puleio, R.; Nocera, F.D.; Lucifora, G.; et al. Epidemiological and genetic analysis of Cetacean Morbillivirus circulating on the Italian coast between 2018 and 2021. Front. Vet. Sci. 2023, 10, 1216838. [Google Scholar] [CrossRef]
- Yanagi, Y.; Tatsuo, H.; Ono, N.; Tanaka, K. SLAM (CDw150) is a cellular receptor for measles virus. Nature 2000, 406, 893–897. [Google Scholar]
- Noyce, R.S.; Bondre, D.G.; Ha, M.N.; Lin, L.-T.; Sisson, G.; Tsao, M.-S.; Richardson, C.D. Tumor cell marker PVRL4 (Nectin 4) is an epithelial cell receptor for measles virus. PLoS Pathog. 2011, 7, e1002240. [Google Scholar] [CrossRef]
- de Vries, R.D.; Duprex, W.P.; de Swart, R.L. Morbillivirus infections: An introduction. Viruses 2015, 7, 699–706. [Google Scholar] [CrossRef]
- Saliki, J.T.; Cooper, E.J.; Gustavson, J.P. Emerging morbillivirus infections of marine mammals: Development of two diagnostic approaches. Ann. N. Y. Acad. Sci. 2002, 969, 51–59. [Google Scholar] [CrossRef]
- Shams, F.; Pourtaghi, H. Effect of maternally derived antibodies on two commercial vaccines in changes of serum antibody titres against distemper in puppies. Vet. Med. Sci. 2023, 9, 698–703. [Google Scholar] [CrossRef]
- Bouvier, N.M.; Palese, P. The biology of influenza viruses. Vaccine 2008, 26 (Suppl. S4), D49–D53. [Google Scholar] [CrossRef]
- Ramis, A.J.; van Riel, D.; van de Bildt, M.W.; Osterhaus, A.; Kuiken, T. Influenza A and B virus attachment to respiratory tract in marine mammals. Emerg. Infect. Dis. 2012, 18, 817–820. [Google Scholar] [CrossRef] [PubMed]
- Webster, R.G.; Geraci, J.; Petursson, G.; Skirnisson, K. Conjunctivitis in human beings caused by influenza A virus of seals. N. Engl. J. Med. 1981, 304, 911. [Google Scholar] [PubMed]
- Fereidouni, S.R.; Harder, T.C.; Gaidet, N.; Ziller, M.; Hoffmann, B.; Hammoumi, S.; Globig, A.; Starick, E.; Kaleta, E.F.; Eiden, M.; et al. Influenza Virus Infections in Marine Mammals. Emerg. Infect. Dis. 2016, 22, 839–842. [Google Scholar] [CrossRef] [PubMed]
- Plancarte, M.; Kovalenko, G.; Baldassano, J.; Ramírez, A.L.; Carrillo, S.; Duignan, P.J.; Goodfellow, I.; Bortz, E.; Dutta, J.; van Bakel, H.; et al. Human influenza A virus H1N1 in marine mammals in California, 2019. PLoS ONE 2023, 18, e0283049. [Google Scholar] [CrossRef]
- Plaza, P.I.; Gamarra-Toledo, V.; Euguí, J.R.; Lambertucci, S.A. Recent Changes in Patterns of Mammal Infection with Highly Pathogenic Avian Influenza A(H5N1) Virus Worldwide. Emerg. Infect. Dis. 2024, 30, 444–452. [Google Scholar] [CrossRef]
- Uhart, M.M.; Vanstreels, R.E.T.; Nelson, M.I.; Olivera, V.; Campagna, J.; Zavattieri, V.; Lemey, P.; Campagna, C.; Falabella, V.; Rimondi, A. Epidemiological data of an influenza A/H5N1 outbreak in elephant seals in Argentina indicates mammal-to-mammal transmission. Nat. Commun. 2024, 15, 9516. [Google Scholar] [CrossRef]
- UK Government. Confirmed Findings of Influenza of Avian Origin in Non-Avian Wildlife. 2025. Available online: https://www.gov.uk/government/publications/bird-flu-avian-influenza-findings-in-non-avian-wildlife/confirmed-findings-of-influenza-of-avian-origin-in-non-avian-wildlife (accessed on 9 February 2025).
- Gadzhiev, A.; Petherbridge, G.; Sharshov, K.; Sobolev, I.; Alekseev, A.; Gulyaeva, M.; Litvinov, K.; Boltunov, I.; Teymurov, A.; Zhigalin, A.; et al. Pinnipeds and avian influenza: A global timeline and review of research on the impact of highly pathogenic avian influenza on pinniped populations with particular reference to the endangered Caspian seal (Pusa caspica). Front. Cell. Infect. Microbiol. 2024, 14, 1325977. [Google Scholar] [CrossRef]
- Hinshaw, V.S.; Bean, W.J.; Geraci, J.; Fiorelli, P.; Early, G.; Webster, R.G. Characterization of two influenza A viruses from a pilot whale. J. Virol. 1986, 58, 655–656. [Google Scholar] [CrossRef]
- Murawski, A.; Fabrizio, T.; Ossiboff, R.; Kackos, C.; Jeevan, T.; Jones, J.C.; Kandeil, A.; Walker, D.; Turner, J.C.M.; Patton, C.; et al. Highly pathogenic avian influenza A(H5N1) virus in a common bottlenose dolphin (Tursiops truncatus) in Florida. Commun. Biol. 2024, 7, 476. [Google Scholar] [CrossRef]
- Sevilla, N.; Lizarraga, W.; Jimenez-Vasquez, V.; Hurtado, V.; Molina, I.S.; Huarca, L.; Lope-Pari, P.; Vargas, I.; Arotinco, G.; Padilla-Rojas, C. Highly pathogenic avian influenza A (H5N1) virus outbreak in Peru in 2022–2023. Infect. Med. 2024, 3, 100108. [Google Scholar] [CrossRef]
- Shin, D.L.; Siebert, U.; Lakemeyer, J.; Grilo, M.; Pawliczka, I.; Wu, N.H.; Valentin-Weigand, P.; Haas, L.; Herrler, G. Highly Pathogenic Avian Influenza A(H5N8) Virus in Gray Seals, Baltic Sea. Emerg. Infect. Dis. 2019, 25, 2295–2298. [Google Scholar] [CrossRef]
- Postel, A.; King, J.; Kaiser, F.K.; Kennedy, J.; Lombardo, M.S.; Reineking, W.; de le Roi, M.; Harder, T.; Pohlmann, A.; Gerlach, T.; et al. Infections with highly pathogenic avian influenza A virus (HPAIV) H5N8 in harbor seals at the German North Sea coast, 2021. Emerg. Microbes Infect. 2022, 11, 725–729. [Google Scholar] [CrossRef]
- Kaplan, B.S.; Webby, R.J. The avian and mammalian host range of highly pathogenic avian H5N1 influenza. Virus Res. 2013, 178, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Campagna, C.; Uhart, M.; Falabella, V.; Campagna, J.; Zavattieri, V.; Vanstreels, R.E.T.; Lewis, M.N. Catastrophic mortality of southern elephant seals caused by H5N1 avian influenza. Mar. Mammal Sci. 2024, 40, 322–325. [Google Scholar] [CrossRef]
- Runstadler, J.A.; Puryear, W. A Brief Introduction to Influenza A Virus in Marine Mammals. In Animal Influenza Virus. Methods in Molecular Biology; Spackman, E., Ed.; Humana: New York, NY, USA, 2020; Volume 2123. [Google Scholar] [CrossRef]
- Rimondi, A.; Vanstreels, R.; Olivera, V.; Donini, A.; Lauriente, M.; Uhart, M.M. Highly Pathogenic Avian Influenza A(H5N1) Viruses from Multispecies Outbreak, Argentina, August 2023. Emerg. Infect. Dis. 2024, 30, 812–814. [Google Scholar] [CrossRef]
- Duignan, P.J.; Van Bressem, M.F.; Cortés-Hinojosa, G.A.; Kennedy-Stoskopf, S. Viruses. In CRC Handbook of Marine Mammal Medicine, 3rd ed.; Gulland, F.M.D., Dierauf, L.A., Whitman, K.L., Eds.; CRC Press: Boca Raton, FL, USA, 2018; pp. 331–365. [Google Scholar]
- Schmitt, T.L.; Reidarson, T.H.; St. Leger, J.; Rivera, R.; Nollens, H.H. Novel presentation of San Miguel sea lion virus (Calicivirus) epizootic in adult captive sea lions, Zalophus californianus. In Proceedings of the International Association for Aquatic Animal Medicine, 40th Annual Conference, San Antonio, TX, USA, 2–6 May 2009. [Google Scholar]
- Smith, A.W.; Iversen, P.L.; Skilling, D.E.; Stein, D.A.; Bok, K.; Matson, D.O. Vesivirus viremia and seroprevalence in humans. J. Med. Virol. 2006, 78, 693–701. [Google Scholar] [CrossRef]
- Vinjé, J.; Estes, M.K.; Esteves, P.; Green, K.Y.; Katayama, K.; Knowles, N.J.; L’Homme, Y.; Martella, V.; Vennema, H.; White, P.A. ICTV Virus Taxonomy Profile: Caliciviridae. J. Gen. Virol. 2019, 100, 1469–1470. [Google Scholar] [CrossRef]
- Smith, A.W.; Skilling, D.E.; Prato, C.M.; Bray, H.L. Calicivirus (SMSV-5) infection in experimentally inoculated opaleye fish (Girella nigricans). Arch. Virol. 1981, 67, 165–168. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.W.; Skilling, D.E.; Cherry, N.; Mead, J.H.; Matson, D.O. Calicivirus Emergence from Ocean Reservoirs: Zoonotic and Interspecies Movements. Emerg. Infect. Dis. 1998, 4, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Neill, J.D. The complete genome sequence of the San Miguel sea lion virus-8 reveals that it is not a member of the vesicular exanthema of swine virus/San Miguel sea lion virus species of the Caliciviridae. Genome Announc. 2014, 2, e01286-14. [Google Scholar] [CrossRef]
- Bossart, G.D.; Duignan, P.J. Emerging Viruses in Marine Mammals. CAB Rev. 2018, 13, 052. [Google Scholar] [CrossRef]
- Li, L.; Shan, T.; Delwart, E. The Fecal Viral Flora of California Sea Lions. J. Virol. 2011, 85, 9909–9917. [Google Scholar] [CrossRef]
- de Graaf, M.; Bodewes, R.; Koopmans, M.P.G. Norovirus Infection in Harbor Porpoises. Emerg. Infect. Dis. 2017, 23, 87–91. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, D.H.; Sair, A.; Williams, K.; Papafragkou, E.; Jean, J.; Moore, C.; Jaykus, L. Persistence of caliciviruses on environmental surfaces and their transfer to food. Int. J. Food Microbiol. 2006, 108, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.W.; Skilling, D.E.; Ridgway, S. Calicivirus-Induced Vesicular Disease in Cetaceans and Probable Interspecies Transmission. J. Am. Vet. Med. Assoc. 1983, 183, 1223–1225. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, S.; Kulkarni, A.; Kv, R. Prospects of Vaccination in Crustaceans with Special Reference to Shrimp. In Fish Immune System and Vaccines; Makesh, M., Rajendran, K.V., Eds.; Springer: Singapore, 2022. [Google Scholar] [CrossRef]
- Fredrick, W.S.; Ravichandran, S. Hemolymph proteins in marine crustaceans. Asian Pac. J. Trop. Biomed. 2012, 2, 496–502. [Google Scholar] [CrossRef]
- Russell, F.A.; Hutmacher, D.W.; Dargaville, T.R.; Beagley, K. Vaccine delivery: Overcoming the challenges of vaccinating livestock and wildlife. Vet. Vaccine 2024, 3, 100093. [Google Scholar] [CrossRef]
- Tammas, I.; Bitchava, K.; Gelasakis, A.I. Transforming Aquaculture through Vaccination: A Review on Recent Developments and Milestones. Vaccines 2024, 12, 732. [Google Scholar] [CrossRef]
- Sommerset, I.; Krossøy, B.; Biering, E.; Frost, P. Vaccines for fish in aquaculture. Expert Rev. Vaccines 2005, 4, 89–101. [Google Scholar] [CrossRef]
- van Muiswinkel, W.B.; Pilarczyk, A.; Rehulka, J. Vaccination against Spring Viraemia of Carp (SVC)—From the past till the future. Bull. Eur. Assoc. Fish Pathol. 2018, 38, 254–260. [Google Scholar]
- Tattiyapong, P.; Kitiyodom, S.; Yata, T.; Jantharadej, K.; Adamek, M.; Surachetpong, W. Chitosan nanoparticle immersion vaccine offers protection against tilapia lake virus in laboratory and field studies. Fish Shellfish Immunol. 2022, 131, 972–979. [Google Scholar] [CrossRef]
- Mai, T.T.; Kayansamruaj, P.; Taengphu, S.; Senapin, S.; Costa, J.Z.; Del-Pozo, J.; Thompson, K.D.; Rodkhum, C.; Dong, H.T. Efficacy of heat-killed and formalin-killed vaccines against Tilapia tilapinevirus in juvenile Nile tilapia (Oreochromis niloticus). J. Fish Dis. 2021, 44, 2097–2109. [Google Scholar] [CrossRef]
- Bacharach, E.; Eldar, A. Tilapia Lake Virus Vaccines. U.S. Patent US2016/0354458A1, 9 August 2016. Available online: https://patents.google.com/patent/US20160354458A1/en (accessed on 12 September 2022).
- Biacchesi, S.; Mérour, E.; Chevret, D.; Lamoureux, A.; Bernard, J.; Brémont, M. NV Proteins of Fish Novirhabdovirus Recruit Cellular PPM1Bb Protein Phosphatase and Antagonize RIG-I-Mediated IFN Induction. Sci. Rep. 2017, 7, 44025. [Google Scholar] [CrossRef] [PubMed]
- Chinchilla, B.; Encinas, P.; Coll, J.M.; Gómez-Casado, E. Differential Immune Transcriptome and Modulated Signalling Pathways in Rainbow Trout Infected with Viral Haemorrhagic Septicaemia Virus (VHSV) and Its Derivative Non-Virion (NV) Gene Deleted. Vaccines 2020, 8, 58. [Google Scholar] [CrossRef] [PubMed]
- Souto, S.; Mérour, E.; Le Coupanec, A.; Lamoureux, A.; Bernard, J.; Brémont, M.; Millet, J.K.; Biacchesi, S. Recombinant Viral Hemorrhagic Septicemia Virus with Rearranged Genomes as Vaccine Vectors to Protect against Lethal Betanodavirus Infection. Front. Immunol. 2023, 14, 1138961. [Google Scholar] [CrossRef]
- Edgar Amar, C.; Faisan, J.P. Efficacy of an Inactivated Vaccine and Nutritional Additives Against White Spot Syndrome Virus (WSSV) in Shrimp (Penaeus monodon). Isr. J. Aquac. Bamidgeh 2011, 63, 9. [Google Scholar]
- Syahidah, D.; Elliman, J.; Constantinoiu, C.; Owens, L. Mosquito cells (C6/36) fail to support the complete replication of Penaeus merguiensis hepandensovirus. J. Invertebr. Pathol. 2017, 145, 31–38. [Google Scholar] [CrossRef]
- Jeong, K.H.; Kim, H.J.; Kim, H.J. Current status and future directions of fish vaccines employing virus-like particles. Fish Shellfish Immunol. 2020, 100, 49–57. [Google Scholar] [CrossRef]
- Ma, J.; Bruce, T.J.; Jones, E.M.; Cain, K.D. A Review of Fish Vaccine Development Strategies: Conventional Methods and Modern Biotechnological Approaches. Microorganisms 2019, 7, 569. [Google Scholar] [CrossRef]
- Lorenzen, N.; Olesen, N.J.; Vestergård-Jørgensen, P.E.; Etzerodt, M.; Holtet, T.L.; Thøgersen, H.C. Molecular Cloning and Expression in Escherichia coli of the Glycoprotein Gene of VHS Virus, and Immunization of Rainbow Trout with the Recombinant Protein. J. Gen. Virol. 1993, 74, 623–630. [Google Scholar] [CrossRef]
- Lecocq-Xhonneux, F.; Thiry, M.; Dheur, I.; Rossius, M.; Vanderheijden, N.; Martial, J.; de Kinkelin, P. A Recombinant Viral Haemorrhagic Septicaemia Virus Glycoprotein Expressed in Insect Cells Induces Protective Immunity in Rainbow Trout. J. Gen. Virol. 1994, 75, 1579–1587. [Google Scholar] [CrossRef]
- Thwaite, R.; Ji, J.; Torrealba, D.; Coll, J.; Sabés, M.; Villaverde, A.; Roher, N. Protein nanoparticles made of recombinant viral antigens: A promising biomaterial for oral delivery of fish prophylactics. Front. Immunol. 2018, 9, 1652. [Google Scholar] [CrossRef]
- Rojas-Peña, M.; Aceituno, P.; Salvador, M.E.; Garcia-Ordoñez, M.; Teles, M.; Ortega-Villaizan, M.M.; Perez, L.; Roher, N. How Modular Protein Nanoparticles May Expand the Ability of Subunit Anti-Viral Vaccines: The Spring Viremia Carp Virus (SVCV) Case. Fish Shellfish Immunol. 2022, 131, 1051–1062. [Google Scholar] [CrossRef] [PubMed]
- Embregts, C.W.E.; Rigaudeau, D.; Tacchi, L.; Pijlman, G.P.; Kampers, L.; Veselý, T.; Pokorová, D.; Boudinot, P.; Wiegertjes, G.F.; Forlenza, M. Vaccination of carp against SVCV with an oral DNA vaccine or an insect cell-based subunit vaccine. Fish Shellfish Immunol. 2019, 85, 66–77. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhang, Y.; Yuan, M.; Zhu, W.; Pei, C.; Zhao, X.; Kong, X. An oral vaccine against spring viremia of carp virus induces protective immunity in common carp (Cyprinus carpio L.). Aquaculture 2023, 566, 739167. [Google Scholar] [CrossRef]
- Lueangyangyuen, A.; Senapin, S.; Dong, H.T.; Unajak, S.; Wangkahart, E.; Khunrae, P. Expression and purification of S5196-272 and S6200-317 proteins from Tilapia Lake Virus (TiLV) and their potential use as vaccines. Protein Expr. Purif. 2022, 190, 106013. [Google Scholar] [CrossRef]
- Chamtim, P.; Suwan, E.; Dong, H.T.; Sirisuay, S.; Areechon, N.; Wangkahart, E.; Hirono, I.; Mavichak, R.; Unajak, S. Combining segments 9 and 10 in DNA and recombinant protein vaccines conferred superior protection against tilapia lake virus in hybrid red tilapia (oreochromis sp.) compared to single segment vaccines. Front. Immunol. 2022, 13, 935480. [Google Scholar] [CrossRef]
- Cueva, M.D.; Villena, G.K.; Kitazono, A.A. Efficient cloning of tilapia lake virus complementary DNAs using an in vivo strategy in baker’s yeast. J. World Aquac. Soc. 2021, 52, 1209–1220. [Google Scholar] [CrossRef]
- Bañuelos-Hernández, B.; Rodríguez-Ramírez, T.; Albarrán-Tamayo, F.; Valadez, C.E.A.; Hernández, A.C. Using the TiLV virus genome sequence to develop a recombinant oral vaccine in microalgae. Comment to the article “Complete Genome Sequence of a Tilapia Lake Virus Isolate Obtained from Nile Tilapia (Oreochromis niloticus)”. Nova Sci. 2020, 12, 24. [Google Scholar] [CrossRef]
- Chang, C.J. Development and evaluation of DNA vaccine against salmonid alphavirus. Methods Mol. Biol. 2022, 2411, 205–218. [Google Scholar]
- Thorarinsson, R.; Ramstad, A.; Wolf, J.C.; Sindre, H.; Skjerve, E.; Rimstad, E.; Evensen, Ø.; Rodriguez, J.F. Effect of pancreas disease vaccines on infection levels and virus transmission in Atlantic salmon (Salmo salar) challenged with salmonid alphavirus, genotype 2. Front. Immunol. 2024, 15, 1342816. [Google Scholar] [CrossRef]
- Ahmadivand, S.; Soltani, M.; Behdani, M.; Evensen, Ø.; Alirahimi, E.; Hassanzadeh, R.; Soltani, E. Oral DNA vaccines based on CS-TPP nanoparticles and alginate microparticles confer high protection against infectious pancreatic necrosis virus (IPNV) infection in trout. Dev. Comp. Immunol. 2017, 74, 178–189. [Google Scholar] [CrossRef]
- Zhao, Z.; Jiang, F.Y.; Zhou, G.Q.; Duan, H.X.; Xia, J.Y.; Zhu, B. Protective immunity against spring viremia of carp virus by mannose modified chitosan loaded DNA vaccine. Virus Res. 2022, 320, 198896. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.; Wang, Y.; Chen, X.; Wang, Q.; Bergmann, S.M.; Yang, Y.; Wang, Y.; Li, B.; Lv, Y.; Li, H.; et al. Potency and efficacy of VP20-based vaccine against tilapia lake virus using different prime-boost vaccination regimens in tilapia. Aquaculture 2021, 539, 736654. [Google Scholar] [CrossRef]
- Yu, N.-T.; Zeng, W.-W.; Xiong, Z.; Liu, Z.-X. A high efficacy DNA vaccine against Tilapia Lake Virus in Nile tilapia (Oreochromis niloticus). Aquac. Rep. 2022, 24, 101166. [Google Scholar] [CrossRef]
- Soltani, M.; Ahmadivand, S.; Behdani, M.; Hassanzadeh, R.; Rahmati-Holasoo, H.; Taheri-Mirghaed, A. Transcription of adaptive-immune genes upon challenge with infectious pancreatic necrosis virus (IPNV) in DNA vaccinated rainbow trout. Int. J. Aquat. Biol. 2016, 4, 353–359. [Google Scholar]
- Lundstrom, K. Self-Replicating Alphaviruses: From Pathogens to Therapeutic Agents. Viruses 2024, 16, 1762. [Google Scholar] [CrossRef] [PubMed]
- Abonyi, F.; Eszterbauer, E.; Baska, F.; Hardy, T.; Doszpoly, A. First Experimental Application of DNA-Layered Salmonid Alphavirus-Based Replicon Vaccine in Non-Salmonid Fish: Induced Early Semi-Specific Protection against Spring Viremia of Carp Virus (SVCV) in Common Carp (Cyprinus carpio). Animals 2024, 14, 2698. [Google Scholar] [CrossRef]
- Rout, N.; Kumar, S.; Jaganmohan, S.; Murugan, V. DNA vaccines encoding viral envelope proteins confer protective immunity against WSSV in black tiger shrimp. Vaccine 2007, 25, 2778–2786. [Google Scholar] [CrossRef]
- Ravi, M.; Sudhakar, T.; Hari Haran, S.; Sudhakaran, R.; Stalin Dhas, T. Nanoparticles based DNA vaccine in marine water crabs (Scylla serrata) for protection against white spot syndrome virus (WSSV). Biocatal. Agric. Biotechnol. 2020, 28, 101764. [Google Scholar] [CrossRef]
- Jorgensen, J.B.; Johansen, L.H.; Steiro, K.; Johansen, A. CpG DNA induces protective antiviral immune responses in Atlantic salmon (Salmo salar L.). J. Virol. 2003, 77, 11471–11479. [Google Scholar] [CrossRef]
- Vaughan, K.; Del Crew, J.; Hermanson, G.; Wloch, M.K.; Riffenburgh, R.H.; Smith, C.R.; Van Bonn, W.G. A DNA vaccine against dolphin morbillivirus is immunogenic in bottlenose dolphins. Vet. Immunol. Immunopathol. 2007, 120, 260–266. [Google Scholar] [CrossRef]
- Dahl, L.O.S.; Hak, S.; Braaen, S.; Molska, A.; Rodà, F.; Parot, J.; Wessel, Ø.; Fosse, J.H.; Bjørgen, H.; Borgos, S.E.; et al. Implementation of mRNA-Lipid Nanoparticle Technology in Atlantic Salmon (Salmo salar). Vaccines 2024, 12, 788. [Google Scholar] [CrossRef] [PubMed]
- See, S.A.; Bhassu, S.; Tang, S.S.; Yusoff, K. Newly Developed mRNA Vaccines Induce Immune Responses in Litopenaeus vannamei Shrimps During Primary Vaccination. Dev. Comp. Immunol. 2024, 162, 105264. [Google Scholar] [CrossRef] [PubMed]
- Jorge, S.; Dellagostin, O.A. The Development of Veterinary Vaccines: A Review of Traditional Methods and Modern Biotechnology Approaches. Biotechnol. Res. Innov. 2017, 1, 6–13. [Google Scholar] [CrossRef]
- Maki, J.; Guiot, A.-L.; Aubert, M.; Brochier, B.; Cliquet, F.; Hanlon, C.A.; King, R.; Oertli, E.H.; Rupprecht, C.E.; Schumacher, C. Oral vaccination of wildlife using a vaccinia–rabies-glycoprotein recombinant virus vaccine (RABORAL V-RG®): A global review. Vet. Res. 2017, 48, 57. [Google Scholar] [CrossRef]
- Travieso, T.; Li, J.; Mahesh, S.; Mello, J.D.; Blasi, M. The use of viral vectors in vaccine development. npj Vaccines 2022, 7, 75. [Google Scholar] [CrossRef]
- Baron, M.D.; Iqbal, M.; Nair, V. Recent Advances in Viral Vectors in Veterinary Vaccinology. Curr. Opin. Virol. 2018, 29, 1–7. [Google Scholar] [CrossRef]
- Ding, C.; Ma, J.; Dong, Q.; Liu, Q. Live Bacterial Vaccine Vector and Delivery Strategies of Heterologous Antigen: A Review. Immunol. Lett. 2018, 197, 70–77. [Google Scholar] [CrossRef]
- Boersma, W.J.A.; Shaw, M.; Claassen, E. Probiotic Bacteria as Live Oral Vaccines Lactobacillus as the Versatile Delivery Vehicle. In Probiotics 3; Fuller, R., Perdigon, G., Eds.; Springer: Dordrecht, The Netherlands, 2000. [Google Scholar] [CrossRef]
- Lv, P.; Song, Y.; Liu, C.; Yu, L.; Shang, Y.; Tang, H.; Sun, S.; Wang, F. Application of Bacillus subtilis as a live vaccine vector: A review. J. Vet. Med. Sci. 2020, 82, 1693–1699. [Google Scholar] [CrossRef]
- Soltani, M.; Ahmadivand, S.; Ringø, E. Chapter 11—Bacillus as Probiotics in Shellfish Culture. In Bacillus Probiotics for Sustainable Aquaculture; Soltani, M., Elumalai, P., Ghosh, K., Ringø, E., Eds.; Taylor & Francis’ CRC Press: Boca Raton, FL, USA, 2024; p. 280. [Google Scholar]
- Naderi-Samani, M.; Soltani, M.; Dadar, M.; Taheri-Mirghaed, A.; Zargar, A.; Ahmadivand, S.; Hassanzadeh, R.; Moazami Goudarzi, L. Oral immunization of trout fry with recombinant Lactococcus lactis NZ3900 expressing G gene of viral hemorrhagic septicaemia virus (VHSV). Fish Shellfish Immunol. 2020, 105, 62–70. [Google Scholar] [CrossRef]
- Li, S.; Li, X.; Yuan, R.; Chen, X.; Chen, S.; Qiu, Y.; Yang, Q.; Wang, M.; Shi, J.; Zhang, S. Development of a recombinant adenovirus-vectored vaccine against both infectious hematopoietic necrosis virus and infectious pancreatic necrosis virus in rainbow trout (Oncorhynchus mykiss). Fish Shellfish Immunol. 2023, 132, 108457. [Google Scholar] [CrossRef]
- Wolf, A.; Hodneland, K.; Frost, P.; Hoeijmakers, M.; Rimstad, E. Salmonid Alphavirus-Based Replicon Vaccine against Infectious Salmon Anemia (ISA): Impact of Immunization Route and Interactions of the Replicon Vector. Fish Shellfish Immunol. 2014, 36, 383–392. [Google Scholar] [CrossRef]
- Citarasu, T.; Lelin, C.; Babu, M.M.; Anand, S.B.; Nathan, A.A.; Vakharia, V.N. Oral Vaccination of Macrobrachium Rosenbergii with Baculovirus-Expressed M. Rosenbergii Nodavirus (MrNV) Capsid Protein Induces Protective Immunity against MrNV Challenge. Fish Shellfish Immunol. 2019, 86, 1123–1129. [Google Scholar] [CrossRef]
- Kerstetter, L.J.; Buckley, S.; Bliss, C.M.; Coughlan, L. Adenoviral Vectors as Vaccines for Emerging Avian Influenza Viruses. Front. Immunol. 2021, 11, 607333. [Google Scholar] [CrossRef]
- Hikke, M.C. Next-Generation Salmonid Alphavirus Vaccine Development. Ph.D. Theis, Wageningen University, Wageningen, The Netherlands, 2016. [Google Scholar] [CrossRef][Green Version]
- Tesarova, B.; Musilek, K.; Rex, S.; Heger, Z. Taking advantage of cellular uptake of ferritin nanocages for targeted drug delivery. J. Control Release 2020, 325, 176–190. [Google Scholar] [CrossRef]
- Kibenge, F.S. Emerging viruses in aquaculture. Curr. Opin. Virol. 2019, 34, 97–103. [Google Scholar] [CrossRef]
- Pandey, K.K.; Sahoo, B.R.; Pattnaik, A.K. Protein Nanoparticles as Vaccine Platforms for Human and Zoonotic Viruses. Viruses 2024, 16, 936. [Google Scholar] [CrossRef]
- Putri, R.M.; Allende-Ballestero, C.; Luque, D.; Klem, R.; Rousou, K.-A.; Liu, A.; Traulsen, C.H.-H.; Rurup, W.F.; Koay, M.S.T.; Castón, J.R.; et al. Structural Characterization of Native and Modified Encapsulins as Nanoplatforms for in Vitro Catalysis and Cellular Uptake. ACS Nano 2017, 11, 12796–12804. [Google Scholar] [CrossRef]
- Brune, K.D.; Howarth, M. New routes and opportunities for modular construction of particulate vaccines: Stick, click, and glue. Front. Immunol. 2018, 9, 1432. [Google Scholar] [CrossRef]
- Lamontagne, F.; Khatri, V.; St-Louis, P.; Bourgault, S.; Archambault, D. Vaccination strategies based on bacterial self-assembling proteins as antigen delivery nanoscaffolds. Vaccines 2022, 10, 1920. [Google Scholar] [CrossRef]
- Tang, P.; Cui, E.-H.; Chang, W.-C.; Yu, C.; Wang, H.; Du, E.-Q.; Wang, J.-Y. Nanoparticle-based bivalent swine influenza virus vaccine induces enhanced immunity and effective protection against drifted H1N1 and H3N2 viruses in mice. Viruses 2022, 14, 2443. [Google Scholar] [CrossRef]
- Shrivastava, S.; Carmen, J.M.; Lu, Z.; Basu, S.; Sankhala, R.S.; Chen, W.H.; Nguyen, P.; Chang, W.C.; King, J.; Corbitt, C.; et al. SARS-CoV-2 spike–ferritin–nanoparticle adjuvanted with ALFQ induces long-lived plasma cells and cross-neutralizing antibodies. npj Vaccines 2023, 8, 43. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, B.; Tolia, N.H. Protein-based antigen presentation platforms for nanoparticle vaccines. npj Vaccines 2021, 6, 70. [Google Scholar] [CrossRef] [PubMed]
- Ahmadivand, S.; Fux, R.; Palić, D. Role of T Follicular Helper Cells in Viral Infections and Vaccine Design. Cells 2025, 14, 508. [Google Scholar] [CrossRef]

| Virus (Family) | Genome | Disease | Major Host Species | Geographic Distribution | Transmission Mode | Disease Impact | Licensed Vaccines or Antiviral Drugs |
|---|---|---|---|---|---|---|---|
| IHNV (Rhabdoviridae) | −ssRNA (11.5 Kb) | Infectious hematopoietic necrosis | Salmonids (Trout and Salmon) | North America, Europe, and Asia | Horizontal | Mortality up to 100% in early life stages | DNA vaccine for Salmon in Canada |
| VHSV (Rhabdoviridae) | −ssRNA (11.5 Kb) | Viral hemorrhagic septicemia | Over 80 host fish species | North America, Europe, and Asia | Horizontal | High mortality/Affects all life stages/Emerging hosts | None available (NA) |
| SVCV (Rhabdoviridae) | −ssRNA (11 Kb) | Spring viremia of carp | Carp | Europe and Asia | Horizontal | High mortality (up to 70% in young carp) | NA |
| ISAV (Orthomyxoviridae) | −ssRNA (13.5 Kb) | Infectious salmon anemia | Atlantic salmon | Europe and North and South America | Horizontal | Up to 90% mortality, affects all life stages | Inactivated (IP) |
| SAV (Togaviridae) | +ssRNA (12 Kb) | Pancreatic disease | Salmonids | Northern Europe | Horizontal | Over 50% mortality in severe cases | Inactivated (IP), DNA vaccine(IM) a |
| TiLV (Amnoonviridae) | −ssRNA (10.3 Kb) | Tilapia lake virus disease | Tilapia | Asia, Africa, and South America | Horizontal/Vertical | Up to 90% mortality, affects all life stages | NA |
| YHV (Roniviridae) | +ssRNA (~26.6 Kb) | Yellow head disease | Penaeid shrimp (P. monodon) | Asia, Mozambique, and Mexico | Horizontal/Vertical b | Up to 100% mortality in postlarvae (PL) | NA |
| TSV (Dicistroviridae) | +ssRNA (~10 kb) | Taura Syndrome/Red tail disease | Penaeid shrimp (L. vannamei) | Asia and the Americas | Horizontal | Up to 100% mortality PL, juvenile, subadult | NA |
| IMNV (Totiviridae) | dsRNA (~8 Kb) | Infectious myonecrosis | Penaeid shrimp (L. vannamei) | Brazil and Indonesia | Horizontal/Vertical | Up to 70% mortality, reduced FCR and market value | NA |
| MrNV (Nodaviridae) | +ssRNA (~4.5 kb) | White tail disease | Macrobrachium rosenbergii | Asia-Pacific | Horizontal/Vertical | High mortality in larvae, PL and juveniles | NA |
| Morbilliviruses c (Paramyxoviridae) | −ssRNA (~16 Kb) | Morbillivirosis/Distemper | Marine mammals (phocine and cetacean) | Worldwide | Horizontal | High mortality, mass die-offs, immune suppression | NA |
| Influenza A virus (Orthomyxoviridae) | −ssRNA (~13.5 Kb) | Influenza | Pinnipeds and cetaceans | Europe, Asia, and North and South America | Horizontal | Respiratory disease, high mortality, zoonotic | NA |
| Caliciviruses (Caliciviridae) | +ssRNA (~7–8 kb) | Calicivirus diseases | Sea lions | North America | Horizontal | Gastroenteritis, vesicular disease, contagious | NA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmadivand, S.; Savage, A.C.N.P.; Palic, D. Biosecurity and Vaccines for Emerging Aquatic Animal RNA Viruses. Viruses 2025, 17, 768. https://doi.org/10.3390/v17060768
Ahmadivand S, Savage ACNP, Palic D. Biosecurity and Vaccines for Emerging Aquatic Animal RNA Viruses. Viruses. 2025; 17(6):768. https://doi.org/10.3390/v17060768
Chicago/Turabian StyleAhmadivand, Sohrab, Ayanna Carla N. Phillips Savage, and Dušan Palic. 2025. "Biosecurity and Vaccines for Emerging Aquatic Animal RNA Viruses" Viruses 17, no. 6: 768. https://doi.org/10.3390/v17060768
APA StyleAhmadivand, S., Savage, A. C. N. P., & Palic, D. (2025). Biosecurity and Vaccines for Emerging Aquatic Animal RNA Viruses. Viruses, 17(6), 768. https://doi.org/10.3390/v17060768








