Association of Comorbidities with Adverse Outcomes in Adults Hospitalized with Respiratory Syncytial Virus (RSV) Infection: A Retrospective Cohort Study from Switzerland (2022–2024)
Abstract
1. Introduction
2. Materials and Methods
2.1. Design and Setting
2.2. Inclusion and Exclusion Criteria
2.3. Data Collection
2.4. Outcomes
2.5. Statistical Analysis
2.6. Ethical Considerations
3. Results
3.1. Patient Characteristics
3.2. Comorbidities
3.3. Outcomes of Hospitalized Patients with RSV
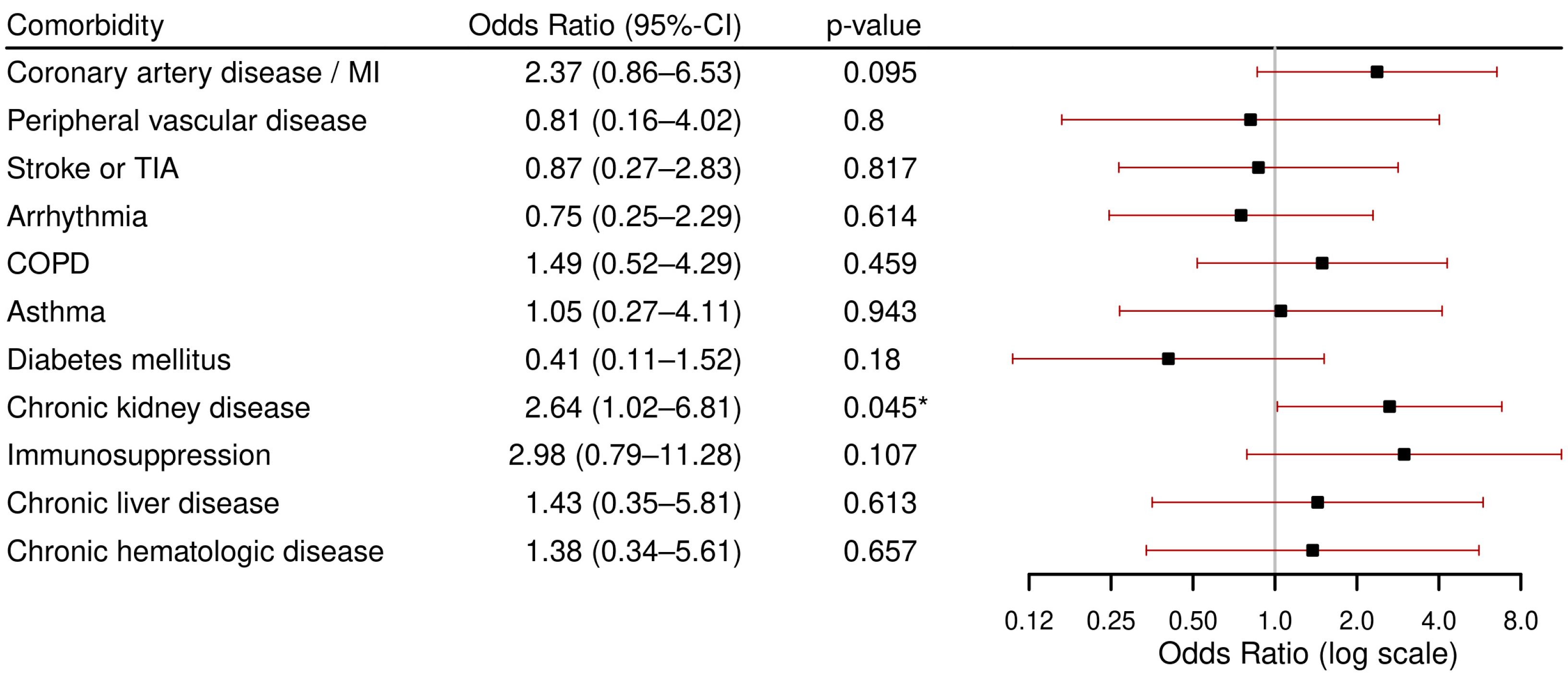
3.3.1. Primary Outcome: Severe Course
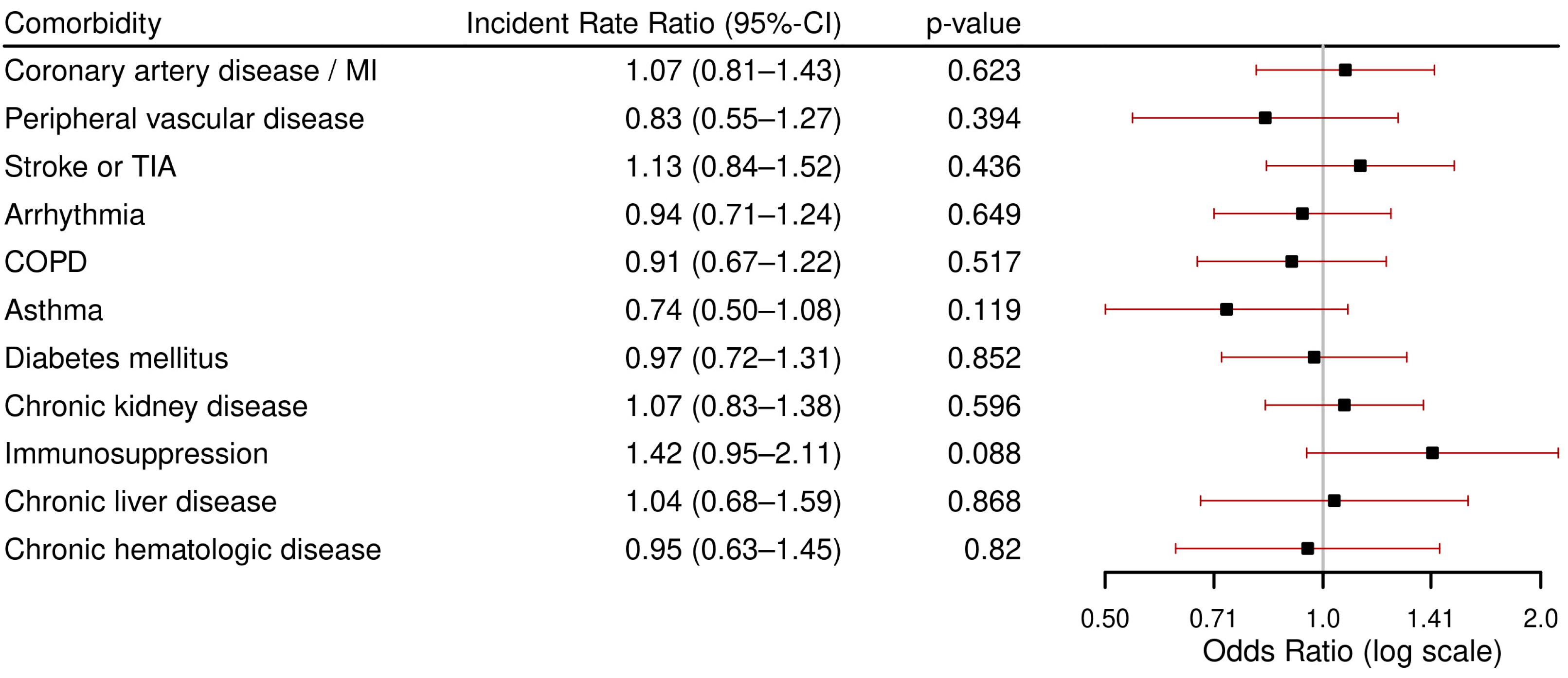
3.3.2. Secondary Outcomes: ICU Admission, in-Hospital Death, and LOHS
4. Discussion
- Patients who tested positively for RSV infection were mainly elderly individuals and nearly all had chronic underlying comorbidities, mainly cardiovascular, respiratory diseases and CKD.
- The highest rates of severe course (ICU admission or in-hospital death) were found in patients with immunosuppression, followed by coronary artery disease/myocardial infarction, chronic hematological disease, CKD and CLD.
- CKD was associated with both severe course and in-hospital mortality.
- Immunosuppression was associated with ICU admission.
4.1. Patient Characteristics
4.2. Comorbidities
4.3. Outcomes of Hospitalized Patients with RSV
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ARI | Acute respiratory infections |
| Brpm | Breaths per minute |
| Bpm | Beats per minute |
| BMI | Body mass index |
| CHF | Congestive heart failure |
| CKD | Chronic kidney disease |
| CLD | Chronic liver disease |
| COPD | Chronic obstructive pulmonary disease |
| CRP | C-reactive protein |
| e-GFR | Estimated glomerular filtration rate |
| ICU | Intensive care unit |
| IQR | Interquartile range |
| PCR | Polymerase chain reaction |
| RSV | Respiratory syncytial virus |
| SD | Standard deviation |
| SpO2 | peripheral capillary oxygen saturation |
| TIA | Transient ischemic attack |
References
- Haynes, A.K.; Manangan, A.P.; Iwane, M.K.; Sturm-Ramirez, K.; Homaira, N.; Brooks, W.A.; Luby, S.; Rahman, M.; Klena, J.D.; Zhang, Y.; et al. Respiratory Syncytial Virus Circulation in Seven Countries with Global Disease Detection Regional Centers. J. Infect. Dis. 2013, 208 (Suppl. 3), S246–S254. [Google Scholar] [CrossRef] [PubMed]
- Meerhoff, T.J.M.; Paget, J.W.; Kimpen, J.L.; Schellevis, F. Variation of Respiratory Syncytial Virus and the Relation with Meteorological Factors in Different Winter Seasons. Pediatr. Infect. Dis. J. 2009, 28, 860–866. [Google Scholar] [CrossRef] [PubMed]
- Broberg, E.K.; Waris, M.; Johansen, K.; Snacken, R.; Penttinen, P. European Influenza Surveillance Network. Seasonality and geographical spread of respiratory syncytial virus epidemics in 15 European countries, 2010 to 2016. Euro Surveill. 2018, 23, 17-00284. [Google Scholar] [CrossRef] [PubMed]
- Kaler, J.; Hussain, A.; Patel, K.; Hernandez, T.; Ray, S. Respiratory Syncytial Virus: A Comprehensive Review of Transmission, Pathophysiology, and Manifestation. Cureus 2023, 15, e36342. [Google Scholar] [CrossRef] [PubMed]
- Shi, T.; McLean, K.; Campbell, H.; Nair, H. Aetiological role of common respiratory viruses in acute lower respiratory infections in children under five years: A systematic review and meta-analysis. J. Glob. Health 2015, 5, 010408. [Google Scholar] [CrossRef] [PubMed]
- Nam, H.H.; Ison, M.G. Respiratory syncytial virus infection in adults. BMJ 2019, 366, l5021. [Google Scholar] [CrossRef] [PubMed]
- Falsey, A.R.; Hennessey, P.A.; Formica, M.A.; Cox, C.; Walsh, E.E. Respiratory Syncytial Virus Infection in Elderly and High-Risk Adults. N. Engl. J. Med. 2005, 352, 1749–1759. [Google Scholar] [CrossRef] [PubMed]
- Falsey, A.R.; McElhaney, J.E.; Beran, J.; van Essen, G.A.; Duval, X.; Esen, M.; Galtier, F.; Gervais, P.; Hwang, S.-J.; Kremsner, P.; et al. Respiratory Syncytial Virus and Other Respiratory Viral Infections in Older Adults with Moderate to Severe Influenza-like Illness. J. Infect. Dis. 2014, 209, 1873–1881. [Google Scholar] [CrossRef] [PubMed]
- Jansen, A.G.S.C.; Sanders, E.A.M.; Hoes, A.W.; van Loon, A.M.; Hak, E. Influenza- and respiratory syncytial virus-associated mortality and hospitalisations. Eur. Respir. J. 2007, 30, 1158–1166. [Google Scholar] [CrossRef] [PubMed]
- Begley, K.M.; Leis, A.M.; Petrie, J.G.; Truscon, R.; Johnson, E.; Lamerato, L.; Wei, M.; Monto, A.S.; Martin, E.T. Epidemiology of Respiratory Syncytial Virus in Adults and Children with Medically Attended Acute Respiratory Illness Over Three Seasons. Clin. Infect. Dis. 2024, 79, 1039–1045. [Google Scholar] [CrossRef] [PubMed]
- Wyffels, V.; Kariburyo, F.; Gavart, S.; Fleischhackl, R.; Yuce, H. A Real-World Analysis of Patient Characteristics and Predictors of Hospitalization Among US Medicare Beneficiaries with Respiratory Syncytial Virus Infection. Adv. Ther. 2020, 37, 1203–1217. [Google Scholar] [CrossRef] [PubMed]
- Kujawski, S.A.; Whitaker, M.; Ritchey, M.D.; Reingold, A.L.; Chai, S.J.; Anderson, E.J.; Openo, K.P.; Monroe, M.; Ryan, P.; Bye, E.; et al. Rates of respiratory syncytial virus (RSV)associated hospitalization among adults with congestive heart failure—United States, 2015–2017. PLoS ONE 2022, 17, e0264890. [Google Scholar] [CrossRef] [PubMed]
- Belongia, E.A.; King, J.P.; Kieke, B.A.; Pluta, J.; Al-Hilli, A.; Meece, J.K.; Shinde, V. Clinical features, severity, and incidence of RSV illness during 12 consecutive seasons in a community cohort of adults ≥60 years old. Open Forum Infect. Dis. 2018, 5, ofy316. [Google Scholar] [CrossRef] [PubMed]
- Fragkou, P.C.; Dimopoulou, D.; Moschopoulos, C.D.; Skevaki, C. Effects of long-term corticosteroid use on susceptibility to respiratory viruses: A narrative review. Clin. Microbiol. Infect. 2025, 31, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Khawaja, F.; Chemaly, R.F. Respiratory syncytial virus in hematopoietic cell transplant recipients and patients with hematologic malignancies. Haematologica 2019, 104, 1322–1331. [Google Scholar] [CrossRef] [PubMed]
- Manuel, O.; Estabrook, M. RNA respiratory viral infections in solid organ transplant recipients: Guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin. Transplant. 2019, 33, e13511. [Google Scholar] [CrossRef] [PubMed]
- Woodruff, R.C.; Melgar, M.; Pham, H.; Sperling, L.S.; Loustalot, F.; Kirley, P.D.; Austin, E.; Yousey-Hindes, K.; Openo, K.P.; Ryan, P.; et al. Acute Cardiac Events in Hospitalized Older Adults with Respiratory Syncytial Virus Infection. JAMA Intern. Med. 2024, 184, 602. [Google Scholar] [CrossRef] [PubMed]
- Coussement, J.; Zuber, B.; Garrigues, E.; Gros, A.; Vandueren, C.; Epaillard, N.; Voiriot, G.; Tandjaoui-Lambiotte, Y.; Lascarrou, J.-B.; Boissier, F.; et al. Characteristics and Outcomes of Patients in the ICU with Respiratory Syncytial Virus Compared with Those with Influenza Infection. Chest 2022, 161, 1475–1484. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.cdc.gov/rsv/hcp/clinical-overview/index.html (accessed on 1 July 2025).
- Boesing, M.; Albrich, W.; Bridevaux, P.-O.; Charbonnier, F.; Clarenbach, C.; Fellrath, J.-M.; Gianella, P.; Kern, L.; Latshang, T.; Pavlov, N.; et al. Vaccination in adult patients with chronic lung diseases. Praxis 2024, 113, 297–305. [Google Scholar] [PubMed]
- Bundesamt für Gesundheit (BAG). Available online: https://www.bag.admin.ch/de/respiratorisches-synzytial-virus-rsv (accessed on 21 July 2025).
- Lüthi-Corridori, G.; Boesing, M.; Roth, A.; Giezendanner, S.; Leuppi-Taegtmeyer, A.B.; Schuetz, P.; Leuppi, J.D. Predictors of Length of Stay, Rehospitalization and Mortality in Community-Acquired Pneumonia Patients: A Retrospective Cohort Study. J. Clin. Med. 2023, 12, 5601. [Google Scholar] [CrossRef] [PubMed]
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009, 42, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Harris, P.A.; Taylor, R.; Minor, B.L.; Elliott, V.; Fernandez, M.; O’Neal, L.; McLeod, L.; Delacqua, F.; Kirby, J.; Duda, S.N.; et al. The REDCap consortium: Building an international community of software platform partners. J. Biomed. Inform. 2019, 95, 103208. [Google Scholar] [CrossRef] [PubMed]
- Osei-Yeboah, R.; Spreeuwenberg, P.; Del Riccio, M.; Fischer, T.K.; Egeskov-Cavling, A.M.; Bøås, H.; van Boven, M.; Wang, X.; Lehtonen, T.; Bangert, M.; et al. Estimation of the Number of Respiratory Syncytial Virus–Associated Hospitalizations in Adults in the European Union. J. Infect. Dis. 2023, 228, 1539–1548. [Google Scholar] [CrossRef] [PubMed]
- Havers, F.P.; Whitaker, M.; Melgar, M.; Pham, H.; Chai, S.J.; Austin, E.; Meek, J.; Openo, K.P.; Ryan, P.A.; Brown, C.; et al. Burden of Respiratory Syncytial Virus–Associated Hospitalizations in US Adults, October 2016 to September 2023. JAMA Netw. Open 2024, 7, e2444756. [Google Scholar] [CrossRef] [PubMed]
- Urchueguía-Fornes, A.; Muñoz-Quiles, C.; Mira-Iglesias, A.; López-Lacort, M.; Mengual-Chuliá, B.; López-Labrador, F.X.; Díez-Domingo, J.; Orrico-Sánchez, A. Ten-Year Surveillance of Respiratory Syncytial Virus Hospitalizations in Adults: Incidence Rates and Case Definition Implications. J. Infect. Dis. 2025, 231, e830–e839. [Google Scholar] [CrossRef] [PubMed]
- Ivey, K.S.; Edwards, K.M.; Talbot, H.K. Respiratory Syncytial Virus and Associations with Cardiovascular Disease in Adults. J. Am. Coll. Cardiol. 2018, 71, 1574–1583. [Google Scholar] [CrossRef] [PubMed]
- Volling, C.; Hassan, K.; Mazzulli, T.; Green, K.; Al-Den, A.; Hunter, P.; Mangat, R.; Ng, J.; McGeer, A. Respiratory syncytial virus infection-associated hospitalization in adults: A retrospective cohort study. BMC Infect. Dis. 2014, 14, 665. [Google Scholar] [CrossRef] [PubMed]
- Prasad, N.; Walker, T.A.; Waite, B.; Wood, T.; Trenholme, A.A.; Baker, M.G.; McArthur, C.; Wong, C.A.; Grant, C.C.; Huang, S.Q.; et al. Respiratory Syncytial Virus–Associated Hospitalizations Among Adults with Chronic Medical Conditions. Clin. Infect. Dis. 2021, 73, e158-63. [Google Scholar] [CrossRef] [PubMed]
- Osei-Yeboah, R.; Johannesen, C.K.; Egeskov-Cavling, A.M.; Chen, J.; Lehtonen, T.; Fornes, A.U.; Paget, J.; Fischer, T.K.; Wang, X.; Nair, H.; et al. Respiratory Syncytial Virus–Associated Hospitalization in Adults with Comorbidities in 2 European Countries: A Modeling Study. J. Infect. Dis. 2024, 229 (Suppl. 1), S70–S77. [Google Scholar] [CrossRef] [PubMed]
- Tseng, H.F.; Sy, L.S.; Ackerson, B.; Solano, Z.; Slezak, J.; Luo, Y.; Fischetti, C.; Shinde, V. Severe Morbidity and Short- and Mid- to Long-term Mortality in Older Adults Hospitalized with Respiratory Syncytial Virus Infection. J. Infect. Dis. 2020, 222, 1298–1310. [Google Scholar] [CrossRef] [PubMed]
- Chuaychoo, B.; Ngamwongwan, S.; Kaewnaphan, B.; Athipanyasilp, N.; Horthongkham, N.; Kantakamalakul, W.; Muangman, N. Clinical manifestations and outcomes of respiratory syncytial virus infection in adult hospitalized patients. J. Clin. Virol. 2019, 117, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Goldman, C.R.; Sieling, W.D.; Alba, L.R.; Francisco, R.A.S.; Vargas, C.Y.; Barrett, A.E.; Phillips, M.; Finelli, L.; Saiman, L. Severe Clinical Outcomes Among Adults Hospitalized with Respiratory Syncytial Virus Infections, New York City, 2017–2019. Public Health Rep. 2022, 137, 929–935. [Google Scholar] [CrossRef] [PubMed]
- Chorazka, M.; Flury, D.; Herzog, K.; Albrich, W.C.; Vuichard-Gysin, D. Clinical outcomes of adults hospitalized for laboratory confirmed respiratory syncytial virus or influenza virus infection. PLoS ONE 2021, 16, e0253161. [Google Scholar] [CrossRef] [PubMed]
- Celante, H.; Oubaya, N.; Fourati, S.; Beaune, S.; Khellaf, M.; Casalino, E.; Ricard, J.-D.; Vieillard-Baron, A.; Heming, N.; Dessap, A.M.; et al. Prognosis of hospitalised adult patients with respiratory syncytial virus infection: A multicentre retrospective cohort study. Clin. Microbiol. Infect. 2023, 29, 943.e1–943.e8. [Google Scholar] [CrossRef] [PubMed]
- Ackerson, B.; Tseng, H.F.; Sy, L.S.; Solano, Z.; Slezak, J.; Luo, Y.; Fischetti, C.; Shinde, V. Severe Morbidity and Mortality Associated with Respiratory Syncytial Virus Versus Influenza Infection in Hospitalized Older Adults. Clin. Infect. Dis. 2019, 69, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Koval, C.E.; Gonzalez, B.E. RSV in transplant and immunocompromised patients. Clevel. Clin. J. Med. 2024, 91 (Suppl. 1), S34–S41. [Google Scholar] [CrossRef] [PubMed]
- Chatzis, O.; Darbre, S.; Pasquier, J.; Meylan, P.; Manuel, O.; Aubert, J.D.; Beck-Popovic, M.; Masouridi-Levrat, S.; Ansari, M.; Kaiser, L.; et al. Burden of severe RSV disease among immunocompromised children and adults: A 10 year retrospective study. BMC Infect. Dis. 2018, 18, 111. [Google Scholar] [CrossRef] [PubMed]
- Gaillet, A.; Layese, R.; Fourati, S.; Celante, H.; Pham, T.; Benghanem, S.; Dessap, A.M.; de Montmollin, E.; Pirault, J.; Vieillard-Baron, A.; et al. Clinical Phenotypes and Outcomes Associated with Respiratory Syncytial Virus Infection in Critically Ill Patients: A Retrospective Multicenter Cohort Study in Greater Paris Area Hospitals, 2017–2023. J. Infect. Dis. 2025, jiaf129. [Google Scholar] [CrossRef] [PubMed]
- Ambrosch, A.; Luber, D.; Klawonn, F.; Kabesch, M. Focusing on severe infections with the respiratory syncytial virus (RSV) in adults: Risk factors, symptomatology and clinical course compared to influenza A/B and the original SARS-CoV-2 strain. J. Clin. Virol. 2023, 161, 105399. [Google Scholar] [CrossRef] [PubMed]
- Havers, F.P.; Whitaker, M.; Melgar, M.; Chatwani, B.; Chai, S.J.; Alden, N.B.; Meek, J.; Openo, K.P.; Ryan, P.A.; Kim, S.; et al. Characteristics and Outcomes Among Adults Aged ≥60 Years Hospitalized with Laboratory-Confirmed Respiratory Syncytial Virus—RSV-NET, 12 States, July 2022–June 2023. Morb. Mortal. Wkly. Rep. 2023, 72, 1075–1082. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.; Lui, G.C.Y.; Wong, K.T.; Li, T.C.M.; Tse, E.C.M.; Chan, J.Y.C.; Yu, J.; Wong, S.S.M.; Choi, K.W.; Wong, R.Y.K.; et al. High Morbidity and Mortality in Adults Hospitalized for Respiratory Syncytial Virus Infections. Clin. Infect. Dis. 2013, 57, 1069–1077. [Google Scholar] [CrossRef] [PubMed]
- Ross, H.S.; Dallas, R.H.; Ferrolino, J.A.; Johnson, M.B.; Allison, K.J.; Cross, S.J.; Hayden, R.T.; Mejias, A.; Hijano, D.R. Clinical Outcomes of Respiratory Syncytial Virus Infection Among Pediatric Immunocompromised Hosts. Pediatr. Blood Cancer 2025, 72, e31484. [Google Scholar] [CrossRef] [PubMed]
- Malik, A.; Szpunar, S.; Sharma, M.; Johnson, L.B.; Saravolatz, L.; Bhargava, A. Predictors of prolonged length of stay in adult patients with respiratory syncytial virus infections—A multi-center historical cohort study. Front. Microbiol. 2024, 15, 1385439. [Google Scholar] [CrossRef] [PubMed]
- Begley, K.M.; Monto, A.S.; E Lamerato, L.; Malani, A.N.; Lauring, A.S.; Talbot, H.K.; Gaglani, M.; McNeal, T.; Silveira, F.P.; Zimmerman, R.K.; et al. Prevalence and Clinical Outcomes of Respiratory Syncytial Virus vs. Influenza in Adults Hospitalized with Acute Respiratory Illness from a Prospective Multicenter Study. Clin. Infect. Dis. 2023, 76, 1980–1988. [Google Scholar] [CrossRef] [PubMed]
- Loubet, P.; Fernandes, J.; de Pouvourville, G.; Sosnowiez, K.; Elong, A.; Guilmet, C.; Omichessan, H.; Bureau, I.; Fagnani, F.; Emery, C.; et al. Respiratory syncytial virus-related hospital stays in adults in France from 2012 to 2021: A national hospital database study. J. Clin. Virol. 2024, 171, 105635. [Google Scholar] [CrossRef] [PubMed]


| All (n = 136) | Missing n (%) | |
|---|---|---|
| Demographic | ||
| Age at admission (years), median (IQR) | 79.50 (72–87) | |
| Female n (%) | 84 (61.8%) | |
| Smoking Status | 99 (72.8%) | |
| Former Smoker, n (%) | 19 (51.4%) | |
| Current smoker, n (%) | 14 (37.8%) | |
| Never smoked, n (%) | 4 (10.8%) | |
| Reason for hospital admission | ||
| Respiratory, n (%) | 105 (77.2%) | |
| Cardiovascular, n (%) | 3 (2.2%) | |
| Gastroenterogical, n (%) | 8 (5.9%) | |
| Orthopedic, n (%) | 5 (3.7%) | |
| Other, n (%) | 15 (11.0%) | |
| Vital Signs at admission | ||
| Respiratory rate, median, brpm (IQR) | 21 (18–25) | 8 (5.8%) |
| SpO2 in %, median (IQR) | 93 (90–95) | |
| Supplemental oxygen, n (%) | 26 (19.1%) | |
| Fever 1, n (%) | 41 (30.4%) | 4 (2.9%) |
| Heartrate, bpm, median (IQR) | 89 (77.75–118.50) | |
| Systolic blood pressure, mmHg, median (IQR) | 142 (120–154) | |
| Diastolic blood pressure, mmHg, median (IQR) | 80 (67–91) | |
| Laboratory values at admission | ||
| Leucocytes (×109), median (IQR) | 10 (7.05–13.90) | 6 (4.4%) |
| Lymphocytes (×109), median (IQR) | 1 (0.70–1.60) | 30 (22%) |
| C-reactive protein (mg/L), median (IQR) | 44 (17.75–118.50) | |
| Neutrophil-Lymphocyte ratio, median (IQR) | 7.51 (3.96–14) |
| Comorbidities | |
|---|---|
| Chronic cardiovascular, n (%) | 103 (75.7%) |
| Hypertension, n (%) | 79 (58.0%) |
| Peripheral arterial disease, n (%) | 12 (8.8%) |
| Coronary artery disease or MI, n (%) | 33 (24.2.%) |
| Previous stroke or TIA, n (%) | 24 (23.3%) |
| Other 1, n (%) | 63 (61.2%) |
| Diabetes mellitus Type 2, n (%) | 27 (19.8%) |
| Chronic respiratory, n (%) | 69 (51%) |
| COPD, n (%) | 25 (18.3%) |
| Asthma, n (%) | 16 (11.7%) |
| Sleep apnea syndrome, n (%) | 8 (5.8%) |
| Other chronic respiratory diseases, n (%) | 14 (10.2%) |
| Chronic kidney disease, n (%) | 50 (36.7%) |
| Active cancer, n (%) | 9 (6.7%) |
| Depression, n (%) | 11 (8%) |
| Dementia, n (%) | 16 (11.7%) |
| Neurological disorders | 27 (19.8%) |
| State of immunosuppression, n (%) | 11 (8.0%) |
| Chronic hematologic disease, n (%) | 13 (9.5%) |
| Gastrointestinal, n (%) | 25 (18.3%) |
| Chronic liver disease, n (%) | 11 (8%) |
| Rheumatological, n (%) | 13 (9.5%) |
| n | Severe Course, n (%) | ICU, n (%) | In-Hospital Death, n (%) | LOHS 1, Median (IQR) | |
|---|---|---|---|---|---|
| Overall | 136 | 25 (18.4) | 19 (14) | 9 (6.6) | 6 (4–10) |
| No comorbidity | 2 | 1 (50) | 1 (50%) | 0 | 3.5 (3.2–3.7) |
| Coronary artery disease/MI | 36 | 10 (27.8) | 7 (19.4) | 5 (13.9) | 8 (4–11.5) |
| Arrythmia | 34 | 5 (14.7) | 4 (11.8) | 3 (8.8) | 7 (3.5–11) |
| Peripheral vascular disease | 12 | 2 (16.7) | 1 (8.3) | 1 (8.3) | 6 (3–9) |
| Diabetes mellitus Type 2 | 27 | 3 (11.1) | 3 (11.1) | 1 (3.7) | 6 (3–10) |
| COPD | 25 | 6 (24) | 6 (24) | 1 (4) | 6 (4–9) |
| Asthma | 16 | 3 (18.8) | 2 (12.5) | 2 (12.5) | 5 (3.2–6.7) |
| Chronic kidney disease | 50 | 13 (26) | 8 (16) | 8 (16) | 6.5 (4–13) |
| Stroke or TIA | 24 | 4 (16.7) | 3 (12.5) | 2 (8.3) | 8 (6–11.7) |
| Chronic liver disease | 12 | 3 (25) | 2 (16.7) | 2 (16.7) | 6 (4.2 –8.7) |
| Immunosuppression | 11 | 4 (36.4) | 4 (36.4) | 1 (9.1) | 9 (6.2–14.2) |
| Chronic hematological disease | 13 | 3 (23.1) | 1 (7.7) | 2 (15.4) | 6 (4.2–8.7) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Joseph, N.; Passavant, E.D.B.-v.; Lüthi-Corridori, G.; Jaun, F.; Mitrovic, S.; Leuppi, J.D.; Boesing, M. Association of Comorbidities with Adverse Outcomes in Adults Hospitalized with Respiratory Syncytial Virus (RSV) Infection: A Retrospective Cohort Study from Switzerland (2022–2024). Viruses 2025, 17, 1030. https://doi.org/10.3390/v17081030
Joseph N, Passavant EDB-v, Lüthi-Corridori G, Jaun F, Mitrovic S, Leuppi JD, Boesing M. Association of Comorbidities with Adverse Outcomes in Adults Hospitalized with Respiratory Syncytial Virus (RSV) Infection: A Retrospective Cohort Study from Switzerland (2022–2024). Viruses. 2025; 17(8):1030. https://doi.org/10.3390/v17081030
Chicago/Turabian StyleJoseph, Neetha, Elisa D. Bally-von Passavant, Giorgia Lüthi-Corridori, Fabienne Jaun, Sandra Mitrovic, Jörg Daniel Leuppi, and Maria Boesing. 2025. "Association of Comorbidities with Adverse Outcomes in Adults Hospitalized with Respiratory Syncytial Virus (RSV) Infection: A Retrospective Cohort Study from Switzerland (2022–2024)" Viruses 17, no. 8: 1030. https://doi.org/10.3390/v17081030
APA StyleJoseph, N., Passavant, E. D. B.-v., Lüthi-Corridori, G., Jaun, F., Mitrovic, S., Leuppi, J. D., & Boesing, M. (2025). Association of Comorbidities with Adverse Outcomes in Adults Hospitalized with Respiratory Syncytial Virus (RSV) Infection: A Retrospective Cohort Study from Switzerland (2022–2024). Viruses, 17(8), 1030. https://doi.org/10.3390/v17081030






