Relationship Between Estimated Drug Distribution of Antiretroviral Therapy and Immune Proteins in Cerebrospinal Fluid During Chronic HIV Suppression
Abstract
1. Introduction
2. Materials and Methods
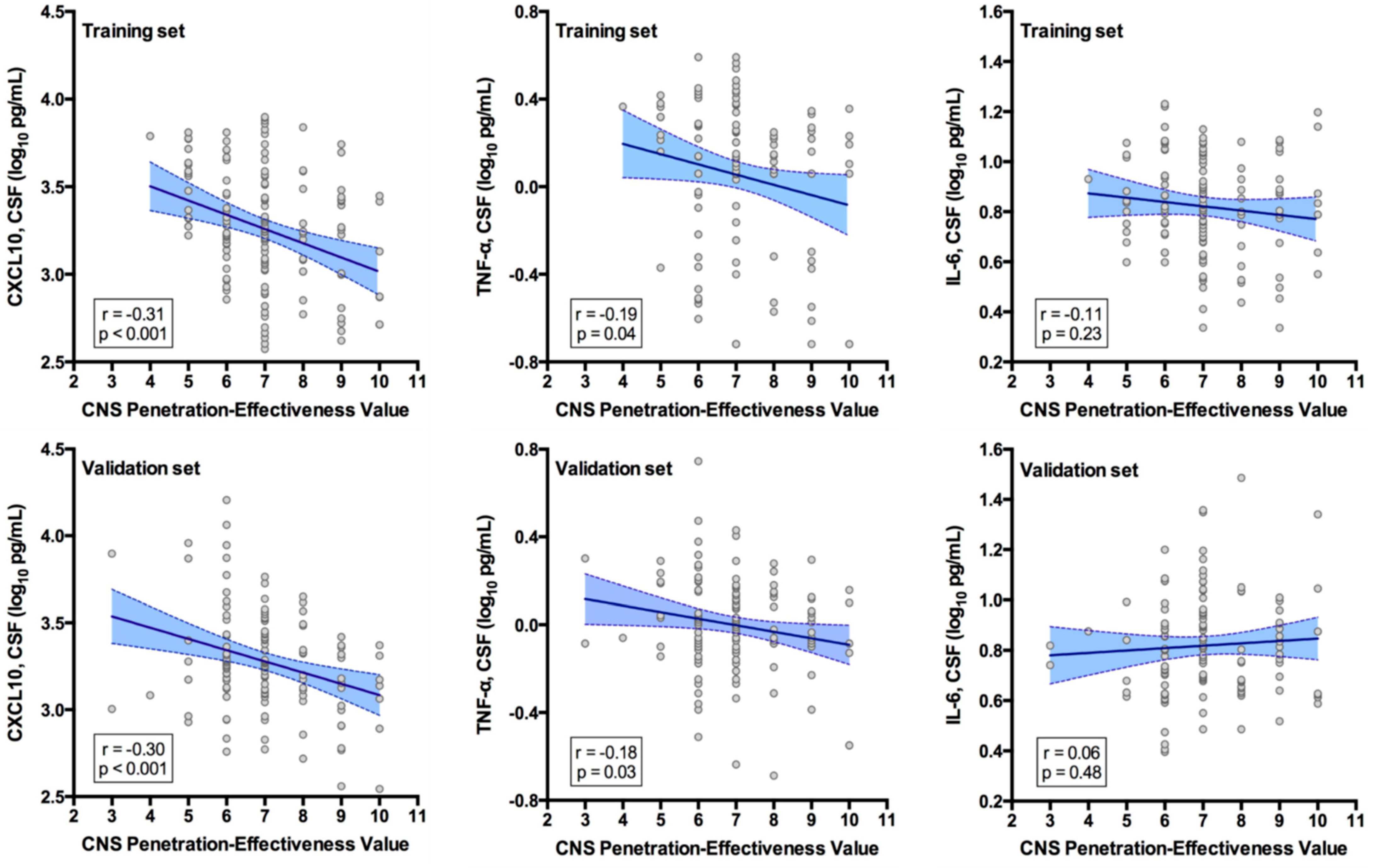
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PWH | People with HIV |
| CPE | Central nervous system effectiveness penetration score |
| CSF | Cerebrospinal fluid |
| CNS | Central nervous system |
| ART | Antiretroviral therapy |
| ARV | Antiretroviral |
| eADDC | Estimated ART drug distribution into the CNS |
| CHARTER | CNS HIV Antiretroviral Therapy Effects Research |
| IL-6 | Interleukin 6 |
| TNF-α | Tumor Necrosis Factor α |
| CXCL10 | C-X-C Motif Chemokine Ligand 10 |
| IFN | Interferon |
| TG | Training/discovery group |
| VG | Validation group |
| St.aβ | Standardized adjusted β coefficient |
| BMI | Body mass index |
| HCV | Hepatitis C virus |
| PI | Protease inhibitor |
| NNRTI | Non-nucleoside reverse transcriptase inhibitor |
| INSTI | Integrase strand transfer inhibitor |
| PBMCs | Peripheral blood mononuclear cells |
| BCa | Bias-corrected accelerated |
References
- Letendre, S.; Marquie-Beck, J.; Capparelli, E.; Best, B.; Clifford, D.; Collier, A.C.; Gelman, B.B.; McArthur, J.C.; McCutchan, J.A.; Morgello, S.; et al. Validation of the CNS Penetration-Effectiveness Rank for Quantifying Antiretroviral Penetration Into the Central Nervous System. Arch. Neurol. 2008, 65, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Cusini, A.; Vernazza, P.L.; Yerly, S.; Decosterd, L.A.; Ledergerber, B.; Fux, C.A.; Rohrbach, J.; Widmer, N.; Hirschel, B.; Gaudenz, R.; et al. Higher CNS Penetration-Effectiveness of Long-Term Combination Antiretroviral Therapy Is Associated with Better HIV-1 Viral Suppression in Cerebrospinal Fluid. J. Acquir. Immune Defic. Syndr. 2013, 62, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Cysique, L.A.; Waters, E.K.; Brew, B.J. Central Nervous System Antiretroviral Efficacy in HIV Infection: A Qualitative and Quantitative Review and Implications for Future Research. BMC Neurol. 2011, 11, 148. [Google Scholar] [CrossRef]
- Trunfio, M.; Pinnetti, C.; Arsuffi, S.; Bai, F.; Celani, L.; D’Ettorre, G.; Vera, J.H.; Monforte, A.D.; Focà, E.; Ghisetti, V.; et al. The Presence of Resistance-Associated Mutations in Reverse Transcriptase Gene Is Associated with Cerebrospinal Fluid HIV-1 Escape: A Multicentric Retrospective Analysis. J. Med. Virol. 2023, 95, e28704. [Google Scholar] [CrossRef]
- Mudra Rakshasa-Loots, A.; Whalley, H.C.; Vera, J.H.; Cox, S.R. Neuroinflammation in HIV-Associated Depression: Evidence and Future Perspectives. Mol. Psychiatry 2022, 27, 3619–3632. [Google Scholar] [CrossRef] [PubMed]
- Ellis, R.J.; Marquine, M.J.; Kaul, M.; Fields, J.A.; Schlachetzki, J.C.M. Mechanisms Underlying HIV-Associated Cognitive Impairment and Emerging Therapies for Its Management. Nat. Rev. Neurol. 2023, 19, 668–687. [Google Scholar] [CrossRef]
- Killingsworth, L.; Spudich, S. Neuropathogenesis of HIV-1: Insights from across the Spectrum of Acute through Long-Term Treated Infection. Semin. Immunopathol. 2022, 44, 709–724. [Google Scholar] [CrossRef]
- Santos, G.M.A.; Locatelli, I.; Métral, M.; Calmy, A.; Lecompte, T.D.; Nadin, I.; Hauser, C.; Cusini, A.; Hasse, B.; Kovari, H.; et al. Cross-Sectional and Cumulative Longitudinal Central Nervous System Penetration Effectiveness Scores Are Not Associated With Neurocognitive Impairment in a Well Treated Aging Human Immunodeficiency Virus-Positive Population in Switzerland. Open Forum Infect. Dis. 2019, 6, ofz277. Available online: https://academic.oup.com/ofid/article/6/7/ofz277/5529443 (accessed on 16 May 2025).
- Arentoft, A.; Troxell, K.; Alvarez, K.; Aghvinian, M.; Rivera Mindt, M.; Cherner, M.; Van Dyk, K.; Razani, J.; Roxas, M.; Gavilanes, M. HIV Antiretroviral Medication Neuropenetrance and Neurocognitive Outcomes in HIV+ Adults: A Review of the Literature Examining the Central Nervous System Penetration Effectiveness Score. Viruses 2022, 14, 1151. [Google Scholar] [CrossRef]
- Vassallo, M.; Durant, J.; Biscay, V.; Lebrun-Frenay, C.; Dunais, B.; Laffon, M.; Harvey-Langton, A.; Cottalorda, J.; Ticchioni, M.; Carsenti, H.; et al. Can High Central Nervous System Penetrating Antiretroviral Regimens Protect against the Onset of HIV-Associated Neurocognitive Disorders? AIDS 2014, 28, 493–501. [Google Scholar] [CrossRef]
- Force, G.; Ghout, I.; Ropers, J.; Carcelain, G.; Marigot-Outtandy, D.; Hahn, V.; Darchy, N.; Defferriere, H.; Bouaziz-Amar, E.; Carlier, R.; et al. Improvement of HIV-Associated Neurocognitive Disorders after Antiretroviral Therapy Intensification: The Neuro+3 Study. J. Antimicrob. Chemother. 2021, 76, 743–752. [Google Scholar] [CrossRef] [PubMed]
- Letendre, S.L.; Chen, H.; McKhann, A.; Roa, J.; Vecchio, A.; Daar, E.S.; Berzins, B.; Hunt, P.W.; Marra, C.M.; Campbell, T.B.; et al. Antiretroviral Therapy Intensification for Neurocognitive Impairment in Human Immunodeficiency Virus. Clin. Infect. Dis. 2023, 77, 866–874. [Google Scholar] [CrossRef] [PubMed]
- Lazzaro, A.; Vai, D.; Barco, A.; Stroffolini, G.; Pirriatore, V.; Guastamacchia, G.; Nigra, M.; Ghisetti, V.; Tettoni, M.C.; Noce, G.; et al. Switching to Low Neurotoxic Antiretrovirals to Improve Neurocognition Among People Living with HIV-1-Associated Neurocognitive Disorder: The MARAND-X Randomized Clinical Trial. J. Acquir. Immune Defic. Syndr. 2024, 97, 180–191. [Google Scholar] [CrossRef]
- De Benedetto, I.; Trunfio, M.; Guastamacchia, G.; Bonora, S.; Calcagno, A. A Review of the Potential Mechanisms of Neuronal Toxicity Associated with Antiretroviral Drugs. J. Neurovirol. 2020, 26, 642–651. [Google Scholar] [CrossRef] [PubMed]
- Lanman, T.; Letendre, S.; Ma, Q.; Bang, A.; Ellis, R. CNS Neurotoxicity of Antiretrovirals. J. Neuroimmune Pharmacol. 2021, 16, 130–143. [Google Scholar] [CrossRef]
- Hughes, S.M.; Levy, C.N.; Calienes, F.L.; Stekler, J.D.; Pandey, U.; Vojtech, L.; Berard, A.R.; Birse, K.; Noël-Romas, L.; Richardson, B.; et al. Treatment with Commonly Used Antiretroviral Drugs Induces a Type I/III Interferon Signature in the Gut in the Absence of HIV Infection. Cell Rep. Med. 2020, 1, 100096. [Google Scholar] [CrossRef]
- Toksoy, A.; Sennefelder, H.; Adam, C.; Hofmann, S.; Trautmann, A.; Goebeler, M.; Schmidt, M. Potent NLRP3 Inflammasome Activation by the HIV Reverse Transcriptase Inhibitor Abacavir. J. Biol. Chem. 2017, 292, 2805–2814. [Google Scholar] [CrossRef]
- Wu, T.; Zhang, J.; Geng, M.; Tang, S.-J.; Zhang, W.; Shu, J. Nucleoside Reverse Transcriptase Inhibitors (NRTIs) Induce Proinflammatory Cytokines in the CNS via Wnt5a Signaling. Sci. Rep. 2017, 7, 4117. [Google Scholar] [CrossRef]
- Korencak, M.; Byrne, M.; Richter, E.; Schultz, B.T.; Juszczak, P.; Ake, J.A.; Ganesan, A.; Okulicz, J.F.; Robb, M.L.; de los Reyes, B.; et al. Effect of HIV Infection and Antiretroviral Therapy on Immune Cellular Functions. JCI Insight 2019, 4, e126675. [Google Scholar] [CrossRef]
- Delmonte, O.M.; Bertolotto, G.; Ricotti, E.; Tovo, P.-A. Immunomodulatory Effects of Two HIV Protease Inhibitors, Saquinavir and Ritonavir, on Lymphocytes from Healthy Seronegative Individuals. Immunol. Lett. 2007, 111, 111–115. [Google Scholar] [CrossRef]
- Gannon, P.J.; Akay-Espinoza, C.; Yee, A.C.; Briand, L.A.; Erickson, M.A.; Gelman, B.B.; Gao, Y.; Haughey, N.J.; Zink, M.C.; Clements, J.E.; et al. HIV Protease Inhibitors Alter Amyloid Precursor Protein Processing via β-Site Amyloid Precursor Protein Cleaving Enzyme-1 Translational Up-Regulation. Am. J. Pathol. 2017, 187, 91–109. [Google Scholar] [CrossRef] [PubMed]
- Soontornniyomkij, V.; Umlauf, A.; Soontornniyomkij, B.; Gouaux, B.; Ellis, R.J.; Levine, A.J.; Moore, D.J.; Letendre, S.L. Association of Antiretroviral Therapy with Brain Aging Changes among HIV-Infected Adults. AIDS 2018, 32, 2005–2015. [Google Scholar] [CrossRef] [PubMed]
- Stern, A.L.; Lee, R.N.; Panvelker, N.; Li, J.; Harowitz, J.; Jordan-Sciutto, K.L.; Akay-Espinoza, C. Differential Effects of Antiretroviral Drugs on Neurons In Vitro: Roles for Oxidative Stress and Integrated Stress Response. J. Neuroimmune Pharmacol. 2018, 13, 64–76. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Minchella, P.; Alvarez-Carbonell, D.; Purandare, N.; Nagampalli, V.K.; Blankenberg, D.; Hulgan, T.; Gerschenson, M.; Karn, J.; Aras, S.; et al. Contemporary Antiretroviral Therapy Dysregulates Iron Transport and Augments Mitochondrial Dysfunction in HIV-Infected Human Microglia and Neural-Lineage Cells. Int. J. Mol. Sci. 2023, 24, 12242. [Google Scholar] [CrossRef]
- Pasternak, A.O.; Vroom, J.; Kootstra, N.A.; Wit, F.W.; de Bruin, M.; De Francesco, D.; Bakker, M.; Sabin, C.A.; Winston, A.; Prins, J.M.; et al. Non-Nucleoside Reverse Transcriptase Inhibitor-Based Combination Antiretroviral Therapy Is Associated with Lower Cell-Associated HIV RNA and DNA Levels Compared to Protease Inhibitor-Based Therapy. eLife 2021, 10, e68174. [Google Scholar] [CrossRef]
- Gray, L.R.; Tachedjian, G.; Ellett, A.M.; Roche, M.J.; Cheng, W.-J.; Guillemin, G.J.; Brew, B.J.; Turville, S.G.; Wesselingh, S.L.; Gorry, P.R.; et al. The NRTIs Lamivudine, Stavudine and Zidovudine Have Reduced HIV-1 Inhibitory Activity in Astrocytes. PLoS ONE 2013, 8, e62196. [Google Scholar] [CrossRef]
- Patel, S.H.; Ismaiel, O.A.; Mylott, W.R.; Yuan, M.; Hauser, K.F.; McRae, M. Simultaneous Determination of Intracellular Concentrations of Tenofovir, Emtricitabine, and Dolutegravir in Human Brain Microvascular Endothelial Cells Using Liquid Chromatography-Tandem Mass Spectrometry (LC-MS/MS). Anal. Chim. Acta 2019, 1056, 79–87. [Google Scholar] [CrossRef]
- Asahchop, E.L.; Meziane, O.; Mamik, M.K.; Chan, W.F.; Branton, W.G.; Resch, L.; Gill, M.J.; Haddad, E.; Guimond, J.V.; Wainberg, M.A.; et al. Reduced Antiretroviral Drug Efficacy and Concentration in HIV-Infected Microglia Contributes to Viral Persistence in Brain. Retrovirology 2017, 14, 47. [Google Scholar] [CrossRef]
- Rose-John, S.; Jenkins, B.J.; Garbers, C.; Moll, J.M.; Scheller, J. Targeting IL-6 Trans-Signalling: Past, Present and Future Prospects. Nat. Rev. Immunol. 2023, 23, 666–681. [Google Scholar] [CrossRef]
- Probert, L. TNF and Its Receptors in the CNS: The Essential, the Desirable and the Deleterious Effects. Neuroscience 2015, 302, 2–22. [Google Scholar] [CrossRef]
- Liu, M.; Guo, S.; Hibbert, J.M.; Jain, V.; Singh, N.; Wilson, N.O.; Stiles, J.K. CXCL10/IP-10 in Infectious Diseases Pathogenesis and Potential Therapeutic Implications. Cytokine Growth Factor. Rev. 2011, 22, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Burlacu, R.; Umlauf, A.; Marcotte, T.D.; Soontornniyomkij, B.; Diaconu, C.C.; Bulacu-Talnariu, A.; Temereanca, A.; Ruta, S.M.; Letendre, S.; Ene, L.; et al. Plasma CXCL10 Correlates with HAND in HIV-Infected Women. J. Neurovirol. 2020, 26, 23–31. [Google Scholar] [CrossRef]
- Williams, M.E.; Stein, D.J.; Joska, J.A.; Naudé, P.J.W. Cerebrospinal Fluid Immune Markers and HIV-Associated Neurocognitive Impairments: A Systematic Review. J. Neuroimmunol. 2021, 358, 577649. [Google Scholar] [CrossRef] [PubMed]
- Mudra Rakshasa-Loots, A.; Bakewell, N.; Sharp, D.J.; Gisslén, M.; Zetterberg, H.; Alagaratnam, J.; Wit, F.W.N.M.; Kootstra, N.A.; Winston, A.; Reiss, P.; et al. Biomarkers of Central and Peripheral Inflammation Mediate the Association between HIV and Depressive Symptoms. Transl. Psychiatry 2023, 13, 190. [Google Scholar] [CrossRef]
- Hart, B.B.; Nordell, A.D.; Okulicz, J.F.; Palfreeman, A.; Horban, A.; Kedem, E.; Neuhaus, J.; Jacobs, D.R.; Duprez, D.A.; Neaton, J.D.; et al. Inflammation-Related Morbidity and Mortality Among HIV-Positive Adults: How Extensive Is It? J. Acquir. Immune Defic. Syndr. 2018, 77, 1–7. [Google Scholar] [CrossRef]
- Yuan, L.; Liu, A.; Qiao, L.; Sheng, B.; Xu, M.; Li, W.; Chen, D. The Relationship of CSF and Plasma Cytokine Levels in HIV Infected Patients with Neurocognitive Impairment. Biomed. Res. Int. 2015, 2015, 506872. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.; Kabwe, C.; Molinaro, M.; Metro, A.; Mbewe, E.; Kabundula, P.; Mweemba, M.; Mwanza-Kabaghe, S.; Adams, H.; Birbeck, G.; et al. Inflammatory and Immunologic Biomarkers Associated with Cognitive Impairment in Pediatric and Adolescent Patients with HIV in Lusaka, Zambia: A Sub-Study of the HIV-Associated Neurocognitive Disorders in Zambia (HANDZ) (P4-8.005). Neurology 2023, 100, 1951. [Google Scholar] [CrossRef]
- Bandera, A.; Taramasso, L.; Bozzi, G.; Muscatello, A.; Robinson, J.A.; Burdo, T.H.; Gori, A. HIV-Associated Neurocognitive Impairment in the Modern ART Era: Are We Close to Discovering Reliable Biomarkers in the Setting of Virological Suppression? Front. Aging Neurosci. 2019, 11, 187. [Google Scholar] [CrossRef]
- Crespo-Bermejo, C.; de Arellano, E.R.; Lara-Aguilar, V.; Valle-Millares, D.; Gómez-Lus, M.L.; Madrid, R.; Martín-Carbonero, L.; Briz, V. Persistent Low-Level Viremia in Persons Living with HIV Undertreatment: An Unresolved Status. Virulence 2021, 12, 2919–2931. [Google Scholar] [CrossRef]
- Kamat, A.; Misra, V.; Cassol, E.; Ancuta, P.; Yan, Z.; Li, C.; Morgello, S.; Gabuzda, D. A Plasma Biomarker Signature of Immune Activation in HIV Patients on Antiretroviral Therapy. PLoS ONE 2012, 7, e30881. [Google Scholar] [CrossRef]
- Rahmat-Zaie, R.; Amini, J.; Haddadi, M.; Beyer, C.; Sanadgol, N.; Zendedel, A. TNF-α/STAT1/CXCL10 Mutual Inflammatory Axis That Contributes to the Pathogenesis of Experimental Models of Multiple Sclerosis: A Promising Signaling Pathway for Targeted Therapies. Cytokine 2023, 168, 156235. [Google Scholar] [CrossRef]
- Lichtblau, N.; Schmidt, F.M.; Schumann, R.; Kirkby, K.C.; Himmerich, H. Cytokines as Biomarkers in Depressive Disorder: Current Standing and Prospects. Int. Rev. Psychiatry 2013, 25, 592–603. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.Y.; Lee, Z.H.; Song, Y.W. The Interaction between CXCL10 and Cytokines in Chronic Inflammatory Arthritis. Autoimmun. Rev. 2013, 12, 554–557. [Google Scholar] [CrossRef] [PubMed]
- Miura, M.; Kitaura, H.; Ohori, F.; Narita, K.; Ren, J.; Noguchi, T.; Marahleh, A.; Ma, J.; Lin, A.; Fan, Z.; et al. Role of CXCL10 Released from Osteocytes in Response to TNF-α Stimulation on Osteoclasts. Sci. Rep. 2025, 15, 3040. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.; Yao, H.; Dhillon, N.K.; Buch, S.J. HIV-1 Tat Co-Operates with IFN-γ and TNF-α to Increase CXCL10 in Human Astrocytes. PLoS ONE 2009, 4, e5709. [Google Scholar] [CrossRef]
- Lawrence, J.M.; Schardien, K.; Wigdahl, B.; Nonnemacher, M.R. Roles of Neuropathology-Associated Reactive Astrocytes: A Systematic Review. Acta Neuropathol. Commun. 2023, 11, 42. [Google Scholar] [CrossRef]
- Roff, S.R.; Noon-Song, E.N.; Yamamoto, J.K. The Significance of Interferon-γ in HIV-1 Pathogenesis, Therapy, and Prophylaxis. Front. Immunol. 2014, 4, 498. [Google Scholar] [CrossRef]
- Erta, M.; Quintana, A.; Hidalgo, J. Interleukin-6, a Major Cytokine in the Central Nervous System. Int. J. Biol. Sci. 2012, 8, 1254–1266. [Google Scholar] [CrossRef]
- McElvaney, O.J.; Curley, G.F.; Rose-John, S.; McElvaney, N.G. Interleukin-6: Obstacles to Targeting a Complex Cytokine in Critical Illness. Lancet Respir. Med. 2021, 9, 643–654. [Google Scholar] [CrossRef]
- Chang, Q.; Bournazou, E.; Sansone, P.; Berishaj, M.; Gao, S.P.; Daly, L.; Wels, J.; Theilen, T.; Granitto, S.; Zhang, X.; et al. The IL-6/JAK/Stat3 Feed-Forward Loop Drives Tumorigenesis and Metastasis. Neoplasia 2013, 15, 848-IN45. [Google Scholar] [CrossRef]
- Kumar, P.; DeJesus, E.; Huhn, G.; Sloan, L.; Small, C.B.; Edelstein, H.; Felizarta, F.; Hao, R.; Ross, L.; Stancil, B.; et al. Evaluation of Cardiovascular Biomarkers in a Randomized Trial of Fosamprenavir/Ritonavir vs. Efavirenz with Abacavir/Lamivudine in Underrepresented, Antiretroviral-Naïve, HIV-Infected Patients (SUPPORT): 96-Week Results. BMC Infect. Dis. 2013, 13, 269. [Google Scholar] [CrossRef] [PubMed]
- McComsey, G.A.; Kitch, D.; Daar, E.S.; Tierney, C.; Jahed, N.C.; Melbourne, K.; Ha, B.; Brown, T.T.; Bloom, A.; Fedarko, N.; et al. Inflammation Markers after Randomization to Abacavir/Lamivudine or Tenofovir/Emtricitabine with Efavirenz or Atazanavir/Ritonavir. AIDS 2012, 26, 1371–1385. [Google Scholar] [CrossRef]
- Okay, G.; Koc, M.M.; Guler, E.M.; Yabaci, A.; Kocyigit, A.; Akkoyunlu, Y. The Effect of Antiretroviral Therapy on IL-6, IL-1β, TNF-α, IFN-γ Levels and Their Relationship with HIV-RNA and CD4+ T Cells in HIV Patients. Curr. HIV Res. 2020, 18, 354–361. [Google Scholar] [CrossRef]
- Qu, Y.; Weinstein, A.; Wang, Z.; Cheng, Y.; Kingsley, L.; Levine, A.; Martin, E.; Munro, C.; Ragin, A.B.; Rubin, L.H.; et al. Legacy Effect on Neuropsychological Function in HIV-Infected Men on Combination Antiretroviral Therapy. AIDS 2022, 36, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Lake, J.E.; Vo, Q.T.; Jacobson, L.P.; Sacktor, N.; Miller, E.N.; Post, W.S.; Becker, J.T.; Palella, F.J.; Ragin, A.; Martin, E.; et al. Adiponectin and Interleukin-6, but Not Adipose Tissue, Are Associated with Worse Neurocognitive Function in HIV-Infected Men. Antivir. Ther. 2015, 20, 235–244. [Google Scholar] [CrossRef]
- Sattler, F.R.; He, J.; Letendre, S.; Wilson, C.; Sanders, C.; Heaton, R.; Ellis, R.; Franklin, D.; Aldrovandi, G.; Marra, C.M.; et al. Abdominal Obesity Contributes to Neurocognitive Impairment in HIV-Infected Patients with Increased Inflammation and Immune Activation. J. Acquir. Immune Defic. Syndr. 2015, 68, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Marconi, V.C.; Moser, C.; Gavegnano, C.; Deeks, S.G.; Lederman, M.M.; Overton, E.T.; Tsibris, A.; Hunt, P.W.; Kantor, A.; Sekaly, R.-P.; et al. Randomized Trial of Ruxolitinib in Antiretroviral-Treated Adults With Human Immunodeficiency Virus. Clin. Infect. Dis. 2022, 74, 95–104. [Google Scholar] [CrossRef]
- Trunfio, M.; Tang, B.; Iudicello, J.E.; Ma, Q.; Franklin, D.R.; Cookson, D.; Riggs, P.K.; Cherner, M.; Moore, D.J.; Heaton, R.K.; et al. Distinct Effects of SSRIs and SNRIs on Soluble Biomarkers in Blood and Cerebrospinal Fluid of People with HIV. J. Infect. Dis. 2023, 229, jiad558. [Google Scholar] [CrossRef]
- Letendre, S.L.; Marquie-Beck, J.; Ellis, R.J.; Woods, S.P.; Best, B.; Clifford, D.B.; Collier, A.C.; Gelman, B.B.; Marra, C.; McArthur, J.C.; et al. The Role of Cohort Studies in Drug Development: Clinical Evidence of Antiviral Activity of Serotonin Reuptake Inhibitors and HMG-CoA Reductase Inhibitors in the Central Nervous System. J. Neuroimmune Pharmacol. 2007, 2, 120–127. [Google Scholar] [CrossRef]
- Calza, L.; Colangeli, V.; Borderi, M.; Beci, G.; Esposito, F.; Bon, I.; Re, M.C.; Viale, P. Rosuvastatin Decreases Serum Inflammatory Markers and Slows Atherosclerosis Progression Rate in Treated HIV-Infected Patients with Metabolic Syndrome. Infect. Dis. 2021, 53, 81–88. [Google Scholar] [CrossRef]
- Edén, A.; Nilsson, S.; Hagberg, L.; Fuchs, D.; Zetterberg, H.; Svennerholm, B.; Gisslén, M. Asymptomatic Cerebrospinal Fluid HIV-1 Viral Blips and Viral Escape During Antiretroviral Therapy: A Longitudinal Study. J. Infect. Dis. 2016, 214, 1822–1825. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Valero, I.; Ellis, R.; Heaton, R.; Deutsch, R.; Franklin, D.; Clifford, D.B.; Collier, A.; Gelman, B.; Marra, C.; McCutchan, J.A.; et al. CSF Viral Escape in Aviremic HIV-Infected Patients Receiving ART: Prevalence, Risk Factors and Neurocognitive Effects. AIDS 2019, 33, 475–481. [Google Scholar] [CrossRef] [PubMed]
- Trunfio, M.; Vuaran, E.; Vai, D.; Quarta, C.; Di Stefano, A.; Imperiale, D.; Cinnirella, G.; Bonora, S.; Di Perri, G.; Letendre, S.L.; et al. Symptomatic and Asymptomatic Neurocognitive Impairment, ART Adherence and HIV Control: A 4-Year Observational Study. AIDS Behav. 2024, 28, 3643–3654. [Google Scholar] [CrossRef]
- Kamal, S.; Locatelli, I.; Wandeler, G.; Sehhat, A.; Bugnon, O.; Metral, M.; Du Pasquier, R.; Gutbrod, K.; Cavassini, M.; Schneider, M.P.; et al. The Presence of Human Immunodeficiency Virus-Associated Neurocognitive Disorders Is Associated With a Lower Adherence to Combined Antiretroviral Treatment. Open Forum Infect. Dis. 2017, 4, ofx070. [Google Scholar] [CrossRef]
- Chou, C.-H.; Chiou, J.-S.; Ho, M.-W.; Tien, N.; Li, T.-M.; Chiu, M.-L.; Tsai, F.-J.; Wu, Y.-C.; Chou, I.-C.; Lu, H.-F.; et al. Association of Combination Antiretroviral Therapy with Risk of Neurological Diseases in Patients with HIV/AIDS in Taiwan: A Nested Case-Control Study. Front. Pharmacol. 2023, 14, 1110605. [Google Scholar] [CrossRef] [PubMed]
- Mukerji, S.S.; Misra, V.; Lorenz, D.R.; Uno, H.; Morgello, S.; Franklin, D.; Ellis, R.J.; Letendre, S.; Gabuzda, D. Impact of Antiretroviral Regimens on Cerebrospinal Fluid Viral Escape in a Prospective Multicohort Study of Antiretroviral Therapy-Experienced Human Immunodeficiency Virus-1-Infected Adults in the United States. Clin. Infect. Dis. 2018, 67, 1182–1190. [Google Scholar] [CrossRef]
- Trunfio, M.; Sacchi, A.; Vai, D.; Pittaluga, F.; Croce, M.; Cavallo, R.; Imperiale, D.; Bonora, S.; Di Perri, G.; Letendre, S.L.; et al. Intrathecal Production of Anti-EBV Viral Capsid Antigen IgG Is Associated with Neurocognition and Tau Pathology in People with HIV. AIDS 2023, 38, 477–486. [Google Scholar] [CrossRef]
- Trunfio, M.; Di Girolamo, L.; Ponzetta, L.; Russo, M.; Burdino, E.; Imperiale, D.; Atzori, C.; Di Perri, G.; Calcagno, A. Seropositivity and Reactivations of HSV-1, but Not of HSV-2 nor VZV, Associate with Altered Blood–Brain Barrier, Beta Amyloid, and Tau Proteins in People Living with HIV. J. Neurovirol. 2022, 29, 100–109. [Google Scholar] [CrossRef]
- Chang, L.; Jiang, C.; Cunningham, E.; Buchthal, S.; Douet, V.; Andres, M.; Ernst, T. Effects of APOE Ε4, Age, and HIV on Glial Metabolites and Cognitive Deficits. Neurology 2014, 82, 2213–2222. [Google Scholar] [CrossRef]
- Cusato, J.; Avataneo, V.; Antonucci, M.; Trunfio, M.; Marinaro, L.; Palermiti, A.; Manca, A.; Di Perri, G.; Mula, J.; Bonora, S.; et al. Antiretroviral Levels in the Cerebrospinal Fluid: The Effect of Inflammation and Genetic Variants. Diagnostics 2023, 13, 295. [Google Scholar] [CrossRef]
- Zeiser, R. Immune Modulatory Effects of Statins. Immunology 2018, 154, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Kamkwalala, A.R.; Matthews, A.; Garg, A.; Roy, U.; Ma, Q.; Karris, M.; Sundermann, E.; Ellis, R.J.; Riggs, P.K.; Trunfio, M.; et al. The Effects of Prescribed Medications on Depressive Symptoms and Neurocognitive Performance in People with HIV. Clin. Infect. Dis. 2025, 80, 871–880. [Google Scholar] [CrossRef] [PubMed]
- Hellmuth, J.; Valcour, V.; Spudich, S. CNS Reservoirs for HIV: Implications for Eradication. J. Virus Erad. 2015, 1, 67–71. [Google Scholar] [CrossRef]
- Fabbiani, M.; Di Giambenedetto, S.; Bracciale, L.; Bacarelli, A.; Ragazzoni, E.; Cauda, R.; Navarra, P.; De Luca, A. Pharmacokinetic Variability of Antiretroviral Drugs and Correlation with Virological Outcome: 2 Years of Experience in Routine Clinical Practice. J. Antimicrob. Chemother. 2009, 64, 109–117. [Google Scholar] [CrossRef]
- Massanella, M.; Fromentin, R.; Chomont, N. Residual Inflammation and Viral Reservoirs: Alliance against an HIV Cure. Curr. Opin. HIV AIDS 2016, 11, 234–241. [Google Scholar] [CrossRef]
- White, J.A.; Simonetti, F.R.; Beg, S.; McMyn, N.F.; Dai, W.; Bachmann, N.; Lai, J.; Ford, W.C.; Bunch, C.; Jones, J.L.; et al. Complex Decay Dynamics of HIV Virions, Intact and Defective Proviruses, and 2LTR Circles Following Initiation of Antiretroviral Therapy. Proc. Natl. Acad. Sci. USA 2022, 119, e2120326119. [Google Scholar] [CrossRef]
- Caligaris, G.; Trunfio, M.; Ghisetti, V.; Cusato, J.; Nigra, M.; Atzori, C.; Imperiale, D.; Bonora, S.; Di Perri, G.; Calcagno, A. Blood-Brain Barrier Impairment in Patients Living with HIV: Predictors and Associated Biomarkers. Diagnostics 2021, 11, 867. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Rademeyer, K.; Namuju, O.C.; Abdusalaamu, K.; Fisher, J.; Meya, D.B.; McRae, M.; Boulware, D.R.; Lukande, R.; Nicol, M.R. Postmortem Analysis of Dolutegravir, Tenofovir, Lamivudine, and Efavirenz Penetration in Multiple Central Nervous System Compartments. J. Infect. Dis. 2024, 230, 1215–1223. [Google Scholar] [CrossRef]
- Calcagno, A.; Moltó, J.; Borghetti, A.; Gervasoni, C.; Milesi, M.; Valle, M.; Avataneo, V.; Alcantarini, C.; Pla-Junca, F.; Trunfio, M.; et al. Older Age Is Associated with Higher Dolutegravir Exposure in Plasma and Cerebrospinal Fluid of People Living with HIV. Clin. Pharmacokinet. 2021, 60, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Trunfio, M.; Chaillon, A.; Beliakova-Bethell, N.; Deiss, R.; Letendre, S.L.; Riggs, P.K.; Higgins, N.; Gianella, S. Beyond the Syndemic of Opioid Use Disorders and HIV: The Impact of Opioids on Viral Reservoirs. Viruses 2023, 15, 1712. [Google Scholar] [CrossRef]
- Cusato, J.; Borghetti, A.; Teti, E.; Milesi, M.; Tettoni, M.C.; Bonora, S.; Trunfio, M.; D’Avolio, A.; Compagno, M.; Di Giambenedetto, S.; et al. Dolutegravir Discontinuation for Neuropsychiatric Symptoms in People Living with HIV and Their Outcomes after Treatment Change: A Pharmacogenetic Study. Metabolites 2022, 12, 1202. [Google Scholar] [CrossRef] [PubMed]
- Srinivas, N.; Rosen, E.P.; Gilliland, W.M.; Kovarova, M.; Remling-Mulder, L.; De La Cruz, G.; White, N.; Adamson, L.; Schauer, A.P.; Sykes, C.; et al. Antiretroviral Concentrations and Surrogate Measures of Efficacy in the Brain Tissue and CSF of Preclinical Species. Xenobiotica 2019, 49, 1192–1201. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, M.; Bumpus, N.N.; Ma, Q.; Ellis, R.J.; Soontornniyomkij, V.; Fields, J.A.; Bharti, A.; Achim, C.L.; Moore, D.J.; Letendre, S.L. Antiretroviral Drug Concentrations in Brain Tissue of Adult Decedents. AIDS 2020, 34, 1907–1914. [Google Scholar] [CrossRef] [PubMed]
- Calcagno, A.; Barco, A.; Trunfio, M.; Bonora, S. CNS-Targeted Antiretroviral Strategies: When Are They Needed and What to Choose. Curr. HIV/AIDS Rep. 2018, 15, 84–91. [Google Scholar] [CrossRef]
| Characteristics | Training Group (n = 144) | Validation Group (n = 131) | p Value |
|---|---|---|---|
| Age, years | 43.8 (±8.3) | 43.8 (±8.0) | 0.94 |
| Male sex, n | 122 (84.7%) | 101 (77.1%) | 0.11 |
| White ethnicity, n | 72 (50.0%) | 57 (43.5%) | 0.28 |
| Body mass index | 26.1 (±5.3) | 26.4 (±4.5) | 0.56 |
| HCV seropositive, n | 23 (16.0%) | 49 (37.4%) | <0.001 |
| Duration of HIV, years | 10.4 (3.6–14.8) | 9.5 (4.9–15.0) | 0.20 |
| AIDS diagnosis, n | 94 (65.3%) | 83 (63.3%) | 0.74 |
| Current CD4+ count, cells/µL | 531 (310–692) | 532 (357–691) | 0.97 |
| CD4/CD8 ratio | 0.58 (0.38–0.91) | 0.61 (0.37–0.90) | 0.82 |
| Nadir CD4+ count, cells/µL | 167 (32–266) | 180 (49–265) | 0.46 |
| Current ART regimen duration, months | 9.2 (5.0–21.3) | 13.0 (4.6–29.8) | 0.36 |
| Lifetime ART regimen duration, months | 47.7 (16.4–76.9) | 54.2 (20.7–90.0) | 0.86 |
| ART backbone, n | |||
| FTC/TDF | 60 (41.7%) | 49 (37.4%) | 0.47 |
| 3TC/ZDV | 21 (14.6%) | 27 (20.6%) | 0.19 |
| 3TC/TDF | 15 (10.4%) | 13 (9.9%) | 0.89 |
| 3TC/ABV | 12 (8.3%) | 8 (6.1%) | 0.48 |
| DDI/TDF | 6 (4.2%) | 9 (6.9%) | 0.32 |
| ABV/TDF | 5 (3.5%) | 4 (3.1%) | 0.84 |
| 3TC/D4T | 3 (2.1%) | 2 (1.5%) | 0.73 |
| Other NRTI combinations | 15 (10.4%) | 12 (9.2%) | 0.73 |
| 3 ARV classes | 7 (4.9%) | 7 (5.3%) | 0.86 |
| Prevalent third antiretrovirals, n | |||
| EFV | 47 (32.6%) | 48 (36.6%) | 0.49 |
| ATV/r | 44 (30.6%) | 30 (22.9%) | 0.15 |
| NVP | 19 (13.2%) | 22 (16.8%) | 0.40 |
| LPV/r | 17 (11.8%) | 18 (13.7%) | 0.63 |
| CPE score | 7.0 (6.0–7.2) | 7.0 (6.0–8.0) | 0.96 |
| CSF CXCL10, pg/mL | 1568.8 (947.5–3508.8) | 1897.2 (1141.0–3465.9) | 0.69 |
| CSF TNFα, pg/mL | 0.36 (0.29–0.49) | 0.44 (0.33–0.59) | 0.18 |
| CSF IL-6, pg/mL | 3.21 (2.43–4.29) | 3.34 (2.20–4.65) | 0.99 |
| CSF CXCL10 (n = 275) | CSF TNF-α (n = 275) | |||
|---|---|---|---|---|
| Model 1 (R2 = 0.16, p < 0.001) aβ (BCa 95%CI), p | Model 2 (R2 = 0.15, p < 0.001) aβ (95%CI), p | Model 1 (R2 = 0.07, p < 0.001) aβ (BCa 95%CI), p | Model 2 (R2 = 0.08, p = 0.004) aβ (95%CI), p | |
| CPE value | −0.18 (−0.28; −0.072), p < 0.001 | −0.20 (−0.29; −0.11) p < 0.001 | −0.074 (−0.13; −0.015), p = 0.014 | −0.075 (−0.14; −0.005), p = 0.036 |
| Age, years | - | 0.007 (−0.008; 0.022), p = 0.352 | - | 0.004 (−0.008; 0.015), p = 0.536 |
| Male sex, ref. female | 0.38 (0.080; 0.68) p = 0.015 | 0.41 (0.086; 0.72), p = 0.013 | - | 0.062 (−0.18; 0.30), p = 0.616 |
| White race, ref. others | - | 0.017 (−0.24; 0.27), p = 0.895 | - | −0.078 (−0.27; 0.12), p = 0.433 |
| CD4/CD8 ratio | −0.039 (−0.47; 0.48), p = 0.863 | Excluded | - | - |
| Nadir CD4+ T cell count, cells/µL | −0.11 (−0.38; 0.15) p = 0.424 | Excluded | - | - |
| AIDS episode, ref. none | 0.16 (−0.14; 0.50), p = 0.304 | 0.26 (0.004; 0.52), p = 0.047 | 0.18 (0.013; 0.36), p = 0.045 | 0.19 (−0.007; 0.39), p = 0.059 |
| PI use, ref. no use | 0.24 (−0.31; 0.83), p = 0.360 | Excluded | - | - |
| NNRTI use, ref. no use | 0.12 (−0.39; 0.72), p = 0.636 | Excluded | - | - |
| Duration of current ART regimen, months | −0.33 (−0.58; −0.086), p = 0.010 | −0.35 (−0.60; −0.11), p = 0.005 | −0.25 (−0.44; −0.075), p = 0.005 | −0.27 (−0.45; −0.086), p = 0.004 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trunfio, M.; Iudicello, J.E.; Riggs, P.K.; Kallianpur, A.R.; Hulgan, T.; Ellis, R.J.; Letendre, S.L. Relationship Between Estimated Drug Distribution of Antiretroviral Therapy and Immune Proteins in Cerebrospinal Fluid During Chronic HIV Suppression. Viruses 2025, 17, 749. https://doi.org/10.3390/v17060749
Trunfio M, Iudicello JE, Riggs PK, Kallianpur AR, Hulgan T, Ellis RJ, Letendre SL. Relationship Between Estimated Drug Distribution of Antiretroviral Therapy and Immune Proteins in Cerebrospinal Fluid During Chronic HIV Suppression. Viruses. 2025; 17(6):749. https://doi.org/10.3390/v17060749
Chicago/Turabian StyleTrunfio, Mattia, Jennifer E. Iudicello, Patricia K. Riggs, Asha R. Kallianpur, Todd Hulgan, Ronald J. Ellis, and Scott L. Letendre. 2025. "Relationship Between Estimated Drug Distribution of Antiretroviral Therapy and Immune Proteins in Cerebrospinal Fluid During Chronic HIV Suppression" Viruses 17, no. 6: 749. https://doi.org/10.3390/v17060749
APA StyleTrunfio, M., Iudicello, J. E., Riggs, P. K., Kallianpur, A. R., Hulgan, T., Ellis, R. J., & Letendre, S. L. (2025). Relationship Between Estimated Drug Distribution of Antiretroviral Therapy and Immune Proteins in Cerebrospinal Fluid During Chronic HIV Suppression. Viruses, 17(6), 749. https://doi.org/10.3390/v17060749







