Supplementary Surveillance of Poliovirus Circulation in the Russian Federation: Results of a Study on Migrant Children of “Risk Group”
Abstract
1. Introduction
2. Materials and Methods
2.1. Organization of Research and Collection of Materials
2.2. Algorithm of Laboratory Investigation
2.3. Ethics of Research
3. Results
3.1. Polioviruses
3.2. Response Measures
3.3. Non-Polio Enteroviruses (NPEVs)
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Polio | Poliomyelitis |
| WHO | World Health Organization |
| GPEI | Global Polio Eradication Initiative |
| PV | Poliovirus |
| wPV | Wild poliovirus |
| VDPV | Vaccine-derived poliovirus |
| Circulating VDPV | cVDPV |
| ITD | Intratypic differentiation |
| NPEV | Non-polio enterovirus |
| HAdV | Human adenovirus |
| AFP | Acute flaccid paralysis |
| OPV | Oral poliovirus vaccine |
| tOPV | Trivalent oral poliovirus vaccine |
| bOPV | Bivalent oral poliovirus vaccine |
| IPV | Inactivated poliovirus vaccine |
| nOPV2 | New oral poliovirus vaccine type 2 |
References
- Sutter, R.W.; Kew, O.M.; Cochi, S.L.; Aylward, R.B. Poliovirus Vaccine–Live. In Vaccines, 7th ed.; Plotkin, S.A., Orenstein, W.A., Offit, P.A., Eds.; Elsevier: Philadelphia, PA, USA, 2018; pp. 866–917. [Google Scholar]
- World Health Organization (WHO). Poliovirus IHR Emergency Committee. Available online: https://www.who.int/groups/poliovirus-ihr-emergency-committee (accessed on 16 April 2025).
- Lee, S.E.; Greene, S.A.; Burns, C.C.; Tallis, G.; Wassilak, S.G.F.; Bolu, O. Progress Toward Poliomyelitis Eradication-Worldwide, January 2021–March 2023. MMWR Morb. Mortal. Wkly. Rep. 2023, 72, 517–522. [Google Scholar] [CrossRef] [PubMed]
- Zuckerman, N.S.; Bar-Or, I.; Sofer, D.; Bucris, E.; Morad, H.; Shulman, L.M.; Levi, N.; Weiss, L.; Aguvaev, I.; Cohen, Z.; et al. Emergence of genetically linked vaccine-originated poliovirus type 2 in the absence of oral polio vaccine, Jerusalem, April to July 2022. Euro Surveill. 2022, 71, 2200694. [Google Scholar] [CrossRef]
- Ryerson, A.B.; Lang, D.; Alazawi, M.A.; Neyra, M.; Hill, D.T.; St George, K.; Fuschino, M.; Lutterloh, E.; Backenson, B.; Rulli, S.; et al. Wastewater testing and detection of poliovirus type 2 genetically linked to virus isolated from a paralytic polio case-New York, March 9–October 11, 2022. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 1418–1424. [Google Scholar] [CrossRef]
- Klapsa, D.; Wilton, T.; Zealand, A.; Bujaki, E.; Saxentoff, E.; Troman, C.; Shaw, A.G.; Tedcastle, A.; Majumdar, M.; Mate, R.; et al. Sustained detection of type 2 poliovirus in London sewage between February and July, 2022, by enhanced environmental surveillance. Lancet 2022, 400, 1531–1538. [Google Scholar] [CrossRef]
- Pan American Health Organization/World Health Organization. Epidemiological Update Detection of Poliovirus in Wastewater: Considerations for the Region of the Americas; PAHO/WHO: Washington, DC, USA, 2022; Available online: https://reliefweb.int/report/world/epidemiological-update-detection-poliovirus-wastewater-considerations-region-americas-30-december-2022 (accessed on 16 April 2025).
- John, T.J.; Dharmapalan, D. Lessons from Vaccine-Related Poliovirus in Israel, UK and USA. Vaccines 2022, 10, 1969. [Google Scholar] [CrossRef]
- Böttcher, S.; Kreibich, J.; Wilton, T.; Saliba, V.; Blomqvist, S.; Al-Hello, H.; Savolainen-Kopra, C.; Wieczorek, M.; Gad, B.; Krzysztoszek, A.; et al. Detection of circulating vaccinederived poliovirus type 2 (cVDPV2) in wastewater samples: A wake-up call, Finland, Germany, Poland, Spain, the United Kingdom, 2024. Euro Surveill. 2025, 30, 2500037. [Google Scholar] [CrossRef]
- The WHO European Region declared free of polio. Euro Surveill. 2002, 7, 76–77. Available online: https://pubmed.ncbi.nlm.nih.gov/12631935/ (accessed on 16 April 2025). [CrossRef]
- World Health Organization (WHO). The Health of Refugees and Migrants in the WHO European Region. Available online: https://www.who.int/europe/news-room/fact-sheets/item/the-health-of-refugees-and-migrants-in-the-who-european-region (accessed on 16 April 2025).
- Deal, A.; Halliday, R.; Crawshaw, A.F.; Hayward, S.E.; Burnard, A.; Rustage, K.; Carter, J.; Mehrotra, A.; Knights, F.; Campos-Matos, I.; et al. Migration and outbreaks of vaccine-preventable disease in Europe: A systematic review. Lancet Infect Dis. 2021, 21, e387–e398. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Polio Outbreak in Ukraine Closed–A Success Story for Public Health Despite Extreme Challenges of War. Available online: https://www.who.int/europe/news/item/21-09-2023-polio-outbreak-in-ukraine-closed-a-success-story-for-public-health-despite-extreme-challenges-of-war (accessed on 16 April 2025).
- McAuliffe, M.; Oucho, L.A. (Eds.) World Migration Report 2024; International Organization for Migration (IOM): Geneva, Switzerland, 2024; Available online: https://publications.iom.int/books/world-migration-report-2024 (accessed on 16 April 2025).
- Yakovenko, M.L.; Gmyl, A.P.; Ivanova, O.E.; Eremeeva, T.P.; Ivanov, A.P.; Prostova, M.A.; Baykova, O.Y.; Isaeva, O.V.; Lipskaya, G.Y.; Shakaryan, A.K.; et al. The 2010 outbreak of poliomyelitis in Tajikistan: Epidemiology and lessons learnt. Euro Surveill. 2014, 19, 20706. [Google Scholar] [CrossRef]
- Romanenkova, N.I.; Bichurina, M.A.; Rozaeva, N.R.; Pogrebnaya, T.N. The role of epidemiologic surveillance of migrants in the system of poliomyelitis control. J. Microbiol. Epidemiol. Immunobiol. 2012, 6, 27–31. (In Russian) [Google Scholar]
- Rospotrebnadzor. Sanitary and Epidemiological Requirements for Prevention of Infectious Diseases, Sanpin 3.3686-21. Available online: https://sanepidservice.ru/assets/files/sanpin-3.3686-21.pdf (accessed on 16 April 2025).
- Methodological Guidelines “Epidemiological Surveillance of Poliomyelitis and Acute Flaccid Paralysis” 3.1.1.4016-24. Moscow. 2024. Available online: https://www.garant.ru/products/ipo/prime/doc/408788411/?ysclid=m8r63f9osr165465796 (accessed on 16 April 2025).
- Methodological Guidelines “Organization and Conduct of Studies of Clinical Materials for Polioviruses” 4.2.4064-24. Moscow. 2024. Available online: https://www.rospotrebnadzor.ru/upload/iblock/848/wrmkgfjbig1bnylkd4834zql3uz2iear/MUK-4.2.4064_24.pdf (accessed on 16 April 2025).
- World Health Organization (WHO). Polio Laboratory Manual, 4th ed.; WHO: Geneva, Switzerland, 2004; Available online: https://polioeradication.org/wp-content/uploads/2017/05/Polio_Lab_Manual04.pdf (accessed on 18 May 2025).
- van der Avoort, H.G.A.M.; Hull, B.P.; Hovi, T.; Pallansch, M.A.; Kew, O.M.; Crainic, R.; Wood, D.J.; Mulders, M.N.; van Loon, A.M. A comparative study of five methods of intratypic differentiation of polioviruses. J. Clin. Microbiol. 1995, 10, 2562–2566. [Google Scholar] [CrossRef] [PubMed]
- Kilpatrick, D.R.; Yang, C.F.; Ching, K.; Vincent, A.; Iber, J.; Campagnoli, R.; Mandelbaum, M.; De, L.; Yang, S.-J.; Nix, A.; et al. Rapid group-, serotype-, and vaccine strain-specific identification of poliovirus isolates by real-time reverse transcription-PCR using degenerate primers and probes containing deoxyinosine residues. J. Clin. Microbiol. 2009, 6, 1939–1941. [Google Scholar] [CrossRef]
- Gerloff, N.; Sun, H.; Mandelbaum, M.; Maher, C.; Nix, W.A.; Zaidi, S.; Shaukat, S.; Seakamela, L.; Nalavade, U.P.; Sharma, D.K.; et al. Diagnostic Assay Development for Poliovirus eradication. J. Clin. Microbiol. 2018, 56, e01624-17. [Google Scholar] [CrossRef]
- Ivanova, O.E.; Eremeeva, T.P.; Baykova, O.Y.; Krasota, A.Y.; Yakovchuk, E.V.; Shustova, E.Y.; Malyshkina, L.P.; Mustafina, A.N.-I.; Mikhailova, Y.M.; Chirova, A.V.; et al. Detection of Polioviruses Type 2 among Migrant Children Arriving to the Russian Federation from a Country with a Registered Poliomyelitis Outbreak. Vaccines 2024, 12, 718. [Google Scholar] [CrossRef]
- Nix, W.A.; Oberste, M.S.; Pallansch, M.A. Sensitive, seminested PCR amplification of VP1 sequences for direct identification of all enterovirus serotypes from original clinical specimens. J. Clin. Microbiol. 2006, 44, 2698–2704. [Google Scholar] [CrossRef]
- Ivanova, O.E.; Yurashko, O.V.; Eremeeva, T.P.; Baikova, O.Y.; Morozova, N.S.; Lukashev, A.N. Adenovirus Isolation Rates in Acute Flaccid Paralysis Patients. J. Med Virol. 2012, 84, 75–80. [Google Scholar] [CrossRef]
- Sutter, R.W.; Platt, L.; Mach, O.; Jafari, H.; Aylward, B.R. The new polio eradication end game: Rationale and supporting evidence. J. Infect Dis. 2014, 210 (Suppl. S1), 434–438. [Google Scholar] [CrossRef]
- Popova, A.Y.; Ezhlova, E.B.; Melnikova, A.A.; Morozova, N.S.; Mikhailova, Y.M.; Ivanova, O.E.; Kozlovskaya, L.I.; Eremeeva, T.P.; Gmyl, A.P.; Korotkova, E.A.; et al. Measures counteracting 2016 spread of vaccine-derived poliomyelitis virus type 2 in Russian Federation. Russ. J. Infect. Immun. = Infektsiya I Immun. 2020, 1, 90–98. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Novel Oral Polio Vaccine Type 2 (nOPV2) Granted EUL Recommendation. 2021. Available online: https://polioeradication.org/news/novel-oral-polio-vaccine-type-2-nopv2-granted-interim-emergency-use-listing-recommendation/ (accessed on 18 May 2025).
- Shabana, M.R.; Zaghloul, A.Y.; El Shaarawy, T.H.; Nooran, S.; Elleboudy, N.S.; Aboshanab, K.M. Monitoring the VDPV2 outbreak in Egypt during 2020–2021 highlights the crucial role of environmental surveillance and boosting immunization in combating Poliovirus. BMC Infect Dis. 2024, 24, 866. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Standard Operating Procedures: Responding to a Poliovirus Event or Outbreak, Version 4; World Health Organization: Geneva, Switzerland, 2022; Available online: https://polioeradication.org/wp-content/uploads/2024/05/9789240049154-eng.pdf (accessed on 16 April 2025).
- World Health Organization (WHO). International Health Regulations (2005), 3rd ed.; World Health Organization: Geneva, Switzerland, 2005; Available online: https://iris.who.int/bitstream/handle/10665/246107/9789241580496-eng.pdf?sequence=1 (accessed on 16 April 2025).
- National Action Plan for Maintaining the Polio-Free Status of the Russian Federation. Available online: https://fcgie.ru/download/koord_tsentr/%D0%9D%D0%B0%D1%86%D0%B8%D0%BE%D0%BD%D0%B0%D0%BB%D1%8C%D0%BD%D1%8B%D0%B9%20%D0%BF%D0%BB%D0%B0%D0%BD_2022-2024%D0%B3.pdf (accessed on 16 April 2025).
- Fischer, T.K.; Johannesen, C.K.; Benschop, K.S.M.; Berginc, N.; Saxentoff, E.V.; Huseynov, S.; Hagan, J.H.; Harvala, H. Poliovirus circulation in the WHO European region, 2015–2022: A review of data from WHO’s three core poliovirus surveillance systems. Lancet Reg. Health Eur. 2024, 47, 101104. [Google Scholar] [CrossRef]
- Böttcher, S.; Neubauer, K.; Baillot, A.; Rieder, G.; Adam, M.; Diedrich, S. Stool screening of Syrian refugees and asylum seekers in Germany, 2013/2014: Identification of Sabin like polioviruses. Int. J. Med Microbiol. 2015, 305, 601–606. [Google Scholar] [CrossRef] [PubMed]
- Ioannidou, C.; Galanis, P.; Voulgari-Kokota, A.; Dikalioti, S.; Papachristidou, S.; Bozas, E.; Mentis, A.; Tsoumakas, K.; Pavlopoulou, I. Suboptimal Serologic Immunity Against Poliomyelitis Among New Migrant Children in Greece Calls for Organized Action. Lancet Public Health 2022, 7, e606–e615. [Google Scholar] [CrossRef] [PubMed]
- Hvass, A.M.F.; Wejse, C. High coverage of polio immunization program in refugees resettling in Denmark. A cross-sectional study of polio serology in newly arrived refugees. Expert Rev. Vaccines 2019, 18, 1317–1322. [Google Scholar] [CrossRef]
- Veronesi, L.; Colucci, M.E.; Capobianco, E.; Bracchi, M.T.; Zoni, R.; Palandri, L.; Affanni, P. Immunity status against poliomyelitis in young migrants: A seroprevalence study. Acta Biomed. 2019, 90, 28–34. [Google Scholar] [CrossRef]
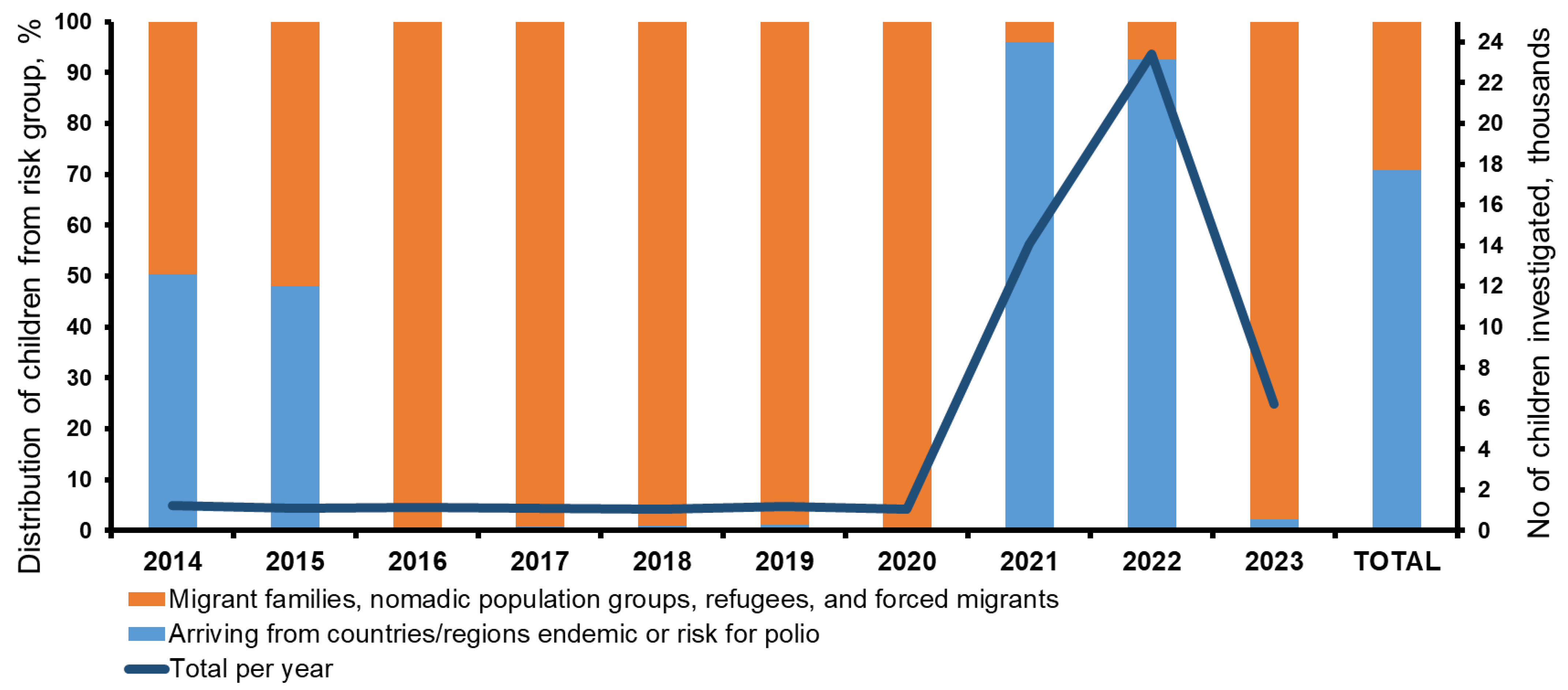

| Year | No of Children Investigated | No. of Isolates | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | Positive | PV Total | PV1 | PV2 | PV3 | Mix | Non-PVs | ||||||||
| No | % | No | % | No | % | No | % | No | % | No | % | No | % | ||
| 2014 | 1231 | 71 | 5.8 | 13 | 18.3 | 1 | 7.7 | 4 | 30.8 | 5 | 38.5 | 3 | 23.1 | 58 | 81.7 |
| 2015 | 1099 | 54 | 4.9 | 14 | 25.9 | 2 | 14.3 | 2 | 14.3 | 8 | 57.1 | 2 | 14.3 | 40 | 74.1 |
| 2016 | 1127 | 63 | 5.6 | 19 | 30.2 | 0 | 0 | 7 | 36.8 | 12 | 63.2 | 0 | 0 | 44 | 69.8 |
| 2017 | 1071 | 52 | 4.9 | 7 | 13.5 | 6 | 85.7 | 0 | 0 | 0 | 0 | 1 | 14.3 | 45 | 86.5 |
| 2018 | 1036 | 47 | 4.5 | 3 | 6.4 | 0 | 0 | 0 | 0 | 3 | 100.0 | 0 | 0 | 44 | 93.6 |
| 2019 | 1202 | 83 | 6.9 | 15 | 18.1 | 8 | 53.3 | 0 | 0 | 6 | 40.0 | 1 | 6.7 | 68 | 81.9 |
| 2020 | 1067 | 15 | 1.4 | 4 | 26.7 | 0 | 0 | 0 | 0 | 4 | 100.0 | 0 | 0 | 11 | 73.3 |
| 2021 | 14,094 | 712 | 5.1 | 205 | 28.8 | 28 | 13.7 | 109 | 53.2 | 53 | 25.9 | 15 | 7.3 | 507 | 71.2 |
| 2022 | 23,425 | 667 | 2.8 | 134 | 20.1 | 33 | 24.6 | 0 | 0 | 77 | 57.5 | 24 | 17.9 | 533 | 79.9 |
| 2023 | 6196 | 313 | 5.1 | 18 | 5.8 | 3 | 16.7 | 0 | 0 | 12 | 66.7 | 3 | 16.7 | 295 | 94.2 |
| Total | 51,548 | 2077 | 4.0 | 432 | 20.8 | 81 | 18.8 | 122 | 28.2 | 180 | 41.7 | 49 | 11.3 | 1.645 | 79.2 |
| Virus | Source/ Gender/Age, Years | Child Country/ Region of Origin | Vaccination Status | Immunity Status | Place and Time of Detection | % nt sub/VP1 | Reference |
|---|---|---|---|---|---|---|---|
| VDPV2 | Healthy/m/1 | Russia, Chechen Republic | Not vaccinated | Normal | Moscow, September 2016 | 1.12 | [28] |
| VDPV2 | Healthy/f/1 | Russia, permanent residence Chechen Republic | Not vaccinated | Transient immuno-deficiency | Chechen Republic, December 2016 | 1.33 | [28] |
| cVDPV2 | Healthy/f/3 | Tajikistan | 1 bOPV | Normal | Voronezh, September 2021 | 4.32 | [24] |
| cVDPV2 | Healthy/f/3 | Tajikistan | 2 IPV | Normal | Moscow region, October 2021 | 3.10 | [24] |
| Sabin-like PV2 | Healthy/m/0.75 | Egypt | 3 OPV, 1 IPV | Normal | Zabaikalsky Krai, June 2021 | 0.33 | – |
| E. alphacoxsakie n = 107 | E. betacoxsakie n = 428 | E. coxsackiepol n = 45 | |||
|---|---|---|---|---|---|
| Type | n | Type | n | Type | n |
| CVA4 | 45 | CVB1–6 | 206 | CVA19 | 12 |
| CVA10 | 20 | E11 | 52 | CVA24 | 12 |
| CVA2 | 11 | E6 | 44 | CVA1 | 6 |
| CVA7 | 9 | E13 | 29 | CVA11 | 4 |
| EV-A71 | 7 | E7 | 22 | CVA20 | 4 |
| CVA5 | 6 | E14 | 11 | EV-C99 | 3 |
| CVA3 | 2 | E25 | 9 | CVA13 | 2 |
| CVA6 | 2 | E3 | 7 | EV-C96 | 2 |
| CVA16 | 2 | E18 | 6 | ||
| EV-A90 | 2 | E29 | 6 | ||
| CVA14 | 1 | CVA9 | 6 | ||
| E30 | 5 | ||||
| E4 | 3 | ||||
| E12 | 3 | ||||
| E17 | 3 | ||||
| E21 | 3 | ||||
| E9 | 2 | ||||
| E19 | 2 | ||||
| E20 | 2 | ||||
| EV-B75 | 2 | ||||
| E1 | 1 | ||||
| E24 | 1 | ||||
| E26 | 1 | ||||
| E31 | 1 | ||||
| EV-B83 | 1 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ivanova, O.E.; Mikhailova, Y.M.; Morozova, N.S.; Chirova, A.V.; Cherepanova, E.A.; Golitsyna, L.N.; Baikova, O.Y.; Yakovchuk, E.V.; Karpova, E.V.; Kozlovskaya, L.I. Supplementary Surveillance of Poliovirus Circulation in the Russian Federation: Results of a Study on Migrant Children of “Risk Group”. Viruses 2025, 17, 746. https://doi.org/10.3390/v17060746
Ivanova OE, Mikhailova YM, Morozova NS, Chirova AV, Cherepanova EA, Golitsyna LN, Baikova OY, Yakovchuk EV, Karpova EV, Kozlovskaya LI. Supplementary Surveillance of Poliovirus Circulation in the Russian Federation: Results of a Study on Migrant Children of “Risk Group”. Viruses. 2025; 17(6):746. https://doi.org/10.3390/v17060746
Chicago/Turabian StyleIvanova, Olga E., Yulia M. Mikhailova, Nadezhda S. Morozova, Alina V. Chirova, Evgeniya A. Cherepanova, Lyudmila N. Golitsyna, Olga Y. Baikova, Elizaveta V. Yakovchuk, Evgenia V. Karpova, and Liubov I. Kozlovskaya. 2025. "Supplementary Surveillance of Poliovirus Circulation in the Russian Federation: Results of a Study on Migrant Children of “Risk Group”" Viruses 17, no. 6: 746. https://doi.org/10.3390/v17060746
APA StyleIvanova, O. E., Mikhailova, Y. M., Morozova, N. S., Chirova, A. V., Cherepanova, E. A., Golitsyna, L. N., Baikova, O. Y., Yakovchuk, E. V., Karpova, E. V., & Kozlovskaya, L. I. (2025). Supplementary Surveillance of Poliovirus Circulation in the Russian Federation: Results of a Study on Migrant Children of “Risk Group”. Viruses, 17(6), 746. https://doi.org/10.3390/v17060746







