Respiratory Syncytial Virus Elicits Glycolytic Metabolism in Pediatric Upper and Lower Airways
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects and Study Procedures
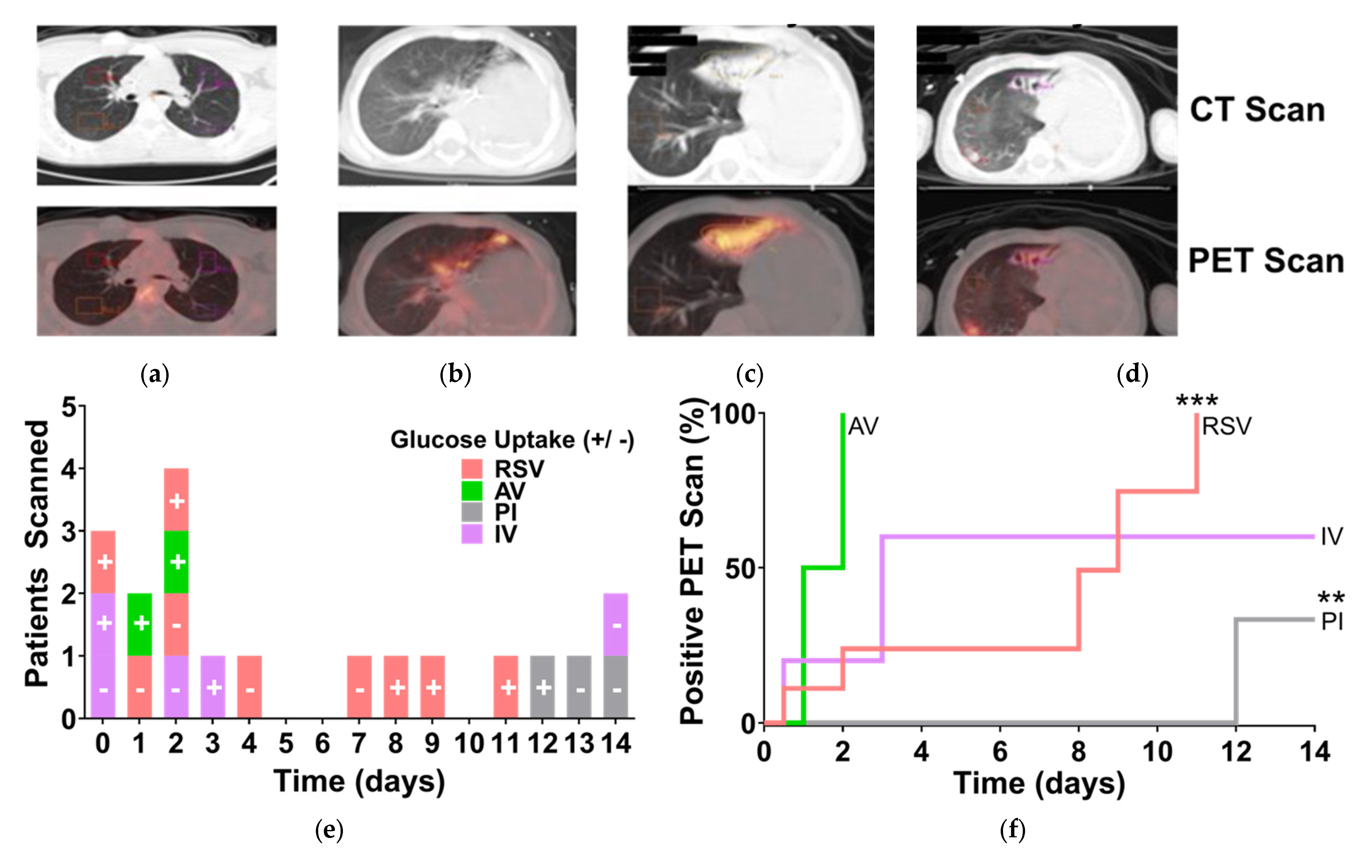
2.2. Infected Patient PET Scans
2.3. Pediatric Nasal Pharyngeal Aspirates
2.4. Cells and Virus
2.5. Pediatric Nasopharynx Tissue-Derived Multicellular Epithelium Cultures (TEpiCs)
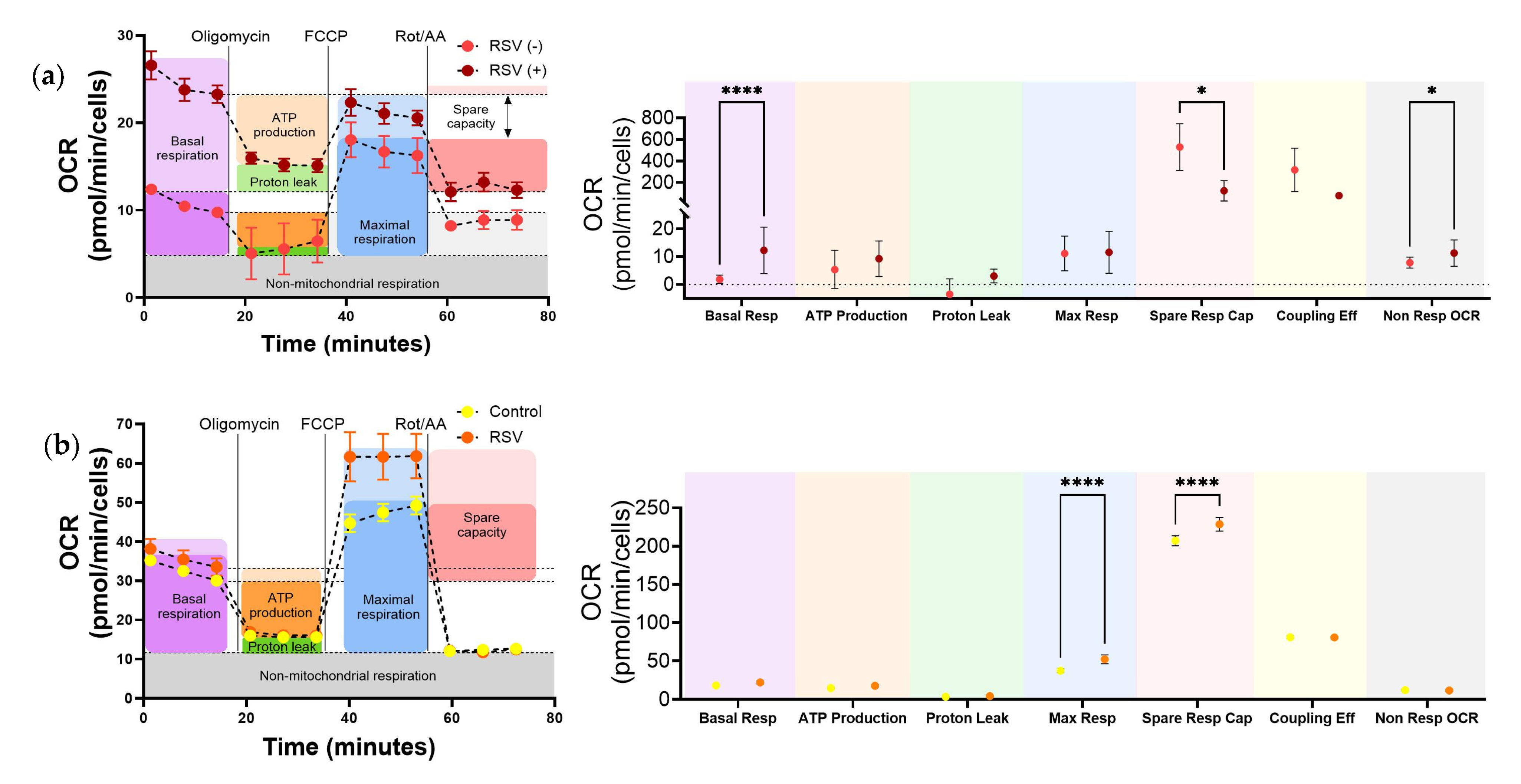
2.6. Cell Bioenergetics
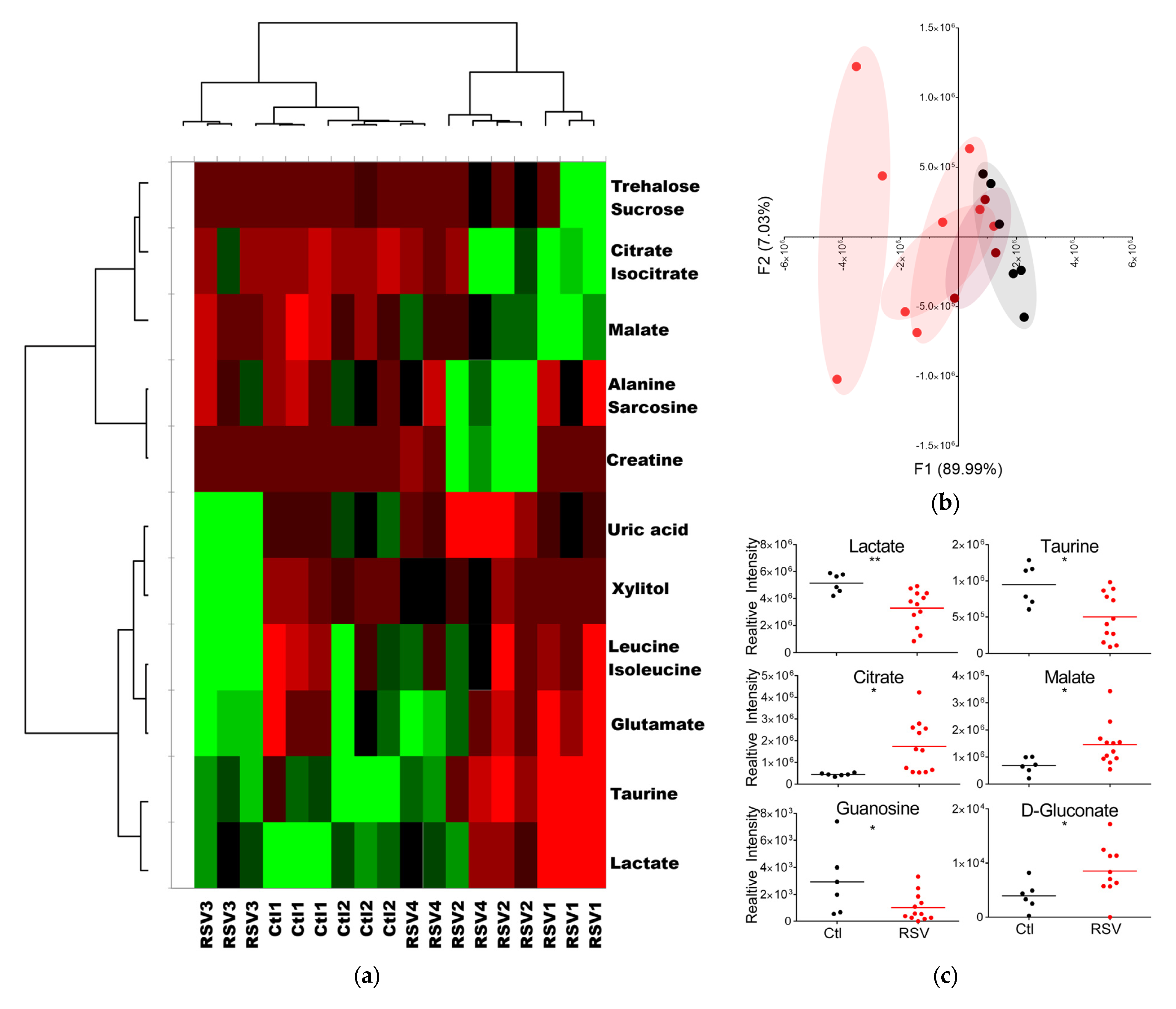
2.7. Metabolomics of Nasopharyngeal Fluids
2.8. Statistical Analysis
3. Results
3.1. RSV-Induced Hypermetabolism in the Lungs of Pediatric Patients
3.2. RSV Infection Alters Metabolite Levels and Profiles in Upper Airway Fluids
3.3. Increased Glycolysis in Upper Airways Cells Infected by RSV In Situ
3.4. In Vitro RSV Infection Significantly Increased Glycolysis in Upper Airway Epithelium Cultures and Lower Airway Bronchial Epithelial Cells
3.5. RSV Increases Mitochondrial Respiration in Upper Respiratory Cells Infected In Situ
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
Abbreviations
| ATP | Adenosine Triphosphate |
| ECAR | Extracellular Acidification Rate |
| FDG | Fluorodeoxyglucose |
| HBECs | Human Bronchial Epithelial Cells |
| IL-1β | Interleukin-1 Beta |
| IL-33 | Interleukin-33 |
| NPA | Nasopharyngeal Aspirates |
| OCR | Oxygen Consumption Rate |
| OXPHOS | Oxidative Phosphorylation |
| PET | Positron Emission Tomography |
| RSV | Respiratory Syncytial Virus |
| RT-qPCR | Reverse Transcription Quantitative Polymerase Chain Reaction |
| TEpiCs | Tissue-Derived Multicellular Epithelial Cultures |
| TCA | Tricarboxylic Acid |
| URCs | Upper Respiratory Cells |
References
- Borchers, A.T.; Chang, C.; Gershwin, M.E.; Gershwin, L.J. Respiratory syncytial virus—A comprehensive review. Clin. Rev. Allergy Immunol. 2013, 45, 331–379. [Google Scholar] [CrossRef] [PubMed]
- Miyairi, I.; DeVincenzo, J.P. Human genetic factors and respiratory syncytial virus disease severity. Clin. Microbiol. Rev. 2008, 21, 686–703. [Google Scholar] [CrossRef] [PubMed]
- Grad, Y.H.; Newman, R.; Zody, M.; Yang, X.; Murphy, R.; Qu, J.; Malboeuf, C.M.; Levin, J.Z.; Lipsitch, M.; DeVincenzo, J. Within-host whole-genome deep sequencing and diversity analysis of human respiratory syncytial virus infection reveals dynamics of genomic diversity in the absence and presence of immune pressure. J. Virol. 2014, 88, 7286–7293. [Google Scholar] [CrossRef] [PubMed]
- Kurt-Jones, E.A.; Popova, L.; Kwinn, L.; Haynes, L.M.; Jones, L.P.; Tripp, R.A.; Walsh, E.E.; Freeman, M.W.; Golenbock, D.T.; Anderson, L.J.; et al. Pattern recognition receptors TLR4 and CD14 mediate response to respiratory syncytial virus. Nat. Immunol. 2000, 1, 398–401. [Google Scholar] [CrossRef]
- Haynes, L.M.; Moore, D.D.; Kurt-Jones, E.A.; Finberg, R.W.; Anderson, L.J.; Tripp, R.A. Involvement of toll-like receptor 4 in innate immunity to respiratory syncytial virus. J. Virol. 2001, 75, 10730–10737. [Google Scholar] [CrossRef]
- Murawski, M.R.; Bowen, G.N.; Cerny, A.M.; Anderson, L.J.; Haynes, L.M.; Tripp, R.A.; Kurt-Jones, E.A.; Finberg, R.W. Respiratory syncytial virus activates innate immunity through Toll-like receptor 2. J. Virol. 2009, 83, 1492–1500. [Google Scholar] [CrossRef]
- You, D.; Marr, N.; Saravia, J.; Shrestha, B.; Lee, G.I.; Turvey, S.E.; Brombacher, F.; Herbert, D.R.; Cormier, S.A. IL-4Ralpha on CD4+ T cells plays a pathogenic role in respiratory syncytial virus reinfection in mice infected initially as neonates. J. Leukoc. Biol. 2013, 93, 933–942. [Google Scholar] [CrossRef]
- Becnel, D.; You, D.; Erskin, J.; Dimina, D.M.; Cormier, S.A. A role for airway remodeling during respiratory syncytial virus infection. Respir. Res. 2005, 6, 122. [Google Scholar] [CrossRef]
- Derscheid, R.J.; Ackermann, M.R. The innate immune system of the perinatal lung and responses to respiratory syncytial virus infection. Vet. Pathol. 2013, 50, 827–841. [Google Scholar] [CrossRef]
- Fauroux, B.; Simoes, E.A.F.; Checchia, P.A.; Paes, B.; Figueras-Aloy, J.; Manzoni, P.; Bont, L.; Carbonell-Estrany, X. The Burden and Long-term Respiratory Morbidity Associated with Respiratory Syncytial Virus Infection in Early Childhood. Infect. Dis. Ther. 2017, 6, 173–197. [Google Scholar] [CrossRef]
- Singanayagam, A.; Joshi, P.V.; Mallia, P.; Johnston, S.L. Viruses exacerbating chronic pulmonary disease: The role of immune modulation. BMC Med. 2012, 10, 27. [Google Scholar] [CrossRef] [PubMed]
- Flores-Torres, A.S.; Samarasinghe, A.E. Impact of Therapeutics on Unified Immunity During Allergic Asthma and Respiratory Infections. Front. Allergy 2022, 3, 852067. [Google Scholar] [CrossRef] [PubMed]
- Topalidou, X.; Kalergis, A.M.; Papazisis, G. Respiratory Syncytial Virus Vaccines: A Review of the Candidates and the Approved Vaccines. Pathogens 2023, 12, 1259. [Google Scholar] [CrossRef]
- Bahadoran, A.; Bezavada, L.; Smallwood, H.S. Fueling influenza and the immune response: Implications for metabolic reprogramming during influenza infection and immunometabolism. Immunol. Rev. 2020, 295, 140–166. [Google Scholar] [CrossRef]
- Kleinehr, J.; Wilden, J.J.; Boergeling, Y.; Ludwig, S.; Hrincius, E.R. Metabolic Modifications by Common Respiratory Viruses and Their Potential as New Antiviral Targets. Viruses 2021, 13, 2068. [Google Scholar] [CrossRef]
- Adamko, D.J.; Saude, E.; Bear, M.; Regush, S.; Robinson, J.L. Urine metabolomic profiling of children with respiratory tract infections in the emergency department: A pilot study. BMC Infect. Dis. 2016, 16, 439. [Google Scholar] [CrossRef]
- Stewart, C.J.; Hasegawa, K.; Wong, M.C.; Ajami, N.J.; Petrosino, J.F.; Piedra, P.A.; Espinola, J.A.; Tierney, C.N.; Camargo, C.A., Jr.; Mansbach, J.M. Respiratory Syncytial Virus and Rhinovirus Bronchiolitis Are Associated With Distinct Metabolic Pathways. J. Infect. Dis. 2018, 217, 1160–1169. [Google Scholar] [CrossRef]
- Stewart, C.J.; Mansbach, J.M.; Piedra, P.A.; Toivonen, L.; Camargo, C.A.; Hasegawa, K. Association of respiratory viruses with serum metabolome in infants with severe bronchiolitis. Pediatr. Allergy Immunol. 2019, 30, 848–851. [Google Scholar] [CrossRef]
- Turi, K.N.; Romick-Rosendale, L.; Gebretsadik, T.; Watanabe, M.; Brunwasser, S.; Anderson, L.J.; Moore, M.L.; Larkin, E.K.; Peebles, R.S., Jr.; Hartert, T.V. Using urine metabolomics to understand the pathogenesis of infant respiratory syncytial virus (RSV) infection and its role in childhood wheezing. Metabolomics 2018, 14, 135. [Google Scholar] [CrossRef]
- Martin-Vicente, M.; Gonzalez-Riano, C.; Barbas, C.; Jimenez-Sousa, M.A.; Brochado-Kith, O.; Resino, S.; Martinez, I. Metabolic changes during respiratory syncytial virus infection of epithelial cells. PLoS ONE 2020, 15, e0230844. [Google Scholar] [CrossRef]
- Lu, Y.; Xu, S.; Sun, H.; Shan, J.; Shen, C.; Ji, J.; Lin, L.; Xu, J.; Peng, L.; Dai, C.; et al. Analysis of temporal metabolic rewiring for human respiratory syncytial virus infection by integrating metabolomics and proteomics. Metabolomics 2023, 19, 30. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Schulze, K.E.; Ghildyal, R.; Henstridge, D.C.; Kolanowski, J.L.; New, E.J.; Hong, Y.; Hsu, A.C.; Hansbro, P.M.; Wark, P.A.; et al. Respiratory syncytial virus co-opts host mitochondrial function to favour infectious virus production. Elife 2019, 8, e42448. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.F.; Cai, J.X.; Zhang, J.J.; Tang, Y.J.; Chen, J.Y.; Xiong, S.; Li, Y.L.; Zhang, H.; Liu, Z.; Li, M.M. Respiratory syncytial virus co-opts hypoxia-inducible factor-1alpha-mediated glycolysis to favor the production of infectious virus. mBio 2023, 14, e0211023. [Google Scholar] [CrossRef]
- Dettmer, K.; Vogl, F.C.; Ritter, A.P.; Zhu, W.; Nurnberger, N.; Kreutz, M.; Oefner, P.J.; Gronwald, W.; Gottfried, E. Distinct metabolic differences between various human cancer and primary cells. Electrophoresis 2013, 34, 2836–2847. [Google Scholar] [CrossRef]
- Marchetti, P.; Fovez, Q.; Germain, N.; Khamari, R.; Kluza, J. Mitochondrial spare respiratory capacity: Mechanisms, regulation, and significance in non-transformed and cancer cells. FASEB J. 2020, 34, 13106–13124. [Google Scholar] [CrossRef]
- Morris, D.R.; Qu, Y.; Agrawal, A.; Garofalo, R.P.; Casola, A. HIF-1alpha Modulates Core Metabolism and Virus Replication in Primary Airway Epithelial Cells Infected with Respiratory Syncytial Virus. Viruses 2020, 12, 1088. [Google Scholar] [CrossRef]
- Connelly, A.R.; Jeong, B.M.; Coden, M.E.; Cao, J.Y.; Chirkova, T.; Rosas-Salazar, C.; Cephus, J.Y.; Anderson, L.J.; Newcomb, D.C.; Hartert, T.V.; et al. Metabolic Reprogramming of Nasal Airway Epithelial Cells Following Infant Respiratory Syncytial Virus Infection. Viruses 2021, 13, 2055. [Google Scholar] [CrossRef]
- Smallwood, H.S.; Duan, S.; Morfouace, M.; Rezinciuc, S.; Shulkin, B.L.; Shelat, A.; Zink, E.E.; Milasta, S.; Bajracharya, R.; Oluwaseum, A.J.; et al. Targeting Metabolic Reprogramming by Influenza Infection for Therapeutic Intervention. Cell Rep. 2017, 19, 1640–1653. [Google Scholar] [CrossRef]
- Okda, F.; Sakr, A.; Webster, R.; Webby, R. Tropism, susceptibility, infectivity, and cytokine releases of differentiated human tonsillar epithelial cells by different Influenza viruses. bioRixv 2021. preprint. [Google Scholar] [CrossRef]
- Lu, W.; Clasquin, M.F.; Melamud, E.; Amador-Noguez, D.; Caudy, A.A.; Rabinowitz, J.D. Metabolomic analysis via reversed-phase ion-pairing liquid chromatography coupled to a stand alone orbitrap mass spectrometer. Anal. Chem. 2010, 82, 3212–3221. [Google Scholar] [CrossRef]
- Martens, L.; Chambers, M.; Sturm, M.; Kessner, D.; Levander, F.; Shofstahl, J.; Tang, W.H.; Rompp, A.; Neumann, S.; Pizarro, A.D.; et al. mzML—A community standard for mass spectrometry data. Mol. Cell Proteom. 2011, 10, R110 000133. [Google Scholar] [CrossRef] [PubMed]
- Chambers, M.C.; Maclean, B.; Burke, R.; Amodei, D.; Ruderman, D.L.; Neumann, S.; Gatto, L.; Fischer, B.; Pratt, B.; Egertson, J.; et al. A cross-platform toolkit for mass spectrometry and proteomics. Nat. Biotechnol. 2012, 30, 918–920. [Google Scholar] [CrossRef] [PubMed]
- Melamud, E.; Vastag, L.; Rabinowitz, J.D. Metabolomic analysis and visualization engine for LC-MS data. Anal. Chem. 2010, 82, 9818–9826. [Google Scholar] [CrossRef] [PubMed]
- Clasquin, M.F.; Melamud, E.; Rabinowitz, J.D. LC-MS data processing with MAVEN: A metabolomic analysis and visualization engine. Curr. Protoc. Bioinform. 2012, 37, 14.11.1–14.11.23. [Google Scholar] [CrossRef]
- Hofman, M.S.; Hicks, R.J. How We Read Oncologic FDG PET/CT. Cancer Imaging 2016, 16, 35. [Google Scholar] [CrossRef]
- Shi, T.; McAllister, D.A.; O’Brien, K.L.; Simoes, E.A.F.; Madhi, S.A.; Gessner, B.D.; Polack, F.P.; Balsells, E.; Acacio, S.; Aguayo, C.; et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: A systematic review and modelling study. Lancet 2017, 390, 946–958. [Google Scholar] [CrossRef]
- Shrestha, B.; You, D.; Saravia, J.; Siefker, D.T.; Jaligama, S.; Lee, G.I.; Sallam, A.A.; Harding, J.N.; Cormier, S.A. IL-4Ralpha on dendritic cells in neonates and Th2 immunopathology in respiratory syncytial virus infection. J. Leukoc. Biol. 2017, 102, 153–161. [Google Scholar] [CrossRef]
- Goyal, P.; Rajala, M.S. Reprogramming of glucose metabolism in virus infected cells. Mol. Cell Biochem. 2023, 478, 2409–2418. [Google Scholar] [CrossRef]
- Laure, M.; Hamza, H.; Koch-Heier, J.; Quernheim, M.; Muller, C.; Schreiber, A.; Muller, G.; Pleschka, S.; Ludwig, S.; Planz, O. Antiviral efficacy against influenza virus and pharmacokinetic analysis of a novel MEK-inhibitor, ATR-002, in cell culture and in the mouse model. Antivir. Res. 2020, 178, 104806. [Google Scholar] [CrossRef]
- Koch-Heier, J.; Schonsiegel, A.; Waidele, L.M.; Volk, J.; Full, Y.; Wallasch, C.; Canisius, S.; Burnet, M.; Planz, O. Pharmacokinetics, Pharmacodynamics and Antiviral Efficacy of the MEK Inhibitor Zapnometinib in Animal Models and in Humans. Front. Pharmacol. 2022, 13, 893635. [Google Scholar] [CrossRef]
- Rohde, G.; Stenglein, S.; Prozesky, H.; Manudhane, G.; Sandulescu, O.; Bauer, M.; Overend, T.; Koch, W.; Neuschwander, D.; Planz, O.; et al. Efficacy and safety of zapnometinib in hospitalised adult patients with COVID-19 (RESPIRE): A randomised, double-blind, placebo-controlled, multicentre, proof-of-concept, phase 2 trial. EClinicalMedicine 2023, 65, 102237. [Google Scholar] [CrossRef] [PubMed]
- Jimenez de Oya, N.; Esler, W.P.; Huard, K.; El-Kattan, A.F.; Karamanlidis, G.; Blazquez, A.B.; Ramos-Ibeas, P.; Escribano-Romero, E.; Louloudes-Lazaro, A.; Casas, J.; et al. Targeting host metabolism by inhibition of acetyl-Coenzyme A carboxylase reduces flavivirus infection in mouse models. Emerg. Microbes Infect. 2019, 8, 624–636. [Google Scholar] [CrossRef] [PubMed]
- Munday, D.C.; Howell, G.; Barr, J.N.; Hiscox, J.A. Proteomic analysis of mitochondria in respiratory epithelial cells infected with human respiratory syncytial virus and functional implications for virus and cell biology. J. Pharm. Pharmacol. 2015, 67, 300–318. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.; Bicker, J.; Falcao, A.; Fortuna, A. Air-liquid interface (ALI) impact on different respiratory cell cultures. Eur. J. Pharm. Biopharm. 2023, 184, 62–82. [Google Scholar] [CrossRef]
- Teoh, S.T.; Leimanis-Laurens, M.L.; Comstock, S.S.; Winters, J.W.; Vandenbosch, N.L.; Prokop, J.W.; Bachmann, A.S.; Lunt, S.Y.; Rajasekaran, S. Combined Plasma and Urinary Metabolomics Uncover Metabolic Perturbations Associated with Severe Respiratory Syncytial Viral Infection and Future Development of Asthma in Infant Patients. Metabolites 2022, 12, 178. [Google Scholar] [CrossRef]
- Alsuwaidi, A.R.; Benedict, S.; Kochiyil, J.; Mustafa, F.; Hartwig, S.M.; Almarzooqi, S.; Albawardi, A.; Rizvi, T.A.; Varga, S.M.; Souid, A.K. Bioenergetics of murine lungs infected with respiratory syncytial virus. Virol. J. 2013, 10, 22. [Google Scholar] [CrossRef]
- Hui, S.; Ghergurovich, J.M.; Morscher, R.J.; Jang, C.; Teng, X.; Lu, W.; Esparza, L.A.; Reya, T.; Le, Z.; Yanxiang Guo, J.; et al. Glucose feeds the TCA cycle via circulating lactate. Nature 2017, 551, 115–118. [Google Scholar] [CrossRef]
- Thyrsted, J.; Storgaard, J.; Blay-Cadanet, J.; Heinz, A.; Thielke, A.L.; Crotta, S.; de Paoli, F.; Olagnier, D.; Wack, A.; Hiller, K.; et al. Influenza A induces lactate formation to inhibit type I IFN in primary human airway epithelium. iScience 2021, 24, 103300. [Google Scholar] [CrossRef]
- Thomas, T.; Stefanoni, D.; Reisz, J.A.; Nemkov, T.; Bertolone, L.; Francis, R.O.; Hudson, K.E.; Zimring, J.C.; Hansen, K.C.; Hod, E.A.; et al. COVID-19 infection alters kynurenine and fatty acid metabolism, correlating with IL-6 levels and renal status. JCI Insight 2020, 5, e140327. [Google Scholar] [CrossRef]
- Wang, K.; Khoramjoo, M.; Srinivasan, K.; Gordon, P.M.K.; Mandal, R.; Jackson, D.; Sligl, W.; Grant, M.B.; Penninger, J.M.; Borchers, C.H.; et al. Sequential multi-omics analysis identifies clinical phenotypes and predictive biomarkers for long COVID. Cell Rep. Med. 2023, 4, 101254. [Google Scholar] [CrossRef]
- Lopez-Hernandez, Y.; Monarrez-Espino, J.; Lopez, D.A.G.; Zheng, J.; Borrego, J.C.; Torres-Calzada, C.; Elizalde-Diaz, J.P.; Mandal, R.; Berjanskii, M.; Martinez-Martinez, E.; et al. The plasma metabolome of long COVID patients two years after infection. Sci. Rep. 2023, 13, 12420. [Google Scholar] [CrossRef] [PubMed]
- Jong, C.J.; Sandal, P.; Schaffer, S.W. The Role of Taurine in Mitochondria Health: More Than Just an Antioxidant. Molecules 2021, 26, 4913. [Google Scholar] [CrossRef] [PubMed]
- Horvath, K.M.; Herbst, M.; Zhou, H.; Zhang, H.; Noah, T.L.; Jaspers, I. Nasal lavage natural killer cell function is suppressed in smokers after live attenuated influenza virus. Respir. Res. 2011, 12, 102. [Google Scholar] [CrossRef] [PubMed]
- Fransson, M.; Benson, M.; Erjefalt, J.S.; Jansson, L.; Uddman, R.; Bjornsson, S.; Cardell, L.O.; Adner, M. Expression of Toll-like receptor 9 in nose, peripheral blood and bone marrow during symptomatic allergic rhinitis. Respir. Res. 2007, 8, 17. [Google Scholar] [CrossRef]
- Arambula, A.; Brown, J.R.; Neff, L. Anatomy and physiology of the palatine tonsils, adenoids, and lingual tonsils. World J. Otorhinolaryngol. Head. Neck Surg. 2021, 7, 155–160. [Google Scholar] [CrossRef]
- Proenca-Modena, J.L.; Pereira Valera, F.C.; Jacob, M.G.; Buzatto, G.P.; Saturno, T.H.; Lopes, L.; Souza, J.M.; Escremim Paula, F.; Silva, M.L.; Carenzi, L.R.; et al. High rates of detection of respiratory viruses in tonsillar tissues from children with chronic adenotonsillar disease. PLoS ONE 2012, 7, e42136. [Google Scholar] [CrossRef]
- Jartti, T.; Palomares, O.; Waris, M.; Tastan, O.; Nieminen, R.; Puhakka, T.; Ruckert, B.; Aab, A.; Vuorinen, T.; Allander, T.; et al. Distinct regulation of tonsillar immune response in virus infection. Allergy 2014, 69, 658–667. [Google Scholar] [CrossRef]
- Proenca-Modena, J.L.; Buzatto, G.P.; Paula, F.E.; Saturno, T.H.; Delcaro, L.S.; Prates, M.C.; Tamashiro, E.; Valera, F.C.; Arruda, E.; Anselmo-Lima, W.T. Respiratory viruses are continuously detected in children with chronic tonsillitis throughout the year. Int. J. Pediatr. Otorhinolaryngol. 2014, 78, 1655–1661. [Google Scholar] [CrossRef]
- Mookerjee, S.A.; Nicholls, D.G.; Brand, M.D. Determining Maximum Glycolytic Capacity Using Extracellular Flux Measurements. PLoS ONE 2016, 11, e0152016. [Google Scholar] [CrossRef]
- Alsuwaidi, A.R.; Albawardi, A.; Almarzooqi, S.; Benedict, S.; Othman, A.R.; Hartwig, S.M.; Varga, S.M.; Souid, A.K. Respiratory syncytial virus increases lung cellular bioenergetics in neonatal C57BL/6 mice. Virology 2014, 454–455, 263–269. [Google Scholar] [CrossRef]
- Brand, M.D.; Nicholls, D.G. Assessing mitochondrial dysfunction in cells. Biochem. J. 2011, 435, 297–312. [Google Scholar] [CrossRef] [PubMed]
- Hill, B.G.; Benavides, G.A.; Lancaster, J.R., Jr.; Ballinger, S.; Dell’Italia, L.; Jianhua, Z.; Darley-Usmar, V.M. Integration of cellular bioenergetics with mitochondrial quality control and autophagy. Biol. Chem. 2012, 393, 1485–1512. [Google Scholar] [CrossRef] [PubMed]
- Vu, L.D.; Phan, A.T.Q.; Hijano, D.R.; Siefker, D.T.; Tillman, H.; Cormier, S.A. IL-1β Promotes Expansion of IL-33(+) Lung Epithelial Stem Cells after Respiratory Syncytial Virus Infection during Infancy. Am. J. Respir. Cell Mol. Biol. 2022, 66, 312–322. [Google Scholar] [CrossRef]
- Vu, L.D.; Siefker, D.; Jones, T.L.; You, D.; Taylor, R.; DeVincenzo, J.; Cormier, S.A. Elevated Levels of Type 2 Respiratory Innate Lymphoid Cells in Human Infants with Severe Respiratory Syncytial Virus Bronchiolitis. Am. J. Respir. Crit. Care Med. 2019, 200, 1414–1423. [Google Scholar] [CrossRef]
- Qian, X.; Aboushousha, R.; van de Wetering, C.; Chia, S.B.; Amiel, E.; Schneider, R.W.; van der Velden, J.L.J.; Lahue, K.G.; Hoagland, D.A.; Casey, D.T.; et al. IL-1/inhibitory κB kinase ε-induced glycolysis augment epithelial effector function and promote allergic airways disease. J. Allergy Clin. Immunol. 2018, 142, 435–450.e410. [Google Scholar] [CrossRef]
- Finucane, O.M.; Sugrue, J.; Rubio-Araiz, A.; Guillot-Sestier, M.-V.; Lynch, M.A. The NLRP3 inflammasome modulates glycolysis by increasing PFKFB3 in an IL-1β-dependent manner in macrophages. Sci. Rep. 2019, 9, 4034. [Google Scholar] [CrossRef]
- Ben-Shlomo, I.; Kol, S.; Roeder, L.M.; Resnick, C.E.; Hurwitz, A.; Payne, D.W.; Adashi, E.Y. Interleukin (IL)-1beta increases glucose uptake and induces glycolysis in aerobically cultured rat ovarian cells: Evidence that IL-1beta may mediate the gonadotropin-induced midcycle metabolic shift. Endocrinology 1997, 138, 2680–2688. [Google Scholar] [CrossRef]
- Liang, Y.; Wang, X.; Wang, H.; Yang, W.; Yi, P.; Soong, L.; Cong, Y.; Cai, J.; Fan, X.; Sun, J. IL-33 activates mTORC1 and modulates glycolytic metabolism in CD8(+) T cells. Immunology 2022, 165, 61–73. [Google Scholar] [CrossRef]
- Scott, L.M.; Vincent, E.E.; Hudson, N.; Neal, C.; Jones, N.; Lavelle, E.C.; Campbell, M.; Halestrap, A.P.; Dick, A.D.; Theodoropoulou, S. Interleukin-33 regulates metabolic reprogramming of the retinal pigment epithelium in response to immune stressors. JCI Insight 2021, 6, e129429. [Google Scholar] [CrossRef]
- Caslin, H.L.; Taruselli, M.T.; Haque, T.; Pondicherry, N.; Baldwin, E.A.; Barnstein, B.O.; Ryan, J.J. Inhibiting Glycolysis and ATP Production Attenuates IL-33-Mediated Mast Cell Function and Peritonitis. Front. Immunol. 2018, 9, 3026. [Google Scholar] [CrossRef]
- Lin, J.; Liu, J.; Ma, R.; Hao, J.; Liang, Y.; Zhao, J.; Zhang, A.; Meng, H.; Lu, J. Interleukin-33: Metabolic checkpoints, metabolic processes, and epigenetic regulation in immune cells. Front. Immunol. 2022, 13, 900826. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flores-Torres, A.S.; Rezinciuc, S.; Bezavada, L.; Shulkin, B.L.; Cormier, S.A.; Smallwood, H.S. Respiratory Syncytial Virus Elicits Glycolytic Metabolism in Pediatric Upper and Lower Airways. Viruses 2025, 17, 703. https://doi.org/10.3390/v17050703
Flores-Torres AS, Rezinciuc S, Bezavada L, Shulkin BL, Cormier SA, Smallwood HS. Respiratory Syncytial Virus Elicits Glycolytic Metabolism in Pediatric Upper and Lower Airways. Viruses. 2025; 17(5):703. https://doi.org/10.3390/v17050703
Chicago/Turabian StyleFlores-Torres, Armando S., Svetlana Rezinciuc, Lavanya Bezavada, Barry L. Shulkin, Stephania A. Cormier, and Heather S. Smallwood. 2025. "Respiratory Syncytial Virus Elicits Glycolytic Metabolism in Pediatric Upper and Lower Airways" Viruses 17, no. 5: 703. https://doi.org/10.3390/v17050703
APA StyleFlores-Torres, A. S., Rezinciuc, S., Bezavada, L., Shulkin, B. L., Cormier, S. A., & Smallwood, H. S. (2025). Respiratory Syncytial Virus Elicits Glycolytic Metabolism in Pediatric Upper and Lower Airways. Viruses, 17(5), 703. https://doi.org/10.3390/v17050703





