Thermal Inactivation of Hepatitis E Virus: A Narrative Review
Abstract
1. Introduction
2. Thermal Stability of Hepatitis E Virus in Cell Culture Systems
| HEV Genotypes | Cell Lines | Detection Methods | Treatment Temperature and Duration | Completely Inactivation | Refs. |
|---|---|---|---|---|---|
| HEV-1/4 | A549 | TCID50/0.025 mL, CPE | 56 °C for 30 min | Yes | [51] |
| HEV-1 | HepG2/C3A | Focus-forming units (ffu) | 4 °C for 1 h | No | [52] |
| HEV-1 | HepG2/C3A | Focus-forming units (ffu) | 50 °C for 1 h | No | [52] |
| HEV-1 | HepG2/C3A | Focus-forming units (ffu) | 56 °C for 1 h | No | [52] |
| HEV-1 | HepG2/C3A | Focus-forming units (ffu) | 60 °C for 1 h | No | [52] |
| HEV-2 | HepG2/C3A | Focus-forming units (ffu) | 4 °C for 1 h | No | [52] |
| HEV-2 | HepG2/C3A | Focus-forming units (ffu) | 50 °C for 1 h | No | [52] |
| HEV-2 | HepG2/C3A | Focus-forming units (ffu) | 56 °C for 1 h | No | [52] |
| HEV-2 | HepG2/C3A | Focus-forming units (ffu) | 60 °C for 1 h | No | [52] |
| HEV-3b | PLC/PRF/5 | Real-time RT-PCR for HEV RNA in CM | 56 °C for 30 min | No | [53] |
| HEV-3b | PLC/PRF/5 | Real-time RT-PCR for HEV RNA in CM | 70 °C for 10 min | Yes | [53] |
| HEV-3b | PLC/PRF/5 | Real-time RT-PCR for HEV RNA in CM | 95 °C for 1 min | Yes | [53] |
| HEV-3b | PLC/PRF/5 | Real-time RT-PCR for HEV RNA in CM | 95 °C for 10 min | Yes | [53] |
| HEV-3 | A549 | Real-time RT-PCR for cellular HEV RNA | 80 °C for 24 h | Yes | [18] |
| HEV-3 | A549 | Real-time RT-PCR for cellular HEV RNA | 60 °C for 72 h | No | [18] |
| HEV-3i | PLC/PRF/5, A549, HepG2 | Real-time RT-PCR, Focus-forming units (ffu) | 95 °C for 1 min | No | [54] |
| HEV-3i | PLC/PRF/5, A549, HepG2 | Real-time RT-PCR, Focus-forming units (ffu) | 90 °C for 1 min | No | [54] |
| HEV-3i | PLC/PRF/5, A549, HepG2 | Real-time RT-PCR, Focus-forming units (ffu) | 85 °C for 1 min | No | [54] |
| HEV-3i | PLC/PRF/5, A549, HepG2 | Real-time RT-PCR, Focus-forming units (ffu) | 80 °C for 1 min | No | [54] |
| HEV-3i | PLC/PRF/5, A549, HepG2 | Real-time RT-PCR, Focus-forming units (ffu) | 75 °C for 1 min | No | [54] |
| HEV-3i | PLC/PRF/5, A549, HepG2 | Real-time RT-PCR, Focus-forming units (ffu) | 70 °C for 1 min | No | [54] |
| HEV-3i | PLC/PRF/5, A549, HepG2 | Real-time RT-PCR, Focus-forming units (ffu) | No heat | No | [54] |
| HEV-3c | A549/D3 | Focus-forming units (ffu) | 37 °C for 21 days | No | [28] |
| HEV-3c | A549/D3 | Focus-forming units (ffu) | Room temperature for 28 days | No | [28] |
| HEV-3c | A549/D3 | Focus-forming units (ffu) | 4 °C for 56 days | No | [28] |
| HEV-3c | A549/D3 | Focus-forming units (ffu) | No heat for 1 min | No | [28] |
| HEV-3c | A549/D3 | Focus-forming units (ffu) | 37 °C for 1 min | No | [28] |
| HEV-3c | A549/D3 | Focus-forming units (ffu) | 50 °C for 1 min | No | [28] |
| HEV-3c | A549/D3 | Focus-forming units (ffu) | 55 °C for 1 min | No | [28] |
| HEV-3c | A549/D3 | Focus-forming units (ffu) | 60 °C for 1 min | No | [28] |
| HEV-3c | A549/D3 | Focus-forming units (ffu) | 65 °C for 1 min | No | [28] |
| HEV-3c | A549/D3 | Focus-forming units (ffu) | 70 °C for 1 min | No | [28] |
| HEV-3c | A549/D3 | Focus-forming units (ffu) | 75 °C for 1 min | No | [28] |
| HEV-3c | A549/D3 | Focus-forming units (ffu) | 80 °C for 1 min | Yes | [28] |
| HEV-3c | A549/D3 | Focus-forming units (ffu) | 85 °C for 1 min | Yes | [28] |
| HEV-3c | A549/D3 | Focus-forming units (ffu) | 90 °C for 1 min | Yes | [28] |
| HEV-3c | A549/D3 | Focus-forming units (ffu) | 70 °C for 1.5 min | No | [28] |
| HEV-3c | A549/D3 | Focus-forming units (ffu) | 70 °C for 2 min and longer | Yes | [28] |
| HEV-3k | PLC/PRF/5 | Real-time RT-PCR for HEV RNA in CM | 56 °C for 1 h | No | [55] |
| HEV-3k | PLC/PRF/5 | Real-time RT-PCR for HEV RNA in CM | 58 °C for 30 min | No | [55] |
| HEV-3k | PLC/PRF/5 | Real-time RT-PCR for HEV RNA in CM | 58 °C for 1 h | Yes | [55] |
| HEV-3k | PLC/PRF/5 | Real-time RT-PCR for HEV RNA in CM | 60 °C for 5 min | No | [55] |
| HEV-3k | PLC/PRF/5 | Real-time RT-PCR for HEV RNA in CM | 60 °C for 10 min and longer | Yes | [55] |
| HEV-3k | PLC/PRF/5 | Real-time RT-PCR for HEV RNA in CM | 62 °C for 60 min | Yes | [55] |
| HEV-3k | PLC/PRF/5 | Real-time RT-PCR for HEV RNA in CM | 63 °C for 1 min | No | [55] |
| HEV-3k | PLC/PRF/5 | Real-time RT-PCR for HEV RNA in CM | 63 °C for 5 min | No | [55] |
| HEV-3k | PLC/PRF/5 | Real-time RT-PCR for HEV RNA in CM | 63 °C for 30 min | Yes | [55] |
| HEV-3k | PLC/PRF/5 | Real-time RT-PCR for HEV RNA in CM | 65 °C for 1 min | No | [55] |
| HEV-3k | PLC/PRF/5 | Real-time RT-PCR for HEV RNA in CM | 65 °C for 5 min | Yes | [55] |
| HEV-3k | PLC/PRF/5 | Real-time RT-PCR for HEV RNA in CM | 70 °C for 1 min | Yes | [55] |
| HEV-3k | PLC/PRF/5 | Real-time RT-PCR for HEV RNA in CM | 70 °C for 5 min | Yes | [55] |
| HEV-3k | PLC/PRF/5 | Real-time RT-PCR for HEV RNA in CM | 75 °C for 1 min | Yes | [55] |
| HEV-3k | PLC/PRF/5 | Real-time RT-PCR for HEV RNA in CM | 80 °C for 1 min | Yes | [55] |
| HEV-4 | PLC/PRF/5 | Real-time RT-PCR for HEV RNA in CM | 60 °C for 1 min | No | [55] |
| HEV-4 | PLC/PRF/5 | Real-time RT-PCR for HEV RNA in CM | 63 °C for 1 min | No | [55] |
| HEV-4 | PLC/PRF/5 | Real-time RT-PCR for HEV RNA in CM | 63 °C for 5 min | No | [55] |
| HEV-4 | PLC/PRF/5 | Real-time RT-PCR for HEV RNA in CM | 63 °C for 30 min | Yes | [55] |
| HEV-4 | PLC/PRF/5 | Real-time RT-PCR for HEV RNA in CM | 65 °C for 1 min | No | [55] |
| HEV-4 | PLC/PRF/5 | Real-time RT-PCR for HEV RNA in CM | 65 °C for 5 min | Yes | [55] |
| HEV-4 | PLC/PRF/5 | Real-time RT-PCR for HEV RNA in CM | 70 °C for 1 min | No | [55] |
| HEV-4 | PLC/PRF/5 | Real-time RT-PCR for HEV RNA in CM | 70 °C for 5 min | Yes | [55] |
| HEV-4 | PLC/PRF/5 | Real-time RT-PCR for HEV RNA in CM | 75 °C for 1 min | Yes | [55] |
| HEV-4 | PLC/PRF/5 | Real-time RT-PCR for HEV RNA in CM | 80 °C for 1 min | Yes | [55] |
| HEV-3c/3e | A549/D3 | Focus-forming units (ffu) | 4 °C for 1 week | No | [56] |
| HEV-3c/3e | A549/D3 | Focus-forming units (ffu) | 10 °C for 1 week | No | [56] |
| HEV-3c/3e | A549/D3 | Focus-forming units (ffu) | 21 °C for 1 week | No | [56] |
| HEV-3c/3e | A549/D3 | Focus-forming units (ffu) | 21 °C for 2 weeks | Yes | [56] |
| HEV-3c/3e | A549/D3 | Focus-forming units (ffu) | 65 °C for 10 min | Yes | [56] |
| HEV-3c/3e | A549/D3 | Focus-forming units (ffu) | 65 °C for 20 min | Yes | [56] |
| HEV-3c/3e | A549/D3 | Focus-forming units (ffu) | 71 °C for 10 min | Yes | [56] |
| HEV-3c/3e | A549/D3 | Focus-forming units (ffu) | 71 °C for 20 min | Yes | [56] |
| HEV-3e | A549/D3 | Focus-forming units (ffu) | 80 °C for 10 min | Yes | [56] |
| HEV-3e | A549/D3 | Focus-forming units (ffu) | 80 °C for 20 min | Yes | [56] |
| HEV-3c/3e | A549 | Real-time RT-PCR for HEV RNA in CM | 4 °C for 12 weeks | No | [57] |
| HEV-3c/3e | A549 | Real-time RT-PCR for HEV RNA in CM | -20 °C for 12 weeks | No | [57] |
| HEV-3c/3e | A549 | Real-time RT-PCR for HEV RNA in CM | 56 °C for 1 h | No | [57] |
| HEV-3c/3e | A549 | Real-time RT-PCR for HEV RNA in CM | 65 °C for 1 h | No | [57] |
| HEV-3c/3e | A549 | Real-time RT-PCR for HEV RNA in CM | 72 °C for 12 min | Yes | [57] |
| HEV-3c/3e | A549 | Real-time RT-PCR for HEV RNA in CM | 72 °C for 1 h | Yes | [57] |
| HEV-3e | A549 | Real-time RT-PCR for HEV RNA in CM | 95 °C for 1 min | No | [57] |
| HEV-3c/3e | A549 | Real-time RT-PCR for HEV RNA in CM | 95 °C for 3 min | Yes | [57] |
| HEV-3c | A549 | Focus-forming units (ffu) | (Sausage) 70 °C for 21 min in water bath | No | [58] |
| HEV-3c | A549 | Focus-forming units (ffu) | (Sausage Core) 70 °C for 23 min in water bath | Yes | [58] |
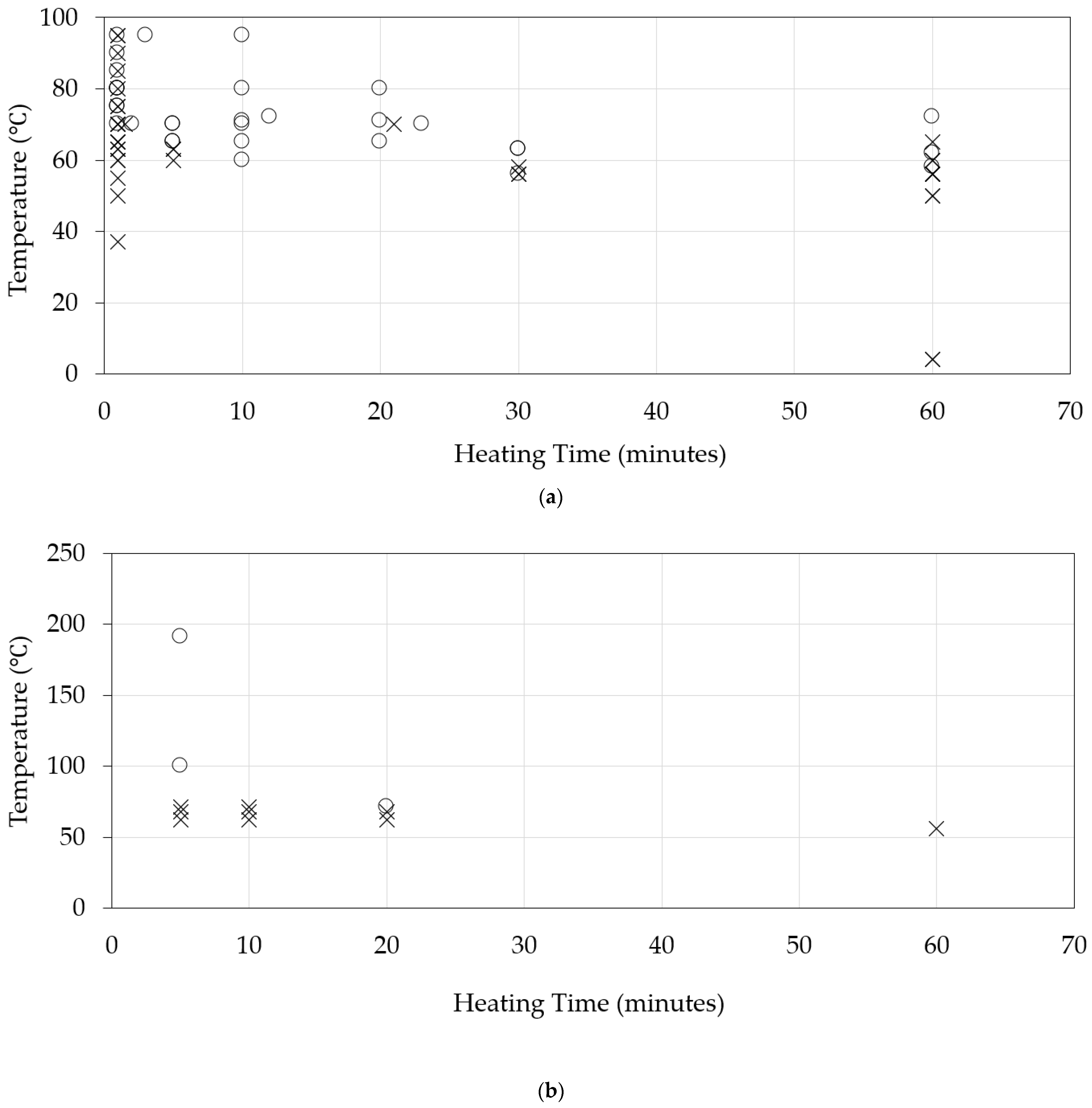
3. Thermal Stability of Hepatitis E Virus in Pig Bioassays
4. Thermal Stability of Hepatitis E Virus in Plasma Products
5. Discussion
6. Future Perspectives
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| HEV | hepatitis E virus |
| NAT | Nucleic acid testing |
| CPE | cytopathic effect |
| ffu | focus-forming units |
| GEs | genome equivalents |
| RT-PCR | Reverse transcription–polymerase chain reaction |
| TCID50 | A 50% tissue culture infective dose |
References
- Balayan, M.S.; Andjaparidze, A.G.; Savinskaya, S.S.; Ketiladze, E.S.; Braginsky, D.M.; Savinov, A.P.; Poleschuk, V.F. Evidence for a virus in non-A, non-B hepatitis transmitted via the fecal-oral route. Intervirology 1983, 20, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Reyes, G.R.; Purdy, M.A.; Kim, J.P.; Luk, K.C.; Young, L.M.; Fry, K.E.; Bradley, D.W. Isolation of a cDNA from the virus responsible for enterically transmitted non-A, non-B hepatitis. Science 1990, 247, 1335–1339. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Iwata, K.; Watanabe, N.; Hatahara, T.; Ohta, Y.; Baba, K.; Mishiro, S. Full-genome nucleotide sequence of a hepatitis E virus strain that may be indigenous to Japan. Virology 2001, 287, 9–12. [Google Scholar] [CrossRef] [PubMed]
- Kamar, N.; Selves, J.; Mansuy, J.M.; Ouezzani, L.; Péron, J.M.; Guitard, J.; Cointault, O.; Esposito, L.; Abravanel, F.; Danjoux, M.; et al. Hepatitis E virus and chronic hepatitis in organ-transplant recipients. N. Engl. J. Med. 2008, 358, 811–817. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; de Man, R.A.; Kamar, N.; Pan, Q. Chronic hepatitis E: Advancing research and patient care. J. Hepatol. 2022, 77, 1109–1123. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Beniwal, M.; Kar, P.; Sharma, J.B.; Murthy, N.S. Hepatitis E in pregnancy. Int. J. Gynaecol. Obstet. 2004, 85, 240–244. [Google Scholar] [CrossRef] [PubMed]
- Ohnishi, S.; Kang, J.H.; Maekubo, H.; Takahashi, K.; Mishiro, S. A case report: Two patients with fulminant hepatitis E in Hokkaido, Japan. Hepatol. Res. 2003, 25, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Fukae, J.; Tsugawa, J.; Ouma, S.; Umezu, T.; Kusunoki, S.; Tsuboi, Y. Guillain-Barré and Miller Fisher syndromes in patients with anti-hepatitis E virus antibody: A hospital-based survey in Japan. Neurol. Sci. 2016, 37, 1849–1851. [Google Scholar] [CrossRef] [PubMed]
- Kamar, N.; Rostaing, L.; Abravanel, F.; Garrouste, C.; Lhomme, S.; Esposito, L.; Basse, G.; Cointault, O.; Ribes, D.; Nogier, M.B.; et al. Ribavirin therapy inhibits viral replication on patients with chronic hepatitis e virus infection. Gastroenterology 2010, 139, 1612–1618. [Google Scholar] [CrossRef] [PubMed]
- Kamar, N.; Abravanel, F.; Garrouste, C.; Cardeau-Desangles, I.; Mansuy, J.M.; Weclawiak, H.; Izopet, J.; Rostaing, L. Three-month pegylated interferon-alpha-2a therapy for chronic hepatitis E virus infection in a haemodialysis patient. Nephrol. Dial. Transplant. 2010, 25, 2792–2795. [Google Scholar] [CrossRef] [PubMed]
- Kanda, T.; Li, T.C.; Takahashi, M.; Nagashima, S.; Primadharsini, P.P.; Kunita, S.; Sasaki-Tanaka, R.; Inoue, J.; Tsuchiya, A.; Nakamoto, S.; et al. Recent advances in hepatitis E virus research and the Japanese clinical practice guidelines for hepatitis E virus infection. Hepatol. Res. 2024, 54, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wu, W.; Chen, M.; Teng, Z. Prevalence of hepatitis E virus in domestic animals in the Chinese mainland: A systematic review and meta-analysis. BMC Vet. Res. 2025, 21, 136. [Google Scholar] [CrossRef] [PubMed]
- Haase, J.A.; Schlienkamp, S.; Ring, J.J.; Steinmann, E. Transmission patterns of hepatitis E virus. Curr. Opin. Virol. 2025, 70, 101451. [Google Scholar] [CrossRef] [PubMed]
- Bienz, M.; Renaud, C.; Liu, J.R.; Wong, P.; Pelletier, P. Hepatitis E Virus in the United States and Canada: Is It Time to Consider Blood Donation Screening? Transfus. Med. Rev. 2024, 38, 150835. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Hepatitis E. Available online: https://www.who.int/news-room/fact-sheets/detail/hepatitis-e (accessed on 21 March 2025).
- Flores, G.; Juárez, J.C.; Montoro, J.B.; Tusell, J.M.; Altisent, C.; Juste, C.; Jardí, R. Seroprevalence of parvovirus B19, cytomegalovirus, hepatitis A virus and hepatitis E virus antibodies in haemophiliacs treated exclusively with clotting-factor concentrates considered safe against human immunodeficiency and hepatitis C viruses. Haemophilia 1995, 1, 115–117. [Google Scholar] [CrossRef] [PubMed]
- Toyoda, H.; Honda, T.; Hayashi, K.; Katano, Y.; Goto, H.; Kumada, T.; Takahashi, K.; Abe, N.; Mishiro, S.; Takamatsu, J. Prevalence of hepatitis E virus IgG antibody in Japanese patients with hemophilia. Intervirology 2008, 51, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Yunoki, M.; Yamamoto, S.; Tanaka, H.; Nishigaki, H.; Tanaka, Y.; Nishida, A.; Adan-Kubo, J.; Tsujikawa, M.; Hattori, S.; Urayama, T.; et al. Extent of hepatitis E virus elimination is affected by stabilizers present in plasma products and pore size of nanofilters. Vox Sang. 2008, 95, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Owada, T.; Kaneko, M.; Matsumoto, C.; Sobata, R.; Igarashi, M.; Suzuki, K.; Matsubayashi, K.; Mio, K.; Uchida, S.; Satake, M.; et al. Establishment of culture systems for Genotypes 3 and 4 hepatitis E virus (HEV) obtained from human blood and application of HEV inactivation using a pathogen reduction technology system. Transfusion 2014, 54, 2820–2827. [Google Scholar] [CrossRef] [PubMed]
- Praditya, D.; Friesland, M.; Gravemann, U.; Handke, W.; Todt, D.; Behrendt, P.; Müller, T.H.; Steinmann, E.; Seltsam, A. Hepatitis E virus is effectively inactivated in platelet concentrates by ultraviolet C light. Vox Sang. 2020, 115, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Kapsch, A.M.; Farcet, M.R.; Wieser, A.; Ahmad, M.Q.; Miyabayashi, T.; Baylis, S.A.; Blümel, J.; Kreil, T.R. Antibody-enhanced hepatitis E virus nanofiltration during the manufacture of human immunoglobulin. Transfusion 2020, 60, 2500–2507. [Google Scholar] [CrossRef] [PubMed]
- Feagins, A.R.; Opriessnig, T.; Guenette, D.K.; Halbur, P.G.; Meng, X.J. Inactivation of infectious hepatitis E virus present in commercial pig livers sold in local grocery stores in the United States. Int. J. Food Microbiol. 2008, 123, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Barnaud, E.; Rogée, S.; Garry, P.; Rose, N.; Pavio, N. Thermal inactivation of infectious hepatitis E virus in experimentally contaminated food. Appl. Environ. Microbiol. 2012, 78, 5153–5159. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Lee, S.; Jung, U.S.; Lee, S.; Hong, G.P.; Choi, M.J. Effect of Ultraviolet-C (UV-C) Irradiation on Foodborne Pathogen Inactivation in Prosciutto and Changes on Its Physicochemical Properties. Food Sci. Anim. Resour. 2025, 45, 535–552. [Google Scholar] [CrossRef] [PubMed]
- Girones, R.; Carratalà, A.; Calgua, B.; Calvo, M.; Rodriguez-Manzano, J.; Emerson, S. Chlorine inactivation of hepatitis E virus and human adenovirus 2 in water. J. Water Health 2014, 12, 436–442. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Latorre, L.; Gonzales-Gustavson, E.; Hundesa, A.; Sommer, R.; Rosina, G. Thermal Stability of Hepatitis E Virus as Estimated by a Cell Culture Method. Int. J. Hyg. Environ. Health 2016, 219, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Devhare, P.B.; Chatterjee, S.N.; Arankalle, V.A.; Lole, K.S. Analysis of antiviral response in human epithelial cells infected with hepatitis E virus. PLoS ONE 2013, 8, e63793. [Google Scholar] [CrossRef] [PubMed]
- Johne, R.; Trojnar, E.; Filter, M.; Hofmann, J. Thermal Stability of Hepatitis E Virus as Estimated by a Cell Culture Method. Appl. Environ. Microbiol. 2016, 82, 4225–4231. [Google Scholar] [CrossRef] [PubMed]
- Nasheri, N.; Doctor, T.; Chen, A.; Harlow, J.; Gill, A. Evaluation of High-Pressure Processing in Inactivation of the Hepatitis E Virus. Front. Microbiol. 2020, 11, 461. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, C.; Liu, X.; Huang, X.; Lu, J.; Zhu, K.; Liao, M.; Chen, L.; Jiang, H.; Zang, X.; Wang, Y.; et al. Effectiveness of a hepatitis E vaccine against medically-attended symptomatic infection in HBsAg-positive adults from a test-negative design study. Nat. Commun. 2025, 16, 1699. [Google Scholar] [CrossRef] [PubMed]
- Baylis, S.A.; Hanschmann, K.M.; Blümel, J.; Nübling, C.M.; HEV Collaborative Study Group. Standardization of hepatitis E virus (HEV) nucleic acid amplification technique-based assays: An initial study to evaluate a panel of HEV strains and investigate laboratory performance. J. Clin. Microbiol. 2011, 49, 1234–1239. [Google Scholar] [CrossRef] [PubMed]
- Vollmer, T.; Diekmann, J.; Johne, R.; Eberhardt, M.; Knabbe, C.; Dreier, J. Novel approach for detection of hepatitis E virus infection in German blood donors. J. Clin. Microbiol. 2012, 50, 2708–2713. [Google Scholar] [CrossRef] [PubMed]
- Baylis, S.A.; Blümel, J.; Mizusawa, S.; Matsubayashi, K.; Sakata, H.; Okada, Y.; Nübling, C.M.; Hanschmann, K.M.; HEV Collaborative Study Group. World Health Organization International Standard to harmonize assays for detection of hepatitis E virus RNA. Emerg. Infect. Dis. 2013, 19, 729–735. [Google Scholar] [CrossRef] [PubMed]
- Baylis, S.A.; Terao, E.; Blümel, J.; Hanschmann, K.O. Collaborative study for the establishment of the Ph. Eur. Hepatitis E virus RNA for NAT testing biological reference preparation batch 1. Pharmeur Bio Sci. Notes. 2017, 2017, 12–28. [Google Scholar] [PubMed]
- Baylis, S.A.; Hanschmann, K.O.; Matsubayashi, K.; Sakata, H.; Roque-Afonso, A.M.; Kaiser, M.; Corman, V.M.; Kamili, S.; Aggarwal, R.; Trehanpati, N.; et al. Development of a World Health Organization International Reference Panel for different genotypes of hepatitis E virus for nucleic acid amplification testing. J. Clin. Virol. 2019, 119, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Sakata, H.; Matsubayashi, K.; Iida, J.; Nakauchi, K.; Kishimoto, S.; Sato, S.; Ikuta, K.; Satake, M.; Kino, S. Trends in hepatitis E virus infection: Analyses of the long-term screening of blood donors in Hokkaido, Japan, 2005–2019. Transfusion 2021, 61, 3390–3401. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, A.; Matsubayashi, K.; Odajima, T.; Sakata, H.; Iida, J.; Kai, K.; Goto, N.; Satake, M. Universal nucleic acid donor screening revealed epidemiological features of hepatitis E and prevented transfusion-transmitted infection in Japan. Transfusion 2024, 64, 335–347. [Google Scholar] [CrossRef] [PubMed]
- Yazaki, Y.; Mizuo, H.; Takahashi, M.; Nishizawa, T.; Sasaki, N.; Gotanda, Y.; Okamoto, H. Sporadic acute or fulminant hepatitis E in Hokkaido, Japan, may be food-borne, as suggested by the presence of hepatitis E virus in pig liver as food. J. Gen. Virol. 2003, 84, 2351–2357. [Google Scholar] [CrossRef] [PubMed]
- Mizuo, H.; Yazaki, Y.; Sugawara, K.; Tsuda, F.; Takahashi, M.; Nishizawa, T.; Okamoto, H. Possible risk factors for the transmission of hepatitis E virus and for the severe form of hepatitis E acquired locally in Hokkaido, Japan. J. Med. Virol. 2005, 76, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Okano, H.; Takahashi, M.; Isono, Y.; Tanaka, H.; Nakano, T.; Oya, Y.; Sugimoto, K.; Ito, K.; Ohmori, S.; Maegawa, T.; et al. Characterization of sporadic acute hepatitis E and comparison of hepatitis E virus genomes in acute hepatitis patients and pig liver sold as food in Mie, Japan. Hepatol. Res. 2014, 44, E63–E76. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, J.J.; Preiss, J.; Schemmerer, M.; Huber, B.; Plentz, A.; Jilg, W. Detection of hepatitis E virus (HEV) from porcine livers in Southeastern Germany and high sequence homology to human HEV isolates. J. Clin. Virol. 2011, 52, 50–54. [Google Scholar] [CrossRef] [PubMed]
- Pallerla, S.R.; Schembecker, S.; Meyer, C.G.; Linh, L.T.K.; Johne, R.; Wedemeyer, H.; Bock, C.T.; Kremsner, P.G.; Velavan, T.P. Hepatitis E virus genome detection in commercial pork livers and pork meat products in Germany. J. Viral Hepat. 2021, 28, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Di Bartolo, I.; Angeloni, G.; Ponterio, E.; Ostanello, F.; Ruggeri, F.M. Detection of hepatitis E virus in pork liver sausages. Int. J. Food Microbiol. 2015, 193, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Vergara, C.; Quintero, J.; Duarte, J.F.; Suescún, J.P.; López-Herrera, A. Detection of hepatitis E virus genome in pig livers in Antioquia, Colombia. Genet. Mol. Res. 2015, 14, 2890–2899. [Google Scholar] [CrossRef] [PubMed]
- Feagins, A.R.; Opriessnig, T.; Guenette, D.K.; Halbur, P.G.; Meng, X.J. Detection and characterization of infectious Hepatitis E virus from commercial pig livers sold in local grocery stores in the USA. J. Gen. Virol. 2007, 88, 912–917. [Google Scholar] [CrossRef] [PubMed]
- Cossaboom, C.M.; Heffron, C.L.; Cao, D.; Yugo, D.M.; Houk-Miles, A.E.; Lindsay, D.S.; Zajac, A.M.; Bertke, A.S.; Elvinger, F.; Meng, X.J. Risk factors and sources of foodborne hepatitis E virus infection in the United States. J. Med. Virol. 2016, 88, 1641–1645. [Google Scholar] [CrossRef] [PubMed]
- Bouwknegt, M.; Lodder-Verschoor, F.; van der Poel, W.H.; Rutjes, S.A.; de Roda Husman, A.M. Hepatitis E virus RNA in commercial porcine livers in The Netherlands. J. Food Prot. 2007, 70, 2889–2895. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, M.A.; Arankalle, V.A. The detection and characterization of hepatitis E virus in pig livers from retail markets of India. J. Med. Virol. 2008, 80, 1387–1390. [Google Scholar] [CrossRef] [PubMed]
- Colson, P.; Borentain, P.; Queyriaux, B.; Kaba, M.; Moal, V.; Gallian, P.; Heyries, L.; Raoult, D.; Gerolami, R. Pig liver sausage as a source of hepatitis E virus transmission to humans. J. Infect. Dis. 2010, 202, 825–834. [Google Scholar] [CrossRef] [PubMed]
- Bennett, C.; Coughlan, S.; Hunt, K.; Butler, F.; Fanning, S.; Ryan, E.; De Gascun, C.; O’Gorman, J. Detection of hepatitis E RNA in pork products at point of retail in Ireland—Are consumers at risk? Int. J. Food Microbiol. 2024, 410, 110492. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Li, D.; Wei, S.; Li, Q.; Yuan, X.; Geng, L.; Li, X.; Liu, M. Cell culture of sporadic hepatitis E virus in China. Clin. Diagn. Lab. Immunol. 1999, 6, 729–733. [Google Scholar] [CrossRef] [PubMed]
- Emerson, S.U.; Arankalle, V.A.; Purcell, R.H. Thermal stability of hepatitis E virus. J. Infect. Dis. 2005, 192, 930–933. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Takahashi, M.; Kusano, E.; Okamoto, H. Development and evaluation of an efficient cell-culture system for Hepatitis E virus. J. Gen. Virol. 2007, 88, 903–911. [Google Scholar] [CrossRef] [PubMed]
- Schielke, A.; Filter, M.; Appel, B.; Johne, R. Thermal stability of hepatitis E virus assessed by a molecular biological approach. Virol. J. 2011, 8, 487. [Google Scholar] [CrossRef] [PubMed]
- Imagawa, T.; Sugiyama, R.; Shiota, T.; Li, T.C.; Yoshizaki, S.; Wakita, T.; Ishii, K. Evaluation of Heating Conditions for Inactivation of Hepatitis E Virus Genotypes 3 and 4. J. Food Prot. 2018, 81, 947–952. [Google Scholar] [CrossRef] [PubMed]
- Stunnenberg, M.; Huizen, S.C.V.; Swart, A.; Lodder, W.J.; Boxman, I.L.A.; Rutjes, S.A. Thermal Inactivation of Hepatitis E Virus in Pork Products Estimated with a Semiquantitative Infectivity Assay. Microorganisms 2023, 11, 2451. [Google Scholar] [CrossRef] [PubMed]
- Monini, M.; Ianiro, G.; De Sabato, L.; Bivona, M.; Ostanello, F.; Di Bartolo, I. Persistence of hepatitis E virus (HEV) subtypes 3c and 3e: Long-term cold storage and heat treatments. Food Microbiol. 2024, 121, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Schilling-Loeffler, K.; Meyer, D.; Wolff, A.; Santamaría-Palacios, J.; Reich, F.; Johne, R. Determination of hepatitis E virus inactivation during manufacturing of spreadable pork liver sausage and salami-like raw pork sausage. Int. J. Food Microbiol. 2025, 429, 111018. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.B.; Simmonds, P.; Izopet, J.; Oliveira-Filho, E.F.; Ulrich, R.G.; Johne, R.; Koenig, M.; Jameel, S.; Harrison, T.J.; Meng, X.J.; et al. Proposed reference sequences for hepatitis E virus subtypes. J. Gen. Virol. 2016, 97, 537–542. [Google Scholar] [CrossRef] [PubMed]
- Randazzo, W.; Vasquez-García, A.; Aznar, R.; Sánchez, G. Viability RT-qPCR to Distinguish Between HEV and HAV With Intact and Altered Capsids. Front. Microbiol. 2018, 9, 1973. [Google Scholar] [CrossRef] [PubMed]
- Roberts, P.L.; Dunkerley, C.; McAuley, A.; Winkelman, L. Effect of manufacturing process parameters on virus inactivation by dry heat treatment at 80 degrees C in factor VIII. Vox Sang. 2007, 92, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Yunoki, M.; Tanaka, H.; Takahashi, K.; Urayama, T.; Hattori, S.; Ideno, S.; Furuki, R.; Sakai, K.; Hagiwara, K.; Ikuta, K. Hepatitis E virus derived from different sources exhibits different behaviour in virus inactivation and/or removal studies with plasma derivatives. Biologicals 2016, 44, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Farcet, M.R.; Lackner, C.; Antoine, G.; Rabel, P.O.; Wieser, A.; Flicker, A.; Unger, U.; Modrof, J.; Kreil, T.R. Hepatitis E virus and the safety of plasma products: Investigations into the reduction capacity of manufacturing processes. Transfusion 2016, 56, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Satake, M.; Matsubayashi, K.; Hoshi, Y.; Taira, R.; Furui, Y.; Kokudo, N.; Akamatsu, N.; Yoshizumi, T.; Ohkohchi, N.; Okamoto, H.; et al. Unique clinical courses of transfusion-transmitted hepatitis E in patients with immunosuppression. Transfusion 2017, 57, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Dähnert, L.; Schlosser, J.; Fast, C.; Fröhlich, A.; Gröner, A.; Lange, E.; Roth, N.J.; Schäfer, W.; Schröder, C.; Eiden, M.; et al. Hepatitis E virus: Efficacy of pasteurization of plasma-derived VWF/FVIII concentrate determined by pig bioassay. Transfusion 2021, 61, 1266–1277. [Google Scholar] [CrossRef] [PubMed]
- Gallian, P.; Pouchol, E.; Djoudi, R.; Lhomme, S.; Mouna, L.; Gross, S.; Bierling, P.; Assal, A.; Kamar, N.; Mallet, V.; et al. Transfusion-Transmitted Hepatitis E Virus Infection in France. Transfus. Med. Rev. 2019, 33, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Gallian, P.; Lhomme, S.; Morel, P.; Gross, S.; Mantovani, C.; Hauser, L.; Tinard, X.; Pouchol, E.; Djoudi, R.; Assal, A.; et al. Risk for Hepatitis E Virus Transmission by Solvent/Detergent-Treated Plasma. Emerg. Infect. Dis. 2020, 26, 2881–2886. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.H.; Tan, B.H.; Teo, E.C.; Lim, S.G.; Dan, Y.Y.; Wee, A.; Aw, P.P.; Zhu, Y.; Hibberd, M.L.; Tan, C.K.; et al. Chronic Infection with Camelid Hepatitis E Virus in a Liver Transplant Recipient Who Regularly Consumes Camel Meat and Milk. Gastroenterology 2016, 150, 355–357.e3. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Yang, F.; Xu, X.; Feng, M.; Liao, Y.; He, Z.; Takeda, N.; Muramatsu, M.; Li, Q.; Li, T.C. Immunization of human hepatitis E viruses conferred protection against challenge by a camel hepatitis E virus. Vaccine 2020, 38, 7316–7322. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, H. Culture systems for hepatitis E virus. J. Gastroenterol. 2013, 48, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Fantilli, A.; López Villa, S.D.; Zerega, A.; Di Cola, G.; López, L.; Wassaf Martínez, M.; Pisano, M.B.; Ré, V.E. Hepatitis E virus infection in a patient with alcohol related chronic liver disease: A case report of acute-on-chronic liver failure. Virol. J. 2021, 18, 245. [Google Scholar] [CrossRef] [PubMed]
- Wichmann, O.; Schimanski, S.; Koch, J.; Kohler, M.; Rothe, C.; Plentz, A.; Jilg, W.; Stark, K. Phylogenetic and case-control study on hepatitis E virus infection in Germany. J. Infect. Dis. 2008, 198, 1732–1741. [Google Scholar] [CrossRef] [PubMed]
- Sasaki-Tanaka, R.; Kanda, T.; Yokoo, T.; Abe, H.; Hayashi, K.; Sakamaki, A.; Kamimura, H.; Terai, S. Hepatitis A and E Viruses Are Important Agents of Acute Severe Hepatitis in Asia: A Narrative Review. Pathogens 2025, 14, 454. [Google Scholar] [CrossRef]
- Kabiraz, M.P.; Majumdar, P.R.; Mahmud, M.M.C.; Bhowmik, S.; Ali, A. Conventional and advanced detection techniques of foodborne pathogens: A comprehensive review. Heliyon 2023, 9, e15482. [Google Scholar] [CrossRef] [PubMed]
- Johne, R.; Scholz, J.; Falkenhagen, A. Heat stability of foodborne viruses—Findings, methodological challenges and current developments. Int. J. Food Microbiol. 2024, 413, 110582. [Google Scholar] [CrossRef] [PubMed]
- Velusamy, V.; Arshak, K.; Korostynska, O.; Oliwa, K.; Adley, C. An overview of foodborne pathogen detection: In the perspective of biosensors. Biotechnol. Adv. 2010, 28, 232–254. [Google Scholar] [CrossRef] [PubMed]
| Countries | Foodstuffs | Prevalence (%)/Total N | Year, Refs. |
|---|---|---|---|
| Japan | Raw pig liver | 7 (1.9%)/363 | 2003, [38] |
| Japan | Raw pig liver | 12 (4.9%)/243 | 2014, [40] |
| United States | Pig liver | 14 (11%)/127 | 2007, [45] |
| Netherlands | Pig liver | 4 (6.5%)62 | 2007, [47] |
| India | Pig liver | 2 (0.8%)/240 | 2008, [48] |
| Germany | Pig liver | 8 (4%)/200 | 2011, [41] |
| Germany | Pig products (liver, liver sausages, liver pate samples, etc.) | 13 (10%)/130 | 2021, [42] |
| France | Raw figatellu (a traditional pig liver sausage) | 7 (58.3%)/12 | 2010, [49] |
| Italy | Raw pig liver sausages | 10 (22.2%)/45 | 2015, [43] |
| Italy | Dry pig liver sausages | 1 (4.3%)/23 | 2015, [43] |
| Colombia | Pig liver from slaughterhouses | 62 (41.3%)/150 | 2015, [44] |
| Columbia | Pig liver from grocery stores | 25 (25%)/100 | 2015, [44] |
| Ireland | Pig products | 9 (4.8%)/188 | 2024, [50] |
| Ireland | Pig liver | 6 (24%)/25 | 2024, [50] |
| Ireland | Fermented sausage (pig) | 1 (2.0%)/49 | 2024, [50] |
| Ireland | Pig sausage (27 g) | 1 (1.5%)/65 | 2024, [50] |
| Ireland | Pig sausage (72 g) | 1 (2.0%)/49 | 2024, [50] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kanda, T.; Okamoto, H. Thermal Inactivation of Hepatitis E Virus: A Narrative Review. Viruses 2025, 17, 702. https://doi.org/10.3390/v17050702
Kanda T, Okamoto H. Thermal Inactivation of Hepatitis E Virus: A Narrative Review. Viruses. 2025; 17(5):702. https://doi.org/10.3390/v17050702
Chicago/Turabian StyleKanda, Tatsuo, and Hiroaki Okamoto. 2025. "Thermal Inactivation of Hepatitis E Virus: A Narrative Review" Viruses 17, no. 5: 702. https://doi.org/10.3390/v17050702
APA StyleKanda, T., & Okamoto, H. (2025). Thermal Inactivation of Hepatitis E Virus: A Narrative Review. Viruses, 17(5), 702. https://doi.org/10.3390/v17050702







