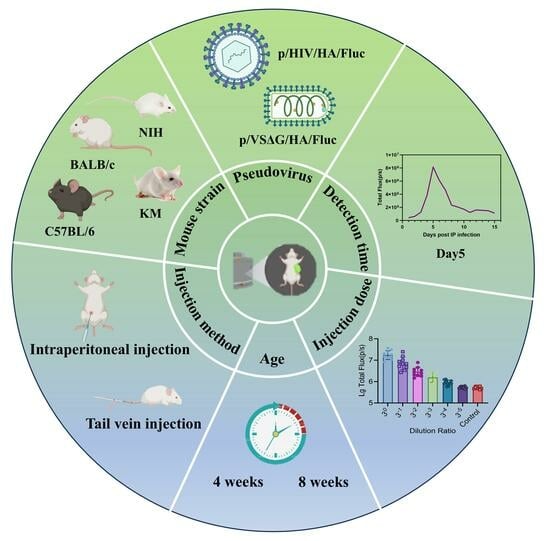
A Bioluminescent Imaging Mouse Model for Seasonal Influenza Virus Infection Based on a Pseudovirus System
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells, Plasmids, Vaccines, Strains, and Animals
2.2. Influenza Pseudovirus Packaging and Titration
2.3. Pseudovirus-Based Neutralization Assay
2.4. Influenza Vaccine Immunization in Mice
2.5. Influenza Pseudovirus and Live Virus Challenge
2.6. Bioluminescence Imaging
2.7. Passive Immunological Evaluation
2.8. Statistical Analysis of Data
3. Results
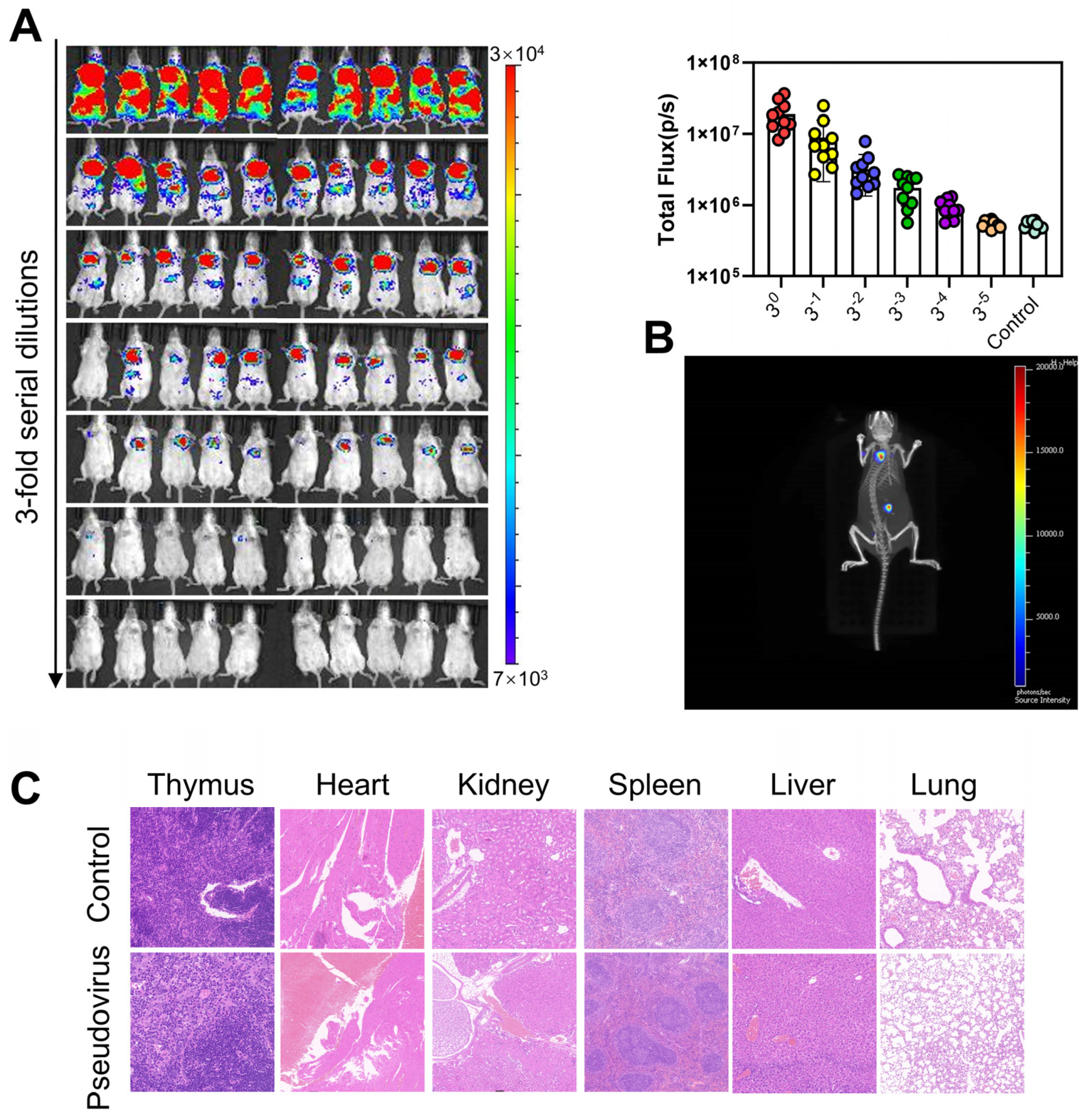
3.1. Construction and Optimization of Mouse Models for Bioluminescence Imaging
3.2. Evaluation of Vaccine Protection Using a Live Virus Infection Animal Model
3.3. Evaluation of Vaccine Protection Using a Pseudoviral Infection Animal Model
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Name | Isolate ID | Year | Egg/Cell | Abbreviation |
|---|---|---|---|---|
| A/Victoria/2570/2019 (H1N1) | EPI_ISL_417210 | 2022~2023 | egg | VI19 |
| B/Austria/1359417/2021 | EPI_ISL_1519459 | 2022~2023 | egg | BV21 |
| A/Victoria/4897/2022 (H1N1) | EPI_ISL_16714268 | 2023~2024 | egg | VI22 |
| A/Wisconsin/67/2022 (H1N1) | (EPI_ISL_15928563 | 2023~2024 | cell | WI22 |
| A/Guangdong-Maonan/SWL1536/2019 | / | 2020~2021 | egg | GD19 |
References
- Liang, Y. Pathogenicity and virulence of influenza. Virulence 2023, 14, 2223057. [Google Scholar] [CrossRef] [PubMed]
- Peteranderl, C.; Herold, S.; Schmoldt, C. Human Influenza Virus Infections. Semin. Respir. Crit. Care Med. 2016, 37, 487–500. [Google Scholar] [CrossRef] [PubMed]
- Keilman, L.J. Seasonal Influenza (Flu). Nurs. Clin. 2019, 54, 227–243. [Google Scholar] [CrossRef] [PubMed]
- Ly, H. Recent global outbreaks of highly pathogenic and low-pathogenicity avian influenza A virus infections. Virulence 2024, 15, 2383478. [Google Scholar] [CrossRef]
- Fodor, E.; Te Velthuis, A.J.W. Structure and Function of the Influenza Virus Transcription and Replication Machinery. Cold Spring Harb. Perspect. Med. 2020, 10, a038398. [Google Scholar] [CrossRef]
- Dadonaite, B.; Gilbertson, B.; Knight, M.L.; Trifkovic, S.; Rockman, S.; Laederach, A.; Brown, L.E.; Fodor, E.; Bauer, D.L.V. The structure of the influenza A virus genome. Nat. Microbiol. 2019, 4, 1781–1789. [Google Scholar] [CrossRef]
- Zhu, M.; Anirudhan, V.; Du, R.; Rong, L.; Cui, Q. Influenza virus cell entry and targeted antiviral development. J. Med. Virol. 2023, 95, e29181. [Google Scholar] [CrossRef]
- Zhu, Z.; Fodor, E.; Keown, J.R. A structural understanding of influenza virus genome replication. Trends Microbiol. 2023, 31, 308–319. [Google Scholar] [CrossRef]
- Liu, M.; Huang, L.Z.X.; Smits, A.A.; Büll, C.; Narimatsu, Y.; van Kuppeveld, F.J.M.; Clausen, H.; de Haan, C.A.M.; de Vries, E. Human-type sialic acid receptors contribute to avian influenza A virus binding and entry by hetero-multivalent interactions. Nat. Commun. 2022, 13, 4054. [Google Scholar] [CrossRef]
- Xue, K.S.; Moncla, L.H.; Bedford, T.; Bloom, J.D. Within-Host Evolution of Human Influenza Virus. Trends Microbiol. 2018, 26, 781–793. [Google Scholar] [CrossRef]
- Chauhan, R.P.; Gordon, M.L. An overview of influenza A virus genes, protein functions, and replication cycle highlighting important updates. Virus Genes 2022, 58, 255–269. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Ma, H.; Wang, X.; Bao, Z.; Tang, S.; Yi, C.; Sun, B. Broadly neutralizing antibodies to combat influenza virus infection. Antivir. Res. 2024, 221, 105785. [Google Scholar] [CrossRef] [PubMed]
- Zost, S.J.; Wu, N.C.; Hensley, S.E.; Wilson, I.A. Immunodominance and Antigenic Variation of Influenza Virus Hemagglutinin: Implications for Design of Universal Vaccine Immunogens. J. Infect. Dis. 2019, 219, S38–S45. [Google Scholar] [CrossRef] [PubMed]
- Kallewaard, N.L.; Corti, D.; Collins, P.J.; Neu, U.; McAuliffe, J.M.; Benjamin, E.; Wachter-Rosati, L.; Palmer-Hill, F.J.; Yuan, A.Q.; Walker, P.A.; et al. Structure and Function Analysis of an Antibody Recognizing All Influenza A Subtypes. Cell 2016, 166, 596–608. [Google Scholar] [CrossRef]
- Li, S.; Qiao, Y.; Xu, Y.; Li, P.; Nie, J.; Zhao, Q.; Chai, W.; Shi, Y.; Kong, W.; Shan, Y. Identification of Linear Peptide Immunogens with Verified Broad-spectrum Immunogenicity from the Conserved Regions within the Hemagglutinin Stem Domain of H1N1 Influenza Virus. Immunol. Investig. 2022, 51, 411–424. [Google Scholar] [CrossRef]
- Wu, N.C.; Wilson, I.A. Influenza Hemagglutinin Structures and Antibody Recognition. Cold Spring Harb. Perspect. Med. 2020, 10, a038778. [Google Scholar] [CrossRef]
- Kosik, I.; Angeletti, D.; Gibbs, J.S.; Angel, M.; Takeda, K.; Kosikova, M.; Nair, V.; Hickman, H.D.; Xie, H.; Brooke, C.B.; et al. Neuraminidase inhibition contributes to influenza A virus neutralization by anti-hemagglutinin stem antibodies. J. Exp. Med. 2019, 216, 304–316. [Google Scholar] [CrossRef]
- Sánchez-de Prada, L.; Iván, S.-M.; Ortiz, d.L.R.; María, E.J.; Adolfo, G.-S.; Aydillo, T. Immunodominance hierarchy after seasonal influenza vaccination. Emerg. Microbes Infect. 2022, 11, 2670–2679. [Google Scholar] [CrossRef]
- Trombetta, C.M.; Perini, D.; Mather, S.; Temperton, N.; Montomoli, E. Overview of Serological Techniques for Influenza Vaccine Evaluation: Past, Present and Future. Vaccines 2014, 2, 707–734. [Google Scholar] [CrossRef]
- Segovia, K.M.; França, M.S.; Bahnson, C.S.; Latorre-Margalef, N.; Stallknecht, D.E. Are Microneutralization and Hemagglutination Inhibition Assays Comparable? Serological Results from Influenza Experimentally Infected Mallard Ducks. Avian Dis. 2019, 63, 138–144. [Google Scholar] [CrossRef]
- Trombetta, C.M.; Remarque, E.J.; Mortier, D.; Montomoli, E. Comparison of hemagglutination inhibition, single radial hemolysis, virus neutralization assays, and ELISA to detect antibody levels against seasonal influenza viruses. Influenza Other Respir. Viruses 2018, 12, 675–686. [Google Scholar] [CrossRef] [PubMed]
- Carnell, G.W.; Trombetta, C.M.; Ferrara, F.; Montomoli, E.; Temperton, N.J. Correlation of influenza B haemagglutination inhibiton, single-radial haemolysis and pseudotype-based microneutralisation assays for immunogenicity testing of seasonal vaccines. Vaccines 2021, 9, 100. [Google Scholar] [CrossRef] [PubMed]
- Heeringa, M.; Leav, B.; Smolenov, I.; Palladino, G.; Isakov, L.; Matassa, V. Comparability of titers of antibodies against seasonal influenza virus strains as determined by hemagglutination inhibition and microneutralization assays. J. Clin. Microbiol. 2020, 58, e00750-20. [Google Scholar] [CrossRef]
- Nguyen, T.Q.; Rollon, R.; Choi, Y.K. Animal Models for Influenza Research: Strengths and Weaknesses. Viruses 2021, 13, 1011. [Google Scholar] [CrossRef]
- Kirk, N.M.; Liang, Y.; Ly, H. Comparative Pathology of Animal Models for Influenza A Virus Infection. Pathogens 2023, 13, 35. [Google Scholar] [CrossRef]
- Kimble, J.B.; Wymore Brand, M.; Kaplan, B.S.; Gauger, P.; Coyle, E.M.; Chilcote, K.; Khurana, S.; Vincent, A.L. Vaccine-Associated Enhanced Respiratory Disease following Influenza Virus Infection in Ferrets Recapitulates the Model in Pigs. J. Virol. 2022, 96, e0172521. [Google Scholar] [CrossRef]
- Roubidoux, E.K.; Schultz-Cherry, S. Animal Models Utilized for the Development of Influenza Virus Vaccines. Vaccines 2021, 9, 787. [Google Scholar] [CrossRef]
- Wyde, P.R.; Couch, R.B.; Mackler, B.F.; Cate, T.R.; Levy, B.M. Effects of low- and high-passage influenza virus infection in normal and nude mice. Infect. Immun. 1977, 15, 221–229. [Google Scholar] [CrossRef]
- Wasik, B.R.; Voorhees, I.E.H.; Barnard, K.N.; Alford-Lawrence, B.K.; Weichert, W.S.; Hood, G.; Nogales, A.; Martínez-Sobrido, L.; Holmes, E.C.; Parrish, C.R. Influenza Viruses in Mice: Deep Sequencing Analysis of Serial Passage and Effects of Sialic Acid Structural Variation. J. Virol. 2019, 93, e01039-19. [Google Scholar] [CrossRef]
- Tran, V.; Moser, L.A.; Poole, D.S.; Mehle, A. Highly Sensitive Real-Time In Vivo Imaging of an Influenza Reporter Virus Reveals Dynamics of Replication and Spread. J. Virol. 2013, 87, 13321–13329. [Google Scholar] [CrossRef]
- Nogales, A.; Ávila-Pérez, G.; Rangel-Moreno, J.; Chiem, K.; DeDiego, M.L.; Martínez-Sobrido, L. A Novel Fluorescent and Bioluminescent Bireporter Influenza A Virus To Evaluate Viral Infections. J. Virol. 2019, 93, 10-1128. [Google Scholar] [CrossRef] [PubMed]
- Czakó, R.; Vogel, L.; Lamirande, E.W.; Bock, K.W.; Moore, I.N.; Ellebedy, A.H.; Ahmed, R.; Mehle, A.; Subbarao, K. In Vivo Imaging of Influenza Virus Infection in Immunized Mice. mBio 2017, 8, 10-1128. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Bryant, H.; Fiedler, E.; Cao, T.; Rayner, J.O. Real-time tracking of bioluminescent influenza A virus infection in mice. Sci. Rep. 2022, 12, 3152. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhao, C.; Liu, Q.; Huang, W.; Wang, Y. Development and application of a bioluminescent imaging mouse model for Chikungunya virus based on pseudovirus system. Vaccine 2017, 35, 6387–6394. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Q.; Liu, Q.; Huang, W.; Nie, J.; Wang, Y. A bioluminescent imaging mouse model for Marburg virus based on a pseudovirus system. Hum. Vaccin. Immunother. 2017, 13, 1811–1817. [Google Scholar] [CrossRef]
- Chen, Q.; Tang, K.; Zhang, X.; Chen, P.; Guo, Y. Establishment of pseudovirus infection mouse models for in vivo pharmacodynamics evaluation of filovirus entry inhibitors. Acta Pharm. Sin. B 2018, 8, 200–208. [Google Scholar] [CrossRef]
- Allen, J.D.; Ross, T.M. Evaluation of Next-Generation H3 Influenza Vaccines in Ferrets Pre-Immune to Historical H3N2 Viruses. Front. Immunol. 2021, 12, 707339. [Google Scholar] [CrossRef]
- Bodewes, R.; Rimmelzwaan, G.F.; Osterhaus, A.D. Animal models for the preclinical evaluation of candidate influenza vaccines. Expert Rev. Vaccines 2010, 9, 59–72. [Google Scholar] [CrossRef]
- Al Farroukh, M.; Kiseleva, I.; Stepanova, E.; Bazhenova, E.; Krutikova, E.; Tkachev, A.; Chistyakova, A.; Rekstin, A.; Puchkova, L.; Rudenko, L. The Effect of Mice Adaptation Process on the Pathogenicity of Influenza A/South Africa/3626/2013 (H1N1)pdm09 Model Strain. Int. J. Mol. Sci. 2023, 24, 17386. [Google Scholar] [CrossRef]
- Meng, F.; Chen, C.; Wan, H.; Zhou, Q. Advances of lentiviral vectors. Zhongguo Fei Ai Za Zhi 2014, 17, 870–876. [Google Scholar] [CrossRef]
- Xiang, Q.; Li, L.; Wu, J.; Tian, M.; Fu, Y. Application of pseudovirus system in the development of vaccine, antiviral-drugs, and neutralizing antibodies. Microbiol. Res. 2022, 258, 126993. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Guerrero, A.; Cosset, F.L.; Verhoeyen, E. Lentiviral Vector Pseudotypes: Precious Tools to Improve Gene Modification of Hematopoietic Cells for Research and Gene Therapy. Viruses 2020, 12, 1016. [Google Scholar] [CrossRef] [PubMed]
- Belser, J.A.; Pulit-Penaloza, J.A.; Maines, T.R. Ferreting Out Influenza Virus Pathogenicity and Transmissibility: Past and Future Risk Assessments in the Ferret Model. Cold Spring Harb. Perspect. Med. 2020, 10, a038323. [Google Scholar] [CrossRef]
- Tseng, S.-H.; Lam, B.; Kung, Y.J.; Lin, J.; Liu, L.; Tsai, Y.C.; Ferrall, L.; Roden, R.B.S.; Wu, T.C.; Hung, C.-F. A novel pseudovirus-based mouse model of SARS-CoV-2 infection to test COVID-19 interventions. J. Biomed. Sci. 2021, 28, 34. [Google Scholar] [CrossRef]
- Tim, F.; David, F.B.; Victoria, A.M.; Paul, G.T.; Stacey, S.C. Influenza virus and SARS-CoV-2: Pathogenesis and host responses in the respiratory tract. Nat. Rev. Microbiol. 2021, 19, 425–441. [Google Scholar] [CrossRef]
- Kumar, R.; Srivastava, Y.; Muthuramalingam, P.; Singh, S.K.; Verma, G.; Tiwari, S.; Tandel, N.; Beura, S.K.; Panigrahi, A.R.; Maji, S.; et al. Understanding Mutations in Human SARS-CoV-2 Spike Glycoprotein: A Systematic Review & Meta-Analysis. Viruses 2023, 15, 856. [Google Scholar] [CrossRef]
- Rudraraju, R.; Subbarao, K. Passive immunization with influenza haemagglutinin specific monoclonal antibodies. Hum. Vaccin. Immunother. 2018, 14, 2728–2736. [Google Scholar] [CrossRef]
- Koudstaal, W.; Koldijk, M.H.; Brakenhoff, J.P.; Cornelissen, L.A.; Weverling, G.J.; Friesen, R.H.; Goudsmit, J. Pre- and postexposure use of human monoclonal antibody against H5N1 and H1N1 influenza virus in mice: Viable alternative to oseltamivir. J. Infect. Dis. 2009, 200, 1870–1873. [Google Scholar] [CrossRef][Green Version]
- Avci, P.; Karimi, M.; Sadasivam, M.; Antunes-Melo, W.C.; Carrasco, E.; Hamblin, M.R. In-vivo monitoring of infectious diseases in living animals using bioluminescence imaging. Virulence 2018, 9, 28–63. [Google Scholar] [CrossRef]
- Smith, S.; Rayner, J.O.; Kim, J.H. Fluorofurimazine, a novel NanoLuc substrate, enhances real-time tracking of influenza A virus infection without altering pathogenicity in mice. Microbiol. Spectr. 2025, 13, e0268924. [Google Scholar] [CrossRef]
- Coleman, S.M.; McGregor, A. A bright future for bioluminescent imaging in viral research. Future Virol. 2015, 10, 169–183. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Su, Y.; Lin, M.Z.; Ronald, J.A. Brightening up Biology: Advances in Luciferase Systems for in Vivo Imaging. ACS Chem. Biol. 2021, 16, 2707–2718. [Google Scholar] [CrossRef] [PubMed]
- Saito-Moriya, R.; Nakayama, J.; Kamiya, G.; Kitada, N.; Obata, R.; Maki, S.A.; Aoyama, H. How to Select Firefly Luciferin Analogues for In Vivo Imaging. Int. J. Mol. Sci. 2021, 22, 1848. [Google Scholar] [CrossRef]
- Lin, X.; Zhu, M.; Zhao, X.; Si, L.; Dong, M.; Anirudhan, V.; Cui, Q.; Rong, L.; Du, R. Optimization and applications of an in vivo bioluminescence imaging model of influenza A virus infections. Virol. Sin. 2023, 38, 631–634. [Google Scholar] [CrossRef]
- Pan, W.; Dong, J.; Chen, P.; Zhang, B.; Li, Z.; Chen, L. Development and application of bioluminescence imaging for the influenza A virus. J. Thorac. Dis. 2018, 10, S2230–S2237. [Google Scholar] [CrossRef]
- Chen, K.-Y.; Karuppusamy, J.; O’Neill, M.B.; Opuu, V.; Bahin, M.; Foulon, S.; Ibanez, P.; Quintana-Murci, L.; Ozawa, T.; van der Werf, S.; et al. High-throughput droplet-based analysis of influenza A virus genetic reassortment by single-virus RNA sequencing. Proc. Natl. Acad. Sci. USA 2023, 120, e2211098120. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Zhang, M.; An, Y.; Li, L.; Wu, H.; Cheng, Z.; Pan, L.; Yang, C.; Huang, W.; Geng, Y.; et al. A Bioluminescent Imaging Mouse Model for Seasonal Influenza Virus Infection Based on a Pseudovirus System. Viruses 2025, 17, 686. https://doi.org/10.3390/v17050686
Wang Y, Zhang M, An Y, Li L, Wu H, Cheng Z, Pan L, Yang C, Huang W, Geng Y, et al. A Bioluminescent Imaging Mouse Model for Seasonal Influenza Virus Infection Based on a Pseudovirus System. Viruses. 2025; 17(5):686. https://doi.org/10.3390/v17050686
Chicago/Turabian StyleWang, Yifei, Mengyi Zhang, Yimeng An, Lanshu Li, Hao Wu, Ziqi Cheng, Ling Pan, Chaoying Yang, Weijin Huang, Yansheng Geng, and et al. 2025. "A Bioluminescent Imaging Mouse Model for Seasonal Influenza Virus Infection Based on a Pseudovirus System" Viruses 17, no. 5: 686. https://doi.org/10.3390/v17050686
APA StyleWang, Y., Zhang, M., An, Y., Li, L., Wu, H., Cheng, Z., Pan, L., Yang, C., Huang, W., Geng, Y., & Zhao, C. (2025). A Bioluminescent Imaging Mouse Model for Seasonal Influenza Virus Infection Based on a Pseudovirus System. Viruses, 17(5), 686. https://doi.org/10.3390/v17050686