Investigation of β-Carboline Alkaloid Harmaline Against Cyvirus cyprinidallo3 Infection In Vitro and In Vivo
Abstract
1. Introduction
2. Materials and Methods
2.1. Viruses, Cells, and Chemicals
2.2. CyHV-3 Plaque Reduction Assay Following Drug Treatment
2.3. Harmaline Cytotoxicity Assay In Vitro
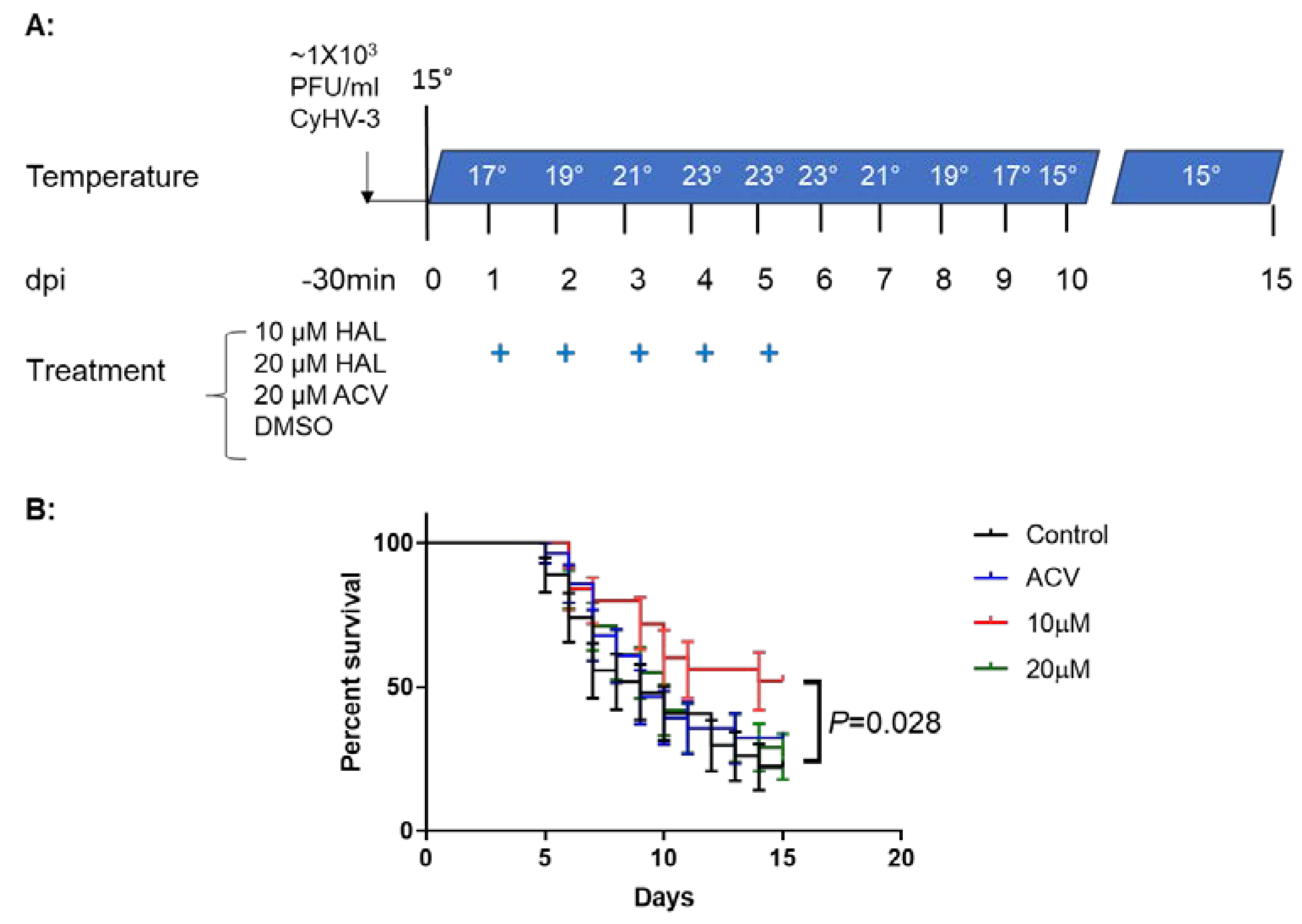
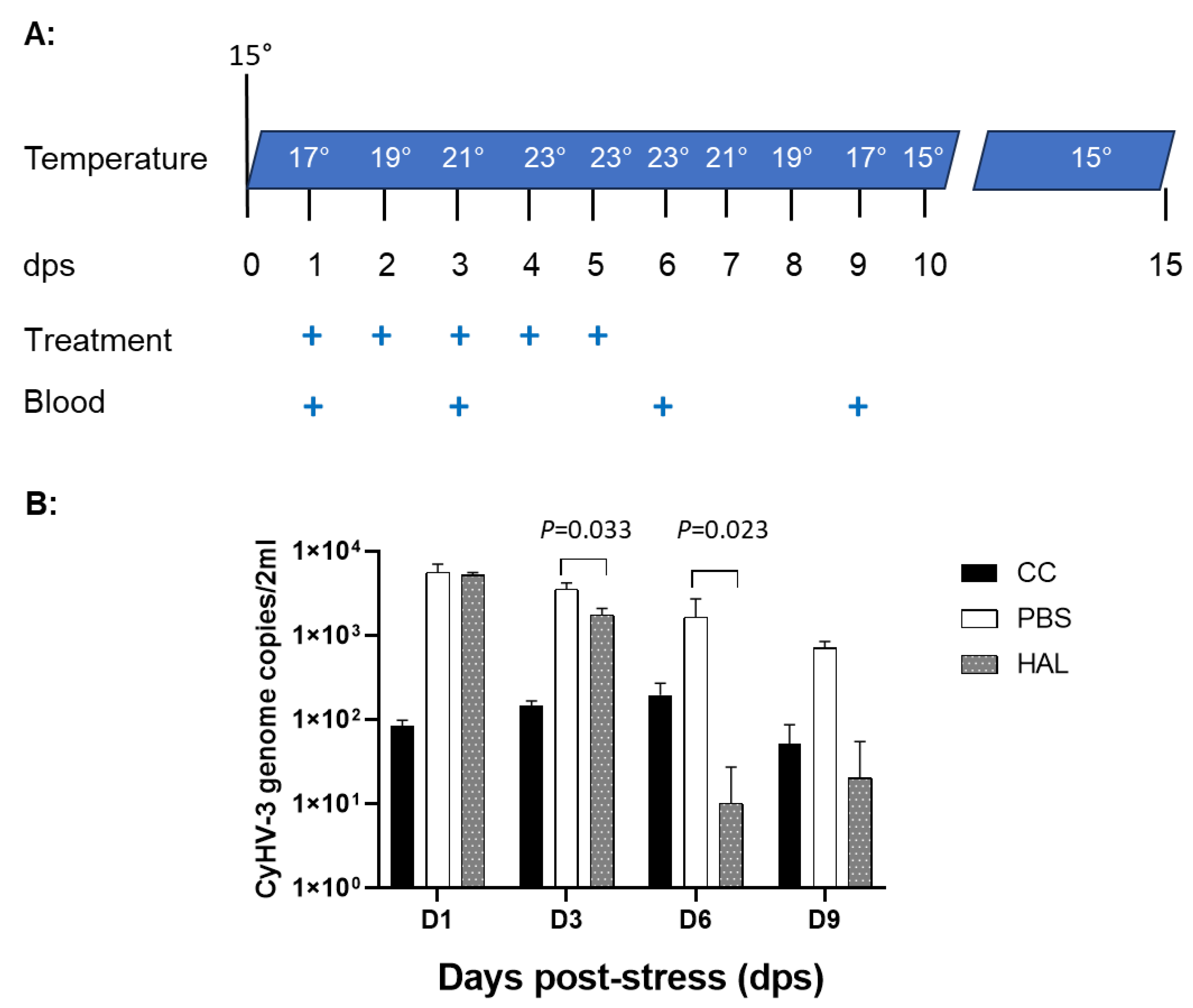
2.4. Koi Infection and Drug Treatment
2.5. CyHV-3 Reactivation and Harmaline Treatment in CyHV-3 Latently Infected Koi
2.6. Total White Blood Cell (WBC) Isolation and Total DNA Extraction
2.7. CyHV-3 DNA Real-Time PCR
2.8. Statistical Analysis
3. Results
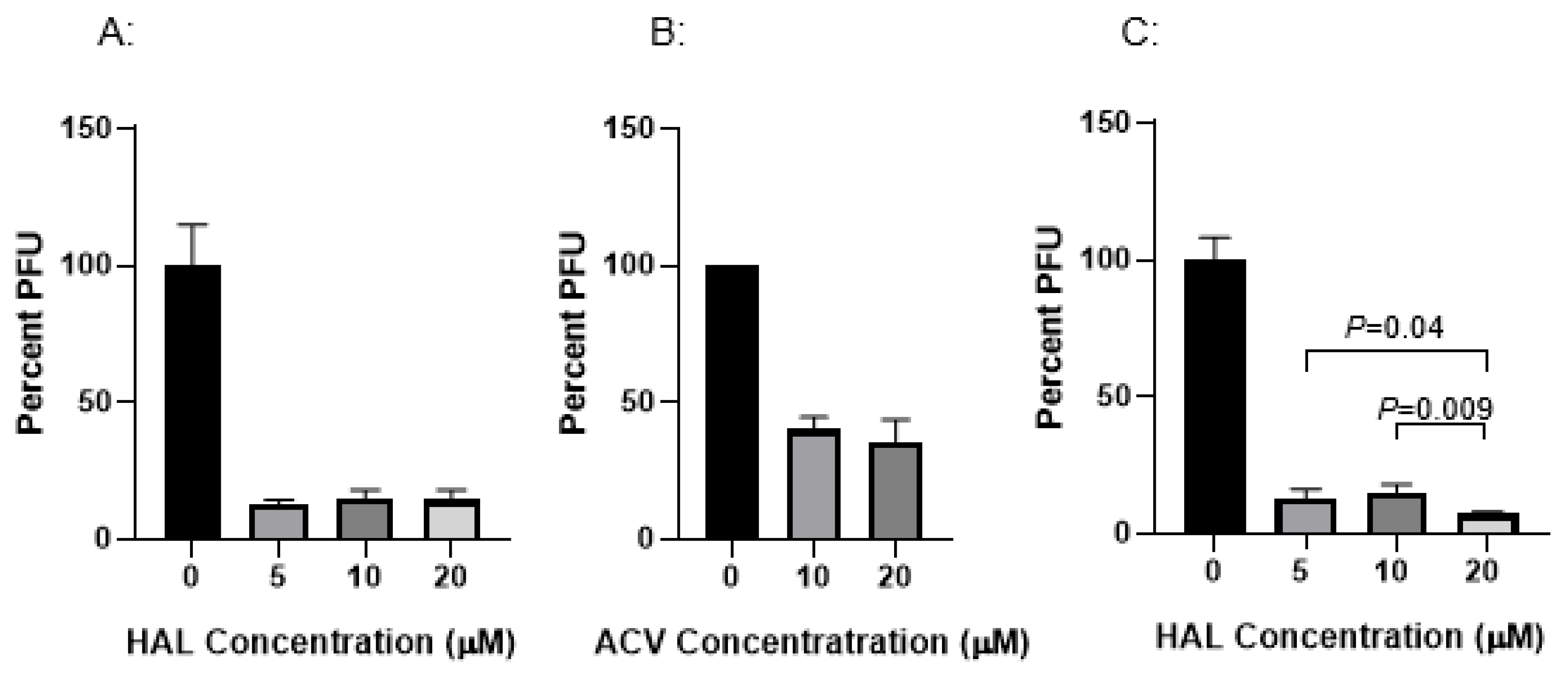
3.1. HAL Reduces CyHV-3 Replication In Vitro
3.2. HAL Treatment Effect Is Different with Treatment Time Against Different Viral Dose
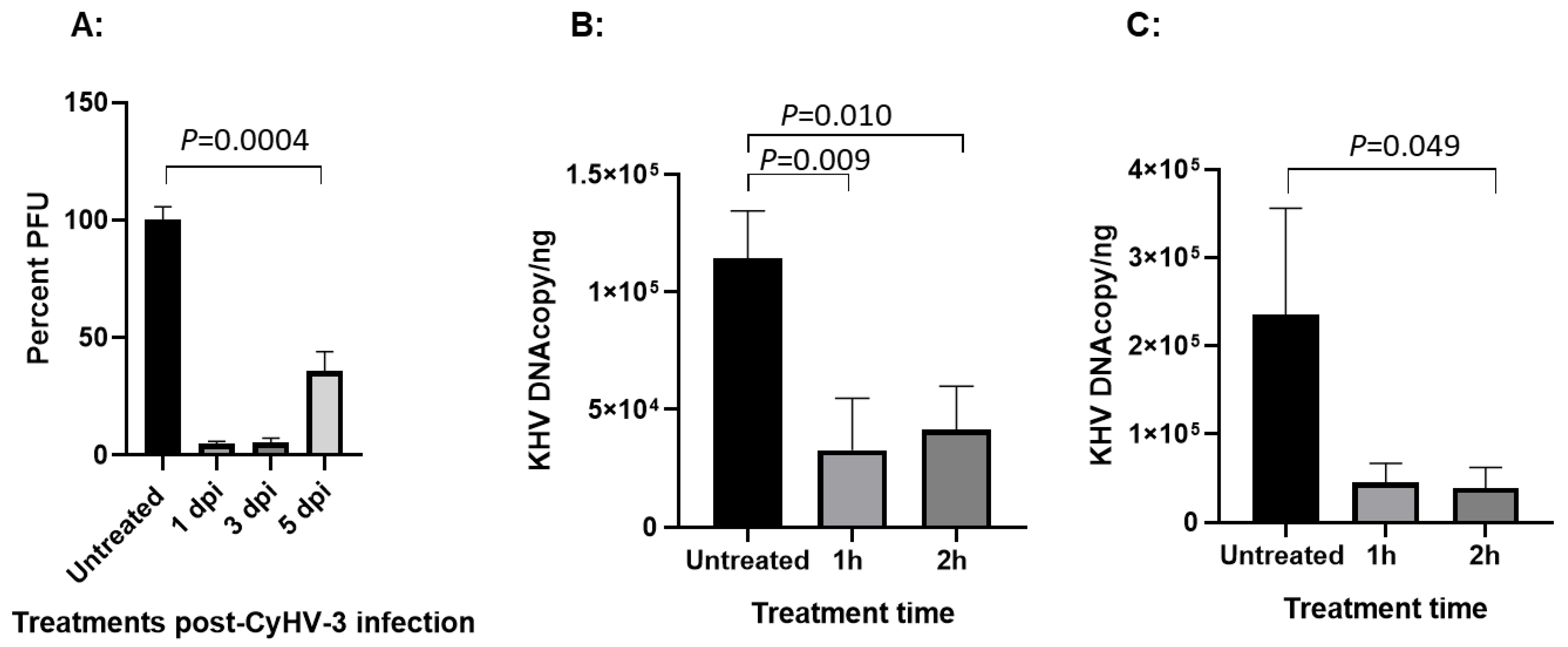
3.3. HAL Has a Treatment Effect Within the First 5 Days Post-Infection
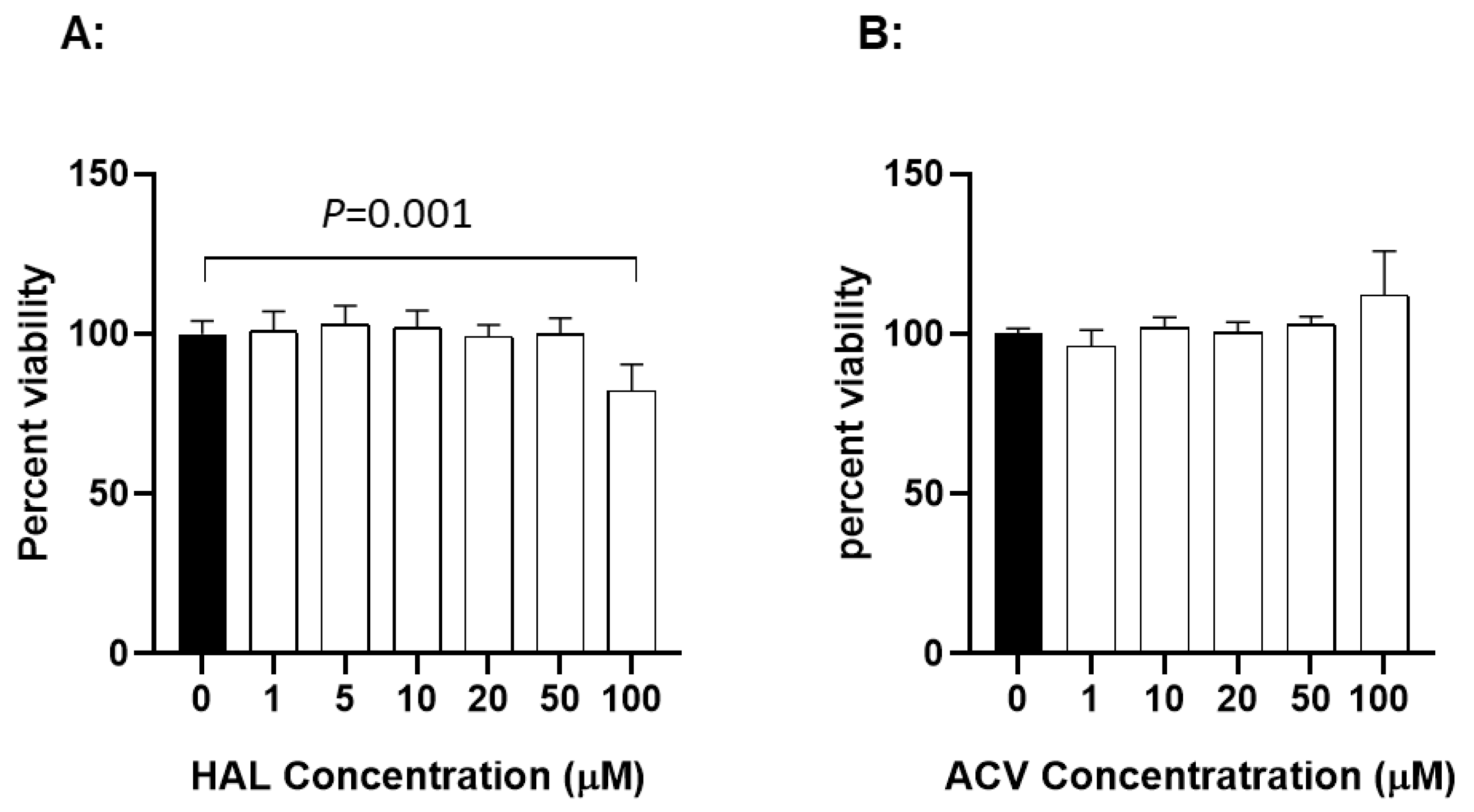
3.4. Cytotoxicity of HAL
3.5. HAL Reduced Mortality in CyHV-3 Infected Younger Koi
3.6. HAL Reduced CyHV-3 Reactivation from Temperature Stress
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gilad, O.; Yun, S.; Andree, K.B.; Adkison, M.A.; Zlotkin, A.; Bercovier, H.; Eldar, A.; Hedrick, R.P. Initial characteristics of koi herpesvirus and development of a polymerase chain reaction assay to detect the virus in koi, Cyprinus carpio koi. Dis. Aquat. Organ. 2002, 48, 101–108. [Google Scholar] [CrossRef]
- Hedrick, R.P.; Gilad, O.; Yun, S.; Spangenberg, J.V.; Marty, G.D.; Nordhausen, R.W.; Kebus, M.J.; Bercovier, H.; Eldar, A. A Herpesvirus Associated with Mass Mortality of Juvenile and Adult Koi, a Strain of Common Carp. J. Aquat. Anim. Health 2000, 12, 44–57. [Google Scholar] [CrossRef]
- Cheng, L.; Chen, C.Y.; Tsai, M.A.; Wang, P.C.; Hsu, J.P.; Chern, R.S.; Chen, S.C. Koi herpesvirus epizootic in cultured carp and koi, Cyprinus carpio L., in Taiwan. J. Fish. Dis. 2011, 34, 547–554. [Google Scholar] [CrossRef]
- Gilad, O.; Yun, S.; Zagmutt-Vergara, F.J.; Leutenegger, C.M.; Bercovier, H.; Hedrick, R.P. Concentrations of a Koi herpesvirus (KHV) in tissues of experimentally infected Cyprinus carpio koi as assessed by real-time TaqMan PCR. Dis. Aquat. Organ. 2004, 60, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Eide, K.; Miller-Morgan, T.; Heidel, J.; Bildfell, R.; Jin, L. Results of total DNA measurement in koi tissue by Koi Herpes Virus real-time PCR. J. Virol. Methods 2011, 172, 81–84. [Google Scholar] [CrossRef] [PubMed]
- Waltzek, T.B.; Kelley, G.O.; Stone, D.M.; Way, K.; Hanson, L.; Fukuda, H.; Hirono, I.; Aoki, T.; Davison, A.J.; Hedrick, R.P. Koi herpesvirus represents a third cyprinid herpesvirus (CyHV-3) in the family Herpesviridae. J. Gen. Virol. 2005, 86 Pt 6, 1659–1667. [Google Scholar] [CrossRef] [PubMed]
- St-Hilaire, S.; Beevers, N.; Joiner, C.; Hedrick, R.P.; Way, K. Antibody response of two populations of common carp, Cyprinus carpio L., exposed to koi herpesvirus. J. Fish. Dis. 2009, 32, 311–320. [Google Scholar] [CrossRef]
- Eide, K.E.; Miller-Morgan, T.; Heidel, J.R.; Kent, M.L.; Bildfell, R.J.; Lapatra, S.; Watson, G.; Jin, L. Investigation of koi herpesvirus latency in koi. J. Virol. 2011, 85, 4954–4962. [Google Scholar] [CrossRef]
- Xu, J.R.; Bently, J.; Beck, L.; Reed, A.; Miller-Morgan, T.; Heidel, J.R.; Kent, M.L.; Rockey, D.D.; Jin, L. Analysis of koi herpesvirus latency in wild common carp and ornamental koi in Oregon, USA. J. Virol. Methods 2013, 187, 372–379. [Google Scholar] [CrossRef]
- Amin, M.; Adrianti, D.N.; Lasmika, N.L.A.; Ali, M. Detection of koi herpesvirus in healthy common carps, Cyprinus carpio L. Virus Dis. 2018, 29, 445–452. [Google Scholar] [CrossRef]
- Lin, L.; Chen, S.; Russell, D.S.; Lohr, C.V.; Milston-Clements, R.; Song, T.; Miller-Morgan, T.; Jin, L. Analysis of stress factors associated with KHV reactivation and pathological effects from KHV reactivation. Virus Res. 2017, 240, 200–206. [Google Scholar] [CrossRef]
- Li, S.; Teng, L.; Liu, W.; Cheng, X.; Jiang, B.; Wang, Z.; Wang, C. Interspecies metabolic diversity of harmaline and harmine in in vitro 11 mammalian liver microsomes. Drug Test. Anal. 2017, 9, 754–768. [Google Scholar] [CrossRef] [PubMed]
- Li, S.P.; Wang, Y.W.; Qi, S.L.; Zhang, Y.P.; Deng, G.; Ding, W.Z.; Ma, C.; Lin, Q.Y.; Guan, H.D.; Liu, W.; et al. Analogous beta-Carboline Monoamine Oxidase-A Inhibition and Associated Antioxidant Activity in Plant Extracts with Potential Antidepressant Actiosfunction by Attenuating Acetylcholinesterase Activity, Oxidative Stress, and Inflammation in Mice. Front. Pharmacol. 2018, 9, 346. [Google Scholar] [CrossRef]
- Herraiz, T.; Guillen, H. Monoamine Oxidase-A Inhibition and Associated Antioxidant Activity in Plant Extracts with Potential Antidepressant Actions. BioMed Res. Int. 2018, 2018, 4810394. [Google Scholar] [CrossRef]
- Gonzalez, M.M.; Vizoso-Pinto, M.G.; Erra-Balsells, R.; Gensch, T.; Cabrerizo, F.M. In Vitro Effect of 9,9′-Norharmane Dimer against Herpes Simplex Viruses. Int. J. Mol. Sci. 2024, 25, 4966. [Google Scholar] [CrossRef]
- Kobayashi, J.H.; Harbour, G.C.; Gilmore, J.; Rinehart, K.L., Jr. Eudistomins A, D, G, H, I, J, M, N, O, P, and Q, bromo, hydroxy, pyrrolyl and iminoazepino. beta.-carbolines from the antiviral Caribbean tunicate Eudistoma olivaceum. J. Am. Chem. Soc. 1984, 106, 1526–1528. [Google Scholar] [CrossRef]
- Wang, C.; Wang, T.; Hu, R.; Duan, L.; Hou, Q.; Han, Y.; Dai, J.; Wang, W.; Ren, S.; Liu, H.; et al. 9-Butyl-Harmol Exerts Antiviral Activity against Newcastle Disease Virus through Targeting GSK-3beta and HSP90beta. J. Virol. 2023, 97, e0198422. [Google Scholar] [CrossRef]
- Quintana, V.M.; Piccini, L.E.; Panozzo Zenere, J.D.; Damonte, E.B.; Ponce, M.A.; Castilla, V. Antiviral activity of natural and synthetic beta-carbolines against dengue virus. Antivir. Res. 2016, 134, 26–33. [Google Scholar] [CrossRef]
- Chen, D.; Su, A.; Fu, Y.; Wang, X.; Lv, X.; Xu, W.; Xu, S.; Wang, H.; Wu, Z. Harmine blocks herpes simplex virus infection through downregulating cellular NF-kappaB and MAPK pathways induced by oxidative stress. Antivir. Res. 2015, 123, 27–38. [Google Scholar] [CrossRef]
- Chen, D.; Tian, X.; Zou, X.; Xu, S.; Wang, H.; Zheng, N.; Wu, Z. Harmine, a small molecule derived from natural sources, inhibits enterovirus 71 replication by targeting NF-kappaB pathway. Int. Immunopharmacol. 2018, 60, 111–120. [Google Scholar] [CrossRef]
- Dahal, S.; Clayton, K.; Cabral, T.; Cheng, R.; Jahanshahi, S.; Ahmed, C.; Koirala, A.; Villasmil Ocando, A.; Malty, R.; Been, T.; et al. On a path toward a broad-spectrum anti-viral: Inhibition of HIV-1 and coronavirus replication by SR kinase inhibitor harmine. J. Virol. 2023, 97, e0039623. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, L.K.; Smyth, G.K.; Greenfield, P.F. Accuracy of the endpoint assay for virus titration. Cytotechnology 1992, 8, 231–236. [Google Scholar] [CrossRef]
- St-Hilaire, S.; Beevers, N.; Way, K.; Le Deuff, R.M.; Martin, P.; Joiner, C. Reactivation of koi herpesvirus infections in common carp Cyprinus carpio. Dis. Aquat. Organ. 2005, 67, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Reed, A.N.; Izume, S.; Dolan, B.P.; LaPatra, S.; Kent, M.; Dong, J.; Jin, L. Identification of B cells as a major site for cyprinid herpesvirus 3 latency. J. Virol. 2014, 88, 9297–9309. [Google Scholar] [CrossRef] [PubMed]
- Yuasa, K. Effect of Water Temperature onMortality and Virus Sheddingin Carp ExperimentallyInfected with KoiHerpesvirus. Jpn. Soc. Fish Pathol. 2008, 43, 83–85. [Google Scholar] [CrossRef]
- Matsuoka, S.; Petri, G.; Larson, K.; Behnke, A.; Wang, X.; Peng, M.; Spagnoli, S.; Lohr, C.; Milston-Clements, R.; Divilov, K.; et al. Evaluation of Histone Demethylase Inhibitor ML324 and Acyclovir against Cyprinid herpesvirus 3 Infection. Viruses 2023, 15, 163. [Google Scholar] [CrossRef]
- Choi, D.L.; Sohn, S.G.; Bang, J.D.; Do, J.W.; Park, M.S. Ultrastructural identification of a herpes-like virus infection in common carp Cyprinus carpio in Korea. Dis. Aquat. Organ. 2004, 61, 165–168. [Google Scholar] [CrossRef]
- Minamoto, T.; Honjo, M.N.; Uchii, K.; Yamanaka, H.; Suzuki, A.A.; Kohmatsu, Y.; Iida, T.; Kawabata, Z. Detection of cyprinid herpesvirus 3 DNA in river water during and after an outbreak. Vet. Microbiol. 2009, 135, 261–266. [Google Scholar] [CrossRef]
- Minamoto, T.; Honjo, M.N.; Kawabata, Z. Seasonal distribution of cyprinid herpesvirus 3 in Lake Biwa, Japan. Appl. Environ. Microbiol. 2009, 75, 6900–6904. [Google Scholar] [CrossRef]
- Boutier, M.; Gao, Y.; Donohoe, O.; Vanderplasschen, A. Current knowledge and future prospects of vaccines against cyprinid herpesvirus 3 (CyHV-3). Fish Shellfish Immunol. 2019, 93, 531–541. [Google Scholar] [CrossRef]
- Perelberg, A.; Ilouze, M.; Kotler, M.; Steinitz, M. Antibody response and resistance of Cyprinus carpio immunized with cyprinid herpes virus 3 (CyHV-3). Vaccine 2008, 26, 3750–3756. [Google Scholar] [CrossRef] [PubMed]
- Embregts, C.W.E.; Tadmor-Levi, R.; Vesely, T.; Pokorova, D.; David, L.; Wiegertjes, G.F.; Forlenza, M. Intra-muscular and oral vaccination using a Koi Herpesvirus ORF25 DNA vaccine does not confer protection in common carp (Cyprinus carpio L.). Fish. Shellfish Immunol. 2019, 85, 90–98. [Google Scholar] [CrossRef]
- Quijano Carde, E.M.; Yazdi, Z.; Yun, S.; Hu, R.; Knych, H.; Imai, D.M.; Soto, E. Pharmacokinetic and Efficacy Study of Acyclovir Against Cyprinid Herpesvirus 3 in Cyprinus carpio. Front. Vet. Sci. 2020, 7, 587952. [Google Scholar] [CrossRef] [PubMed]
- Troszok, A.; Kolek, L.; Szczygiel, J.; Wawrzeczko, J.; Borzym, E.; Reichert, M.; Kaminska, T.; Ostrowski, T.; Jurecka, P.; Adamek, M.; et al. Acyclovir inhibits Cyprinid herpesvirus 3 multiplication in vitro. J. Fish. Dis. 2018, 41, 1709–1718. [Google Scholar] [CrossRef]
- Troszok, A.; Kolek, L.; Szczygiel, J.; Ostrowski, T.; Adamek, M.; Irnazarow, I. Anti-CyHV-3 Effect of Fluorescent, Tricyclic Derivative of Acyclovir 6-(4-MeOPh)-TACV in vitro. J. Vet. Res. 2019, 63, 513–518. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, D.; Yu, S. Pharmacological effects of harmine and its derivatives: A review. Arch. Pharmacal Res. 2020, 43, 1259–1275. [Google Scholar] [CrossRef]
- Hegazy, A.; Mahmoud, S.H.; Elshaier, Y.; Shama, N.M.A.; Nasr, N.F.; Ali, M.A.; El-Shazly, A.M.; Mostafa, I.; Mostafa, A. Antiviral activities of plant-derived indole and beta-carboline alkaloids against human and avian influenza viruses. Sci. Rep. 2023, 13, 1612. [Google Scholar] [CrossRef]
- Benzekri, R.; Bouslama, L.; Papetti, A.; Hammami, M.; Smaoui, A.; Limam, F. Anti HSV-2 activity of Peganum harmala (L.) and isolation of the active compound. Microb. Pathog. 2018, 114, 291–298. [Google Scholar] [CrossRef]
- Hudson, J.B.; Graham, E.A.; Towers, G.H. Antiviral effect of harmine, a photoactive beta-carboline alkaloid. Photochem. Photobiol. 1986, 43, 21–26. [Google Scholar] [CrossRef]
- Kavouras, J.H.; Prandovszky, E.; Valyi-Nagy, K.; Kovacs, S.K.; Tiwari, V.; Kovacs, M.; Shukla, D.; Valyi-Nagy, T. Herpes simplex virus type 1 infection induces oxidative stress and the release of bioactive lipid peroxidation by-products in mouse P19N neural cell cultures. J. Neurovirol. 2007, 13, 416–425. [Google Scholar] [CrossRef]
- Zhu, L.; Yuan, C.; Zhang, D.; Ma, Y.; Ding, X.; Zhu, G. BHV-1 induced oxidative stress contributes to mitochondrial dysfunction in MDBK cells. Vet. Res. 2016, 47, 47. [Google Scholar] [CrossRef] [PubMed]
- Arima, K.; Kakinuma, A.; Tamura, G. Surfactin, a crystalline peptidelipid surfactant produced by Bacillus subtilis: Isolation, characterization and its inhibition of fibrin clot formation. Biochem. Biophys. Res. Commun. 1968, 31, 488–494. [Google Scholar] [CrossRef] [PubMed]
- Gain, C.; Song, S.; Angtuaco, T.; Satta, S.; Kelesidis, T. The role of oxidative stress in the pathogenesis of infections with coronaviruses. Front. Microbiol. 2022, 13, 1111930. [Google Scholar] [CrossRef] [PubMed]
- Sutter, J.; Bruggeman, P.J.; Wigdahl, B.; Krebs, F.C.; Miller, V. Manipulation of Oxidative Stress Responses by Non-Thermal Plasma to Treat Herpes Simplex Virus Type 1 Infection and Disease. Int. J. Mol. Sci. 2023, 24, 4673. [Google Scholar] [CrossRef]
- Georgiadis, M.P.; Hedrick, R.P.; Johnson, W.O.; Yun, S.; Gardner, I.A. Risk factors for outbreaks of disease attributable to white sturgeon iridovirus and white sturgeon herpesvirus-2 at a commercial sturgeon farm. Am. J. Vet. Res. 2000, 61, 1232–1240. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manes, C.; Larson, K.; Matsuoka, S.; Wang, X.; Milston-Clements, R.; Jin, L. Investigation of β-Carboline Alkaloid Harmaline Against Cyvirus cyprinidallo3 Infection In Vitro and In Vivo. Viruses 2025, 17, 687. https://doi.org/10.3390/v17050687
Manes C, Larson K, Matsuoka S, Wang X, Milston-Clements R, Jin L. Investigation of β-Carboline Alkaloid Harmaline Against Cyvirus cyprinidallo3 Infection In Vitro and In Vivo. Viruses. 2025; 17(5):687. https://doi.org/10.3390/v17050687
Chicago/Turabian StyleManes, Clement, Kristen Larson, Shelby Matsuoka, Xisheng Wang, Ruth Milston-Clements, and Ling Jin. 2025. "Investigation of β-Carboline Alkaloid Harmaline Against Cyvirus cyprinidallo3 Infection In Vitro and In Vivo" Viruses 17, no. 5: 687. https://doi.org/10.3390/v17050687
APA StyleManes, C., Larson, K., Matsuoka, S., Wang, X., Milston-Clements, R., & Jin, L. (2025). Investigation of β-Carboline Alkaloid Harmaline Against Cyvirus cyprinidallo3 Infection In Vitro and In Vivo. Viruses, 17(5), 687. https://doi.org/10.3390/v17050687







