Australian Cool-Season Pulse Seed-Borne Virus Research: 2. Bean Yellow Mosaic Virus
Abstract
1. Introduction
2. Background Information
3. BYMV Symptom and Sequence Variants
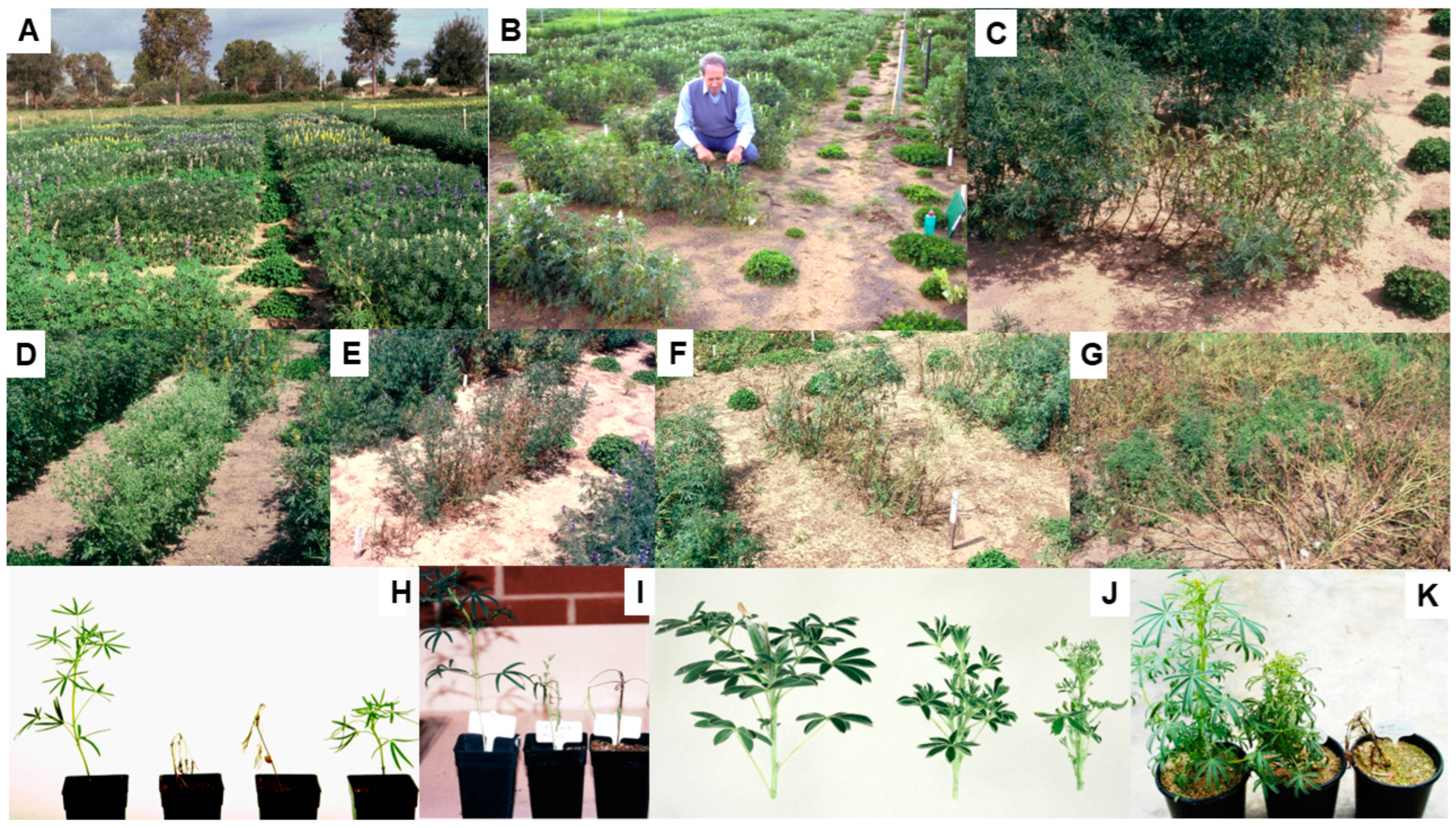
4. Alternative Hosts
5. Lupins
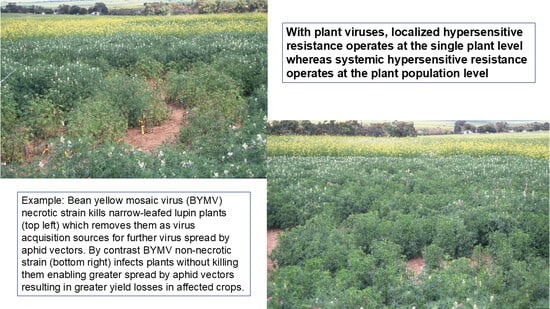
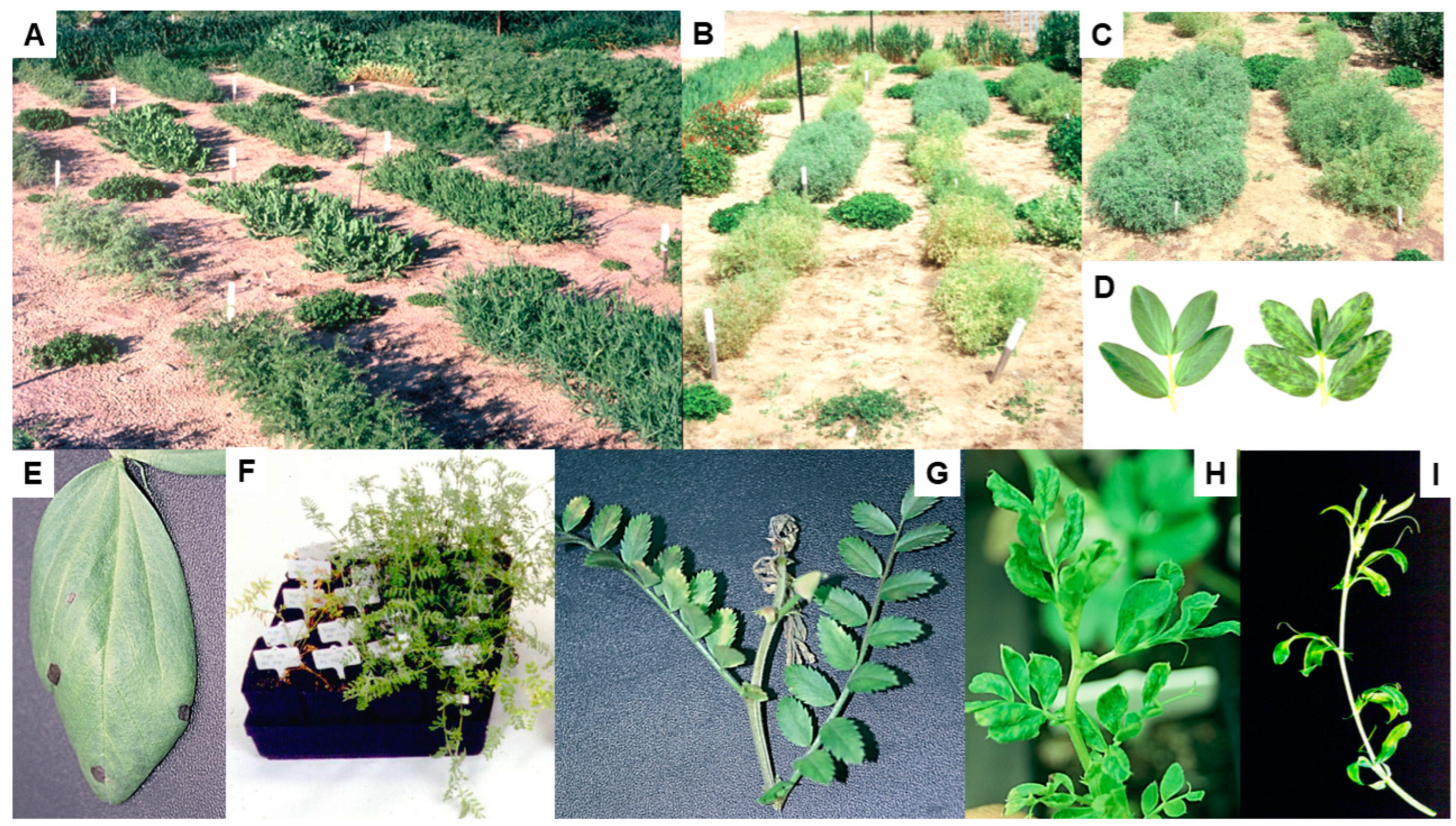
5.1. Necrotic and Non-Necrotic Strains
5.2. Black Pod Syndrome
5.3. Cultural Control
5.4. Host Resistance
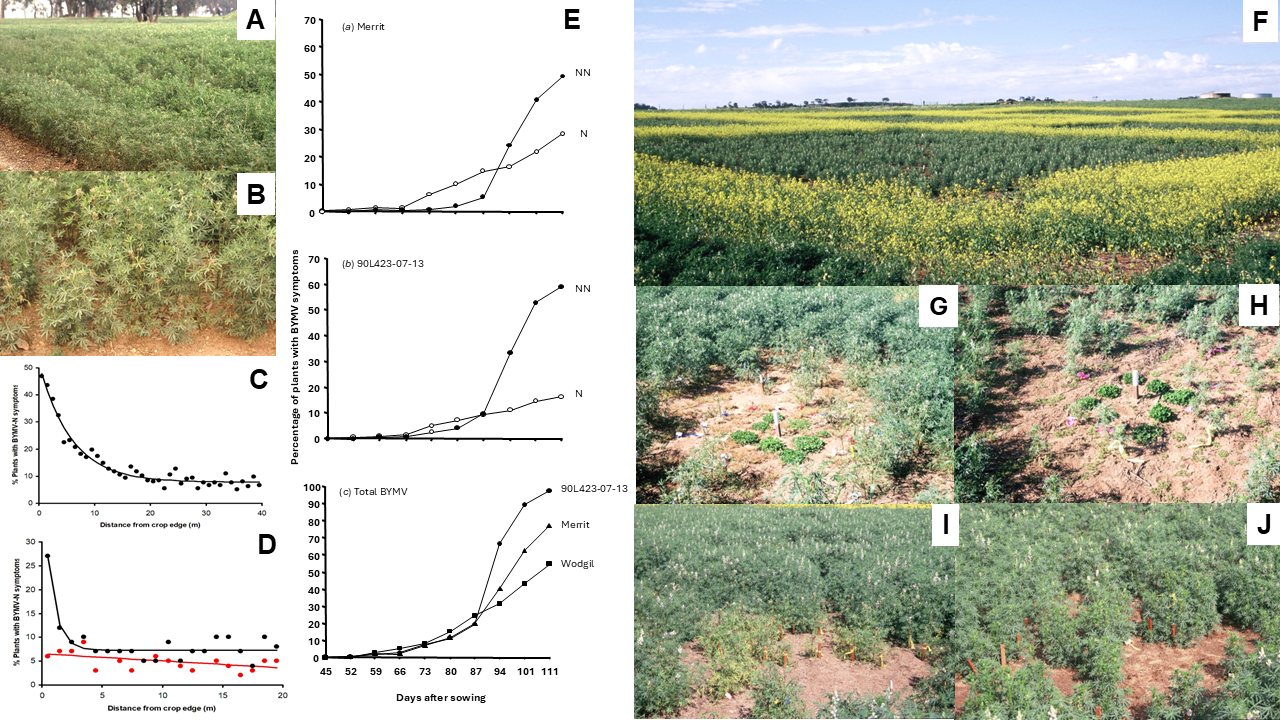
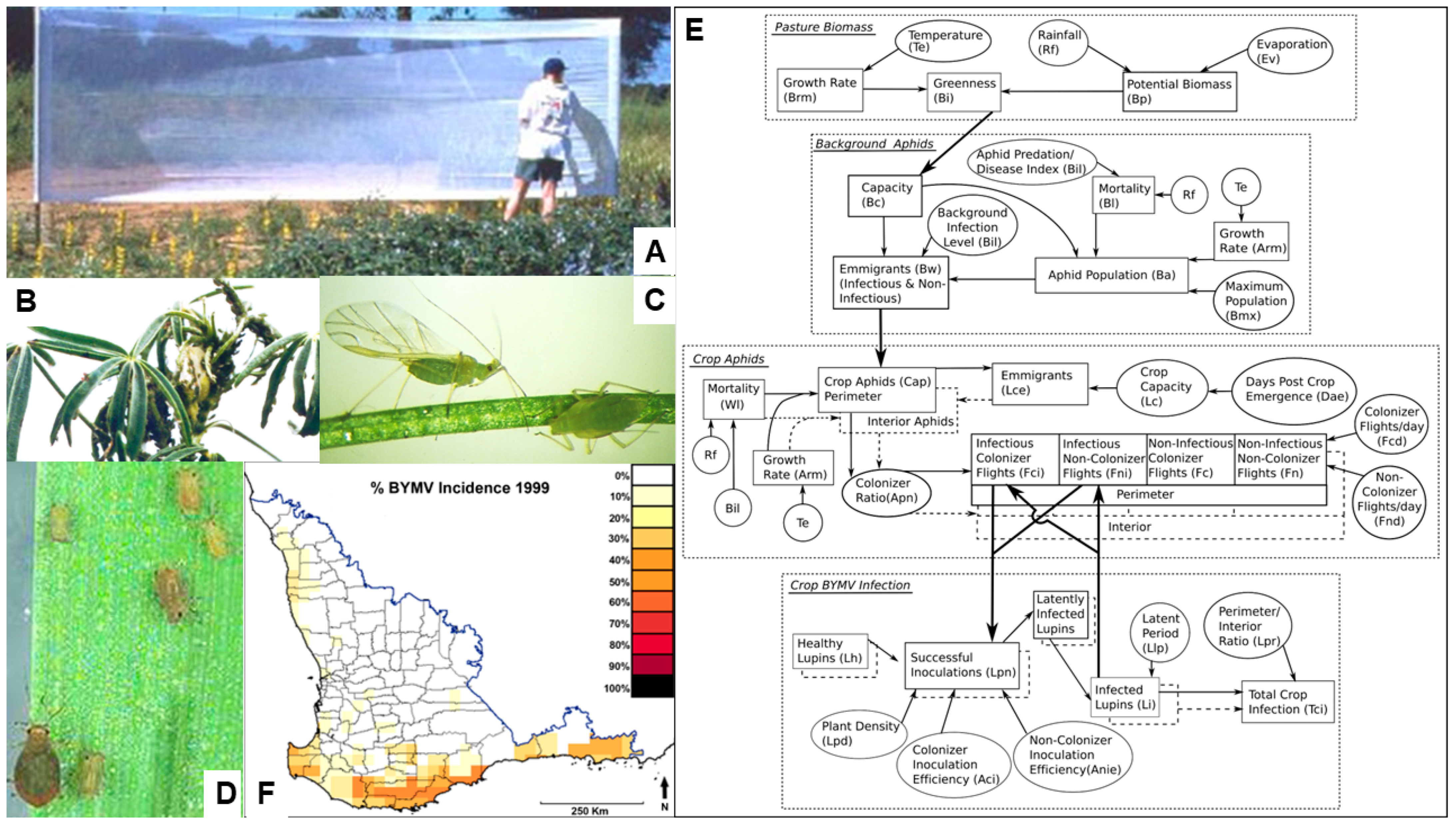
5.5. Temporal and Spatial Patterns of Spread
5.6. Epidemic Drivers and Forecasting
5.7. Integrated Disease Management
- Sow a perimeter non-host barrier crop between the adjacent subterranean clover pasture and the lupin crop to provide a ‘virus cleansing’ barrier that diminishes BYMV spread from external sources into the crop (cultural).
- Avoid fields with a large perimeter: area ratios adjacent to pastures to diminish BYMV spread from external sources into the crop (phytosanitary).
- Sow seeds at high seeding rates to generate high plant densities and early canopy closure to (i) shade out early–current season infected plants, thereby minimizing the early internal secondary infection source for subsequent BYMV spread by aphid vectors, and (ii) diminish aphid landing rates, thereby further diminishing BYMV spread (cultural).
- Sow seeds at narrow row spacing to generate early canopy cover, thereby diminishing aphid landing rates and the extent of BYMV spread before canopy closure (cultural).
- Sow early maturing cultivars to diminish late BYMV spread by vector aphids, especially in prolonged growing seasons (cultural).
- Maximize stubble groundcover using minimum tillage procedures that minimize soil cultivation to diminish aphid landing rates, thereby reducing BYMV spread before canopy closure (cultural).
- Wherever possible, ensure isolation from neighboring subterranean clover pastures to avoid ingress of BYMV from vector aphids flying from external pasture sources (phytosanitary).
- Ensure isolation from neighboring pulse (including lupin) crops to avoid any ingress of BYMV from vector aphids flying from external crop sources (phytosanitary).
- Maximize weed control using selective herbicide to minimize potential clover or other weed infection sources of BYMV within the lupin crop (chemical).
- Spray adjacent pasture with pyrethroid insecticide to suppress both aphid vectors directly and BYMV spread indirectly within the main BYMV source for spread to lupins (chemical)*.
- Mixed cropping with a non-host (e.g., cereal) to diminish BYMV spread to lupins grown for hay production (cultural).
5.8. Main Research Achievements
- The foliage symptom types and extent of yield losses that BYMV infection causes in different lupin crop species.
- Subterranean clover pasture is the most important external source for BYMV spread to lupin crops, although other alternative BYMV host species can sometimes also act in this way.
- The lupin-colonizing species Myzus persicae and Acyrthosiphon kondoi and non-lupin-colonizing species R. padi are the most important BYMV vectors in lupin crops, but A. craccivora, L. pseudobrassicae, R. maidis, R. padi, T. trifolii and Sitobion miscanthi can sometimes also paly significant roles as its vectors.
- The widespread occurrence of necrotic and non-necrotic BYMV strains in narrow-leafed lupin, the seed yield losses they cause and their lack of seed-to-seedling transmission despite BYMV being seed-borne in yellow and white lupins.
- Late infection (i.e., after first flowering) with BYMV necrotic strain causes BPS in narrow-leafed lupin.
- Reflective mulch reduces BYMV spread to narrow-leafed lupin breeder’s single-row plots.
- Non-host borders around crop perimeters provide a ‘virus-cleansing’ barrier which diminishes the BYMV spread from external virus sources into lupin crops. Similarly, growing a non-host crop in a mixture with lupin decreases the BYMV spread to the lupin plants.
- Cereal straw spread on the soil surface to simulate stubble retention and sowing the seed at narrow row spacing using high seeding rates to achieve early canopy closure both diminish aphid vector landings and the consequent BYMV spread, with the straw acting before canopy closure takes over.
- Two independently inherited strain-specific BYMV hypersensitivity genes occur in narrow-leafed lupin, one controlled by a single dominant hypersensitivity gene, Nbm-1, and the other by an, as yet uncharacterized, hypersensitivity gene. Differential genotypes with or without these genes distinguish four biological strain groups (=pathotypes) of BYMV.
- Strain-specific systemic hypersensitive resistance controlled by the gene Nbm-1 operates at the plant population level, limiting BYMV necrotic strain spread by killing infected plants, which removes them as internal virus infection sources for its spread by aphid vectors. This resistance is ineffective when the non-necrotic (i.e., resistance breaking) BYMV strain is present as the infected plants remain alive, providing infection foci for further spread, resulting in greater overall yield losses.
- The delayed BPS development trait in cv. Jenabillup could be important in future breeding for resistance to BPS in narrow-leafed lupin, so knowledge of its likely nature (resistance to systemic infection via the phloem or mature plant resistance, etc.) and its genetic basis (likely to be polygenic) warrant further investigation.
- Narrow-leafed lupin breeding line 84A086-5-20-31 has a high level of BYMV ‘infection resistance’. Therefore, it is recommended for use as a parent in crosses aimed at producing new lupin cultivars suitable for BYMV-prone high-rainfall regions.
- Attempts to introduce BYMV resistance to narrow-leafed and yellow lupin using viral protease (NIa) and replicase (NIb) genes proved unsuccessful despite initially encouraging findings.
- Diverse factors influence the temporal and spatial dynamics of BYMV spread in narrow-leafed lupin stands in different ways depending on the circumstances. These include whether its spread is from external or internal virus sources, necrotic or non-necrotic strains (or both) are present, first aphid vector arrival is early or late, straw is present or absent on the soil surface, row spacing is narrow or wide, seeding rates are low or high and non-host borders are present or absent.
- BYMV epidemics and the resulting seed yield losses in narrow-leafed lupin crops differ widely with the year, growing season, rainfall zone and geographical region. The main epidemic drivers are (i) the size of the primary external virus source and its proximity; (ii) the crop growth stage when aphid vectors first arrive, the size of the aphid population and the extent of their activity; (iii) the aphid species that visit the crop and whether they colonize it; (iv) weather parameters, especially the temperature, rainfall and evaporation; and (v) cultural practices which influence the amount of groundcover, plant density and stage of crop growth when canopy closure first develops.
- A hybrid mechanistic/statistical model was developed which forecasts aphid vector activity and BYMV epidemics in narrow-leafed lupin crops. It uses daily rainfall and the mean temperature in late summer and early autumn to predict aphid population increase, flights from clover pasture and BYMV spread.
- The IDM strategy devised for lupin crops includes phytosanitary, cultural, chemical and host resistance control measures that act in different ways and target either the primary BYMV source (internal or external) or virus spread (early or later phases). Its main components were sowing a non-host perimeter strip in between adjacent pasture and the lupin crop, maximizing the stubble groundcover within the crop and promoting rapid canopy closure by sowing at high seeding rates with narrow row spacing.
5.9. Further Research
6. Pulses Other than Lupins
6.1. Occurrence in Plots, Crops and Seed Stocks
6.2. Seed Yield Losses and Patterns of Spread
6.3. Chemical and Cultural Control
6.4. Host Resistance
6.5. Integrated Disease Management
6.6. Main Research Achievements
- The considerable diversity of foliage symptom types ranging from mild to very severe that BYMV infection causes in different cool-season pulse species other than lupin in Australia. Seed quality defects sometimes also develop, mostly in faba bean.
- BYMV occurrence in commercial crops and experimental or breeding plots of faba bean, field pea and lentil varied widely between different years in different Australian states and regions. Faba bean was infected most often. Chickpea was rarely infected. BYMV was seed-borne in seed stocks of faba bean, field pea and lentil. In northern NSW, a very high natural BYMV incidence was recorded in field pea breeding plots in 2007 and in commercial faba bean crops in 2020. In WA, BYMV infection was also found infecting experimental or breeding plots of the minor pulses narbon bean, grass pea, dwarf chickling, bitter vetch and L. clymenum and a dwarf chickling crop.
- Seed yield losses caused by early BYMV infection were up to 70% with faba bean and 100% in narbon bean and dwarf chickling. ‘Very poor’ seed production was recorded in lentil and L. ochrus.
- Clumping of newly BYMV-infected plants in faba bean plots occurred firstly around original virus source plants, but later around secondary infection foci that developed as migrant aphid vectors spread BYMV further.
- Non-host borders (e.g., of cereals) around faba bean stands suppressed BYMV spread by acting as ‘virus cleansing’ barriers, but applying insecticides to control BYMV spread was ineffective.
- All genotypes of narbon bean, dwarf chickling, L. ochrus and, most of lentil, were ranked as both susceptible and sensitive to infection in field screening experiments with BYMV isolate MI in WA. However, lentil ILL7163 was ranked as highly resistant and subsequent glasshouse tests found it had extreme BYMV resistance suitable for use by Australian lentil breeders. Field pea, faba bean, common vetch and grass pea genotypes were all ranked as having resistance, whereas six out of eight chickpea genotypes were in the highly resistant category (0% infection). The two chickpea genotypes ranked as resistant were always highly sensitive, but the field pea, faba bean, chickpea, common vetch and grass pea sensitivity rankings were all genotype-dependent.
- Field and glasshouse studies with field pea in NSW reported extreme resistance to BYMV in cvs Bundi, Cressy Blue, Glenroy, Moonlight, Mukta and Santi and breeding lines G-1000 and OZP819. As G-1000 also carries extreme resistance to PSbMV, it was the most suitable for use in PSbMV-resistance breeding.
6.7. Further Research
7. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boswell, K.F.; Gibbs, A.J. Viruses of Legumes 1983; Descriptions and Keys from VIDE; Research School of Biological Science, Australian National University: Canberra, Australia, 1983; 139p. [Google Scholar]
- Buchen-Osmond, C.; Crabtree, K.; Gibbs, A.J.; McLean, G.D. Viruses of Plants in Australia: Descriptions and Lists from the VIDE Database (No. 632.8 V821v); Australian National University: Canberra, ACT, Australia, 1988; 590p. [Google Scholar]
- Jones, R.A.C.; McLean, G.D. Virus diseases of lupins. Ann. Appl. Biol. 1989, 114, 609–637. [Google Scholar] [CrossRef]
- Salam, M.U.; Davidson, J.A.; Thomas, G.J.; Ford, R.; Jones, R.A.C.; Lindbeck, K.D.; Macleod, W.J.; Kimber, R.B.; Galloway, J.; Mantri, N.; et al. Advances in winter pulse pathology research in Australia. Australas. Plant Pathol. 2011, 40, 549–567. [Google Scholar] [CrossRef]
- Jones, R.A.C.; Congdon, B.S. Australian cool-season pulse seed-borne virus research: 1. Alfalfa and cucumber mosaic viruses and less important viruses. Viruses 2024, 16, 144. [Google Scholar] [CrossRef] [PubMed]
- Doolittle, S.P.; Jones, F.R. The mosaic disease in the garden pea and other legumes. Phytopathology 1925, 15, 763–772. [Google Scholar]
- Pierce, W.H. Viroses of the bean. Phytopathology 1934, 24, 87–115. [Google Scholar]
- Aitkin, Y.; Grieve, B.J. A mosaic virus of subterranean clover. J. Aust. Inst. Agric. Sci. 1943, 9, 81–82. [Google Scholar]
- Norris, D.O. Pea mosaic on Lupinus varius L. and other species in Western Australia. Bull. Counc. Sci. Ind. Res. 1943, 170, 27. [Google Scholar]
- Bos, L. Bean Yellow Mosaic Virus; Descriptions of Plant Viruses No. 40; Association of Applied Biologists: Wellesbourne, Warwick, UK, 1970; Available online: https://www.dpvweb.net/dpv/showdpv/?dpvno=40 (accessed on 5 February 2025).
- Bos, L.; Hampton, R.O.; Makkouk, K.M. Viruses and virus diseases of pea, lentil, faba bean and chickpea. In World Crops: Cool Season Food Legumes; Summerfield, R.J., Ed.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1988; pp. 591–615. [Google Scholar]
- Edwardson, J.R.; Christie, R.G. CRC Handbook of Viruses Infecting Legumes; CRC Press: Boca Raton, FL, USA, 1991; 564p. [Google Scholar]
- Bos, L. Legume virology. In Encyclopedia of Virology, 3rd ed.; Mahy, W.J., Van Regenmortel, M.H.N., Eds.; Academic Press: Amsterdam, The Netherlands, 2008; pp. 212–220. [Google Scholar]
- Swenson, K.G. Bean yellow mosaic virus transmission by Myzus persicae. Aust. J. Biol. Sci. 1962, 15, 468–482. [Google Scholar] [CrossRef]
- Grylls, N.E.; Peak, J.W. A virus complex of subterranean clover. Aust. J. Aric. Res. 1969, 20, 37–45. [Google Scholar] [CrossRef]
- Johnstone, G.R.; McLean, D.G. Virus diseases of subterranean clover. Ann. Appl. Biol. 1987, 110, 421–440. [Google Scholar] [CrossRef]
- Srithongchai, W. Virus Diseases of Trifolium subterraneum and Vicia faba in Tasmania. Ph.D. Thesis, University of Tasmania, Hobart, Australia, 1990; pp. 1–217. [Google Scholar] [CrossRef]
- Berlandier, F.A.; Thackray, D.J.; Jones, R.A.C.; Latham, L.J.; Cartwright, L. Determining the relative roles of different aphid species as vectors of cucumber mosaic and bean yellow mosaic viruses in lupins. Ann. Appl. Biol. 1997, 131, 297–314. [Google Scholar] [CrossRef]
- Jones, R.A.C. Developing integrated disease management strategies against non-persistently aphid-borne viruses: A model programme. Integr. Pest Man. Rev. 2001, 6, 15–46. [Google Scholar] [CrossRef]
- Maling, T.; Diggle, A.J.; Thackray, D.J.L.; Siddique, K.H.M.; Jones, R.A.C. An epidemiological model for externally sourced vector-borne viruses applied to Bean yellow mosaic virus in lupin crops in a Mediterranean-type environment. Phytopathology 2008, 98, 1280–1290. [Google Scholar] [CrossRef] [PubMed]
- Schwinghamer, M.W.; Nicholas, A.H.; Schilg, M.A. Three aphid vectors of faba bean (Vicia faba) viruses in northern New South Wales and occurrence of Acyrthosiphon pisum-transmitted isolates of Soybean dwarf virus. Australas. Plant Pathol. 2009, 38, 262–269. [Google Scholar] [CrossRef]
- Harvey, H.L. Bean, subterranean clover and lupin diseases caused by bean yellow mosaic virus in Western Australia. J. Agric. Res. West. Aust. Third Ser. 1956, 5, 329–338. [Google Scholar]
- Jayasena, K.W.; Randles, J.W. Patterns of spread of the non-persistently transmitted bean yellow mosaic virus and the persistently transmitted subterranean clover red leaf virus in Vicia faba. Ann. Appl. Biol. 1984, 104, 249–260. [Google Scholar] [CrossRef]
- McKirdy, S.J.; Jones, R.A.C. Bean yellow mosaic potyvirus infection of alternative hosts associated with subterranean clover (Trifolium subterraneum) and narrow-leafed lupins (Lupinus angustifolius): Field screening procedure, relative susceptibility/resistance rankings, seed transmission and persistence between growing seasons. Aust. J. Agric. Res. 1995, 46, 135–152. [Google Scholar]
- McKirdy, S.J.; Jones, R.A.C.; Latham, L.J.; Coutts, B.A. Bean yellow mosaic potyvirus infection of alternative annual pasture, forage, and cool season crop legumes: Susceptibility, sensitivity, and seed transmission. Aust. J. Agric. Res. 2000, 51, 325–346. [Google Scholar] [CrossRef]
- Latham, L.J.; Jones, R.A.C. Incidence of virus infection in experimental plots, commercial crops and seed stocks of cool season crop legumes. Aust. J. Agric. Res. 2001, 52, 397–413. [Google Scholar] [CrossRef]
- Watson, M.A. Some notes on plant virus diseases in South Australia. J. Aust. lnst. Agric. Sci. 1949, 15, 76–81. [Google Scholar]
- Hutton, E.M.; Peak, J.W. Varietal reactions of Trifolium subterraneum L. to Phaseolus virus 2 Pierce. Aust. J. Agric. Res. 1954, 5, 598–607. [Google Scholar] [CrossRef]
- Goodchild, D.J. Relationships of legume viruses in Australia i. strains of bean yellow mosaic virus and pea mosaic virus. Aust. J. Biol. Sci. 1956, 9, 213–230. [Google Scholar] [CrossRef]
- Goodchild, D.J. Relationships of legume viruses in Australia ii. Serological relationships of bean yellow mosaic virus and pea mosaic virus. Aust. J. Biol. Sci. 1956, 9, 231–237. [Google Scholar] [CrossRef]
- Taylor, R.H.; Smith, P.R. The relationship between bean yellow mosaic virus and pea mosaic virus. Aust. J. Biol. Sci. 1968, 21, 429–438. [Google Scholar] [CrossRef]
- Moghal, S.M.; Francki, R.I.B. Towards a system for the identification and classification of potyviruses: I. Serology and amino acid composition of six distinct viruses. Virology 1976, 73, 350–362. [Google Scholar] [CrossRef] [PubMed]
- Moghal, S.M.; Francki, R.I.B. Towards a system for the identification and classification of potyviruses: II. Virus particle length, symptomatology, and cytopathology of six distinct viruses. Virology 1981, 112, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Randles, J.W.; Davies, C.; Gibbs, A.J.; Hatta, T. Amino acid composition of capsid protein as a taxonomic criterion for classifying the atypical S strain of bean yellow mosaic virus. Aust. J. Biol. Sci. 1980, 33, 245–254. [Google Scholar] [CrossRef]
- Abu-Samah, N.; Randles, J.W. A comparison of the nucleotide sequence homologies of three isolates of bean yellow mosaic virus and their relationship to other potyviruses. Virology 1981, 110, 436–444. [Google Scholar] [CrossRef]
- Abu-Samah, N.; Randles, J.W. A comparison of Australian bean yellow mosaic virus isolates using molecular hybridisation analysis. Ann. Appl. Biol. 1983, 103, 97–107. [Google Scholar] [CrossRef]
- Tracy, S.L.; Frenkel, M.J.; Gough, K.H.; Hanna, P.J.; Shukla, D.D. Bean yellow mosaic, clover yellow vein, and pea mosaic are distinct potyviruses: Evidence from coat protein gene sequences and molecular hybridization involving the 3′ non-coding regions. Arch. Virol. 1992, 122, 249–261. [Google Scholar] [CrossRef]
- Xiao, X.W.; Frenkel, M.J.; Ward, C.W.; Shukla, D.D. Nucleotide sequence of the 3′-terminal region of the genome confirms that pea mosaic virus is a strain of bean yellow mosaic potyvirus. Arch. Virol. 1994, 136, 381–387. [Google Scholar] [CrossRef]
- Wylie, S.J.; Coutts, B.A.; Jones, M.G.K.; Jones, R.A.C. Phylogenetic analysis of bean yellow mosaic virus isolates from four continents: Relationship between the seven groups found and their hosts and origins. Plant Dis. 2008, 92, 1596–1603. [Google Scholar] [CrossRef] [PubMed]
- Wylie, S.J.; Jones, R.A.C. Role of Recombination in the evolution of host specialization within bean yellow mosaic virus. Phytopathology 2009, 99, 512–518. [Google Scholar] [CrossRef] [PubMed]
- Mathews, A.; Dwyer, G.; Wylie, S.J.; Jones, M.G.K. Nucleotide and deduced amino acid sequence of the 3′ end of the BYMV-MI genome. Arch. Virol. 1995, 140, 2269–2272. [Google Scholar] [CrossRef] [PubMed]
- Webster, C.G.; Coutts, B.A.; Jones, R.A.C.; Jones, M.G.K.; Wylie, S.J. Impact at the interface of an ancient ecosystem and a recent agroecosystem: Studies on three legume-infecting potyviruses in the southwest Australian floristic region. Plant Pathol. 2007, 56, 729–742. [Google Scholar] [CrossRef]
- Wylie, S.J.; Kueh, J.; Welsh, B.; Smith, L.J.; Jones, M.G.K.; Jones, R.A.C. A non-aphid-transmissible isolate of bean yellow mosaic potyvirus has an altered NAG motif in its coat protein. Arch. Virol. 2002, 147, 1813–1820. [Google Scholar] [CrossRef]
- Gibbs, A.J.; Mackenzie, A.; Blanchfield, A.; Cross, P.; Wilson, C.; Kitajima, E.; Nightingale, M.; Clements, M. Viruses of orchids in Australia: Their identification, biology and control. Aust. Orchid Rev. 2000, 65, 10–21. [Google Scholar]
- Kehoe, M.A.; Buirchell, B.J.; Coutts, B.A.; Jones, R.A.C. Black Pod Syndrome of Lupinus angustifolius is caused by late infection with bean yellow mosaic virus. Plant Dis. 2014, 98, 739–745. [Google Scholar] [CrossRef]
- Kehoe, M.A.; Coutts, B.A.; Buirchell, B.J.; Jones, R.A.C. Plant virology and next generation sequencing: Experiences with a potyvirus. PLoS ONE 2014, 9, e104580. [Google Scholar] [CrossRef][Green Version]
- Cheng, Y.; Jones, R.A.C. Biological properties of necrotic and non-necrotic strains of bean yellow mosaic virus in cool season grain legumes. Ann. Appl. Biol. 2000, 136, 215–227. [Google Scholar] [CrossRef]
- McKirdy, S.J.; Jones, R.A.C. Further studies on the incidence of virus infection in white clover pastures. Aust. J. Agric. Res. 1997, 48, 31–38. [Google Scholar] [CrossRef]
- Kehoe, M.A.; Coutts, B.A.; Buirchell, B.J.; Jones, R.A.C. Split personality of a potyvirus: To specialize or not to specialize? PLoS ONE 2014, 9, e105770. [Google Scholar] [CrossRef]
- Jones, R.A.C. Seed-borne virus diseases in annual pasture legumes. J. Agric. West. Aust. Fourth Ser. 1988, 29, 58–61. [Google Scholar]
- McKirdy, S.J.; Coutts, B.A.; Jones, R.A.C. Occurrence of bean yellow mosaic virus in subterranean clover pastures and perennial native legumes. Aust. J. Agric. Res. 1994, 45, 183–194. [Google Scholar] [CrossRef]
- Jones, R.A.C. Virus diseases of annual pasture legumes: Incidences, losses, epidemiology and management. Crop Past. Sci. 2012, 63, 399–418. [Google Scholar] [CrossRef]
- Jones, R.A.C. Effects of cereal borders, admixture with cereals and plant density on the spread of bean yellow mosaic potyvirus into narrow-leafed lupins (Lupinus angustifolius). Ann. Appl. Biol. 1993, 122, 501–518. [Google Scholar] [CrossRef]
- McKirdy, S.J.; Jones, R.A.C.; Barbetti, M.J. Emerging disease problems in new pasture species. In Cereal Research and Industry Development in Western Australia, Crop Updates, Rendezvous Observation City Hotel, Perth, February 1998; Loss, S., Siddique, K., Eds.; Western Australian Department of Agriculture: Perth, WA, Australia, 1998; pp. 54–55. [Google Scholar]
- Jones, R.A.C. Bean yellow mosaic virus infection of alternative pasture legume species. In Cereals Research and Industry Development in Western Australia, 1999 Crop Updates. Perth, 17–18 February 1999; Zaichou, C., Kerr, N., Eds.; Western Australian Department of Agriculture: Perth, WA, Australia, 1999; pp. 33–34. [Google Scholar]
- Vincent, S.J.; Coutts, B.A.; Jones, R.A.C. Effects of introduced and indigenous viruses on native plants: Exploring their disease causing potential at the agro-ecological interface. PLoS ONE 2014, 9, e91224. [Google Scholar] [CrossRef]
- Pathipanawat, W.; Jones, R.A.C.; Sivasithamparam, K. Studies on seed and pollen transmission of alfalfa mosaic, cucumber mosaic and bean yellow mosaic viruses in cultivars and accessions of annual Medicago species. Aust. J. Agric. Res. 1995, 153–165. [Google Scholar] [CrossRef]
- Jones, R.A.C. Alteration of plant species mixtures by virus infection: Managed pastures the forgotten dimension. Plant Pathol. 2022, 71, 1255–1281. [Google Scholar] [CrossRef]
- Cheng, Y.; Jones, R.A.C. Distribution and incidence of necrotic and non-necrotic strains of bean yellow mosaic virus in wild and crop lupins. Aust. J. Agric. Res. 1999, 50, 589–600. [Google Scholar] [CrossRef]
- Gladstones, J.S. Natural resistance to escape from BYMV in Lupinus angustifolius. In Highlights of Lupin Research and Development in Western Australia; Shea, G., Ed.; Agriculture Western Australia: Perth, WA, Australia, 1998; pp. 31–32. [Google Scholar]
- Jones, R.A.C.; Coutts, B.A. Screening for resistance to bean yellow mosaic virus in lupins using a disease nursery. In Highlights of Lupin Research and Development in Western Australia; Shea, G., Ed.; Agriculture Western Australia: Perth, WA, Australia, 1998; pp. 29–30. [Google Scholar]
- White, P.; Baker, M. Black pod syndrome in lupins can be reduced by regular insecticide sprays. In Proceedings of the Agribusiness Crop Updates: Crop Specific Disease 24th February 2009; Penny, S., Shea, G., French, B., Douglas, A., Paterson, J., Eds.; Department of Agriculture and Food: South Perth, WA, Australia, 2009; pp. 65–68. [Google Scholar]
- Jones, R.A.C. Bean yellow mosaic virus in lupins. J. Agric. Sci. West. Aust. Fourth Ser. 1997, 38, 42–48. [Google Scholar]
- Jones, R.A.C. Effects of mulching with cereal straw and row spacing on spread of bean yellow mosaic potyvirus into narrow-leafed lupins (Lupinus angustifolius). Ann. Appl. Biol. 1994, 124, 45–58. [Google Scholar] [CrossRef]
- Harrison, B.D. Virus variation in relation to resistance-breaking in plants. Euphytica 2002, 124, 181–192. [Google Scholar] [CrossRef]
- Thomas-Carroll, M.L.; Jones, R.A.C. Selection, biological properties and fitness of resistance-breaking strains of tomato spotted wilt virus in pepper. Ann. Appl. Biol. 2023, 142, 235–243. [Google Scholar] [CrossRef]
- Cheng, Y.; Jones, R.A.C. Temporal and spatial patterns of spread with the necrotic and non-necrotic strains of bean yellow mosaic virus in narrow-leafed lupins. In Proceedings of the 15th Meeting of the International Working Group Legume Viruses, Fremantle, Australia, 15–17 August 1999; Jones, R.A.C., Ed.; Centre for Legumes in Mediterranean Agriculture: Fremantle, WA, Australia, 1999; p. 48. [Google Scholar]
- Jones, R.A.C.; Coutts, B.A.; Cheng, Y. Yield limiting potential of necrotic and non-necrotic strains of bean yellow mosaic virus in narrow-leafed lupin (Lupinus angustifolius). Aust. J. Agric. Res. 2003, 54, 849–859. [Google Scholar] [CrossRef]
- van Leur, J.; George, J.; Sharman, M.; Congdon, B.; Kehoe, M.A. Seed transmission of bean yellow mosaic virus (BYMV) in albus lupin (Lupinus albus). In Proceedings of the Australian Pulse Conference, Toowoomba, QLD, Australia, 21–23 March 2023. [Google Scholar]
- Buirchell, B.J. Narrow-leafed lupin breeding in Australia—Where to from here? In Lupins for Health and Wealth, Proceedings of the 12th International Lupin Conference, Fremantle, Western Australia, 14–18 September 2008; International Lupin Association: Canterbury, New Zealand, 2008; pp. 14–18. [Google Scholar]
- White, P.; Buirchell, B.; Baker, M. New lupins adapted to the south coast. In Proceedings of the Agribusiness Crop Updates: Lupin, Pulses and Oilseeds; Parker, W., Ed.; Department of Agriculture and Food: South Perth, WA, Australia, 2007; pp. 13–17. [Google Scholar]
- Jones, R.A.C. Reflective mulch decreases the spread of two non-persistently aphid transmitted viruses to narrow-leafed lupin (Lupinus angustifolius). Ann. Appl. Biol. 1991, 118, 79–85. [Google Scholar] [CrossRef]
- Thresh, J.M. Cropping practices and virus spread. Annu. Rev. Phytopathol. 1982, 20, 193–218. [Google Scholar] [CrossRef]
- Irwin, M.E.; Kampmeier, G.E. Vector behaviour, environmental stimuli, and the dynamics of plant virus epidemics. In Spatial Components of Plant Disease Epidemics; Jeger, M.J., Ed.; Prentice-Hall International, Inc.: Englewood Cliffs, NJ, USA, 1989; pp. 14–39. [Google Scholar]
- Jones, R.A.C. Host resistance to virus diseases provides a key enabler towards fast tracking gains in grain lupin breeding. Plants 2023, 12, 2521. [Google Scholar] [CrossRef]
- Jones, R.A.C.; Smith, L. Inheritance of hypersensitive resistance to bean yellow mosaic virus in narrow-leafed lupin (Lupinus angustifolius). Ann. Appl. Biol. 2005, 146, 539–543. [Google Scholar] [CrossRef]
- Cheng, Y.; Jones, R.A.C.; Thackray, D.J. Deploying strain specific hypersensitive resistance to diminish temporal virus spread. Ann. Appl. Biol. 2002, 140, 69–79. [Google Scholar] [CrossRef]
- Thackray, D.J.; Smith, L.J.; Cheng, Y.; Perry, J.N.; Jones, R.A.C. Effect of strain-specific hypersensitive resistance on spatial patterns of virus spread. Ann. Appl. Biol. 2002, 141, 45–59. [Google Scholar] [CrossRef]
- Nyalugwe, E.P.; Barbetti, M.J.; Jones, R.A.C. Studies on resistance phenotypes to turnip mosaic virus in five species of Brassicaceae, and identification of a virus resistance gene in Brassica juncea. Eur. J. Plant Pathol. 2015, 141, 647–666. [Google Scholar] [CrossRef]
- Nyalugwe, E.P.; Barbetti, M.J.; Clode, P.L.; Jones, R.A.C. Systemic hypersensitive resistance to turnip mosaic virus in Brassica juncea is associated with multiple defense responses, especially phloem necrosis and xylem occlusion. Plant Dis. 2016, 100, 1261–1270. [Google Scholar] [CrossRef]
- Nyalugwe, E.P.; Barbetti, M.J.; Jones, R.A.C. Strain specificity of turnip mosaic virus resistance gene TuRBJU 01 in Brassica juncea. Eur. J. Plant Pathol. 2016, 145, 209–213. [Google Scholar] [CrossRef]
- Wylie, S.J. Characterisation of the NIa Gene of Bean Yellow Mosaic Virus, MI isolate, and Its Use for Pathogen-Derived Resistance. Doctoral Dissertation, Murdoch University, Perth, WA, Australia, 1996. [Google Scholar]
- Li, D.A.; Wylie, S.J.; Jones, M.G.K. Preliminary assessment for resistance to bean yellow mosaic virus in transgenic lupins. In Proceedings of the 15th International Working Group on Legume Viruses, Fremantle, Australia, 15–17 August 1999; Centre for Legumes in Mediterranean Agriculture: Perth, WA, Australia, 1999; p. 66. [Google Scholar]
- Smith, L.J.; Jones, R.A.C. Evaluation of transgenic lupin plants containing pathogen-derrived constructs for bean yellow mosaic virus. In Proceedings of the 15th International Working Group on Legume Viruses, Fremantle, WA, Australia, 15–17 August 1999; p. 76. [Google Scholar]
- Jones, R.A.C.; Coutts, B.A.; Buirchell, G.M.; Wylie, S.J. Bean yellow mosaic virus in lupins: Strains, losses, epidemiology andcontrol. In Lupins for Health and Wealth, Proceedings of the 12th International Lupin Conference, Fremantle, Western Australia, 14–18 September 2008; International Lupin Association: Canterbury, New Zealand, 2008; pp. 420–424. [Google Scholar]
- Wylie, S.J.; Welsh, B.; Hodgson, L.; Chapple, S.; Fletcher, N.; Barker, S.; Smith, P.; Jones, M.G.K. Transgenic lupin cultivar immune to bean yellow mosaic virus. In Research Repository; Murdoch University: Perth, WA, Australia, 2004; Available online: https://researchrepository.murdoch.edu.au/id/eprint/19710/1/WYLIE-20040624162812.pdf (accessed on 10 May 2024).
- Taliansky, M.; Samarskaya, V.; Zavriev, S.K.; Fesenko, I.; Kalinina, N.O.; Love, A.J. RNA-based technologies for engineering plant virus resistance. Plants 2021, 10, 82. [Google Scholar] [CrossRef]
- Tripathi, L.; Ntui, V.O.; Tripathi, J.N. RNA interference and CRISPR/Cas9 applications for virus resistance. In CRISPR and RNAi Systems; Abd-Elsalam, K.A., Lim, K.-T., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 163–182. [Google Scholar]
- Ding, S.W. Transgene silencing, RNA interference, and the antiviral defense mechanism directed by small interfering RNAs. Phytopathology 2023, 113, 616–625. [Google Scholar] [CrossRef]
- Jones, R.A.C. Patterns of spread of two non-persistently aphid-borne viruses in lupin stands under four different infection scenarios. Ann. Appl. Biol. 2005, 146, 337–350. [Google Scholar] [CrossRef]
- Jones, R.A.C.; Prince, R. Controlling non-necrotic strains of bean yellow mosaic virus in lupins by cultural methods. In Crop Update Conference, Lupin Update. Sheraton Hotel, Perth, 19–20 February 2003; McLarty, A., Ed.; Agriculture Western Australia: Perth, WA, Australia, 2003; pp. 41–42. [Google Scholar]
- Thackray, D.J.; Jones, R.A.C. Forecasting aphid and virus risk in lupins. In Crop Update Conference, 1999 Lupin Update. Rendezvous Observation City Hotel, Perth, 17–18 February 1999; Shea, G., Ed.; Agriculture Western Australia: Perth, WA, Australia, 1999; pp. 24–26. [Google Scholar]
- Thackray, D.J.; Hawkes, J.; Jones, R.A.C. Forecasting aphid and virus risk in lupins. In Crop Update Conference, 2000 Lupin Update. Rendezvous Observation City Hotel, Perth, 16–17 February 2000; O’Niel, B., Ed.; Agriculture Western Australia: Perth, WA, Australia, 2000; pp. 19–20. [Google Scholar]
- Maling, T.; Diggle, A.J.; Thackray, D.J.; Siddique, K.; Jones, R.A.C. Forecasting green-bridge mediated diseases in the south-west of Australia: Bean yellow mosaic virus in lupins. In Lupins for Health and Wealth. Proceedings of the 12th International Lupin Conference, Fremantle, Western Australia, 14–18 September 2008; International Lupin Association: Fremantle, WA, Australia, 2008; pp. 434–438. [Google Scholar]
- Jones, R.A.C.; Salam, M.; Maling, T.; Diggle, A.J.; Thackray, D.J. Principles of predicting plant virus disease epidemics. Annu. Rev. Phytopathol. 2010, 48, 179–203. [Google Scholar] [CrossRef]
- Jones, R.A.C. Using epidemiological information to develop effective integrated virus disease management strategies. Virus Res. 2004, 100, 5–30. [Google Scholar] [CrossRef]
- Jones, R.A.C. Control of plant virus diseases. Adv. Virus Res. 2006, 67, 205–244. [Google Scholar]
- Jones, R.A.C.; Naidu, R.A. Global dimensions of plant virus diseases: Current status and future perspectives. Annu. Rev. Virol. 2019, 6, 387–409. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.A.C.; Ferris, D.G. Suppressing spread of alfalfa mosaic virus in grazed legume pasture swards using insecticides and admixture with grass, and effects of insecticides on numbers of aphids and three other pasture pests. Ann. Appl. Biol. 2000, 137, 259–271. [Google Scholar] [CrossRef]
- Stace-Smith, R.; Hamilton, R.I. Inoculum thresholds of seedborne pathogens: Viruses. Phytopathology 1988, 78, 875–880. [Google Scholar] [CrossRef]
- Jones, R.A.C. Determining ‘threshold’ levels for seed-borne virus infection in seed stocks. Virus Res. 2000, 71, 171–183. [Google Scholar] [CrossRef]
- Bwye, A.M.; Jones, R.A.C.; Proudlove, W. Effects of sowing seed with different levels of infection, plant density and the growth stage at which plants first develop symptoms on cucumber mosaic-virus infection of narrow-leafed lupins (Lupinus angustifolius). Aust. J. Agric. Res. 1994, 45, 1395–1412. [Google Scholar] [CrossRef]
- Coutts, B.A.; Prince, R.T.; Jones, R.A.C. Quantifying effects of seedborne inoculum on virus spread, yield losses, and seed infection in the pea seed-borne mosaic virus–field pea pathosystem. Phytopathology 2009, 99, 1156–1167. [Google Scholar] [CrossRef]
- Ksiazkiewicz, M.; Yang, H.A. Molecular marker resources supporting the Australian lupin breeding program. In The Lupin Genome, Compendium of Plant Genomes; Kole, C., Ed.; Springer Nature: Berlin, Germany, 2020; pp. 73–86. [Google Scholar]
- Croser, J.S.; Pazos-Navarro, M.; Bennett, R.G.; Tschirren, S.; Edwards, K.; Erskine, W.; Creasy, R.; Ribalta, F.M. Time to flowering of temperate pulses in vivo and generation turnover in vivo–in vitro of narrow-leaf lupin accelerated by low red to far-red ratio and high intensity in the far-red region. Plant Cell Tissue Org. Cult. 2016, 127, 591–599. [Google Scholar] [CrossRef]
- Kaiser, W.J. Biology of bean yellow mosaic and pea leaf roll viruses affecting Vicia faba in Iran. Phytopathol. Z. 1973, 78, 253–263. [Google Scholar] [CrossRef]
- van Leur, J.A.G.; Kumari, S.G. Bean yellow mosaic virus studies in faba bean. In Proceedings of the 15th Biennial Conference of the Australasian Plant Pathology Society, Geelong, VIC, Australia, 26–29 September 2005; p. 311. [Google Scholar]
- Makkouk, K.M.; Kumari, S.G.; van Leur, J.A.; Jones, R.A.C. Control of plant virus diseases in cool-season grain legume crops. Adv. Virus Res. 2014, 90, 207–253. [Google Scholar]
- Jones, R.A.C.; Coutts, B.A. Alfalfa mosaic and cucumber mosaic virus infection in chickpea and lentil: Incidence and seed transmission. Ann. Appl. Biol. 1996, 129, 491–506. [Google Scholar] [CrossRef]
- Aftab, M.; Freeman, A.J.; Davidson, J. Pulse virus surveys in south eastern Australia (2003–2004). In Proceedings of the Australasian Plant Pathology Society 15th Biennial Conference, Geelong, VIC, Australia, 24–29 September 2005; p. 338. Available online: https://catalogue.nla.gov.au/catalog/4494494 (accessed on 3 February 2025).
- Freeman, A.J.; Aftab, M. Surveying for and mapping of viruses in pulse crops in south-eastern Australia. In Proceedings of the Australasian Plant Pathology Society 13th Biennial Conference, Cairns, QLD, Australia, 24–27 September 2001; p. 149. [Google Scholar]
- Freeman, A.J.; Aftab, M.; Dobson, V. Surveying for viruses in pulse crops in Victoria. In Proceedings of the 8th International Congress of Plant Pathology, Christchurch, New Zealand, 2–7 February 2003; Volume 2, p. 261. [Google Scholar]
- Freeman, A.J.; Aftab, M.; McQueen, V.; Davidson, J. The occurrence of common viruses in pulse crops in south eastern Australia. In Proceedings of the 15th Biennial Australasian Plant Pathology Society Conference, Geelong, VIC, Australia, 26–30 September 2005; p. 339. [Google Scholar]
- Freeman, A.J.; Spackman, M.E.; Aftab, M.; McQueen, V.; King, S.; van Leur, J.A.; Loh, M.H.; Rodoni, B. Comparison of tissue blot immunoassay and reverse transcription polymerase chain reaction assay for virus-testing pulse crops from a South-Eastern Australia survey. Australas. Plant Pathol. 2013, 42, 675–683. [Google Scholar] [CrossRef]
- van Leur, J.A.G.; Aftab, M.; Manning, W.; Bowring, A.; Riley, M.J. A severe outbreak of chickpea viruses in northern New South Wales, Australia, during 2012. Australas. Plant Dis. Notes 2013, 8, 49–53. [Google Scholar] [CrossRef][Green Version]
- van Leur, J.A.G.; Duric, Z. Viral Diseases in Faba Bean, Chickpeas, Lentil and Lupins. Impacts, Vectors/Causes and Management Strategies for 2021; Update Paper; Grains Research and Development Corporation: Dubbo, NSW, Australia; pp. 147–155. Available online: https://grdc.com.au/resources-and-publications/grdc-update-papers/tab-content/grdc-update-papers/2021/02/viral-diseases-in-faba-bean,-chickpeas,-lentil-and-lupins.-impacts,-vectorscauses-and-management-strategies-for-2021 (accessed on 20 February 2025).
- Frowd, J.A.; Bernier, C.C. Virus diseases of faba beans in Manitoba and their effects on plant growth and yield. Can. J. Plant Sci. 1977, 57, 845–852. [Google Scholar] [CrossRef]
- Kumari, S.G.; Makkouk, K.M.; Ismail, I.D. Seed transmission and yield loss induced in lentil (Lens culinaris Med.) by bean yellow mosaic potyvirus. LENS Newsl. 1994, 21, 42–44. [Google Scholar]
- Tapio, E. Virus diseases of legumes in Finland and in the Scandinavian countries. Ann. Agric. Finland 1970, 9, 1–96. [Google Scholar]
- Jayasena, K.W.; Randles, J.W. The effect of insecticides and a plant barrier row on aphid populations and the spread of bean yellow mosaic potyvirus and subterranean clover red leaf luteovirus in Vicia faba in South Australia. Ann. Appl. Biol. 1985, 107, 355–364. [Google Scholar] [CrossRef]
- van Leur, J.A.G.; Kumari, S.G.; Aftab, M.; Leonforte, A.; Moore, S. Virus resistance of Australian pea (Pisum sativum) varieties. N. Z. J. Crop Hort. Sci. 2013, 41, 86–101. [Google Scholar] [CrossRef]
- Kanawaty, A.; Kumari, S.G.; van Leur, J.A.G.; Hammadi, H. Screening of lentil genotypes for resistance to bean yellow mosaic virus and effect of mixed infection on the susceptibility of some resistant lentil genotypes. Arab. J. Plant Prot. 2017, 35, 171–177. [Google Scholar] [CrossRef]
- Jones, R.A.C.; Latham, L.J.; Coutts, B.A. Devising integrated disease management tactics against plant viruses from ‘generic’ information on control measures. Agric. Sci. Aust. 2004, 17, 10–18. [Google Scholar]
- Sasaya, T.; Iwasaki, M.; Yamomoto, T. Seed transmission of bean yellow mosaic virus in broad bean (Vicia faba). J. Phytopathol. Jpn. 1993, 59, 559–562. [Google Scholar] [CrossRef]
- Hampton, R.O. Natural spread of viruses infectious to beans. Phytopathology 1967, 57, 476–481. [Google Scholar]
- Thresh, J.M. Gradients of plant virus diseases. Ann. Appl. Biol. 1976, 82, 381–406. [Google Scholar] [CrossRef]
- Jones, R.A.C.; Smith, L.J.; Gajda, B.E.; Latham, L.J. Patterns of spread of Carrot virus Y in carrot plantings and validation of control measures. Ann. Appl. Biol. 2005, 147, 57–67. [Google Scholar] [CrossRef]
- Jones, R.A.C. Plant virus ecology and epidemiology: Historical perspectives, recent progress and future prospects. Ann. Appl. Biol. 2014, 164, 320–347. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jones, R.A.C. Australian Cool-Season Pulse Seed-Borne Virus Research: 2. Bean Yellow Mosaic Virus. Viruses 2025, 17, 668. https://doi.org/10.3390/v17050668
Jones RAC. Australian Cool-Season Pulse Seed-Borne Virus Research: 2. Bean Yellow Mosaic Virus. Viruses. 2025; 17(5):668. https://doi.org/10.3390/v17050668
Chicago/Turabian StyleJones, Roger A. C. 2025. "Australian Cool-Season Pulse Seed-Borne Virus Research: 2. Bean Yellow Mosaic Virus" Viruses 17, no. 5: 668. https://doi.org/10.3390/v17050668
APA StyleJones, R. A. C. (2025). Australian Cool-Season Pulse Seed-Borne Virus Research: 2. Bean Yellow Mosaic Virus. Viruses, 17(5), 668. https://doi.org/10.3390/v17050668