Changes in Body Composition During Intensive Care Unit Stay and Outcomes in Patients with Severe COVID-19 Pneumonia: A Retrospective Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patients
2.2. Statistical Analysis
3. Results
3.1. Body Composition at Admission and Subsequent Rate of Change.
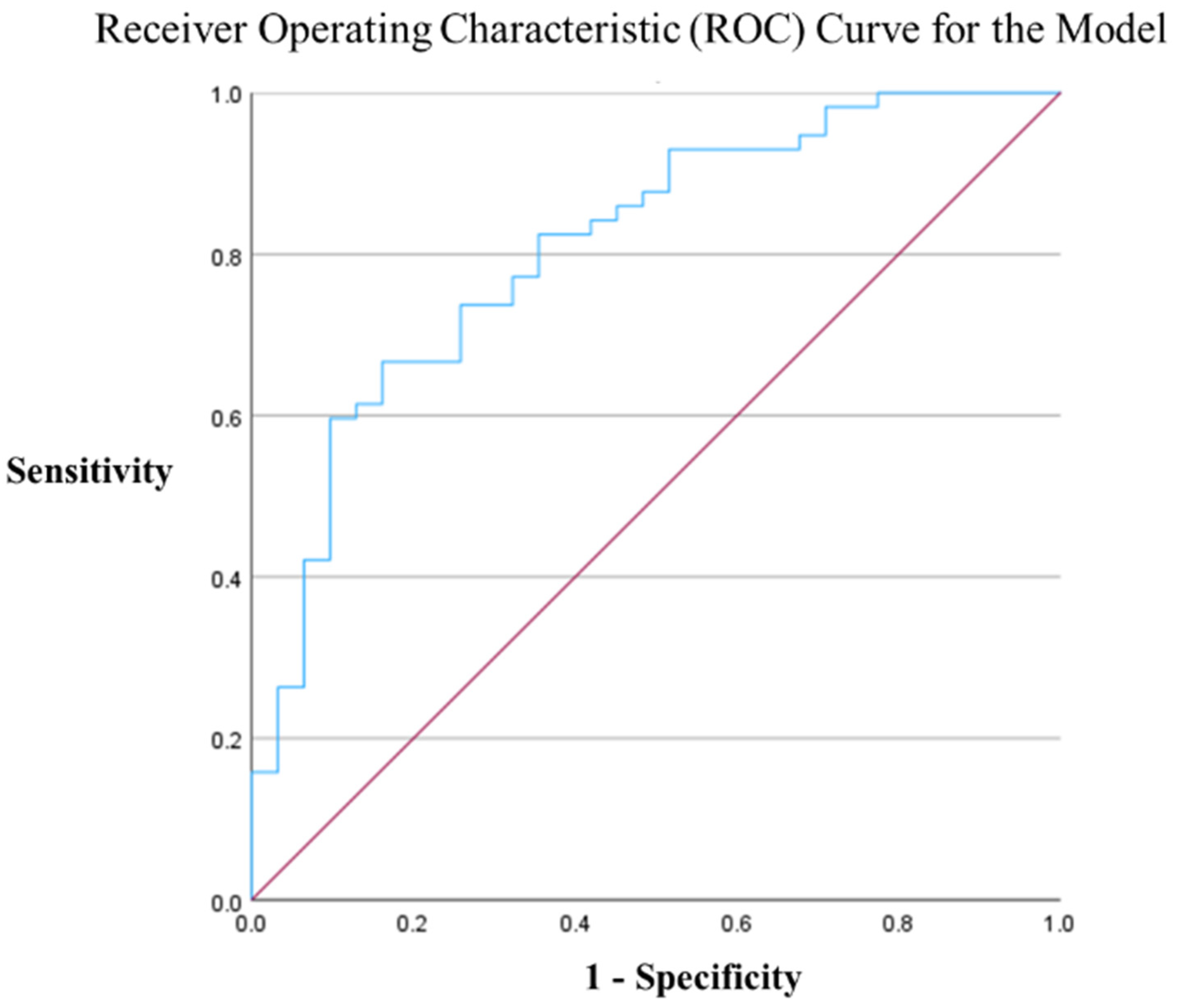
3.2. Multivariate Analysis (Logistic Regression)
4. Discussion
4.1. Low ESM Area as an Independent Factor for Poor Outcomes
4.2. Mechanisms of Muscle Loss in COVID-19 Pneumonia: Acute Sarcopenia, ICU-Acquired Weakness (ICU-AW), and Renin–Angiotensin–Aldosterone System (RAAS) Dysregulation
4.3. Inflammation Accelerates Suppression of Lipolysis as a Mechanism for Subcutaneous Fat Accumulation in Severe Cases
4.4. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACE II | Angiotensin-converting enzyme type II |
| ADL | Activities of daily living |
| APACHE | Acute Physiology and Chronic Health Evaluation |
| AUROC | Area under the receiver operating characteristic curve |
| BMI | Body mass index |
| CCI | Charlson Comorbidity Index |
| CI | Confidence interval |
| CONUT | Controlling nutritional status |
| COVID-19 | Coronavirus disease |
| CT | Computed tomography |
| ESM | Erector spinae muscle |
| ICU | Intensive care unit |
| ICU-AW | ICU-acquired weakness |
| IL-6 | Interleukin-6 |
| Non-Survivors | Non-survivors at the time of discharge from the ICU or transfer |
| OR | Odds ratios |
| P/F ratio | Partial pressure of arterial oxygen/fraction of inspired oxygen ratio |
| RAAS | Renin–angiotensin–aldosterone system |
| ROC | Receiver operating characteristic |
| SARS-CoV-2 | Severe acute respiratory syndrome coronavirus 2 |
| SAT | Subcutaneous adipose tissue |
| SOFA | Sequential Organ Failure Assessment |
| Survivors | Survivors at the time of discharge from the ICU or transfer |
| VAT | Visceral adipose tissue |
References
- Matsunaga, N.; Hayakawa, K.; Terada, M.; Ohtsu, H.; Asai, Y.; Tsuzuki, S.; Suzuki, S.; Toyoda, A.; Suzuki, K.; Endo, M.; et al. Clinical epidemiology of hospitalized patients with coronavirus disease 2019 (COVID-19) in Japan: Report of the COVID-19 registry Japan. Clin. Infect. Dis. 2021, 73, e3677–e3689. [Google Scholar] [CrossRef] [PubMed]
- Kragholm, K.; Andersen, M.P.; Gerds, T.A.; Butt, J.H.; Østergaard, L.; Polcwiartek, C.; Phelps, M.; Andersson, C.; Gislason, G.H.; Torp-Pedersen, C.; et al. Association between male sex and outcomes of coronavirus disease 2019 (COVID-19)—A Danish Nationwide, Register-based study. Clin. Infect. Dis. 2021, 73, e4025–e4030. [Google Scholar] [CrossRef] [PubMed]
- National Center for Immunization and Respiratory Diseases (NCIRD), Division of Viral Diseases. Science Brief: Evidence Used to Update the List of Underlying Medical Conditions Associated with Higher Risk for Severe COVID-19. In CDC COVID-19 Science Briefs; Centers for Disease Control and Prevention (US): Atlanta, GA, USA, 2020. [Google Scholar] [PubMed]
- Jaitovich, A.; Khan, M.M.H.S.; Itty, R.; Chieng, H.C.; Dumas, C.L.; Nadendla, P.; Fantauzzi, J.P.; Yucel, R.M.; Feustel, P.J.; Judson, M.A. ICU admission muscle and Fat Mass, survival, and disability at discharge: A prospective cohort study. Chest 2019, 155, 322–330. [Google Scholar] [CrossRef]
- Puthucheary, Z.A.; Rawal, J.; McPhail, M.; Connolly, B.; Ratnayake, G.; Chan, P.; Hopkinson, N.S.; Phadke, R.; Dew, T.; Sidhu, P.S.; et al. Acute skeletal muscle wasting in critical illness. JAMA 2013, 310, 1591–1600. [Google Scholar] [CrossRef]
- Ali, N.A.; O’Brien, J.M., Jr.; Hoffmann, S.P.; Phillips, G.; Garland, A.; Finley, J.C.W.; Almoosa, K.; Hejal, R.; Wolf, K.M.; Lemeshow, S.; et al. Acquired weakness, handgrip strength, and mortality in critically ill patients. Am. J. Respir. Crit. Care Med. 2008, 178, 261–268. [Google Scholar] [CrossRef]
- Joskova, V.; Patkova, A.; Havel, E.; Najpaverova, S.; Uramova, D.; Kovarik, M.; Zadak, Z.; Hronek, M. Critical evaluation of muscle mass loss as a prognostic marker of morbidity in critically ill patients and methods for its determination. J. Rehabil. Med. 2018, 50, 696–704. [Google Scholar] [CrossRef]
- Jaitovich, A.; Dumas, C.L.; Itty, R.; Chieng, H.C.; Khan, M.M.H.S.; Naqvi, A.; Fantauzzi, J.; Hall, J.B.; Feustel, P.J.; Judson, M.A. ICU admission body composition: Skeletal muscle, bone, and fat effects on mortality and disability at hospital discharge—A prospective, cohort study. Crit. Care 2020, 24, 566. [Google Scholar] [CrossRef]
- Popkin, B.M.; Du, S.; Green, W.D.; Beck, M.A.; Algaith, T.; Herbst, C.H.; Alsukait, R.F.; Alluhidan, M.; Alazemi, N.; Shekar, M. Individuals with obesity and COVID-19: A global perspective on the epidemiology and biological relationships. Obes. Rev. 2020, 21, e13128. [Google Scholar] [CrossRef]
- Rossi, A.P.; Muollo, V.; Dalla Valle, Z.; Urbani, S.; Pellegrini, M.; El Ghoch, M.; Mazzali, G. The role of obesity, body composition, and nutrition in COVID-19 pandemia: A narrative review. Nutrients 2022, 14, 3493. [Google Scholar] [CrossRef]
- Wang, Y.; Tan, S.; Yan, Q.; Gao, Y. Sarcopenia and COVID-19 outcomes. Clin. Interv. Aging 2023, 18, 359–373. [Google Scholar] [CrossRef]
- das Virgens, I.P.A.; Sousa, I.M.; Bezerra, A.D.L.; Fayh, A.P.T. Assessment of body composition in adults hospitalized with acute COVID-19: A scoping review. Front. Nutr. 2023, 10, 1176441. [Google Scholar] [CrossRef] [PubMed]
- Basty, N.; Sorokin, E.P.; Thanaj, M.; Srinivasan, R.; Whitcher, B.; Bell, J.D.; Cule, M.; Thomas, E.L. Abdominal imaging associates body composition with COVID-19 severity. PLoS ONE 2023, 18, e0283506. [Google Scholar] [CrossRef] [PubMed]
- Branea, O.E.; Vlad, A.G.; Pui, M.; Becica, D.A.; Becica, M.E.; Budeanu, A.R.; Budeanu, R.G.; Stoica, F.; Lazar, A.E. Total psoas area and psoas density assessment in COVID-19 patients using CT imaging—Could muscle mass alteration during intensive care hospitalization be determined? J. Crit. Care Med. 2023, 9, 218–229. [Google Scholar] [CrossRef] [PubMed]
- Gualtieri, P.; Falcone, C.; Romano, L.; Macheda, S.; Correale, P.; Arciello, P.; Polimeni, N.; Lorenzo, A.D. Body composition findings by computed tomography in SARS-CoV-2 patients: Increased risk of muscle wasting in obesity. Int. J. Mol. Sci. 2020, 21, 4670. [Google Scholar] [CrossRef]
- Hoyois, A.; Ballarin, A.; Thomas, J.; Lheureux, O.; Preiser, J.C.; Coppens, E.; Perez-Bogerd, S.; Taton, O.; Farine, S.; Van Ouytsel, P.; et al. Nutrition evaluation and management of critically ill patients with COVID-19 during post-intensive care rehabilitation. JPEN J. Parenter Enter Nutr. 2021, 45, 1153–1163. [Google Scholar] [CrossRef]
- Rossi, A.P.; Gottin, L.; Donadello, K.; Schweiger, V.; Brandimarte, P.; Zamboni, G.A.; Florio, A.; Boetti, R.; Pavan, G.; Zamboni, M.; et al. Intermuscular adipose tissue as a risk factor for mortality and muscle injury in critically ill patients affected by COVID-19. Front. Physiol. 2021, 12, 651167. [Google Scholar] [CrossRef]
- Kolck, J.; Rako, Z.A.; Beetz, N.L.; Auer, T.A.; Segger, L.K.; Pille, C.; Penzkofer, T.; Fehrenbach, U.; Geisel, D. Intermittent body composition analysis as monitoring tool for muscle wasting in critically ill COVID-19 patients. Ann. Intensive Care 2023, 13, 61. [Google Scholar] [CrossRef]
- Knaus, W.A.; Draper, E.A.; Wagner, D.P.; Zimmerman, J.E. Apache II: A severity of disease classification system. Crit. Care Med. 1985, 13, 818–829. [Google Scholar] [CrossRef]
- Vincent, J.L.; Moreno, R.; Takala, J.; Willatts, S.; De Mendonça, A.; Bruining, H.; Reinhart, C.K.; Suter, P.M.; Thijs, L.G. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996, 22, 707–710. [Google Scholar] [CrossRef]
- Vincent, J.L.; de Mendonça, A.; Cantraine, F.; Moreno, R.; Takala, J.; Suter, P.M.; Sprung, C.L.; Colardyn, F.; Blecher, S. Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: Results of a multicenter, prospective study. Working group on ‘sepsis-related problems’ of the European Society of Intensive Care Medicine. Crit. Care Med. 1998, 26, 1793–1800. [Google Scholar] [CrossRef]
- Malik, P.; Patel, U.; Mehta, D.; Patel, N.; Kelkar, R.; Akrmah, M.; Gabrilove, J.L.; Sacks, H. Biomarkers and outcomes of COVID-19 hospitalisations: Systematic review and meta-analysis. BMJ Evid. Based Med. 2021, 26, 107–108. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, J.; Kinoshita, K.; Nakagawa, K.; Mizuochi, M. Undernutrition scored using the CONUT score with hypoglycemic status in ICU-admitted elderly patients with sepsis shows increased ICU mortality. Diagnostics 2023, 13, 762. [Google Scholar] [CrossRef] [PubMed]
- Guidelines for nutrition management in critically ill patients. J. Jpn. Soc. Intensive Care Med. 2016, 23, 185–281. [CrossRef]
- Singer, P.; Blaser, A.R.; Berger, M.M.; Alhazzani, W.; Calder, P.C.; Casaer, M.P.; Hiesmayr, M.; Mayer, K.; Montejo, J.C.; Pichard, C.; et al. ESPEN guideline on clinical nutrition in the intensive care unit. Clin. Nutr. 2019, 38, 48–79. [Google Scholar] [CrossRef]
- Ignacio de Ulíbarri, J.; González-Madroño, A.; de Villar, N.G.; González, P.; González, B.; Mancha, A.; Rodríguez, F.; Fernández, G. CONUT: A tool for controlling nutritional status. First validation in a hospital population. Nutr. Hosp. 2005, 20, 38–45. [Google Scholar]
- McNelly, A.S.; Bear, D.E.; Connolly, B.A.; Arbane, G.; Allum, L.; Tarbhai, A.; Cooper, J.A.; Hopkins, P.A.; Wise, M.P.; Brealey, D.; et al. Effect of intermittent or continuous feed on muscle wasting in critical illness: A Phase 2 clinical trial. Chest 2020, 158, 183–194. [Google Scholar] [CrossRef]
- González-Madroño, A.; Mancha, A.; Rodríguez, F.J.; Culebras, J.; de Ulibarri, J.I. Confirming the validity of the CONUT system for early detection and monitoring of clinical undernutrition: Comparison with two logistic regression models developed using SGA as the gold standard. Nutr. Hosp. 2012, 27, 564–571. [Google Scholar]
- Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transpl. 2013, 48, 452–458. [Google Scholar] [CrossRef]
- Maldonado, G.; Greenland, S. Simulation study of confounder-selection strategies. Am. J. Epidemiol. 1993, 138, 923–936. [Google Scholar] [CrossRef]
- van Heusden, H.C.; Swartz, J.E.; Chargi, N.; de Jong, P.A.; van Baal, M.C.P.M.; Wegner, I.; de Bree, R. Feasibility of assessment of skeletal muscle mass on a single cross-sectional image at the level of the fourth thoracic vertebra. Eur. J. Radiol. 2021, 142, 109879. [Google Scholar] [CrossRef]
- Ishida, Y.; Maeda, K.; Yamanaka, Y.; Matsuyama, R.; Kato, R.; Yamaguchi, M.; Nonogaki, T.; Shimizu, A.; Ueshima, J.; Murotani, K.; et al. Formula for the cross-sectional area of the muscles of the third lumbar vertebra level from the twelfth thoracic vertebra level slice on computed tomography. Geriatrics 2020, 5, 47. [Google Scholar] [CrossRef] [PubMed]
- Buchman, A.S.; Boyle, P.A.; Wilson, R.S.; Gu, L.; Bienias, J.L.; Bennett, D.A. Pulmonary function, muscle strengthandmortalityinoldage. Mech. Ageing Dev. 2008, 129, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Shiokawa, N.; Okazaki, T.; Suzukamo, Y.; Miyatake, M.; Kogure, M.; Nakaya, N.; Hozawa, A.; Ebihara, S.; Izumi, S.I. Association between Low Forced Vital Capacity and High Pneu-monia Mortality, and Impact of Muscle Power. J. Clin. Med. 2023, 12, 3272. [Google Scholar] [CrossRef]
- Hill, N.S. Noninvasive ventilation for chronic obstructive pulmonary disease. Respir. Care 2004, 49, 72–87, discussion 87. [Google Scholar] [CrossRef]
- Park, J.W.; Kweon, M.; Hong, S. The influences of position and forced respiratory maneuvers on spinal stability muscles. J. Phys. Ther. Sci. 2015, 27, 491–493. [Google Scholar] [CrossRef]
- Yoshikawa, H.; Komiya, K.; Yamamoto, T.; Fujita, N.; Oka, H.; Okabe, E.; Yamasue, M.; Umeki, K.; Rubin, B.K.; Hiramatsu, K.; et al. Quantitative assessment of erector spinae muscles and prognosis in elderly patients with pneumonia. Sci. Rep. 2021, 11, 4319. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef]
- Biolo, G.; Pišot, R.; Mazzucco, S.; Di Girolamo, F.G.; Situlin, R.; Lazzer, S.; Grassi, B.; Reggiani, C.; Passaro, A.; Rittweger, J.; et al. Anabolic resistance assessed by oral stable isotope ingestion following bed rest in young and older adult volunteers: Relationships with changes in muscle mass. Clin. Nutr. 2017, 36, 1420–1426. [Google Scholar] [CrossRef]
- Wall, B.T.; Dirks, M.L.; van Loon, L.J.C. Skeletal muscle atrophy during short-term disuse: Implications for age-related sarcopenia. Ageing Res. Rev. 2013, 12, 898–906. [Google Scholar] [CrossRef]
- Liu, M.; Luo, J.; Zhou, J.; Zhu, X. Intervention effect of neuromuscular electrical stimulation on ICU acquired weakness: A meta-analysis. Int. J. Nurs. Sci. 2020, 7, 228–237. [Google Scholar] [CrossRef]
- Bax, F.; Lettieri, C.; Marini, A.; Pellitteri, G.; Surcinelli, A.; Valente, M.; Budai, R.; Patruno, V.; Gigli, G.L. Clinical and neurophysiological characterization of muscular weakness in severe COVID-19. Neurol. Sci. 2021, 42, 2173–2178. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, A.; Orozco-Aguilar, J.; Achiardi, O.; Simon, F.; Cabello-Verrugio, C. SARS-CoV-2/renin-angiotensin system: Deciphering the clues for a couple with potentially harmful effects on skeletal muscle. Int. J. Mol. Sci. 2020, 21, 7904. [Google Scholar] [CrossRef] [PubMed]
- Yates, T.; Summerfield, A.; Razieh, C.; Banerjee, A.; Chudasama, Y.; Davies, M.J.; Gillies, C.; Islam, N.; Lawson, C.; Mirkes, E.; et al. A population-based cohort study of obesity, ethnicity and COVID-19 mortality in 12.6 million adults in England. Nat. Commun. 2022, 13, 624. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Zhang, F.; Tong, N. The association of visceral adipose tissue and subcutaneous adipose tissue with metabolic risk factors in a large population of Chinese adults. Clin. Endocrinol. 2016, 85, 46–53. [Google Scholar] [CrossRef]
- Hosoya, T.; Oba, S.; Komiya, Y.; Kawata, D.; Kamiya, M.; Iwai, H.; Miyamoto, S.; Kataoka, M.; Tobiume, M.; Kanno, T.; et al. Apple-shaped obesity: A risky soil for cytokine-accelerated severity in COVID-19. Proc. Natl. Acad. Sci. USA 2023, 120, e2300155120. [Google Scholar] [CrossRef]
- Santa Cruz, A.; Mendes-Frias, A.; Oliveira, A.I.; Dias, L.; Matos, A.R.; Carvalho, A.; Capela, C.; Pedrosa, J.; Castro, A.G.; Silvestre, R. Interleukin-6 Is a Biomarker for the Development of Fatal Severe Acute Respiratory Syndrome Coronavirus 2 Pneumonia. Front. Immunol. 2021, 12, 613422. [Google Scholar] [CrossRef]
- Rotter, V.; Nagaev, I.; Smith, U. Interleukin-6 (IL-6) induces insulin resistance in 3T3-L1 adipocytes and is, like IL-8 and tumor necrosis factor-alpha, overexpressed in human fat cells from insulin-resistant subjects. J. Biol. Chem. 2003, 278, 45777–45784. [Google Scholar] [CrossRef]

| Factors | All (n= 89) | Survivors (n = 57) | Non-Survivors (n = 32) | p-Value * |
|---|---|---|---|---|
| (A) Parameters | ||||
| Age (years) | 65.4 (57–76) | 60 (54–73) | 74.5 (66.5–78.3) | <0.001 |
| Male (%) | 77.5% | 73.7% | 84.4% | 0.246 |
| APACHE Ⅱ score | 16 (12–21) | 14 (11–18) | 20 (16–24.3) | <0.001 |
| SOFA score | 4 (3–6) | 4 (3–5) | 5 (4–8) | 0.040 |
| Presence of Shock, n (%) | 2:87 | 1:56 | 1:31 | 0.675 |
| Lactate (mmol/L) | 1.4 (1.1–2.0) | 1.3 (1.0–1.9) | 1.65 (1.2–2.6) | 0.043 |
| ICU stay (days) | 5 (2–11) | 3 (2–7) | 10 (4–23) | <0.001 |
| P/F Ratio | 90.5 (65.6–134.9) | 80.6 (65.6–142.5) | 99 (67–132.7) | 0.752 |
| D–Dimer (μg/mL) | 1.7 (1.0–7.8) | 1.2 (1.0–2.7) | 5.6 (1.7–11.8) | 0.001 |
| Ferritin (ng/mL) | 679.9 (447.0–1406) | 763.1 (467.0–1435) | 629.3 (366.6–1305) | 0.350 |
| KL–6 (U/mL) | 430.0 (236–740) | 502.5 (290.8–817) | 355 (197.5–556) | 0.229 |
| Comorbidities | ||||
| CCI [min, max] | 1 [0, 9] | 0 [0, 9] | 2 [0, 6] | 0.01 |
| Agea-djusted CCI [min, max] | 4 [0, 11] | [3, 11] | [0, 10] | 0.01 |
| Smoking History n (%) | 41:48 | 23:34 | 18:14 | 0.149 |
| Hypertension n (%) | 47:42 | 30:27 | 17:15 | 0.964 |
| Diabetes Mellitus n (%) | 39:65 | 24:41 | 15:24 | 0.875 |
| Chronic Heart Failure n (%) | 13:76 | 4:53 | 9:23 | 0.012 |
| Malignancy n (%) | 13:76 | 6:51 | 7:25 | 0.146 |
| Respiratory Disease n (%) | 7:82 | 3:54 | 4:28 | 0.224 |
| End–Stage Renal Disease n (%) | 3:86 | 2:55 | 1:31 | 0.923 |
| (B) Treatment | ||||
| Mechanical Ventilation n (%) | 32:57 | 14:43 | 18:14 | 0.003 |
| HFNCO, n (%) | 30:59 | 22:35 | 8:24 | 0.193 |
| drugs | ||||
| Favipiravir, n (%) | 33:56 | 18:39 | 15:17 | 0.152 |
| Remdesivir, n (%) | 59:30 | 41:16 | 18:14 | 0.133 |
| Nafamostat, n (%) | 21:68 | 9:48 | 12:20 | 0.021 |
| Tocilizumab, n (%) | 55:34 | 33:24 | 22:10 | 0.312 |
| Baricitinib, n (%) | 11:78 | 8:49 | 3:29 | 0.522 |
| Anticoagulants, n (%) | 57:32 | 49:8 | 31:1 | 0.101 |
| Steroids, n (%) | 72:17 | 45:12 | 27:5 | 0.532 |
| (C) Nutrition and Glycemic status | ||||
| Height (cm) | 166.9 (159.7–170) | 167.0 (159.8–171) | 165.6 (159.8–170.0) | 0.715 |
| Weight (kg) | 69.0 (58.5–78.0) | 70.6 (58.8–80.7) | 62.5 (56.8–70.4) | 0.059 |
| BMI (kg/m2) | 24.8 (22.3–27.7) | 25.5 (22.7–29.1) | 24.0 (22.1–26.2) | 0.038 |
| CONUT score | 7 (5–9) | 7 (5–8) | 7.0 (5.0–9.0) | 0.493 |
| Lymphocyte Count (cells/µL) | 600 (400–1000) | 600 (400–900) | 700 (475–1100) | 0.412 |
| T–Cho (mg/dL) | 150.5 (123.8–184.5) | 151.5 (125.3–186.3) | 145.0 (123.5–178.3) | 0.402 |
| Albumin (g/dL) | 2.9 (2.6–3.2) | 3.0 (2.6–3.3) | 2.9 (2.6–3.2) | 0.278 |
| Total Caloric Intake over 7 Days (kcal) | 6090 (3840–8000) | 7020 (3840–8400) | 5572 (4080–7375) | 0.320 |
| Total Protein Intake over 7 Days (g) | 280 (161–342) | 301 (150–378) | 268 (182–320) | 0.209 |
| Blood Glucose (mg/dL) | 162 (119–224) | 152 (115–205) | 204 (134–273) | 0.089 |
| HbA1c (NGSP (%)) | 6.5 (6.0–7.3) | 6.4 (6.0–7.2) | 6.6 (6.0–7.5) | 0.739 |
| Variables | All (n = 89) | Survivors (n = 57) | Non-Survivors (n = 32) | p-Value |
|---|---|---|---|---|
| Psoas muscle volume (cm3) | 281.6 (201.3–405.6) | 311.4 (231.8–417.2) | 227.5 (182.1–298.7) | 0.019 |
| Combined pectoralis major and minor muscle areas (mm2) | 2949 (2339–3837) | 3304 (2507–4210) | 2740 (2222–3329) | 0.011 |
| Erector spinae muscle area (mm2) | 3046 (2437–3641) | 3352 (2653–3893) | 2467 (2102–3446) | 0.001 |
| Subcutaneous fat volume (cm3) | 4362 (3254–5546) | 4928 (3426–6681) | 3735 (2759–4855) | 0.003 |
| Visceral fat volume (cm3) | 4471 (3474–6227) | 4475 (3512–6254) | 4151 (3063–6149) | 0.531 |
| Variable | Total (n = 30) | Survivors (n = 15) | Non-Survivors (n = 15) | p-Value |
|---|---|---|---|---|
| Change rate of pectoralis muscle area (%/day), median (IQR) | −0.246 (−1.355–0.150) | −0.434 (−1.355–−0.092) | −0.156 (−1.514–2.110) | 0.064 |
| Change rate of erector spinae area (%/day), median (IQR) | −0.631 (−1.364–−0.076) | −0.586 (−1.247–−0.115) | −1.007 (−1.672–0.392) | 0.202 |
| Change rate of psoas muscle volume (%/day), median (IQR) | −0.911 (−1.404–−0.075) | −0.826 (−1.022–−0.067) | −1.305 (−1.424–−0.200) | 1.00 |
| Change rate of subcutaneous fat volume (%/day), median (IQR) | −0.174 (−0.618–0.751) | −0.550 (−0.768–−0.236) | 0.792 (−0.137–1.388) | 0.043 |
| Change rate of visceral fat volume (%/day), median (IQR) | −0.254 (−0.844–0.180) | −0.226 (−0.783–−0.057) | −0.403 (−0.990–−0.199) | 0.829 |
| Explanatory Variable | Odds Ratio | 95% CI | p-Value |
|---|---|---|---|
| APACHE II score | 0.834 | 0.741–0.938 | 0.002 |
| Psoas muscle volume (cm3) | |||
| Total area of the pectoralis major and minor muscles (mm2) | |||
| Erector spinae muscle area (mm2) | 1.001 | 1.000–1.002 | 0.031 |
| BMI | |||
| Blood glucose (mg/dL) | |||
| D-dimer (μg/mL) | |||
| Lactate (mmol/L) | |||
| Use of mechanical ventilation | |||
| Use of nafamostat |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakabayashi, H.; Yamaguchi, J.; Takahashi, K.; Kai, Y.; Kinoshita, K. Changes in Body Composition During Intensive Care Unit Stay and Outcomes in Patients with Severe COVID-19 Pneumonia: A Retrospective Cohort Study. Viruses 2025, 17, 643. https://doi.org/10.3390/v17050643
Nakabayashi H, Yamaguchi J, Takahashi K, Kai Y, Kinoshita K. Changes in Body Composition During Intensive Care Unit Stay and Outcomes in Patients with Severe COVID-19 Pneumonia: A Retrospective Cohort Study. Viruses. 2025; 17(5):643. https://doi.org/10.3390/v17050643
Chicago/Turabian StyleNakabayashi, Hayato, Junko Yamaguchi, Ken Takahashi, Yasuyoshi Kai, and Kosaku Kinoshita. 2025. "Changes in Body Composition During Intensive Care Unit Stay and Outcomes in Patients with Severe COVID-19 Pneumonia: A Retrospective Cohort Study" Viruses 17, no. 5: 643. https://doi.org/10.3390/v17050643
APA StyleNakabayashi, H., Yamaguchi, J., Takahashi, K., Kai, Y., & Kinoshita, K. (2025). Changes in Body Composition During Intensive Care Unit Stay and Outcomes in Patients with Severe COVID-19 Pneumonia: A Retrospective Cohort Study. Viruses, 17(5), 643. https://doi.org/10.3390/v17050643






