Sequences and Structures of Viral Proteins Linked to the Genomes (VPg) of RNA Viruses
Abstract
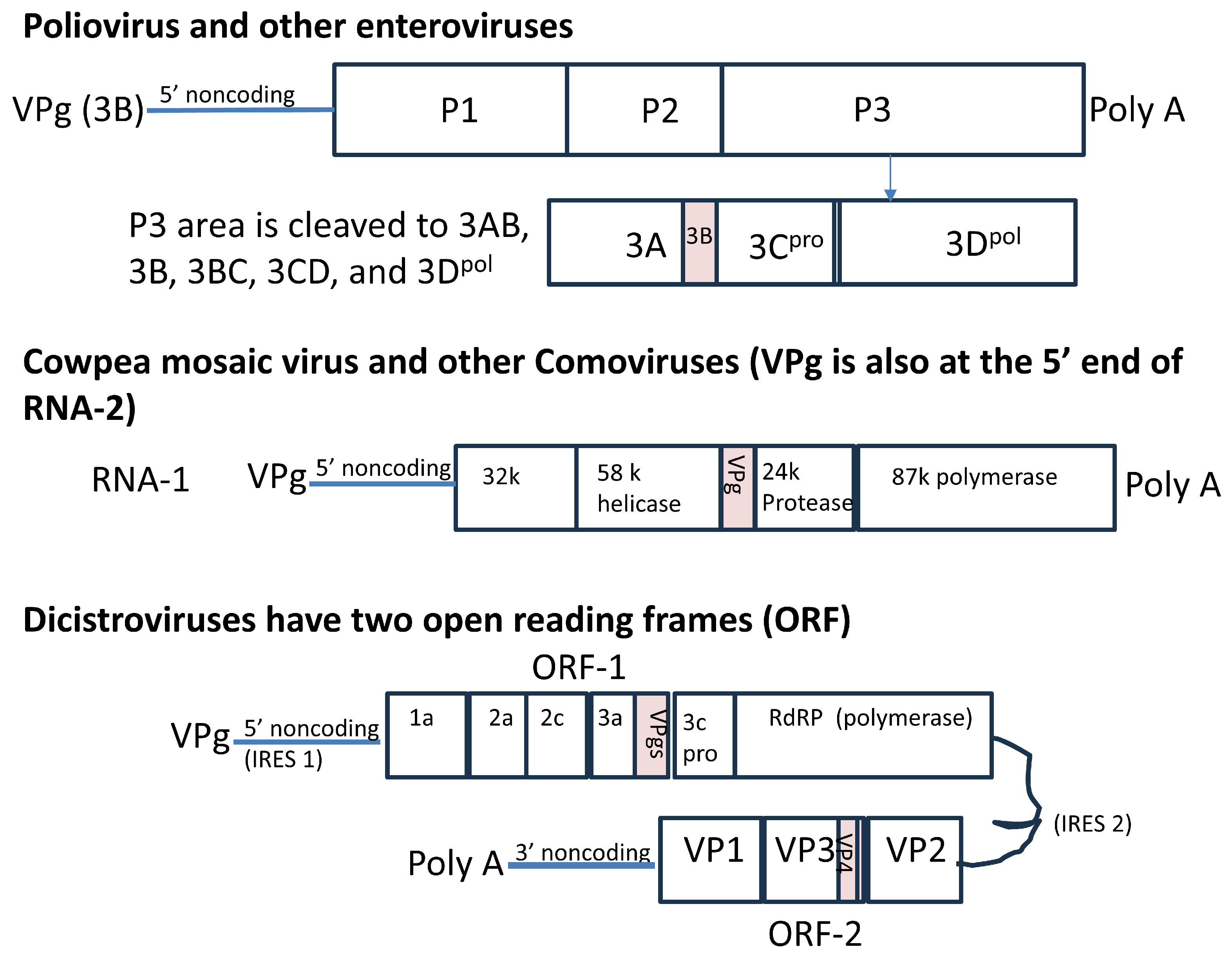
1. Introduction
1.1. Picornavirus VPgs Are Essential for Transcription
1.2. Relationship Between Sequence and Structure of Picornaviral VPg
1.3. VPgs Have Co-Evolved with Their Proteases and Polymerases
1.4. The Short VPgs of Plant Comoviruses and Insect Discistroviruses Have Distinct Sequences and Predicted Structures
1.5. Current Experimental Structures for VPgs
1.6. How Many VPg Genes/Proteins Does One Virus Need?
1.7. What Is the Role of Free VPg, VPgpUpU and Polyuridylylated VPg?
1.8. Larger VPgs Have Quite Different Sequences and an Expanded Role in Viral Replication
2. Conclusions
Future Perspective
Funding
Acknowledgments
Conflicts of Interest
References
- Flanegan, J.B.; Petterson, R.F.; Ambros, V.; Hewlett, N.J.; Baltimore, D. Covalent linkage of a protein to a defined nucleotide sequence at the 5’-terminus of virion and replicative intermediate RNAs of poliovirus. Proc. Natl. Acad. Sci. USA 1977, 74, 961–965. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.F.; Nomoto, A.; Detjen, B.M.; Wimmer, E. A protein covalently linked to poliovirus genome RNA. Proc. Natl. Acad. Sci. USA 1977, 74, 59–63. [Google Scholar] [CrossRef]
- Daubert, S.D.; Bruening, G. Detection of Genome-Linked Protein of Plant and Animal Viruses. Methods Virol. 1984, VIII, 347–379. [Google Scholar]
- Avila-Bonilla, R.G.; Macias, S. The molecular language of RNA 5’ ends: Guardians of RNA identity and immunity. RNA 2024, 30, 327–336. [Google Scholar] [CrossRef]
- Coutinho de Oliveira, L.; Volpon, L.; Rahardjo, A.K.; Osborne, M.J.; Culjkovic-Kraljacic, B.; Trahan, C.; Oeffinger, M.; Kwok, B.H.; Borden, K.L.B. Structural studies of the eIF4E-VPg complex reveal a direct competition for capped RNA: Implications for translation. Proc. Natl. Acad. Sci. USA 2019, 116, 24056–24065. [Google Scholar] [CrossRef] [PubMed]
- Young, V.L.; McSweeney, A.M.; Edwards, M.J.; Ward, V.K. The Disorderly Nature of Caliciviruses. Viruses 2024, 16, 1324. [Google Scholar] [CrossRef] [PubMed]
- Granoff, A.; Webster, R.G. COXSACKIEVIRUSES (PICORNAVIRIDAE). In Encyclopedia of Virology; Elsevier: Amsterdam, The Netherlands, 1999; pp. 305–311. [Google Scholar]
- Bonning, B.C.; Miller, W.A. Dicistroviruses. Annu. Rev. Entomol. 2010, 55, 129–150. [Google Scholar] [CrossRef]
- Oba, M.; Sakaguchi, S.; Teshima, N.; Yokota, T.; Takemae, H.; Tohei, M.; Shimokawa, F.; Murakami, M.; Mizuno, S.; Ishida, H.; et al. Metatranscriptomic identification of novel RNA viruses from raccoon dog (Nyctereutes procyonoides) feces in Japan. Sci. Rep. 2025, 15, 7100. [Google Scholar] [CrossRef]
- Chiquito-Almanza, E.; Zamora-Aboytes, J.M.; Medina, H.R.; Acosta-Gallegos, J.A.; Anaya-Lopez, J.L. Complete genome sequence of a novel comovirus infecting common bean. Arch. Virol. 2020, 165, 1505–1509. [Google Scholar] [CrossRef]
- Mason, P.W.; Bezborodova, S.V.; Henry, T.M. Identification and characterization of a cis-acting replication element (cre) adjacent to the internal ribosome entry site of foot-and-mouth disease virus. J. Virol. 2002, 76, 9686–9694. [Google Scholar] [CrossRef]
- Avanzino, B.C.; Fuchs, G.; Fraser, C.S. Cellular cap-binding protein, eIF4E, promotes picornavirus genome restructuring and translation. Proc. Natl. Acad. Sci. USA 2017, 114, 9611–9616. [Google Scholar] [CrossRef]
- Flather, D.; Semler, B.L. Picornaviruses and nuclear functions: Targeting a cellular compartment distinct from the replication site of a positive-strand RNA virus. Front. Microbiol. 2015, 6, 594. [Google Scholar] [CrossRef]
- Carette, J.E.; Kujawa, A.; Guhl, K.; Verver, J.; Wellink, J.; Van Kammen, A. Mutational analysis of the genome-linked protein of cowpea mosaic virus. Virology 2001, 290, 21–29. [Google Scholar] [CrossRef]
- Warsaba, R.; Stoynov, N.; Moon, K.M.; Flibotte, S.; Foster, L.; Jan, E. Multiple Viral Protein Genome-Linked Proteins Compensate for Viral Translation in a Positive-Sense Single-Stranded RNA Virus Infection. J. Virol. 2022, 96, e0069922. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Laliberte, J.F. The genome-linked protein VPg of plant viruses-a protein with many partners. Curr. Opin. Virol. 2011, 1, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Goodfellow, I. The genome-linked protein VPg of vertebrate viruses — a multifaceted protein. Curr. Opin. Virol. 2011, 1, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wimmer, E.; Paul, A.V. Cis-acting RNA elements in human and animal plus-strand RNA viruses. Biochim. Biophys. Acta 2009, 1789, 495–517. [Google Scholar] [CrossRef]
- van Ackeren, V.S.S.; Pichler, I.; Ziltener, G.; Zaheri, M.; Kufner, V.; Huber, M. Retrospective Genotyping of Enteroviruses Using a Diagnostic Nanopore Sequencing Workflow. Pathogens 2024, 12, 390. [Google Scholar] [CrossRef]
- Stanway, G.; Hove, T.; Knowles, N.J.; Hyypuia, T. Molecular and Biological Basis of Picornavirus Taxonomy. In Molecular Biology of Picornaviruses; Semler, B.L., Wimmer, E., Eds.; ASM Press: Washington, DC, USA, 2002; pp. 17–24. [Google Scholar]
- Palmenberg, A.C.; Gern, J.E. Classification and evolution of human rhinoviruses. Methods Mol. Biol. 2015, 1221, 1–10. [Google Scholar]
- Blomqvist, S.; Savolainen, C.; Raman, L.; Roivainen, M.; Hovi, T. Human rhinovirus 87 and enterovirus 68 represent a unique serotype with rhinovirus and enterovirus features. J. Clin. Microbiol. 2002, 40, 4218–4223. [Google Scholar] [CrossRef]
- Leser, J.S.; Frost, J.L.; Wilson, C.J.; Rudy, M.J.; Clarke, P.; Tyler, K.L. VP1 is the primary determinant of neuropathogenesis in a mouse model of enterovirus D68 acute flaccid myelitis. J. Virol. 2024, 98, e0039724. [Google Scholar] [CrossRef] [PubMed]
- Schein, C.H.; Ye, M.; Paul, A.V.; Oberste, M.S.; Chapman, N.; van der Heden van Noort, G.J.; Filippov, D.V.; Choi, K.H. Sequence specificity for uridylylation of the viral peptide linked to the genome (VPg) of enteroviruses. Virology 2015, 484, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Reuter, G.; Boros, A.; Kiss, T.; Delwart, E.; Pankovics, P. Complete genome characterization of mosavirus (family Picornaviridae) identified in droppings of a European roller (Coracias garrulus) in Hungary. Arch. Virol. 2014, 159, 2723–2729. [Google Scholar] [CrossRef]
- Kapoor, A.; Victoria, J.; Simmonds, P.; Wang, C.; Shafer, R.W.; Nims, R.; Nielsen, O.; Delwart, E. A highly divergent picornavirus in a marine mammal. J. Virol. 2008, 82, 311–320. [Google Scholar] [CrossRef]
- Warsaba, R.; Salcedo-Porras, N.; Flibotte, S.; Jan, E. Expansion of viral genomes with viral protein genome linked copies. Virology 2022, 577, 174–184. [Google Scholar] [CrossRef] [PubMed]
- Schein, C.H.; Danecek, P. Physicochemical (PCP) Based Consensus Sequences and Uses Thereof. U.S. Patent 8,900,596B2, 2 December 2014. [Google Scholar]
- Venkatarajan, M.; Braun, W. New quantitative descriptors of amino acids based on multidimensional scaling of a large number of physical–chemical properties. J. Mol. Model. 2001, 7, 445–453. [Google Scholar]
- Schein, C.H.; Oezguen, N.; Volk, D.E.; Garimella, R.; Paul, A.; Braun, W. NMR structure of the viral peptide linked to the genome (VPg) of poliovirus. Peptides 2006, 27, 1676–1684. [Google Scholar] [CrossRef]
- Schein, C.H.; Volk, D.E.; Oezguen, N.; Paul, A. Novel, structure-based mechanism for uridylylation of the genome-linked peptide (VPg) of picornaviruses. Proteins 2006, 63, 719–726. [Google Scholar] [CrossRef]
- Goodfellow, I.G.; Kerrigan, D.; Evans, D.J. Structure and function analysis of the poliovirus cis-acting replication element (CRE). RNA 2003, 9, 124–137. [Google Scholar] [CrossRef]
- Schein, C.H.; Oezguen, N.; van der Heden van Noort, G.J.; Filippov, D.V.; Paul, A.; Kumar, E.; Braun, W. NMR solution structure of poliovirus uridylyated peptide linked to the genome (VPgpU). Peptides 2010, 31, 1441–1448. [Google Scholar] [CrossRef]
- Paul, A.V.; Wimmer, E. Initiation of protein-primed picornavirus RNA synthesis. Virus Res. 2015, 206, 12–26. [Google Scholar] [CrossRef] [PubMed]
- Agol, V.I.; Paul, A.V.; Wimmer, E. Paradoxes of the replication of picornaviral genomes. Virus Res. 1999, 62, 129–147. [Google Scholar] [CrossRef]
- Langereis, M.A.; Feng, Q.; Nelissen, F.H.; Virgen-Slane, R.; van der Heden van Noort, G.J.; Maciejewski, S.; Filippov, D.V.; Semler, B.L.; van Delft, F.L.; van Kuppeveld, F.J. Modification of picornavirus genomic RNA using ’click’ chemistry shows that unlinking of the VPg peptide is dispensable for translation and replication of the incoming viral RNA. Nucleic Acids Res. 2014, 42, 2473–2482. [Google Scholar] [CrossRef]
- Holmes, A.C.; Zagnoli-Vieira, G.; Caldecott, K.W.; Semler, B.L. Effects of TDP2/VPg Unlinkase Activity on Picornavirus Infections Downstream of Virus Translation. Viruses 2020, 12, 166. [Google Scholar] [CrossRef] [PubMed]
- Steil, B.P.; Barton, D.J. Conversion of VPg into VPgpUpUOH before and during poliovirus negative-strand RNA synthesis. J. Virol. 2009, 83, 12660–12670. [Google Scholar] [CrossRef]
- Steil, B.P.; Kempf, B.J.; Barton, D.J. Poly(A) at the 3’ end of positive-strand RNA and VPg-linked poly(U) at the 5’ end of negative-strand RNA are reciprocal templates during replication of poliovirus RNA. J. Virol. 2010, 84, 2843–2858. [Google Scholar] [CrossRef]
- Zhao, D.; Li, Y.; Li, Z.; Zhu, L.; Sang, Y.; Zhang, H.; Zhang, F.; Ni, B.; Liu, F. Only fourteen 3’-end poly(A)s sufficient for rescuing Senecavirus A from its cDNA clone, but inadequate to meet requirement of viral replication. Virus Res. 2023, 328, 199076. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Wang, Y.; Shan, C.; Sun, Y.; Xu, P.; Zhou, H.; Yang, C.; Shi, P.-Y.; Rao, Z.; Zhang, B.; et al. Crystal Structure of Enterovirus 71 RNA-Dependent RNA Polymerase Complexed with Its Protein Primer VPg: Implication for a trans Mechanism of VPg Uridylylation. J. Virol. 2013, 87, 5755–5768. [Google Scholar] [CrossRef]
- Gruez, A.; Selisko, B.; Roberts, M.; Bricogne, G.; Bussetta, C.; Jabafi, I.; Coutard, B.; De Palma, A.M.; Neyts, J.; Canard, B. The crystal structure of coxsackievirus B3 RNA-dependent RNA polymerase in complex with its protein primer VPg confirms the existence of a second VPg binding site on Picornaviridae polymerases. J. Virol. 2008, 82, 9577–9590. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, Y.; Shan, C.; Chen, C.; Xu, P.; Song, M.; Zhou, H.; Yang, C.; Xu, W.; Shi, P.-Y.; et al. Enterovirus 71 VPg Uridylation Uses a Two-Molecular Mechanism of 3D Polymerase. J. Virol. 2012, 86, 13662–13671. [Google Scholar] [CrossRef]
- Ferrer-Orta, C.; Ferrero, D.S.; Verdaguer, N. Dual role of the foot-and-mouth disease virus 3B1 protein in the replication complex: As protein primer and as an essential component to recruit 3Dpol to membranes. PLoS Pathog. 2023, 19, e1011373. [Google Scholar] [CrossRef]
- Ambros, V.; Baltimore, D. Protein is linked to the 5’ end of poliovirus RNA by a phosphodiester linkage to tyrosine. J. Biol. Chem. 1978, 253, 5263–5266. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Guo, Y.; Lou, Z. Formation and working mechanism of the picornavirus VPg uridylylation complex. Curr. Opin. Virol. 2014, 9, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Crawford, N.M.; Baltimore, D. Genome-linked protein VPg of poliovirus is present as free VPg and CPgpUpU in poliovirus-infected cells. Proc. Natl. Acad. Sci. USA 1983, 80, 7452–7455. [Google Scholar] [CrossRef] [PubMed]
- Paul, A.V.; Peters, J.; Mugavero, J.; Yin, J.; van Boom, J.H.; Wimmer, E. Biochemical and genetic studies of the VPg uridylylation reaction catalyzed by the RNA polymerase of poliovirus. J. Virol. 2003, 77, 891–904. [Google Scholar] [CrossRef]
- Adeyemi, O.O.; Ward, J.C.; Snowden, J.S.; Herod, M.R.; Rowlands, D.J.; Stonehouse, N.J. Functional advantages of triplication of the 3B coding region of the FMDV genome. FASEB J. 2021, 35, e21215. [Google Scholar] [CrossRef]
- Bowen, D.M.; Lewis, J.A.; Lu, W.; Schein, C.H. Simplifying complex sequence information: A PCP-consensus protein binds antibodies against all four Dengue serotypes. Vaccine 2012, 30, 6081–6087. [Google Scholar] [CrossRef]
- Danecek, P.; Lu, W.; Schein, C.H. PCP consensus sequences of flaviviruses: Correlating variance with vector competence and disease phenotype. J. Mol. Biol. 2010, 396, 550–563. [Google Scholar] [CrossRef]
- Lu, W.; Negi, S.S.; Schein, C.H.; Maleki, S.J.; Hurlburt, B.K.; Braun, W. Distinguishing allergens from non-allergenic homologues using Physical-Chemical Property (PCP) motifs. Mol. Immunol. 2018, 99, 1–8. [Google Scholar] [CrossRef]
- Schein, C.H.; Rafael, G.; Baker, W.S.; Anaya, E.S.; Schmidt, J.G.; Weaver, S.C.; Negi, S.; Braun, W. PCP consensus protein/peptide alphavirus antigens stimulate broad spectrum neutralizing antibodies. Peptides 2022, 157, 170844. [Google Scholar] [CrossRef]
- Braun, B.A.; Schein, C.H.; Braun, W. DGraph Clusters Flaviviruses and beta-Coronaviruses According to Their Hosts, Disease Type, and Human Cell Receptors. Bioinform. Biol. Insights 2021, 15, 11779322211020316. [Google Scholar] [CrossRef] [PubMed]
- Oh, H.S.; Pathak, H.B.; Goodfellow, I.G.; Arnold, J.J.; Cameron, C.E. Insight into poliovirus genome replication and encapsidation obtained from studies of 3B-3C cleavage site mutants. J. Virol. 2009, 83, 9370–9387. [Google Scholar] [CrossRef]
- Bouin, A.; Vu, M.N.; Al-Hakeem, A.; Tran, G.P.; Nguyen, J.H.C.; Semler, B.L. Enterovirus-Cardiomyocyte Interactions: Impact of Terminally Deleted Genomic RNAs on Viral and Host Functions. J. Virol. 2023, 97, e0142622. [Google Scholar] [CrossRef] [PubMed]
- Dubankova, A.; Horova, V.; Klima, M.; Boura, E. Structures of kobuviral and siciniviral polymerases reveal conserved mechanism of picornaviral polymerase activation. J. Struct. Biol. 2019, 208, 92–98. [Google Scholar] [CrossRef]
- Dubankova, A.; Humpolickova, J.; Klima, M.; Boura, E. Negative charge and membrane-tethered viral 3B cooperate to recruit viral RNA dependent RNA polymerase 3D (pol). Sci Rep 2017, 7, 17309. [Google Scholar] [CrossRef] [PubMed]
- Toyoda, H.; Kohara, M.; Kataoka, Y.; Suganuma, T.; Omata, T.; Imura, N.; Nomoto, A. Complete nucleotide sequences of all three poliovirus serotype genomes. Implication for genetic relationship, gene function and antigenic determinants. J. Mol. Biol. 1984, 174, 561–585. [Google Scholar] [CrossRef]
- Mostafa, H.H.; Fall, A.; Norton, J.M.; Sachithanandham, J.; Yunker, M.; Abdullah, O.; Hanlon, A.; Gluck, L.; Morris, C.P.; Pekosz, A.; et al. Respiratory virus disease and outcomes at a large academic medical center in the United States: A retrospective observational study of the early 2023/2024 respiratory viral season. Microbiol Spectr 2024, 12, e0111624. [Google Scholar] [CrossRef]
- Nakashima, N.; Shibuya, N. Multiple coding sequences for the genome-linked virus protein (VPg) in dicistroviruses. J. Invertebr. Pathol. 2006, 92, 100–104. [Google Scholar] [CrossRef]
- Zabel, P.; Moerman, M.; Lomonossoff, G.; Shanks, M.; Beyreuther, K. Cowpea mosaic virus VPg: Sequencing of radiochemically modified protein allows mapping of the gene on B RNA. EMBO J. 1984, 3, 1629–1634. [Google Scholar] [CrossRef]
- Franciosa, G.; Locard-Paulet, M.; Jensen, L.J.; Olsen, J.V. Recent advances in kinase signaling network profiling by mass spectrometry. Curr. Opin. Chem. Biol. 2023, 73, 102260. [Google Scholar] [CrossRef]
- Drygin, Y.F. Natural covalent complexes of nucleic acids and proteins: Some comments on practice and theoryon the path from well-known complexes to new ones. Nucleic Acids Res. 1998, 26, 4791–4796. [Google Scholar] [CrossRef] [PubMed]
- Eruera, A.R.; McSweeney, A.M.; McKenzie-Goldsmith, G.M.; Ward, V.K. Protein Nucleotidylylation in +ssRNA Viruses. Viruses 2021, 13, 1549. [Google Scholar] [CrossRef]
- van der Heden van Noort, G.J.; Schein, C.H.; Overkleeft, H.S.; van der Marel, G.A.; Filippov, D.V. A general synthetic method toward uridylylated picornavirus VPg proteins. J. Pept. Sci. 2013, 19, 333–336. [Google Scholar] [CrossRef]
- Mohamud, Y.; Shi, J.; Tang, H.; Xiang, P.; Xue, Y.C.; Liu, H.; Ng, C.S.; Luo, H. Coxsackievirus infection induces a non-canonical autophagy independent of the ULK and PI3K complexes. Sci. Rep. 2020, 10, 19068. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Fung, G.; Piesik, P.; Zhang, J.; Luo, H. Dominant-negative function of the C-terminal fragments of NBR1 and SQSTM1 generated during enteroviral infection. Cell Death Differ. 2014, 21, 1432–1441. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, R.J.; Tada, H.; Ypma-Wong, M.F.; Semler, B.L.; Wimmer, E. Mutational analysis of the genome-linked protein VPg of poliovirus. J. Virol. 1988, 62, 4207–4215. [Google Scholar] [CrossRef] [PubMed]
- Murray, K.E.; Barton, D.J. Poliovirus CRE-dependent VPg uridylylation is required for positive-strand RNA synthesis but not for negative-strand RNA synthesis. J. Virol. 2003, 77, 4739–4750. [Google Scholar] [CrossRef]
- Schein, C.H.; Rowold, D.; Choi, K.H. Allosteric inhibitors of Coxsackie virus A24 RNA polymerase. Bioorg Med. Chem. 2016, 24, 570–577. [Google Scholar] [CrossRef]
- Falk, M.M.; Sobrino, F.; Beck, E. VPg gene amplification correlates with infective particle formation in foot-and-mouth disease virus. J. Virol. 1992, 66, 2251–2260. [Google Scholar] [CrossRef]
- Nayak, A.; Goodfellow, I.G.; Belsham, G.J. Factors required for the Uridylylation of the foot-and-mouth disease virus 3B1, 3B2, and 3B3 peptides by the RNA-dependent RNA polymerase (3Dpol) in vitro. J. Virol. 2005, 79, 7698–7706. [Google Scholar] [CrossRef]
- Gangaramani, D.R.; Eden, E.L.; Shah, M.; Destefano, J.J. The twenty-nine amino acid C-terminal cytoplasmic domain of poliovirus 3AB is critical for nucleic acid chaperone activity. RNA Biol. 2010, 7, 820–829. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Kuhn, R.J.; Wimmer, E. Replication of poliovirus RNA containing two VPg coding sequences leads to a specific deletion event. J. Virol. 1993, 67, 5572–5578. [Google Scholar] [CrossRef] [PubMed]
- Lyle, J.M.; Clewell, A.; Richmond, K.; Richards, O.C.; Hope, D.A.; Schultz, S.C.; Kirkegaard, K. Similar structural basis for membrane localization and protein priming by an RNA-dependent RNA polymerase. J. Biol. Chem. 2002, 277, 16324–16331. [Google Scholar] [CrossRef]
- Dahmane, S.; Shankar, K.; Carlson, L.A. A 3D view of how enteroviruses hijack autophagy. Autophagy 2023, 19, 2156–2158. [Google Scholar] [CrossRef]
- Maynell, L.A.; Kirkegaard, K.; Klymkowsky, M.W. Inhibition of poliovirus RNA synthesis by brefeldin A. J. Virol. 1992, 66, 1985–1994. [Google Scholar] [CrossRef]
- van der Linden, L.; van der Schaar, H.M.; Lanke, K.H.; Neyts, J.; van Kuppeveld, F.J. Differential effects of the putative GBF1 inhibitors Golgicide A and AG1478 on enterovirus replication. J. Virol. 2010, 84, 7535–7542. [Google Scholar] [CrossRef]
- Jadhav, T.; Wooten, M.W. Defining an Embedded Code for Protein Ubiquitination. J. Proteom. Bioinform. 2009, 2, 316. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Walters, K.J. Multitasking with ubiquitin through multivalent interactions. Trends Biochem. Sci. 2010, 35, 352–360. [Google Scholar] [CrossRef]
- Randles, L.; Walters, K.J. Ubiquitin and its binding domains. Front Biosci (Landmark Ed) 2012, 17, 2140–2157. [Google Scholar] [CrossRef]
- Klionsky, D.J.; Abdel-Aziz, A.K.; Abdelfatah, S.; Abdellatif, M.; Abdoli, A.; Abel, S.; Bartek, J. Guidelines for the use and interpretation of assays for monitoring autophagy (4th edition)1. Autophagy 2021, 17, 1–382. [Google Scholar]
- Nouwen, L.V.; Breeuwsma, M.; Zaal, E.A.; van de Lest, C.H.A.; Buitendijk, I.; Zwaagstra, M.; Balic, P.; Filippov, D.V.; Berkers, C.R.; van Kuppeveld, F.J.M. Modulation of nucleotide metabolism by picornaviruses. PLoS Pathog. 2024, 20, e1012036. [Google Scholar] [CrossRef]
- Backe, S.J.; Sager, R.A.; Heritz, J.A.; Wengert, L.A.; Meluni, K.A.; Aran-Guiu, X.; Panaretou, B.; Woodford, M.R.; Prodromou, C.; Bourboulia, D.; et al. Activation of autophagy depends on Atg1/Ulk1-mediated phosphorylation and inhibition of the Hsp90 chaperone machinery. Cell Rep. 2023, 42, 112807. [Google Scholar] [CrossRef] [PubMed]
- Coutinho de Oliveira, L.; Volpon, L.; Osborne, M.J.; Borden, K.L.B. Chemical shift assignment of the viral protein genome-linked (VPg) from potato virus Y. Biomol. NMR Assign. 2019, 13, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Belliot, G.; Sosnovtsev, S.V.; Chang, K.O.; McPhie, P.; Green, K.Y. Nucleotidylylation of the VPg protein of a human norovirus by its proteinase-polymerase precursor protein. Virology 2008, 374, 33–49. [Google Scholar] [CrossRef]
- McSweeney, A.M.; Young, V.L.; Ward, V.K. Norovirus VPg Binds RNA through a Conserved N-Terminal K/R Basic Patch. Viruses 2021, 13, 1282. [Google Scholar] [CrossRef] [PubMed]
- Rantalainen, K.I.; Uversky, V.N.; Permi, P.; Kalkkinen, N.; Dunker, A.K.; Makinen, K. Potato virus A genome-linked protein VPg is an intrinsically disordered molten globule-like protein with a hydrophobic core. Virology 2008, 377, 280–288. [Google Scholar] [CrossRef]
- Schein, C.H. Solubility as a Function of Protein Structure and Solvent Components. Bio/Technol. 1990, 8, 308–317. [Google Scholar] [CrossRef]
- Schein, C.H. Production of Soluble Recombinant Proteins in Bacteria. Bio/Technol. 1989, 7, 1141–1149. [Google Scholar] [CrossRef]
- Smertina, E.; Hall, R.N.; Urakova, N.; Strive, T.; Frese, M. Calicivirus Non-structural Proteins: Potential Functions in Replication and Host Cell Manipulation. Front. Microbiol. 2021, 12, 712710. [Google Scholar] [CrossRef]
- Arhab, Y.; Miscicka, A.; Pestova, T.V.; Hellen, C.U.T. Horizontal gene transfer as a mechanism for the promiscuous acquisition of distinct classes of IRES by avian caliciviruses. Nucleic Acids Res. 2022, 50, 1052–1068. [Google Scholar] [CrossRef]
- Leen, E.N.; Kwok, K.Y.R.; Birtley, J.R.; Simpson, P.J.; Subba-Reddy, C.V.; Chaudhry, Y.; Sosnovtsev, S.V.; Green, K.Y.; Prater, S.N.; Tong, M.; et al. Structures of the Compact Helical Core Domains of Feline Calicivirus and Murine Norovirus VPg Proteins. J. Virol. 2013, 87, 5318–5330. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.J.; Min, H.J.; Yun, H.; Pelton, J.G.; Wemmer, D.E.; Cho, K.O.; Kim, J.S.; Lee, C.W. Solution structure of the porcine sapovirus VPg core reveals a stable three-helical bundle with a conserved surface patch. Biochem. Biophys. Res. Commun. 2015, 459, 610–616. [Google Scholar] [CrossRef] [PubMed]

| Picornavirus | Sequence | IEP/Net Charge at pH 7 |
|---|---|---|
| Enterovirus VPgs: | ||
| PV1-3 | GAYTGLPNKKPNVPTIRTAKVQ | 10.9/4 |
| HEV71 | GAYSGAPKQVLKKPALRTATVQ | 10.9/4 |
| CVA24 | GAYTGLPNKKPSVPTVRTAKVQ | 10.9/4 |
| CVA21 | GAYTGLPNKKPSVPTIRVAKVQ | 10.9/4 |
| CVB3 | GAYTGVPNQKPRVPTLRQAKVQ | 11.5/4 |
| CVB6 | GAYTGMPNQKPKVPTLRQAKVQ | 10.9/4 |
| EchovirusB | GAYTGMPNQKPKVPTLRQAKVQ | 10.9/4 |
| PCP Consensus | GAYTGLPNQKPKVPTIRTAKVQ | 10.9/4 |
| EV-D68 (HRV87) | GPYTGIPNPKPKVPSLRTAKVQ | 10.9/4 |
| Rhino2 | GPYSGE.PKPKTKVPE.RRIVAQ | 10.4/3 |
| Rhino14 | GPYSGNPPHNKLKAPTLRPVVVQ | 10.7/3 |
| Rhino16 | GPYSGE.PKPKTKVPE.RRVVAQ | 10.4/3 |
| Rhino89 | GPYSGE.PKPKSRAPE.RRVVTQ | 10.7/3 |
| PV1 following | GPGFDYAVAMAKRNIVTATTSKG | 10.2/2 |
| CVB3 following | GPAFEFAVAMMKRNSSTVKTEYG | 9.6/1 |
| Kobuvirus | ||
| Aichi virus | AAYSAISHQKPKPKSQKPVPTRHIQRQ | 11.6/6.7 |
| Sicinivirus (chick) | AAYTG------KPPTRKQKRDPEPQ | 10.1/3 |
| Aphthovirus 3 VPgs: | ||
| FMDV-VPg1 | GPYAGPLERQRPLKVRAKLPRQE | 11.3/4 |
| FMDV-VPg2 | GPYAGPMERQKPLKVKARAPVVKE | 10.6/4 |
| FMDV-VPg3 | GPYAGPVKKPVALKVKAKNLIVTE | 10.5/4 |
| Mosavirus 2 VPgs: | ||
| European Roller | GPYCGACKRKAPVLKKTVAE | 10/4 |
| Picornavirus | GPYSGMPRATPKKLKKVVVQ | 11/5 |
| Aquamavirus 2 VPgs: | ||
| Ringed seal | SAYEGCSTRKTARQLARSVVGE | 9.7/2 |
| Picornavirus | GAYDGNVKRTTARELARKAIPSEQ | 10.2/2 |
| Virus | VPg Sequence | IEP/Net Charge at pH7 Comoviridae (Plant) |
|---|---|---|
| Comoviridae (plant) | ||
| Cowpea mosaic virus | SRKPNRFDMQQYRYNNVPLKRRVWADAQMSLDQ | 10.8/4 * |
| Squash mosaic virus | SRKPNRFDVAQYRYRNVPLKRRQWADAQMSLDH | 11.3/5 |
| Red clover mottle | SRKPNRFEVQQYRYKNVPLTRRSWGNAQMSLDQ | 11.3/5 |
| Bean pod mottle virus | SRKPNRYEVSQYRYRNVPIKRRAWVEGQMSFDQ | 10.9/5 |
| Broad bean true mosaic | SRKPNRHDQEQHRYRNVPLTRRNWATAQMSLHQ | 12.2/5.3 |
| Pepper mild mosaic ** | SKKPNRYDVSSYKYRNVPLRQRAWAQAQMSIDQ | 10.7/5 |
| Dicistroviridae (insect) | ||
| Plautia stali | SQEKEGVISRCKIE (3×) | 6.5/0 |
| intestinal virus (PSIV) | SQEKIGSVSRVRVE | 10/1 |
| SQEKLGAIPAVKIE | 7/0 | |
| Himetobi P virus (HIPV) | SYDVGNIKPTRTE (4×) | 7.0/0 |
| Cricket paralysis (CrPV) | VGSSGDNKTQKISKRVVE (4×) | 10.3/2 |
| VGGSGDVKTTKPAKTAVE | 9.7/1 | |
| VGSSGDSKTMKNKITKVE | 10.2/2 | |
| VGSSGDSKTQKQRNTKVE | 10.3/2 | |
| Solenopsis invicta (SINV-1) | AATSGDCMTKVKPRVILE (5×) | 8.9/1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schein, C.H. Sequences and Structures of Viral Proteins Linked to the Genomes (VPg) of RNA Viruses. Viruses 2025, 17, 645. https://doi.org/10.3390/v17050645
Schein CH. Sequences and Structures of Viral Proteins Linked to the Genomes (VPg) of RNA Viruses. Viruses. 2025; 17(5):645. https://doi.org/10.3390/v17050645
Chicago/Turabian StyleSchein, Catherine H. 2025. "Sequences and Structures of Viral Proteins Linked to the Genomes (VPg) of RNA Viruses" Viruses 17, no. 5: 645. https://doi.org/10.3390/v17050645
APA StyleSchein, C. H. (2025). Sequences and Structures of Viral Proteins Linked to the Genomes (VPg) of RNA Viruses. Viruses, 17(5), 645. https://doi.org/10.3390/v17050645






